Abstract
In view of the rapid extension of offshore wind farms, there is an urgent need to improve our knowledge on possible adverse effects of underwater sound generated by pile-driving. Mortality and injuries have been observed in fish exposed to loud impulse sounds, but knowledge on the sound levels at which (sub-)lethal effects occur is limited for juvenile and adult fish, and virtually non-existent for fish eggs and larvae. A device was developed in which fish larvae can be exposed to underwater sound. It consists of a rigid-walled cylindrical chamber driven by an electro-dynamical sound projector. Samples of up to 100 larvae can be exposed simultaneously to a homogeneously distributed sound pressure and particle velocity field. Recorded pile-driving sounds could be reproduced accurately in the frequency range between 50 and 1000 Hz, at zero to peak pressure levels up to 210 dB re 1µPa2 (zero to peak pressures up to 32 kPa) and single pulse sound exposure levels up to 186 dB re 1µPa2s. The device was used to examine lethal effects of sound exposure in common sole (Solea solea) larvae. Different developmental stages were exposed to various levels and durations of pile-driving sound. The highest cumulative sound exposure level applied was 206 dB re 1µPa2s, which corresponds to 100 strikes at a distance of 100 m from a typical North Sea pile-driving site. The results showed no statistically significant differences in mortality between exposure and control groups at sound exposure levels which were well above the US interim criteria for non-auditory tissue damage in fish. Although our findings cannot be extrapolated to fish larvae in general, as interspecific differences in vulnerability to sound exposure may occur, they do indicate that previous assumptions and criteria may need to be revised.
Introduction
The potential harmful impact of anthropogenic underwater sound on marine life is a growing concern. While most interest has focused on marine mammals, there is an increasing awareness of the possible effects on fish [1]–[4]. Loud impulse sounds, such as pile-driving sounds or seismic airgun blasts, may cause mortality by rupturing the swim bladder or other body parts [2], [5]–[6]. Exposure to anthropogenic sound may also cause permanent or temporary hearing loss [7]–[9], or physiological stress as indicated by increased cortisol levels [9]–[10] or increased heart rates [11]. Furthermore, anthropogenic sound may affect fish behaviour and distribution: avoidance (e.g. [12]), interference with intraspecific communication (e.g. [13]) and alterations of behavioural responses to acoustic signals (e.g. [14]) have been observed.
In view of the rapid extension of offshore wind farms, there is an urgent need to acquire more knowledge on the ecological benefits and adverse effects of offshore wind farm construction and operation [15]. Continuous sounds associated with operational wind farms and, in particular, loud impulse sounds associated with pile-driving for the construction of wind farms may have adverse effects on marine mammals and fish. Concern about the effects of pile-driving sound on fish has led to the formulation of interim criteria for non-auditory tissue damage by the US Fisheries Hydro-acoustic Working Group [16]. The agreed interim criteria define maximum peak sound pressure level at 206 dB re 1 µPa2 for all size of fish, maximum cumulative sound exposure level at 187 dB re 1 µPa2s for fish≥2 gram, and maximum cumulative sound exposure level at 183 dB re 1 µPa2s for fish < 2 gram. However, knowledge on the sound levels at which mortality or injury will occur is limited for juvenile and adult fish, and virtually non-existent for fish eggs and larvae [2]. While juvenile and adult fish may actively swim away from a sound source, planktonic larvae are passively transported by currents and are therefore not capable of avoiding sound exposure. As a result, fish larvae may suffer more from underwater sound than older life stages.
For an impact assessment of Dutch offshore wind farms, the effect of pile-driving sound on the number of larvae that reach the inshore nursery areas was modelled for 3 fish species [17]. An existing egg and larval transport model [18]–[20] was expanded with the assumption that egg and larval mortality might occur in a 1 km radius around a pile-driving site. This assumption was based on the limited information available at that time [17]. The results indicated that offshore pile-driving could cause a significant reduction in the number of fish larvae that reach the inshore nursery areas. The validity of this conclusion depends entirely on the validity of the underlying assumption, yet little is known about the vulnerability of fish eggs and larvae to pile-driving sound and the spatial scale at which mortality or injury may occur [2].
This study examined the effect of pile-driving sound on the survival of common sole (Solea solea) larvae. The first goal was to develop a laboratory set-up in which impulse sounds representative of pile-driving sound could be generated. The second goal was to use this laboratory set-up to determine the sound levels at which mortality in fish larvae might occur. The final series of experiments was preceded by a pilot series, in which the relevant exposure levels were explored and the required number of replicates per treatment was determined.
Materials and Methods
Larvaebrator
Exposure of fish larvae to pile-driving sound in situ is costly and logistically complicated, while reproduction of low frequency sounds in fish tanks or small basins is hampered by distortion due to reverberation and resonances [21]. Therefore, we decided to build a device specifically designed to enable controlled exposure of fish larvae to sound in a laboratory setting. This so-called ‘larvaebrator’ was inspired by an existing laboratory set-up for larger fish called the ‘fishabrator’ or the HICI-FT [22]–[23].
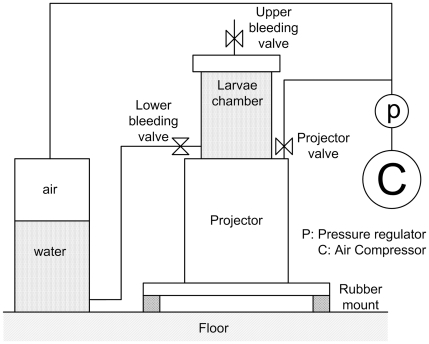
The larvaebrator consists of an underwater sound source (LFPX-4 projector) on which a rigid-walled (28 mm thick steel), cylindrical chamber (110 mm diameter, 160 mm high) is placed (Figure 1). The chamber is filled with sea water (±1.25 litre) and up to 100 fish larvae can be placed in the chamber. The piston of the projector is also the bottom of the chamber and can directly excite the water with a given acoustic signal. Two configurations can be used; the test chamber is either completely filled with water, so that the projector mainly compresses the enclosed volume of water (pressure excitation), or a small layer of air is left at the top of the test chamber, so that the water in the chamber can move while compressing the air volume (velocity excitation). The chamber dimensions are much smaller than the shortest acoustic wavelength of interest (about 1.5 m at the maximum frequency of 1 kHz). Consequently, the larvae in the test chamber are simultaneously exposed to a homogeneously distributed sound pressure and particle velocity field. Sound pressure in the chamber is measured by four pressure transducers, mounted flush in the wall of the chamber. Sound particle velocity is measured by an accelerometer, mounted on the piston of the projector. A static pressure source (an air compressor) is incorporated in the device to enable applying static overpressure inside the chamber (Figure 1). The static overpressure can be varied between 0 and 3 bar, thus simulating a depth range of 0 to 30 m. The experiments in this study were carried out without static overpressure, because the greatest effect of sound pressure is expected to occur at a low static pressure (T. Carlson, unpublished results).
Figure 1. The ‘larvaebrator’ design.
The larvaebrator is a device specifically designed to enable controlled exposure of fish larvae to sound in a laboratory setting.
Pile-driving sound
As it is unclear which characteristics of pile-driving sound could cause mortality, the acoustic signals to which the fish larvae were exposed had to be representative for actual sound exposures in the field. The actual exposure will vary with the properties of the pile-driving project and its environment. ‘Representativeness’ was achieved by playback of recorded pile-driving sound signals. Based on the initially assumed mortality range of 1 km [17], the playback level was adapted to the acoustic levels that were observed at distances between 100 m and 2 km from previous offshore wind farm construction projects in the Dutch part of the North Sea [24]–[25].
The playback level is defined in terms of acoustic metrics that quantify the received signals [26]. Studies on the impact of underwater sound on marine life [16], [23], [27] quantify impulsive sound in terms of sound exposure level (in dB re 1 µPa2s per strike and/or cumulative) and zero to peak sound pressure (value in Pa or level in dB re 1 µPa2). Other possible metrics (impulse, rise time, peak to peak sound pressure, kurtosis, etc.) have sometimes been suggested, but the associated dose-response relations are even less clear than for sound exposure level and zero to peak pressure [2]. Therefore the sound pressure metrics used in the present study were zero to peak sound pressure and sound exposure level. Similar metrics can be derived for acoustic particle velocity. Although sound particle velocity has a direction associated to it, the metrics proposed here only concern the magnitude of particle velocity.
The sound metrics were defined as follows:
• Zero to peak sound pressure is the maximum absolute value of the unweighted instantaneous sound pressure in the measurement bandwidth. Zero to peak sound pressure level (Lz−p) is ten times the logarithm to the base 10 of the ratio of the square of the zero to peak sound pressure to the square of the reference sound pressure of 1 µPa.
• Sound exposure is the time integral of the time-varying square of the unweighted instantaneous sound pressure in the measurement bandwidth over the duration of a single piling impact. Single strike sound exposure level (SELss) is ten times the logarithm to the base 10 of the ratio of the sound exposure of a single piling impact signal to the reference sound exposure of 1µPa2s. Cumulative sound exposure level (SELcum) is the summation over a specified number of piling impacts; SELcum is the average SELss plus ten times the logarithm to the base 10 of the number of strikes.
• Zero to peak sound particle velocity is the maximum absolute value of the unweighted instantaneous total sound particle velocity in the measurement bandwidth. Zero to peak sound particle velocity level (Lv,z−p) is ten times the logarithm to the base 10 of the ratio of the square of the peak sound particle velocity to the square of the reference sound particle velocity of 1 nm/s.
• Sound particle velocity exposure is the time integral of the time-varying square of the unweighted instantaneous sound particle velocity in the measurement bandwidth over the duration of a single piling impact. Single strike sound particle velocity exposure level (VELss) is ten times the logarithm to the base 10 of the ratio of the sound exposure to the reference sound particle velocity exposure of 1 (nm/s)2s. Cumulative sound particle velocity exposure level (VELcum) is the summation over a specified number of piling impacts; VELcum is the average VELss plus ten times the logarithm to the base 10 of the number of strikes.
The sound measured at 100 m from pile-driving events in the North Sea (OWEZ wind farm, 4 m diameter steel monopole, at a water depth of ±20 m, with hammer strike energy of ±800 kJ) had a broadband Lz−p up to 210 dB re 1 µPa2 and a broadband SELss up to 188 dB re 1 µPa2s [25]. Propagation loss to various distances depends in a complex manner on water depth (bathymetry), condition of the water surface (waves) and the acoustic properties of water and sediment. For North Sea conditions in 20–25 m deep water with a sandy bottom, distances between 100 m and 2 km from the pile are approximately in the ‘mode-stripping’ region [28]. In this region, propagation loss for low frequency pile-driving sound approximately varies with distance R as 15log10R. Thus, the levels at 2 km distance are estimated to be about 20 dB lower than the levels at 100 m (i.e. SELss = 168 dB re 1µPa2s and Lz−p = 190 dB re 1 µPa2 at 2 km).
At distances ≥ 100 m from the pile in 20–25 m deep water, the acoustic particle velocity level is roughly proportional to the acoustic pressure level through the characteristic impedance of the medium (ρc): particle velocity level equals pressure level minus 20log10(ρc•(106•10−9)) ≈ 64 dB re 1 (nm/s/µPa)2. This approximation includes a correction factor that accounts for the different reference units for pressure and velocity. Hence, broadband Lv,z−p between 127 and 147 dB re 1 (nm/s)2 and broadband VELss between 104 and 124 dB re 1 (nm/s)2s corresponds with the estimated values for Lz−p and SELss at distances between 100 m and 2 km from the pile.
Two single strike signal recordings were selected for playback, one measured at 100 m and one measured at 800 m distance from the pile. The recorded signals were scaled to different levels to simulate different distances from the pile, the 100 m signal was used for distances between 100 and 800 m, the 800 m signal was used for distances ≥ 800 m.
Typical recorded SELss spectra [25], [29] show that the main (unweighted) energy of underwater pile-driving sound is generated in the 50 Hz to 1 kHz bands. The playback sound was limited to this frequency band, to avoid excitation of spurious resonances in the larvaebrator.
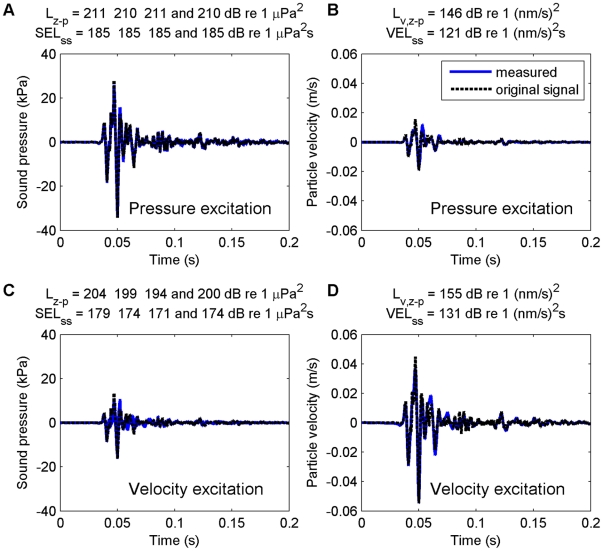
Measurements showed that the projector reproduced the original recorded signal shape quite accurately for sound pressure in the pressure excitation configuration, and for particle velocity in both excitation configurations (Figure 2). The velocity levels were substantially higher for a velocity excitation compared to a pressure excitation. Hence, the effect of particle velocity could be examined decoupled from the effect of sound pressure. In case of pressure excitation, however, the velocity levels were higher than expected from compression of the water volume alone, probably due to remaining flexibility (air/membrane) in the chamber. This means that the set-up does not enable examination of the effect of sound pressure decoupled from particle velocity. The observed pressure to velocity ratio was actually close to the ratio in a plane wave in unbound water. In a plane wave, the acoustic particle velocity and acoustic pressure levels are approximately related through the characteristic impedance of the medium (see above). The measured Lz−p of 211 dB re 1µPa2 (Figure 2a) corresponded with an expected free field Lv,z−p of 147 dB re 1 (nm/s)2 and an observed Lv,z−p of 146 dB re 1 (nm/s)2 (Figure 2b). The measured SELss of 185 dB re 1 µPa2s corresponded with an expected and observed VELss of 121 re 1 (nm/s)2s. Hence, the pressure excitation exposures represent realistic pressure to velocity ratios.
Figure 2. Comparison of the original and measured signal shape.
Comparison of the original signal shape (recorded in the field) and the observed signal shape (measured in the larvaebrator) for a pressure excitation (A, B) and a velocity excitation (C, D), in terms of sound pressure (A, C) and sound particle velocity (B, D). The original signal is scaled to match the peak of the measured signal. The sound levels are given in the header of each panel.
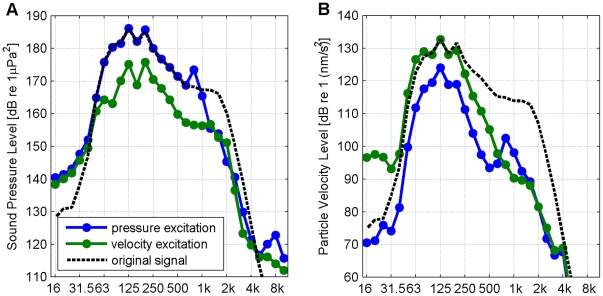
The main characteristics of the frequency spectra of pressure and velocity are reproduced to an acceptable level (Figure 3). The reproduced sound particle velocity spectrum at frequencies above 250 Hz is lower than the spectrum of the recorded sound, but the dominant energy in the range between 63 and 250 Hz is reproduced correctly.
Figure 3. Comparison of the original and measured frequency spectra.
Mean square sound pressure level spectrum (A) and particle velocity level spectrum (B) in 1/3-octave bands (averaged over 0.2 s intervals) for a pressure excitation, a velocity excitation and the original signal scaled to match the peak of the measured signal.
Larvae
Common sole (Solea solea) is a commercially important flatfish species, which was included in the impact assessment of Dutch offshore wind farms [17]. For most marine fish species, it is difficult to obtain eggs or larvae, but common sole eggs and larvae could be obtained throughout the year from a commercial hatchery (SOLEA). Fertilised eggs were purchased from the hatchery and reared to the required larval stage in large cultivation chambers in the laboratory. As the effect of sound exposure may vary between larval stages related to the development of organs, different larval stages were used in the experiments. Stage identification was based on the following classification [30]:
Stage 1: Yolk sac present
Stage 2: Yolk sac absorbed, development of spines and swim bladder.
Stage 3: Swim bladder fully inflated, appearance of fin rays, notochord straight
Stage 4: Onset of asymmetry and eye migration, notochord bent
Stage 4a: Notochord caudally bent upwards by < 45°, eyes symmetrical
Stage 4b–d: Notochord bent by ≥ 45°, onset of eye migration
Stage 5: Completion of metamorphosis, swim bladder resorbed.
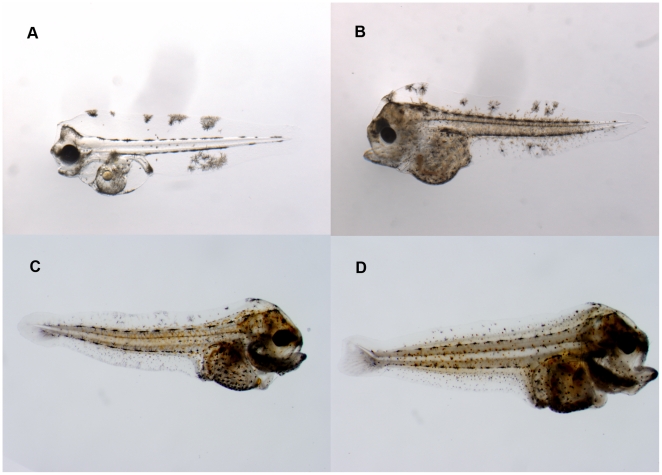
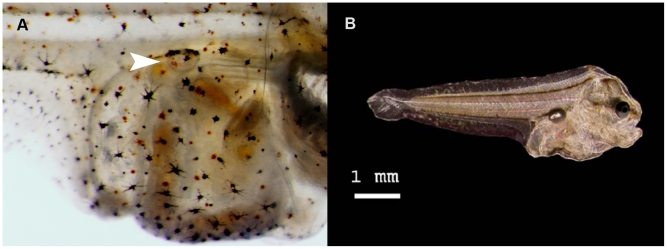
Three (groups of) larval stages were used in the experiments: 1, 2 and 3–4a (Figure 4). The late larval stages were not included because by then the larvae disappear from the water column related to the transition from a pelagic to a demersal life style [31]–[32].
Figure 4. The larval stages of common sole (Solea solea) that were used in the experiments.
The photos show a stage 1 larva of 5.3 mm (A), a stage 2 larva of 6.0 mm (B), a stage 3 larva of 6.5 mm (C) and a stage 4a larva of 7.1 mm (D).
Development rates depend on temperature [33]–[35]. The water temperature in the cultivation chambers was slowly raised from the temperature in the hatchery (12°C) to the ambient temperature in the laboratory (16°C). Within this range, the temperature was manipulated so the majority of larvae would be in the required developmental stage on the days that the treatments were applied. Variations in development rates were observed between larvae that were reared at the same temperature; larvae originating from one spawning event and reared at the same temperature could range from stage 3 to stage 4a.
In stage 3–4a larvae, inflated swim bladders (Figure 5a) were observed in most, but not all larvae. Similar observations were done previously for common sole [36]–[37]. In an aquaculture study [36], inflated swim bladders were observed at 16 days after hatching in larvae reared at 18°C (Figure 5b), but not all larvae of that age had an inflated swim bladder. Histological examination of larvae reared at 19°C showed that the gas gland and bladder are already developed 5 days after hatching, the first inflated swim bladders appear at 10 days after hatching, and not all larvae have an inflated bladder during the inflation period [37]. They observed a dilated pneumatic duct when the swim bladder begins to inflate, indicating passage of gas from the digestive tract to the swim bladder (i.e. a physostomous swim bladder), but they also found indications that inflation may be realised by gas secretion of the gas gland.
Figure 5. The swim bladder in common sole (Solea solea) larvae.
The swim bladder in a stage 4a larva as observed in this study (A) and a published image [36] of the swim bladder in a stage 4a larva (B).
General procedures
Each experiment consisted of a treatment followed by a monitoring period. A treatment was either a sound exposure or a control. The water in the test chamber of the larvaebrator was refreshed before each treatment. Water temperature in the test chamber was the same as in the cultivation chambers. For each experiment, 25 (±5) larvae were taken from the cultivation chambers and subjected to treatment. After treatment, each batch of larvae was transferred to a separate ‘batch-container’ and held during the monitoring period. The control groups underwent the same handling procedures as the exposure groups. The larvae were transferred to and from different water bodies using a plastic pipette, from which the tip was cut off to enlarge the opening. This method minimises mortality due to handling, but it is time consuming as only one or two larvae can be transferred at the same time. The total duration of a treatment including handling of the larvae was 15 (±5) minutes.
From 3–4 days after hatching onwards (i.e. larval stage 2+), the larvae were fed daily and ad libitum with Artemia. The water in the batch-containers was refreshed every day. The response variable that was measured was mortality; the numbers of dead and live larvae in each batch were counted directly after the treatment and daily during the monitoring period. Dead larvae disintegrated completely within 24 hours. Recently dead larvae were visually recognized by their shape or immobility. Within a few hours after death, a larva shrivels up and its shape clearly indicates that it is dead. Immobile larvae were examined using a stereomicroscope to check heart-beat and respiratory activity. Dead larvae were removed from the batch-containers.
The batch-containers were coded and, except for the observations directly after the treatments, the person scoring mortality was not aware of the treatment belonging to the code. The treatments within each replication round were applied in random sequence to avoid bias due to potential serial effects.
This study was performed in accordance with Dutch law concerning animal welfare. The protocol was approved by the Animal Ethical Commission (DEC) of Wageningen UR (experiment code 2010085 under application 2010063.c).
Pilot experiments
In a pilot series of experiments, we maximised the number of treatments and, consequently, minimised the number of replicates per treatment, because very little is known about critical values for sound exposure with regard to larval survival. Each of the three larval stages was subjected to several exposures (Tables 1 and 2) and a control treatment. Two replicates per treatment were carried out for stage 1 larvae, four replicates for stage 2 larvae and five replicates for stage 3–4a larvae. Mortality was recorded directly after the treatment and daily until 10 days after the treatment.
Table 1. Sound levels of the pressure excitation exposures applied in the pilot experiments.
| Stage | Measured sound levels | Strikes | Distance (m) | ||
| Lz−p | SELss | SELcum | |||
| (dB re µPa2) | (dB re1 µPa2s) | (dB re µPa2s) | |||
| 1 | 198 | 175 | 175 | 1 | 800 |
| 211 | 187 | 187 | 1 | 100 | |
| 211 | 187 | 204 | 50 | 100 | |
| 2 | 206 | 181 | 204 | 200 | 200 |
| 210 | 186 | 203 | 50 | 100 | |
| 210 | 186 | 206 | 100 | 100 | |
| 3–4a | 205 | 181 | 206 | 300 | 200 |
| 210 | 186 | 196 | 10 | 100 | |
| 210 | 186 | 206 | 100 | 100 | |
Lz−p = zero to peak sound pressure level, SELss = single strike sound exposure level and SELcum = cumulative sound exposure level, see the text for further explanation. The last 2 columns present the corresponding distance from a ‘typical’ North Sea pile-driving installation and number of strikes.
Table 2. Sound levels of the velocity excitation exposures applied in the pilot experiments.
| Stage | Measured sound levels | Strikes | Distance (m) | ||
| Lv,z−p | VELss | VELcum | |||
| (dB re 1 (nm/s)2) | (dB re 1 (nm/s)2s) | (dB re 1 (nm/s)2s) | |||
| 1 | 133 | 111 | 111 | 1 | 800 |
| 148 | 125 | 125 | 1 | 100 | |
| 147 | 124 | 144 | 100 | 100 | |
| 2 | 142 | 118 | 141 | 200 | 200 |
| 147 | 122 | 142 | 100 | 100 | |
| 3–4a | 145 | 122 | 147 | 300 | 200 |
| 148 | 125 | 145 | 100 | 100 | |
Lv,z−p = zero to peak sound particle velocity level, VELss = single strike sound particle velocity exposure level and VELcum = cumulative sound particle velocity exposure level, see the text for further explanation. The last 2 columns present the corresponding distance from a ‘typical’ North Sea pile-driving installation and number of strikes.
Two types of sound exposure were applied: pressure excitation or velocity excitation (see above). The larvae were exposed to single or multiple strikes at different levels of sound pressure or particle velocity (Tables 1 and 2). The maximum SELcum possible with the larvaebrator (using recorded pile-driving sounds) was 206 dB re 1 µPa2s, which corresponded to 100 strikes at a distance of 100 m from a ‘typical’ (as described above) North Sea pile-driving installation. The strike rate was 50 strikes per minute, so an exposure to 100 strikes lasted 2 minutes.
Final experiments
In the final series of experiments, the number of replicates per treatment was substantially increased, to obtain a higher precision on the estimates of differences in mortality between treatments. The results of the pilot series were used in a power analysis to estimate the number of replicates required for sufficient power (i.e. probability of detecting an effect significantly at the 95% level, given a certain sample size and experimental design) to detect a ‘50% effect’. The % effect was defined as 100% (pe−pc)/(1−pc), in which pe is the estimated mean probability of death in the exposure group and pc is the estimated mean probability of death in the control group. Note that with this definition of the effect to be detected, the difference between the exposure group and control group depends on the mortality in the control group. The analysis showed that doubling the number of replicates increased the power far more than doubling the number of larvae per replicate. Fifteen replicates for each treatment, with 25 larvae per batch, were estimated to give a high probability (≥ 96%) of detecting a 50% effect significantly (at the 95% level) after 5 days.
Given the resources available, it was possible to carry out 3 treatments (2 exposures and 1 control) with 15 replicates for each of the 3 larval stages. We decided to focus on pressure excitation exposures as these appeared to have an effect (although non-significant) in the pilot series. The same two exposures were used for all larval stages: the highest sound pressure exposure possible with the larvaebrator (using recorded pile-driving sounds) and an exposure which was approximately 5 dB lower in both SELcum and Lz−p (Table 3).
Table 3. Sound levels of the pressure excitation exposures applied in the final experiments.
| Stage | Measured sound levels | Strikes | Distance (m) | ||
| Lz−p | SELss | SELcum | |||
| (dB re 1 µPa2) | (dB re 1 µPa2s) | (dB re 1 µPa2s) | |||
| 1 | 205 | 181 | 201 | 100 | 200 |
| 210 | 186 | 206 | 100 | 100 | |
| 2 | 205 | 180 | 200 | 100 | 200 |
| 209 | 185 | 205 | 100 | 100 | |
| 3–4a | 205 | 181 | 201 | 100 | 200 |
| 209 | 185 | 205 | 100 | 100 | |
Lz−p = zero to peak sound pressure level, SELss = single strike sound exposure level and SELcum = cumulative sound exposure level, see the text for further explanation. The last 2 columns present the corresponding distance from a ‘typical’ North Sea pile-driving installation and number of strikes.
As both the absolute level of mortality in the control group and the variation in mortality between batches with the same treatment increased over time, the statistical power to detect a 50% effect decreased with the duration of monitoring. Therefore, the monitoring period was reduced to 7 days in the final series of experiments.
Statistical analysis
Estimates of mortality per larval stage and treatment, as well as the statistical significance of differences between exposure and control groups, were calculated using a generalised linear mixed model. This model treats the data (death or survival of a larva) as outcomes of binomial trials in which the probability of death is a function of treatment, and takes account of possible random variation in mortality between batches (termed ‘batch effect’ hereafter). It is necessary to account for such batch effects because, if present, the assumption (under the binomial distribution) that the outcomes of larvae are determined independently of one another is violated.
The statistical model was formulated as follows:
• The logit transformed probabilities of death pi,j (in treatment i and batch j) were modelled as a function of treatment and random batch effect (αj):
logit(pij) = treatmenti+αj.
• The numbers of dead larvae in batch j from treatment i (kij) were assumed to be binomially distributed depending on the probability of death (pij) and the number of larvae at the beginning of the experiment (Nij, usually 25):
kij ∼ Bin(pij, Nij).
• The random batch effects (αj) were assumed to be normally distributed with mean zero and variance σ2:
αj ∼ N(0, σ2).
The model was fitted and statistical significance tests were performed using the glimmix procedure (with the Kenward-Roger approximation for the degrees of freedom) in SAS (SAS/STAT software. SAS Institute Inc., Cary, North Carolina, USA). The model was fitted separately to the data for each larval stage and for each of two monitoring periods (5 or 7 days in the final series, 5 or 10 days in the pilot series). If, for a given larval stage and monitoring period, the variance of the batch effect was estimated to be (near) zero, then the model was reduced to a generalised linear model without a batch effect.
Results
In the pilot series, no immediate effect of sound exposure (directly after treatment) was observed for any of the three larval stages. Mean mortality in the control group increased from 0% directly after treatment to 67% at the end of the 10 day monitoring period for larvae that were in stage 1 at the time of the treatment. This was 0 to 59% for stage 2 larvae, and 0 to 10% for stage 3–4a larvae. In the case of stage 2 larvae, mortality in the control group was clearly lower than mortality in the highest sound pressure exposure group (SELcum = 206 dB re 1 µPa2s): mean mortality after 10 days was 78% in the exposure group compared to 59% in the control group, that is ±50% less survivors in the exposure group. This difference was not statistically significant, possibly due to low statistical power (i.e. too few replicates). No indications for an effect of sound exposure were observed in the other larval stages or at other sound levels. High variability in mortality between batches with the same treatment was observed.
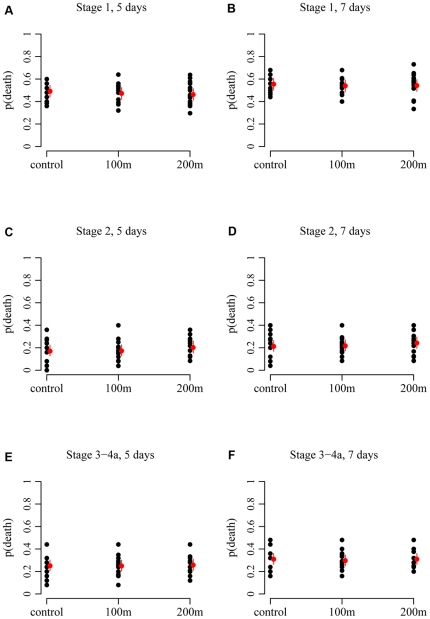
In the final series, as in the pilot series, no immediate effect of sound exposure was observed for any of the three larval stages. Mean mortality in the control group increased from 0% directly after treatment to 55% at the end of the 7 day monitoring period for stage 1 larvae, from 0 to 21% for stage 2 larvae, and from 0 to 31% for stage 3–4a larvae. No clear differences between the exposure groups and the control group were observed for any of the larval stages (Figure 6). The factor treatment was statistically insignificant for all larval stages (Table 4).
Figure 6. Mortality by larval stage and treatment at 5 and 7 days after the treatment.
Estimated mean probability of death with 95% confidence limits (red symbols and bars) and observed mortality for each replicate within each treatment (black symbols). Each replicate consisted of 25 (±5) larvae. The labels of the sound exposure treatments refer to the distance from the pile, the associated sound levels are presented in Table 3.
Table 4. Analysis of variance of the probability of death modelled as a function of treatment and random batch effect.
| Stage | Days | Chi2/DF | Random effect | Fixed effect | |||
| variance | Num DF | Den DF | F value | Pr > F | |||
| 1 | 5 | 0.82 | 0 | 2 | 42 | 0.31 | 0.7 |
| 7 | 0.68 | 0 | 2 | 42 | 0.09 | 0.9 | |
| 2 | 5 | 1.00 | 0.1404 | 2 | 41.90 | 0.48 | 0.6 |
| 7 | 1.00 | 0.0568 | 2 | 41.67 | 0.40 | 0.7 | |
| 3–4a | 5 | 0.99 | 0.0340 | 2 | 42 | 0.03 | 0.9 |
| 7 | 0.99 | 0 | 2 | 42 | 0.10 | 0.9 | |
Model estimates of the 95% confidence interval for the difference between exposure and control were used to estimate the effect that could have been detected with these experiments. Estimates of the upper limit of the 95% confidence interval for effect ranged from 8 to 14% (Table 5). This means that the probability of an effect larger than 14% was insignificant (< 5%). Hence, the detectable effect was substantially smaller than the 50% aimed for in the power analysis.
Table 5. Model estimates of the mean and 95% confidence limits for probability of death in each treatment and for the effect of exposure.
| Stage | Days | Treatment | Estimated probability of death | Estimated effect | |||
| mean | lower limit | upper limit | mean | upper limit | |||
| 1 | 5 | 100 m | 0.47 | 0.42 | 0.52 | −4% | 11% |
| 200 m | 0.46 | 0.41 | 0.51 | −6% | 9% | ||
| control | 0.49 | 0.44 | 0.54 | ||||
| 1 | 7 | 100 m | 0.54 | 0.49 | 0.59 | −3% | 13% |
| 200 m | 0.54 | 0.49 | 0.59 | −3% | 14% | ||
| control | 0.55 | 0.50 | 0.61 | ||||
| 2 | 5 | 100 m | 0.17 | 0.13 | 0.23 | 0% | 10% |
| 200 m | 0.20 | 0.15 | 0.26 | 4% | 14% | ||
| control | 0.17 | 0.13 | 0.22 | ||||
| 2 | 7 | 100 m | 0.22 | 0.17 | 0.27 | 1% | 10% |
| 200 m | 0.24 | 0.19 | 0.30 | 4% | 14% | ||
| control | 0.21 | 0.17 | 0.26 | ||||
| 3–4a | 5 | 100 m | 0.25 | 0.20 | 0.30 | 0% | 10% |
| 200 m | 0.26 | 0.21 | 0.31 | 1% | 11% | ||
| control | 0.25 | 0.21 | 0.30 | ||||
| 3–4a | 7 | 100 m | 0.30 | 0.25 | 0.35 | −2% | 8% |
| 200 m | 0.31 | 0.26 | 0.36 | 0% | 10% | ||
| control | 0.31 | 0.26 | 0.36 | ||||
The effect of exposure was defined as 100% • (pe−pc)/(1−pc), in which pe is the estimated mean probability of death in the exposure group and pc is the estimated mean probability of death in the control group. The labels of the sound exposure treatments refer to the distance from the pile, the associated sound levels are presented in Table 3.
Discussion
Experimental exposure of common sole larvae to pile-driving sound levels up to SELcum = 206 dB re 1 µPa2s and Lz−p = 210 dB re 1 µPa2 did not result in increased mortality during the first 7 days after exposure. No statistically significant differences in mean mortality were found between the control and exposure groups for any of the larval stages. Standard errors on mortality estimates were such that an exposure effect of more than 14% could be excluded at the 95% confidence level.
For larvae not exposed to sound (i.e. the control groups), mean cumulative mortality after 7 days ranged from 8 to 56%. These levels were not considered to be high compared to natural mortality. Natural larval mortality rates are usually expressed in instantaneous daily mortality rates (Z in the equation Nt = N0 • e−Zt, N0 is number of larvae at t = 0 days and Nt is number of larvae after t days). Published estimates for European flatfish species range between 0.035 d−1 [38] for sole in the Bristol Channel and 0.08 d−1 [39] for plaice in the North Sea, that is 22–43% mortality after 7 days. Similar or higher larval mortality rates were estimated for other marine fish species [40]. The differences in control group mortality were not only related to larval stage, but also to spawning stock quality. Clear differences were observed in the viability of eggs and larvae obtained from different spawning events. This was also reported for hatchery reared common sole larvae [36]; mortality ranged from 35 to 80% depending on the spawning group.
The interim SELcum criterion defined by the US Fisheries Hydro-acoustic Working Group for non-auditory tissue damage in small fish (< 2 g) is 183 dB re 1 µPa2s [16]. The highest SELcum used in the present study (206 dB re 1 µPa2s) was much higher than this norm, but no significant effects on the survival of common sole larvae were found. Actually, very little is known on the sound levels that cause damage or mortality in fish eggs and larvae. No studies have addressed the effect of pile-driving sound on fish larvae, and only a few studies have investigated the effect of low frequency, loud impulse sounds on fish larvae [2].
The effect of seismic air gun sounds on eggs and different larval stages of cod (Gadus morhua), saithe (Pollachius virens), herring (Clupea harengus), turbot (Psetta maximus) and plaice (Pleuronectes platessa) was examined in field experiments [41]. Effect was related to the distance from the sound source and the corresponding Lz−p ranged from 220 to 242 dB re 1 µPa2. Cod, turbot and herring larvae were examined in the yolk sac stage: cod showed a small but insignificant effect at 242 dB, herring showed no significant effects due to overall high mortality rates, and turbot showed significant effects at all levels of exposure. Cod and saithe were examined in the post yolk sac larval stages: significant effects were observed for cod at exposures ≥ 223 dB, no significant effects were observed for saithe due to overall high mortality rates. Cod, turbot, herring and plaice were examined in the post-larval stage: cod showed a significant effect at 242 dB, small but insignificant effects were observed at the higher sound levels for the other 3 species. The authors also observed damage to the neuromasts of the lateral line system and to other organs in cod and turbot larvae [41].
Larval and small juvenile spot (Leiostomus xanthurus) and pinfish (Lagodon rhomboides) were exposed to blast shock waves in field experiments [42]. The size of the test animals was 18–20 mm for spot and 16–17 mm for pinfish (note that these larvae/juveniles were larger than the larvae used in the present study). The authors recorded death, lethal and sub-lethal injuries within 24 hours after exposure. For spot, the proportion dead or injured was 0% in the control group and 100% at the highest exposure level: zero to peak pressure = 278−692 kPa (Lz−p ≈ 229−236 dB re 1 µPa2) and energy flux density = 1.096−3.642 J m−2 (SELss ≈ 182−187 dB re 1 µPa2s assuming the impedance of the medium to be 1.53•106 kg/m2s). For pinfish, the proportion dead or injured was 0% in the control group and ranged from 33–100% at the highest exposure level: zero to peak pressure = 558−866 kPa (Lz−p ≈ 235−239 dB re 1 µPa2) and energy flux density = 1.311−2.594 J m−2 (SELss ≈ 183−186 dB re 1 µPa2s ). The blasts applied in this study apparently had a different signal shape compared to our playback of pile-driving sounds; their highest exposures had much higher zero to peak pressure levels then in our study, whereas the single-strike sound exposure levels were comparable.
These two studies show that exposure to loud impulse sounds can cause lethal and sub-lethal effects in fish larvae. The zero to peak pressure levels applied in these studies were 12 to 32 dB higher than in the present study. SELss was only reported in one of the two studies [42] and their highest levels (182–187 dB re 1 µPa2s) were comparable to the levels we used in the final series of experiments (181–186 dB re 1 µPa2s). This indicates that either Lz−p may be a more critical metric for mortality than SELcum, or that common sole larvae are less vulnerable to sound exposure than pinfish and spot larvae/small juveniles.
The swim bladder is an organ which is sensitive to sound pressure and it has been suggested that fish with swim bladders are more vulnerable to sound exposure than species that do not have such air chambers [2]. Common sole larvae only have a swim bladder during a limited period of their larval life [36]–[37]. This may be the reason for the absence of significant effects in stage 1 and 2 larvae. However, significant effects of sound (at higher levels than those used in the present study) have been observed in yolk sac turbot larvae [41], and these larvae do not have a swim bladder either [30]. If the presence of a swim bladder is critical at the sound exposure levels used in this study, then an effect could have been expected in stage 3–4a larvae. Visual inspection before treatment showed that most of these larvae had an inflated swim bladder, but we cannot exclude gas loss from the swim bladder due to handling prior to exposure.
Statistically significant lethal effects of exposure to pile-driving sounds in common sole larvae could occur at higher sound levels than the highest levels used in the present study (SELcum = 206 dB re 1 µPa2s, Lz−p = 210 dB re 1 µPa2). The limited information available to date indicates that interspecific differences in vulnerability to sound exposure may occur. Hence, we would not recommend that the conclusion based on common sole larvae be broadly extrapolated to other fish larvae. However, this study does indicate that the previous assumptions [17] and interim criteria [16] may need to be revised.
Studies on the effects of pile-driving sounds on juvenile salmon also indicate that the US interim criterion for SELcum (set at 187 dB re 1 µPa2s for fish > 2 g) may be relatively low. Field experiments with juvenile steelhead (Oncorhynchus mykiss) [43] and juvenile Coho salmon (O. kisutch) [44] did not show sound-induced injuries or mortality at SELcum exposures up to 194 dB (steelhead) [43] or 207 dB (Coho) [44]. A recent study examined barotrauma injuries in juvenile Chinook salmon (O. tshawytscha) in relation to SELcum, SELss and the number of pile-driving strikes, using the HICI-FT [23]. They developed a ‘Response Weighted Index’ (RWI) to quantify the number and severity of the injuries and recommended a RWI-value to use as biological criterion for juvenile Chinook. The corresponding acceptable exposure bounds include impulsive sounds ≤ 179 dB re 1µPa2s SELss for 1,920 strikes and ≤ 181 dB re 1µPa2s SELss for 960 strikes, combined with a SELcum ≤ 211 dB.
It is important to realise that the present study only focussed on lethal effects of sound exposure. The applied exposures may have caused damage to body tissues or hearing, which did not lead to death within the monitoring period, but could result in lower long-term survival. Sound exposure may also affect physiology or behaviour and hence predation and starvation risks. Besides further research on lethal effects in fish larvae of other species, we recommend future research on sub-lethal effects, varying from injuries to behavioural responses.
A statistically significant effect of sound exposure in experiments does not necessarily indicate a ‘biologically significant’ effect for the entire larval population. To assess the effect of pile-driving sound on the total larval population (in a certain area, at a certain time), dose-response relationships for specific sound metrics (e.g. SELcum, Lz−p) are required as these can be translated to distance from the sound source (using source models and sound propagation models). Furthermore, information on the spatial and temporal distribution of fish larvae in relation to water movements is required; this can be obtained by egg and larval transport modelling (e.g. [20]). We recommend closer examination of the role of different sound metrics and co-variables (e.g. depth), as this enables a better assessment of the impact at the population level.
Acknowledgments
We thank Jan van der Heul, Marco Lohman, Tim Huijer, Dirk Burggraaf, Silja Tribuhl and Ewout Blom for their technical and practical advice and assistance. We thank Frank van den Berg, Tobias van Kooten and Arjen Boon for valuable discussions during the development and execution of this project. We thank Arthur Popper, Audrey Geffen, Michael Ainslie, Mark Dickey-Collas and Jakob Asjes for constructive comments on the report preceding this paper, and Hans Slabbekoorn and two anonymous reviewers for constructive comments on an earlier version of this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was commissioned by the Centre for Water Management, Rijkswaterstaat, the Netherlands, as part of the ‘Shortlist Masterplan Wind’ research programme on the ecological impact of offshore wind farms in the North Sea. This research programme was funded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation, and the Dutch Ministry of Infrastructure and Environment. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Popper AN, Fewtrell J, Smith ME, McCauley RD. Anthropogenic sound: Effects on the behavior and physiology of fishes. Mar Tech Soc J. 2004;37:35–40. [Google Scholar]
- 2.Popper AN, Hastings MC. The effects of anthropogenic sources of sound on fishes. J Fish Biol. 2009;75:455–489. doi: 10.1111/j.1095-8649.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 3.Popper AN, Hastings MC. The effects of human-generated sound on fish. Integr Zool. 2009;4:43–52. doi: 10.1111/j.1749-4877.2008.00134.x. [DOI] [PubMed] [Google Scholar]
- 4.Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C et al. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol Evol. 2010;25:419–427. doi: 10.1016/j.tree.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Caltrans 2001. San Francisco - Oakland Bay Bridge East Span Seismic Safety Project. Pile installation demonstration project, fisheries impact assessment. Caltrans contract 04A0148. San Francisco: Caltrans. Available: http://biomitigation.org/reports/files/PIDP_Fisheries_Impact_Assessment_0_1240.pdf. Accessed: 2011 Jun.
- 6.Dalen J, Dragsund E, Næss A, Sand O. 2007. Effects of seismic surveys on fish, fish catches and sea mammals. Report for the Cooperation group - Fishery Industry and Petroleum Industry. DNV Energy Report no. 2007-0512. Høvik, Oslo: DNV. Available: http://www.olf.no/PageFiles/6574/Effects%20of%20seismic%20surveys%20on%20fish,%20fish%20catches%20and%20sea%20mammals.pdf?epslanguage=no. Accessed: 2011 Jun.
- 7.McCauley RD, Fewtrell J, Popper AN. High intensity anthropogenic sound damages fish ears. J Acoust Soc Am. 2003;113:638–642. doi: 10.1121/1.1527962. [DOI] [PubMed] [Google Scholar]
- 8.Amoser S, Ladich F. Diversity in noise-induced temporary hearing loss in otophysine fishes. J Acoust Soc Am. 2003;113:2170–2179. doi: 10.1121/1.1557212. [DOI] [PubMed] [Google Scholar]
- 9.Smith ME, Kane AS, Popper AN. Noise-induced stress response and hearing loss in goldfish (Carassius auratus). J Exp Biol. 2004;207:427–435. doi: 10.1242/jeb.00755. [DOI] [PubMed] [Google Scholar]
- 10.Wysocki LE, Dittami JP, Ladich F. Ship noise and cortisol secretion in European freshwater fishes. Biol Conserv. 2006;128:501–508. [Google Scholar]
- 11.Graham AL, Cooke SJ. The effects of noise disturbance from various recreational boating activities common to inland waters on the cardiac physiology of a freshwater fish, the largemouth bass (Micropterus salmoides). Aquatic Conserv: Mar. Freshw. Ecosyst. 2008;18:1315–1324. [Google Scholar]
- 12.Slotte A, Hansen K, Dalen J, Ona E. Acoustic mapping of pelagic fish distribution and abundance in relation to a seismic shooting area off the Norwegian west coast. Fish Res. 2004;67:143–150. [Google Scholar]
- 13.Vasconcelos RO, Amorim MCP, Ladich F. Effects of ship noise on the detectability of communication signals in the Lusitanian toadfish. J Exp Biol. 2007;210:2104–2112. doi: 10.1242/jeb.004317. [DOI] [PubMed] [Google Scholar]
- 14.Meager JJ, Rodewald P, Domenici P, Ferno A, Jarvi T et al. Behavioural responses of hatchery-reared and wild cod Gadus morhua to mechano-acoustic predator signals. J Fish Biol. 2011;78:1437–1450. doi: 10.1111/j.1095-8649.2011.02951.x. [DOI] [PubMed] [Google Scholar]
- 15.Inger R, Attrill MJ, Bearhop S, Broderick AC, Grecian WJ et al. Marine renewable energy: potential benefits to biodiversity? An urgent call for research. J Appl Ecol. 2009;46:1145–1153. [Google Scholar]
- 16.Oestman R, Buehler D, Reyff JA, Rodkin R. 2009. Technical Guidance for Assessment and Mitigation of the Hydroacoustic Effects of Pile Driving on Fish. San Francisco: Caltrans. Available: http://www.dot.ca.gov/hq/env/bio/files/Guidance_Manual_2_09. Accessed: 2011 Jun.
- 17.Prins TC, van Beek JKL, Bolle LJ. Deltares report Z4832. Delft: Deltares; 2009. Modelschatting van de effecten van heien voor offshore windmolenparken op de aanvoer van vislarven naar Natura 2000. [Google Scholar]
- 18.Erftemeijer PLA, van Beek JKL, Bolle LJ, Dickey-Collas M, Los HFJ. Variability in transport of fish eggs and larvae. I. Modelling the effects of coastal reclamation. Mar Ecol Prog Ser. 2009;390:167–181. [Google Scholar]
- 19.Dickey-Collas M, Bolle LJ, van Beek JKL, Erftemeijer PLA. Variability in transport of fish eggs and larvae. II. Effects of hydrodynamics on the transport of Downs herring larvae. Mar Ecol Prog Ser. 2009;390:183–194. [Google Scholar]
- 20.Bolle LJ, Dickey-Collas M, van Beek JKL, Erftemeijer PLA, Witte JIJ et al. Variability in transport of fish eggs and larvae. III. Effects of hydrodynamics and larval behaviour on recruitment in plaice. Mar Ecol Prog Ser. 2009;390:195–211. [Google Scholar]
- 21.Akamatsu T, Okunura T, Novarini N, Yan HY. Empirical refinements applicable to the recording of fish sounds in small tanks. J Acoust Soc Am. 2002;112:3073–3082. doi: 10.1121/1.1515799. [DOI] [PubMed] [Google Scholar]
- 22.Martin JS, Rogers PH. Sound exposure chamber for assessing the effects of high-intensity sound on fish. Bioacoustics. 2008;17:331–333. [Google Scholar]
- 23.Halvorsen MB, Casper BM, Woodley CM, Carlson TJ, Popper AN. NCHRP Research Results Digest 363, Project 25-28, National Cooperative Highway Research Program, Transportation Research Board, National Academy of Sciences, Washington; 2011. Predicting and mitigating hydroacoustic impacts on fish from pile installations. [Google Scholar]
- 24.de Jong CAF, Ainslie MA. TNO report MON-RPT-033-DTS-2007-03388. Delft: TNO; 2008. Analysis of the underwater sound during piling activities for the Off-shore Wind Park Q7. [Google Scholar]
- 25.Ainslie MA, de Jong CAF, Dol HS, Blacquière G, Marasini C. 2009. Assessment of natural and anthropogenic sound sources and acoustic propagation in the North Sea. TNO report 2009 - C085. The Hague: TNO. Available: http://www.noordzeeloket.nl/Images/Assessment%20of%20natural%20and%20anthropogenic%20sound%20sources%20and%20acoustic%20propagation%20in%20the%20North%20Sea_tcm14-4113.pdf. Accessed: 2011 Jun.
- 26.Anon 2011. TNO report TNO-DV 2011 C235 Standard for measurement and monitoring of underwater noise, Part I: physical quantities and their units’'. Available: http://www.noordzeeloket.nl/. Accessed: 2012 Feb.
- 27.Southall BL, Bowles AE, Ellison WT, Finneran JJ, Gentry RL et al. Marine Mammal Noise Exposure Criteria: Initial Scientific Recommendations. Aquat Mamm. 2007;33:411–521. [Google Scholar]
- 28.Weston DE. Propagation in water with uniform sound velocity but variable-depth lossy bottom. J Sound Vib. 1976;47:473–483. [Google Scholar]
- 29.Nehls G, Betke K, Eckelmann S, Ros M. 2007. Assessment and costs of potential engineering solutions for the mitigation of the impacts of underwater noise arising from the construction of offshore windfarms. BioConsult SH report on behalf of COWRIE Ltd (COWRIE ENG-01-2007). Husum: BioConsult. Available: http://www.offshorewind.co.uk/Assets/COWRIE-ENGFinal270907.pdf. Accessed: 2011 Jun.
- 30.Al-Maghazachi SJ, Gibson R. The developmental stages of turbot, Scophthalmus maximus. J Exp Mar Biol Ecol. 1984;82:35–51. [Google Scholar]
- 31.Champalbert G, Koutsikopoulos C. Behaviour, transport and recruitment of Bay of Biscay sole (Solea solea): laboratory and field studies. J Mar Biol Ass UK. 1995;75:93–108. [Google Scholar]
- 32.Lagardère F, Amara R, Joassard L. Vertical distribution and feeding activity of metamorphosing sole, Solea solea, before immigration to the Bay of Vilaine nursery (northern Bay of Biscay, France). Environ Biol Fish. 1999;56:213–228. [Google Scholar]
- 33.Fonds M. Laboratory observations on the influence of temperature and salinity on development of the eggs and growth of the larvae of Solea solea (Pisces). Mar Ecol Prog Ser. 1979;1:91–99. [Google Scholar]
- 34.Boulhic M, Galois R, Koutsikopoulos C, Lagardère F, Person-Le Ruyet J. Nutritional status, growth and survival of the pelagic stages of Dover sole Solea solea (L.) in the Bay of Biscay. Ann Inst océanogr Paris Nouv ser. 1992;68:117–139. [Google Scholar]
- 35.Amara R, Lagardère F, Desaunay Y. Seasonal distribution and duration of planktonic stage of Dover sole, Solea solea, larvae in the Bay of Biscay; an hypothesis. J Fish Biol. 1993;43:17–30. [Google Scholar]
- 36.Palazzi R, Richard J, Bozzato G, Zanella L. Larval and juvenile rearing of common sole (Solea solea L.) in the Northern Adriatic (Italy). Aquaculture. 2006;255:495–506. [Google Scholar]
- 37.Boulhic M, Gabaudan J. Histological Study of the Organogenesis of the Digestive-System and Swim Bladder of the Dover Sole, Solea solea (Linnaeus 1758). Aquaculture. 1992;102:373–396. [Google Scholar]
- 38.Horwood J. The Bristol Channel sole (Solea solea (L.)): A fisheries case study. Advances in Marine Biology. 1993;29:215–367. [Google Scholar]
- 39.Harding D, Nichols JH, Tungate DS. The spawning of plaice (Pleuronectes platessa L.) in the Southern Bight. Rapp P-v Cons Int Explor Mer. 1978;172:102–113. [Google Scholar]
- 40.McGurk MD. Natural mortality of marine pelagic fish eggs and larvae - Role of spatial patchiness. Mar Ecol Prog Ser. 1986;34:227–242. [Google Scholar]
- 41.Booman C, Dalen J, Leivestad H, Levsen A, van der Meeren T et al. Undersokelser ved Havforskningsinstituttet og Zoologisk Laboratorium UIB. Rapport Fisken og Havet Nr. 3-1996. Bergen: Havforskningsinstituttet; 1996. Effekter av luftkanonskyting pa egg, larver og yngel. [Google Scholar]
- 42.Govoni JJ, West MA, Settle LR, Lynch RT, Greene MD. Effects of underwater explosions on larval fish: Implications for a coastal engineering project. J Coast Res. 2008;24:228–233. [Google Scholar]
- 43.Caltrans 2010. Mad River Bridges Replacement Project. Effects of pile driving sound on juvenile steelhead. San Francisco: Caltrans. Available: http://www.dot.ca.gov/hq/env/bio/files/madriver_cagedfsh.pdf. Accessed: 2011 Sep.
- 44.Ruggerone GT, Goodman S, Miner R. 2008. Behavioral response and survival of juvenile Coho salmon exposed to pile driving sounds. NRC report prepared for the Port of Seattle. Available: ftp://ftp.odot.state.or.us/techserv/geo-environmental/Biology/Hydroacoustic/References/Literature%20references/GRuggerone.pdf. Accessed: 2011 Sep.