Abstract
We assessed the role of PGC-1α (PPARγ coactivator-1 alpha) in glucose-induced proliferation, migration, and inflammatory gene expression of vascular smooth muscle cells (VSMCs). We carried out phagocytosis studies to assess the role of PGC-1α in transdifferentiation of VSMCs by flow cytometry. We found that high glucose stimulated proliferation, migration and inflammatory gene expression of VSMCs, but overexpression of PGC-1α attenuated the effects of glucose. In addition, overexpression of PGC-1α decreased mRNA and protein level of VSMCs-related genes, and induced macrophage-related gene expression, as well as phagocytosis of VSMCs. Therefore, PGC-1α inhibited glucose-induced proliferation, migration and inflammatory gene expression of VSMCs, which are key features in the pathology of atherosclerosis. More importantly, PGC-1α transdifferentiated VSMCs to a macrophage-like state. Such transdifferentiation possibly increased the portion of VSMCs-derived foam cells in the plaque and favored plaque stability.
1. Introduction
Activation of vascular smooth muscle cells (VSMCs) including proliferation, migration, and inflammatory gene expression plays an important role in the development of atherosclerosis. In early atherosclerosis, VSMCs may contribute to the development of the atheroma through the production of preinflammatory mediators such as monocyte chemoattractant protein-1 (MCP-1) [1]. VSMC-derived foam cells have been demonstrated in vitro [2] and in vivo atherosclerotic plaques [3]. These foam cells assume a macrophage-like state [4].
Hyperglycemia has been implicated as a major contributor to several diabetes complications by inducing key factors including oxidant stress and inflammatory gene expression [5]. Furthermore, these factors may also lead to abnormal proliferation, migration and inflammatory gene expression of VSMCs, which are key features in the pathology of atherosclerosis. Recent studies have demonstrated that treatment of cultured VSMCs in vitro with diabetogenic agents such as high glucose can increase the production of various proinflammatory cytokines and chemokines, which are all associated with vascular inflammation [6].
PPARγ coactivator-1 alpha (PGC-1α) is originally identified as a transcriptional coactivator of PPARγ [7]. PGC-1α can coactivate many nuclear receptors such as liver X receptor [8], and nonnuclear receptors such as Sry-related HMG box-9 [9]. As a metabolic regulator, PGC-1α functions in adaptive thermogenesis in brown fat [7], gluconeogenesis in liver [10], and insulin secretion in islets [11]. Our group has demonstrated that PGC-1α is an important negative regulator for VSMC proliferation and migration under pathophysiological conditions, such as oleic acid stimulation [12]. Additionally, PGC-1α participates in the differentiation procedure including adipocyte differentiation [7] and chondrogenesis [9].
In the present study, we assessed the role of PGC-1α in glucose-induced proliferation, migration, and inflammatory gene expression of VSMCs. We also carried out phagocytosis studies to assess the role of PGC-1α in transdifferentiation of VSMCs. We found that PGC-1α inhibited glucose-induced VSMCs proliferation, migration, and inflammatory gene expression and furthermore resulted in phenotypic changes of VSMCs to a macrophage-like state.
2. Materials and Methods
2.1. Materials
Carboxylate-modified microspheres (F8821) with red fluorescent were purchased from Molecular Probes (Eugene, OR). RNeasy kit (cat. no.74104) and RNase-free DNase I (cat. no. 79254) were from QIAGEN. SYBR green PCR master mix (Part no.: 4309155) was from Applied Biosystems.
2.2. Cell Culture
Rat aortic VSMCs were isolated from the thoracic aortas of 3- to 4-week-old male Sprague-Dawley rats as described previously [13]. Isolated VSMCs were cultured in DMEM with 25 mmol/L or 5.5 mmol/L D-glucose (Gibco-Invitrogen, Carlsbad, USA), supplemented with 10% FCS (GBICO BRL, Rockville, MD), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells after the 4th to 8th passages were used in experiments.
2.3. Adenovirus Infection
Recombinant adenoviruses expressing PGC-1α-GFP (Green Fluorescence Protein) fusion protein and GFP alone were provided by Dr. Dan Kelly (Washington University, Saint Louis, MO, USA). Purified virus stocks were prepared through CsCl density gradient centrifugation [14]. Cells were grown to subconfluence, deprived of serum for 24 hours, and followed by infection with adenovirus at multiplicities of infection (moi) of 50 for 48 hours. The infection efficiency was determined by fluorescence intensity of GFP.
2.4. RNA Analysis
Total RNA of VSMCs was isolated using RNeasy kit and RNase-free DNase I. RNA was reverse transcribed into cDNA with oligo (dT) 18 primers and AMV reverse transcriptase at 42°C for 1 hour in standard buffer (Table 1).
Table 1.
Primer sequences for qRT-PCR, all sequences are from 5′ to 3′.
| Genes | Forward primer | Reverse primer |
|---|---|---|
| β-actin | AGGGAAATCGTGCGTGAC | CGCTCATTGCCGATAGTG |
| Myosin heavy chain | TCCGTGGGTGCAAATAAGG | CCGCTCCCAACCATCAACT |
| SM22α | GAGGACTGTAATGGCTTTGG | GCCTTCCCTTTCTAACTGATG |
| Calponin H1 | GCACCAATAAGTTTGCCAGTC | GAGCGTGTCACAGTGTTCCAT |
| α-actin | TCCTGACCCTGAAGTATCCG | ATCTCCAGAGTCCAGCACAA |
| CD68 | CTGACCTTGCTGGTACTGCT | GGTCGTAGGGCTTGCTGT |
| Mac-2 | CCTACGATATGCCCTTGCC | CCCAGTTATTGTCCTGCTTC |
| ABCA-1 | CCTGCTGAAATACCGACAA | TGAGGGACGATTCCACAT |
| MCP-1 | GCCTG TTGTTCACAGTTGC | TTCTGGACCCATTCCTTATT |
| IL-6 | GAGTTCCGTTTCTACCTG | CTTAGCCACTCCTTCTGT |
Quantitative PCR was performed with the ABI Prism 7000 sequence detection system (ABI, Foster City, CA). SYBR green PCR master mix was used. The reactions were amplified for 30 s at 95°C and 1 min at 60°C for 40 cycles. The thermal denaturation protocol was run at the end of the PCR to determine the number of products that were present in the reaction. All reactions were run in triplicate and included no template and no reverse transcription controls for each gene. The relative amount of each mRNA to β-actin RNA was described using the equation 2-ΔCT where ΔCT = (CTmRNA − CTβ-actin) [15]. Relative gene expression was multiplied by 104 in order to simplify the presentation of the data. The primers used in this study were listed in Table 1.
2.5. Western Blotting Analysis
To obtain total proteins, VSMCs were lysed in a buffer containing 10 mmol/L HEPES (pH 7.9), 1.5 mmol/L MgCl2, 10 mmol/L KCl, and 0.5% NP-40, and cell lysates were centrifuged at 13000 g for 5 min. Supernatants were collected as cytosolic extracts for Western blot. Total proteins were applied to SDS-PAGE gel electrophoresis for proteins detection. The used primary antibodies included anti-pgc-1α (sc-13067) and anti-CD68 (sc-9139) from Santa Cruz Biotechnologies, anti-β-actin (#4967) from Cell Signaling, and anti-α-actin (product no. A2547) from Sigma. After incubation with primary antibodies for 2 h and washing, membranes were incubated with corresponding horseradish-peroxidase (HRP-) conjugated secondary antibody and detected with the ECL Plus Kit (Amersham).
2.6. Migration Assays
Migration assays were performed by the modified Boyden chamber [16]. VSMCs were seeded in 6-well plates (1.5 ×105cells/well) and grew to subconfluence. After 24 h serum deprivation, VSMCs were treated with adenovirus infection for 48 h. Boyden chamber cell migration assay was performed using transwell chambers with fibronectin- (Sigma)-coated 8 μm poresize polycarbonate membranes (BD Biosciences). VSMCs treated were then suspended in low-glucose (5.5 mmol/L) DMEM-0.5% FBS to a concentration of 4 × 105 cells/mL. Low glucose (5.5 mmol/L) or high glucose (25 mmol/L) DMEM (0.6 mL) supplemented with 10% FCS were added to the lower compartment. A 0.1 mL cell suspension (final concentration, 4 × 104 cells/well; diameter, 6.5 μm) was added to the upper compartment, and cells were then incubated at 37°C (95% air, 5% CO2). 6 h later, nonmigrated cells were removed with a cotton swab, and the migrated cells were fixed with paraformaldehyde for 30 min and stained with crystal violet.
2.7. Proliferation and Phagocytosis Assays by Flow Cytometry
Click-iT EdU Flow Cytometry Assay Kits (invitrogen, cat. no. A10202) was used to detect VSMCs proliferation. Cells seeded in 6-well plates (1.5 × 105 cells/well) were cultured up to subconfluence, and the medium was replaced by fresh serum-free medium. The cells were then treated with adenovirus infection for 48 h. Following this, low-glucose (5.5 mmol/L) or high-glucose (25 mmol/L) DMEM supplemented with 10% FCS and 10 μM EdU were added. After 6 h, cells were suspended at 1 × 107 cells/mL in 1% BSA in PBS. 100 μL of cell suspension and 100 μL Click-iT fixative were added to flow tubes, incubated for 15 minutes at room temperature, and protected from light. Cells were washed once with 3 mL 1% BSA in PBS. 100 μL of the 1X saponin-based permeabilization and wash buffer was added, incubated for the 20 minutes at 4°C temperature, and protected from light. Each tube was washed. 0.5 mL Click-iT reaction cocktail were added, mixed well, incubated for 30 minutes at room temperature, and protected from light. Cells were washed once. 0.5 mL 1X saponin-based permeabilization and wash reagent was added. For the detection of EdU, 633/635 nm excitation with red emission filter (i.e., 660/20 nm or similar) was used.
Phagocytotic activity was revealed by microspheres. After 24 h serum deprivation, VSMCs were infected by Ad-PGC-1α or Ad-GFP for 48 hours. Then VSMCs were incubated with 1μm carboxylate-modified microspheres with red fluorescent (2.7 × 106 beads per mL) for 10 h. Cells with the above treatments were suspended with 0.05% trypsin and 0.02% EDTA and then washed three times in PBS. Following this, the cells were analyzed using excitation/emission maxima of 580/605 on FACSCalibur (Becton-Dickinson, San Jose, CA, USA).
2.8. Statistical Analysis
Data are expressed as means ± SEM. Data were analyzed using a one-way ANOVA followed by Fisher's LSD post hoc test. Calculations were performed using SPSS/Windows version 12.5S statistical package (SPSS, Chicago, IL, USA). In all cases, P < 0.05 was taken as statistically significant.
3. Results
3.1. High Glucose Stimulated the Proliferation, Migration, and Inflammatory Gene Expression of VSMCs
VSMCs cultured in low-glucose (5.5 mmol/L) or high-glucose (25 mmol/L) complete media were deprived of serum for 24 h. In proliferation and inflammatory gene expression assays, VSMCs were stimulated by complete media for 6 h, respectively, (Figures 1(a) and 1(c)). In migration assays, VSMCs were suspended, and then low-glucose or high-glucose complete media were added to the lower compartment, respectively, (Figure 1(b)). Results showed that the VSMCs in high glucose were in a preactivated state.
Figure 1.
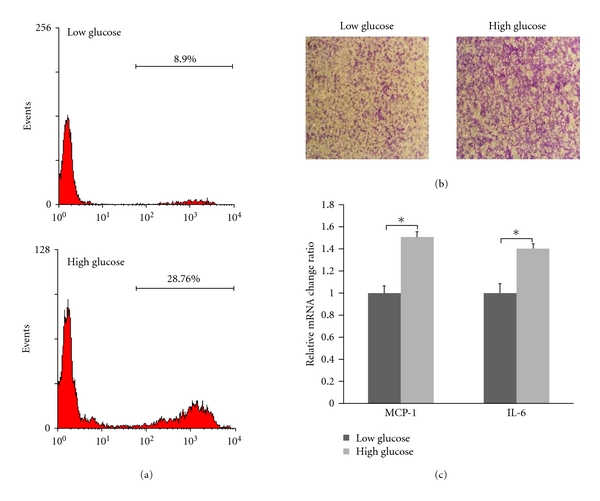
High glucose stimulated the proliferation, migration, and inflammatory gene expression of VSMCs. VSMCs cultured in low-glucose (5.5 mmol/L) or high-glucose (25 mmol/L) complete media were deprived of serum for 24 h. In proliferation and inflammatory gene expression assays, VSMCs were stimulated by complete media for 6 h, respectively. In migration assays, VSMCs were suspended, and then low-glucose or high-glucose complete media were added to the lower compartment respectively. (a) proliferation of VSMCs, (b) migration of VSMCs, and (c) inflammatory gene expression of VSMCs (*P < 0.05).
3.2. Overexpression of PGC-1α Inhibited Proliferation, Migration, and Inflammatory Gene Expression of VSMCs Induced by High Glucose
VSMCs cultured in high glucose (25 mmol/L) complete media were deprived of serum for 24 h. The cells were then treated with adenovirus infection for 48 h. In proliferation and inflammatory gene expression assays, VSMCs were incubated by complete media for 6 h (Figures 2(a) and 2(c)). In migration assays, VSMCs were suspended and then high-glucose complete media was added to the lower compartment (Figure 2(b)). Results showed that proliferation, migration, and inflammatory gene expression of VSMCs were inhibited. Expression of PGC-1α in VSMCs treated with adenovirus infection was showed in Figure 2(d).
Figure 2.
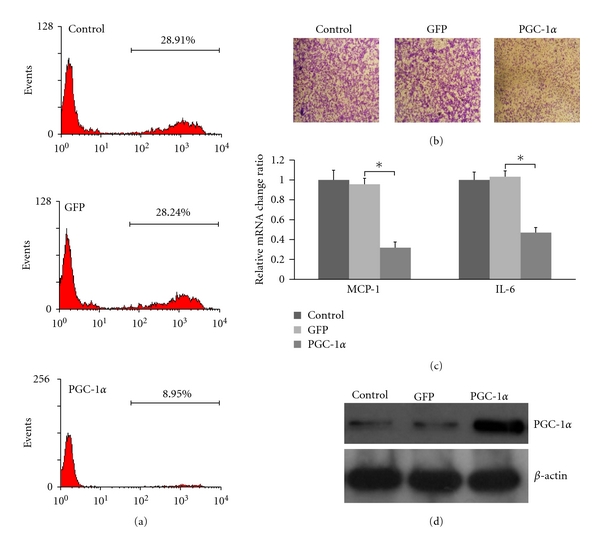
Overexpression of PGC-1α inhibited proliferation, migration, and inflammatory gene expression of VSMCs in high glucose. VSMCs cultured in high-glucose (25 mmol/L) complete media were deprived of serum for 24 h. The cells were then treated with adenovirus infection for 48 h. (a) Proliferation of VSMCs, (b) migration of VSMCs, (c) inflammatory gene expression of VSMCs (*P < 0.05), and (d) Western blotting analysis for PGC-1α in VSMCs.
3.3. Overexpression of PGC-1α Downregulated the VSMCs-Specific Genes Expression and Upregulated the Macrophage-Specific Genes Expression
To characterize the phenotypic changes after overexpression of PGC-1α, we determined the abundance of mRNA for several VSMCs [17] and macrophage-specific genes at the end of the adenovirus treatment for 48 h. As shown in Figure 3(a), VSMCs infected by Ad-PGC-1α had dramatically decreased in mRNA levels of α-actin (57.7% of control, P < 0.05), SM22α (59.5%, P < 0.05), smooth muscle myosin heavy chain (31.5%, P < 0.05), and calponin H1 (57.8%, P < 0.05). At the same time, the mRNA levels of the macrophage-related proteins (CD68) increased (569% of control, P < 0.05). Another macrophage marker, Mac-2, increased to 373% of controls (P < 0.05) (Figure 3(b)). ABCA-1, a key regulator of cholesterol efflux from peripheral cells to high-density lipoprotein [18] and associated predominantly with macrophages in vivo [19], increased to 221% (P < 0.05) in VSMCs infected by Ad-PGC-1α (Figure 3(b)).
Figure 3.
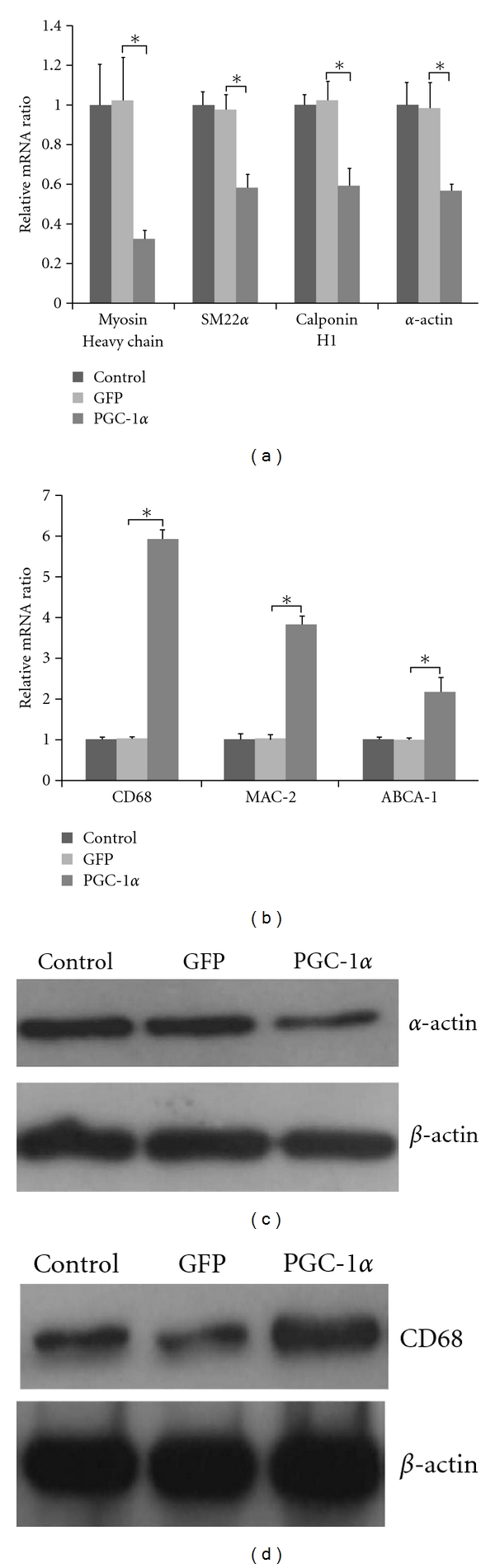
Overexpression of PGC-1α downregulated VSMCs-specific genes and upregulated macrophage-specific genes. (a) Downregulation of VSMCs-specific genes (*P < 0.05), (b) upregulation of macrophage-specific genes (*P < 0.05), (c) Western blotting analysis for α-actin in VSMCs, and (d) Western blotting analysis for CD68 in VSMCs.
Western blot also revealed the presence of specific proteins commonly taken as characteristic for VSMC or macrophage phenotype. Results are showed in Figures 3(c) and 3(d).
3.4. Phagocytosis of VSMCs
To investigate whether VSMCs infected by Ad-PGC-1α acquired functional aspects of macrophages, VSMCs were incubated in 1 μm microspheres with red fluorescent and then assessed by flow cytometry.
As shown in Figure 4, 27.36% of VSMCs infected by Ad-GFP had phagocytotic activity. However, the proportion of VSMCs having phagocytotic activity increased to 77.45% after overexpression of PGC-1α. This finding indicated that the acquisition of functional properties of macrophages existed in VSMCs, in addition to the increases of macrophage-specific gene and protein expression in VSMCs.
Figure 4.
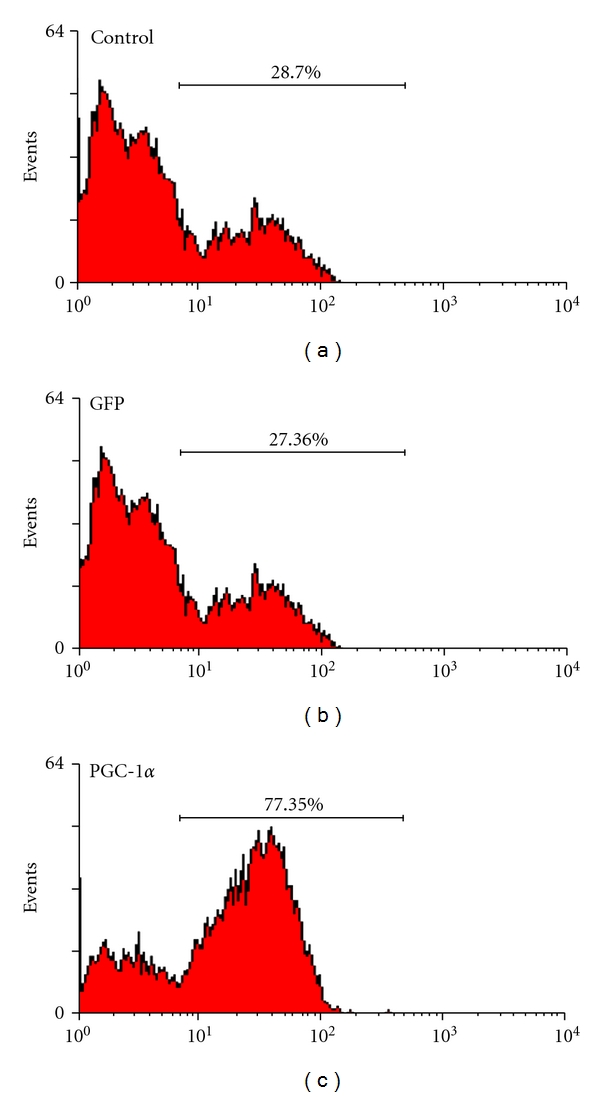
Phagocytosis of VSMCs. VSMCs deprived of serum for 24 h were infected by Ad-PGC-1α or Ad-GFP. After 48 h, VSMCs were incubated with 1 μm microspheres (2.7 × 106 beads per mL) for 10 h. 27.36% of VSMCs infected by Ad-GFP had phagocytotic activity. However, the proportion of VSMCs having phagocytotic activity increased to 77.45% after overexpression of PGC-1α.
4. Discussion
Recent studies showed that VSMCs cultured under high-glucose conditions mimic the diabetic pathophysiological state, which stimulates VSMCs proliferation, migration, and inflammatory genes expression [6, 20]. High glucose stimulates reactive oxygen species production in cultured vascular smooth muscle cells [21]. ROSs have been implicated in all of these above responses including proliferation [22], migration [23], and inflammatory gene expression of VSMCs [24, 25]. PGC-1α is a major transcriptional regulator of the mitochondrial detoxification system [26–29].
In the current study, we found that high-glucose stimulated VSMCs proliferation, migration, and expression of MCP-1 and IL-6. Overexpression of PGC-1α by adenoviruses infection blocked glucose-induced VSMCs proliferation, migration, and expression of MCP-1 and IL-6. These implied that overexpression of PGC-1α could inhibit the preatherogenic responses in VSMCs, which were key features in the pathology of atherosclerosis.
VSMCs maintain considerable plasticity throughout life and can exhibit a diverse range of different phenotypes in response to changes in local environmental cues [17]. In vascular neointimal lesions, VSMCs display diminished expression of a number of proteins that are characteristics of fully differentiated VSMCs [30]. It is well established that the dedifferentiated VSMCs exhibit a number of properties, including enhanced migration, proliferation, and inflammatory gene expression [31].
Given the fact that overexpression of PGC-1α suppressed the main preatherogenic responses in VSMCs, we examined the expression of VSMCs-related genes. All four smooth muscle markers assessed in the present study are related to the contraction function of VSMCs. Myosin heavy chain and α-actin are essential components of smooth muscle contractile machinery [17]. Surprisingly, our results showed that overexpression of PGC-1α rapidly decreased the genes expression of commonly accepted markers of the phenotype of VSMCs. The results were interpreted as evidences for a de-differentiation process of VSMCs.
In further experiments, we found that the expression of macrophage-related genes was induced in VSMCs by overexpression of PGC-1α, and VSMCs acquired the macrophage-like functions assessed by phagocytotic activity. In light of the present results for macrophage-related genes expression of VSMCs, rather than dedifferentiating process, overexpression of PGC-1α in VSMCs may be more appropriately considered as part of a transdifferentiation program that made VSMCs assume macrophage-like states. VSMCs-derived foam cells are usually assumed to occur relatively late in the development of organized atherosclerotic plaque lesion. They retain sufficient phenotypic features to be identifiable by electron microscopy [3]. It has been reported that downregulation of VSMCs-related genes and Up-regulation of macrophage-related genes occurred in VSMCs-derived foam cells [4].
The transdifferentiation was good or bad? Firstly, overexpression of PGC-1α inhibits the expression of MCP-1 and makes it difficult to attract monocytes to bind to VSMCs. Then foam cells derived from macrophages would decrease. Secondly, VSMCs that were transdifferentiated to macrophage-like state compensated for the decrease of macrophage. Thirdly, VSMCs have the relative resistance to cholesterol loading-induced toxicity compared with macrophages, consistent with VSMCs being a more prominent histological feature of the advanced atherosclerotic lesions [32], which reduced the death of VSMCs and then favoured plaque stability.
The mechanisms that overexpression of PGC-1α leads to the conversion of VSMCs to macrophage-like cells remain unknown. Recent studies demonstrated that two transcription factors: Liver X receptor α (LXRα) and PPARγ are strongly regulated by PGC-1α. LXRα is involved in the transcriptional control of a number of genes regulating the transport and catabolism of cholesterol [33]. Identified target genes of LXRα include the ATP-binding cassette transporter A1 (ABCA-1) [34], which is involved in reverse cholesterol transport. In our study, the expression of ABCA-1 is increased by overexpression of PGC-1α in VSMCs. Previous studies have indicated that PPARγ regulates gain of macrophage-like phenotype in primary smooth muscle culture [35] and showed that the uptake of oxidized LDL by macrophages or of cholesterol in endothelial cells could lead to PPARγ activation [36, 37]. Future experiments will be needed to identify the pathways that are responsible for the transdifferentiation to macrophage-like state in VSMCs.
In summary, we demonstrated that PGC-1α inhibited glucose-induced proliferation, migration, and inflammatory gene expression in VSMCs. In addition, overexpression of PGC-1α decreased VSMCs-related genes expression, while it induced macrophage-related genes expression and phagocytosis in VSMCs. PGC-1α transdifferentiated VSMCs to macrophage-like state. Such transdifferentiation increased the portion of VSMCs-derived foam cells in the plaque and then favored plaque stability. PGC-1α may be a potential target for drug development of atherosclerosis.
Sources of Funding
This work was supported by Grants from the National Natural Science Foundation of China to Y. Xiang (nos. 30570731, 30871195, and 81070653).
Acknowledgment
The authors would like to thank Dr. Kelly (Center for Cardiovascular Research, Washington University School of Medicine) for his generous gifts of adenoviral plasmids.
References
- 1.Schwartz SM, deBlois D, O'Brien ER. The intima. Soil for atherosclerosis and restenosis. Circulation Research. 1995;77(3):445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 2.Klouche M, Rose-John S, Schmiedt W, Bhakdi S. Enzymatically degraded, nonoxidized LDL induces human vascular smooth muscle cell activation, foam cell transformation, and proliferation. Circulation. 2000;101(15):1799–1805. doi: 10.1161/01.cir.101.15.1799. [DOI] [PubMed] [Google Scholar]
- 3.Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- 4.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(23):13531–13536. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 6.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor κB in vascular smooth muscle cells. Diabetes. 1999;48(4):855–864. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 7.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 8.Lin JD, Yang RJ, Tarr PT, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell. 2005;120(2):261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami Y, Tsuda M, Takahashi S, et al. Transcriptional coactivator PGC-1α regulates chondrogenesis via association with Sox9. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(7):2414–2419. doi: 10.1073/pnas.0407510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon JC, Puigserver P, Chen GX, et al. Control of hepatic gluconeogenesis through the transcriptional coaotivator PGC-1. Nature. 2001;413(6852):131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 11.Yoon JC, Xu G, Deeney JT, et al. Suppression of β cell energy metabolism and insulin release by PGC-1α. Developmental Cell. 2003;5(1):73–83. doi: 10.1016/s1534-5807(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Liu C, Zhu L, et al. PGC-1α inhibits oleic acid induced proliferation and migration of rat vascular smooth muscle cells. PLoS ONE. 2007;2(11) doi: 10.1371/journal.pone.0001137. Article ID e1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon D, Mohai LG, Schwartz SM. Induction of polyploidy in cultures of neonatal rat aortic smooth muscle cells. Circulation Research. 1986;59(6):633–644. doi: 10.1161/01.res.59.6.633. [DOI] [PubMed] [Google Scholar]
- 14.Miyake S, Makimura M, Kanegae Y, et al. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(3):1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Grotendorst GR, Seppa HE, Kleinman HK, Martin GR. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(6 I):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiological Reviews. 1995;75(3):487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 18.Young SG, Fielding CJ. The ABCs of cholesterol efflux. Nature Genetics. 1999;22(4):316–318. doi: 10.1038/11878. [DOI] [PubMed] [Google Scholar]
- 19.Joseph SB, McKilligin E, Pei L, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(11):7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasunari K, Kano M, Kano H, Yokokawa K, Minami M, Yoshikawa J. Mechanisms of action of troglitazone in the prevention of high glucose- induced migration and proliferation of cultured coronary smooth muscle cells. Circulation Research. 1997;81(6):953–962. doi: 10.1161/01.res.81.6.953. [DOI] [PubMed] [Google Scholar]
- 21.Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49(11):1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 22.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270(5234):296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 23.Weber D, Seshiah P, Taniyama Y, Griendling KK. Src-dependent migration of vascular smooth muscle cells by PDGF is reactive oxygen species dependent. Circulation. 2002;106:260–260. [Google Scholar]
- 24.Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circulation Research. 1998;83(9):952–959. doi: 10.1161/01.res.83.9.952. [DOI] [PubMed] [Google Scholar]
- 25.Han YQ, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-κb transcription factors. Circulation Research. 1999;84(6):695–703. doi: 10.1161/01.res.84.6.695. [DOI] [PubMed] [Google Scholar]
- 26.Kukidome D, Nishikawa T, Sonoda K, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55(1):120–127. [PubMed] [Google Scholar]
- 27.St-Pierre J, Lin J, Krauss S, et al. Bioenergetic analysis of peroxisome proliferator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. Journal of Biological Chemistry. 2003;278(29):26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- 28.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1α regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovascular Research. 2005;66(3):562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 29.St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. Journal of Clinical Investigation. 2000;106(9):1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 32.Rong JX, Kusunoki J, Oelkers P, Sturley SL, Fisher EA. Acyl-coenzymeA (CoA):cholesterol acyltransferase inhibition in rat and human aortic smooth muscle cells is nontoxic and retards foam cell formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(1):122–127. doi: 10.1161/01.ATV.0000148202.49842.3b. [DOI] [PubMed] [Google Scholar]
- 33.Forman BM, Ruan BF, Chen J, Schroepfer GJ, Evans RM. The orphan nuclear receptor LXRa is positively and negatively regulated by distinct products of mevalonate metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10588–10593. doi: 10.1073/pnas.94.20.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. Journal of Biological Chemistry. 2000;275(36):28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto K, Hirano K, Nozaki S, et al. Expression of macrophage (Mφ) scavenger receptor, CD36, in cultured human aortic smooth muscle cells in association with expression of peroxisome proliferator activated receptor-γ, which regulates gain of Mφ-like phenotype in vitro, and its implication in atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(4):1027–1032. doi: 10.1161/01.atv.20.4.1027. [DOI] [PubMed] [Google Scholar]
- 36.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93(2):241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 37.Meerarani P, Smart EJ, Toborek M, Boissonneault GA, Hennig B. Cholesterol attenuates linoleic acid-induced endothelial cell activation. Metabolism. 2003;52(4):493–500. doi: 10.1053/meta.2003.50087. [DOI] [PubMed] [Google Scholar]


