Abstract
Vision provides a primary sensory input for food perception. It raises expectations on taste and nutritional value and drives acceptance or rejection. So far, the impact of visual food cues varying in energy content on subsequent taste integration remains unexplored. Using electrical neuroimaging, we assessed whether high- and low-calorie food cues differentially influence the brain processing and perception of a subsequent neutral electric taste. When viewing high-calorie food images, participants reported the subsequent taste to be more pleasant than when low-calorie food images preceded the identical taste. Moreover, the taste-evoked neural activity was stronger in the bilateral insula and the adjacent frontal operculum (FOP) within 100 ms after taste onset when preceded by high- versus low-calorie cues. A similar pattern evolved in the anterior cingulate (ACC) and medial orbitofrontal cortex (OFC) around 180 ms, as well as, in the right insula, around 360 ms. The activation differences in the OFC correlated positively with changes in taste pleasantness, a finding that is an accord with the role of the OFC in the hedonic evaluation of taste. Later activation differences in the right insula likely indicate revaluation of interoceptive taste awareness. Our findings reveal previously unknown mechanisms of cross-modal, visual-gustatory, sensory interactions underlying food evaluation.
Introduction
One of the properties of food accounting for its palatability is energy density, i.e. calorie content. The energetic content of visually presented food is evaluated in an automatic and fast manner [1], [2] corroborating the greater motivational salience of high-calorie food [3]. There is evidence that the mere viewing of food images activates taste-related brain areas and elicits expectations about the taste and hedonic aspects of the food [1], [3], [4]. This is not surprising because visual cues constitute a primary sensory input indicating the pre-ingestive availability and palatability of food.
Notwithstanding, the sense of taste provides a major input for food perception. When presented alone, taste activates a cortical network including the insula/frontal operculum (FOP), orbitofrontal cortex (OFC), and anterior cingulate cortex (ACC) [5]–[10]. The human insula is involved in the processing of various sensory food properties including taste [11], oral texture [12], and oral temperature [13], but it is also believed to integrate multisensory information to establish an emotionally relevant context for sensory experience [14]. The OFC has been particularly associated with hedonic aspects of sensory experience, the processing of food reward, and positive reinforcement irrespective of stimulus modality [9], [15]–[19].
In humans, responses of the insula are correlated with the subjective intensity of taste, while those of the OFC are correlated with the subjective pleasantness of taste [20]. Yet, when altering the value of a sweet taste stimulus using an aversive cue, taste representation has been found modified already at an early processing level, as indicated by suppressed responses to the tastant in rat primary gustatory cortex [21]. Similarly, it has been proposed that the primary gustatory cortex jointly encodes both the chemical identity and palatability of a tastant [22] suggesting a role of the insula in the evaluation of taste or its precursors beyond mere sensory processing.
The consequences of visual energy cues on taste processing remain unexplored. Therefore, using electro-encephalography (EEG), we investigated whether images of high- and low-calorie food differentially influence the processing of subsequent electric tastes. Electric taste, as generated by a small current on the tongue, elicits event-related potentials (ERPs) with the first positive peak occurring around 130 ms, followed by a central negativity at around 220 ms and a long-lasting central-parietal positivity [23]. Similar waveforms have been described in ERP studies using chemical tastants, with yet a variable timing depending upon the stimulus intensity and quality as well as the device used for its delivery [24]–[29]. Information about topography of the peaks obtained with chemical tastants is scarce as taste ERP studies only used a small number of electrodes distributed on the central midline or in the fronto-central region [30].
Electric taste is unique in that it is hedonically neutral – neither very pleasant nor unpleasant – and its quality is unrelated to any food image content. In addition, when presented at detection threshold level, electric taste activates a dynamic network encompassing key gustatory brain areas, namely the insula, the opercula, and the ventromedial OFC [23]. We hypothesize that high-calorie food cues enhance the hedonic evaluation of the consecutive taste and increase activation in reward-related cortical regions.
Materials and Methods
Participants
Fourteen healthy participants (9 males), aged 22–30 (mean 25.4 yrs), were recruited and paid for participation. Participants went through a medical screening and signed informed consent prior to the experiment. The study protocol was approved by the local ethics board (“Commission cantonale d'éthique de la recherche sur l'être humain”, Lausanne, Switzerland) and conformed to the revised Declaration of Helsinki.
Stimuli and procedures
The visual stimuli were 150 photographs depicting 100 food items and 50 non-food kitchen utensils [2], [31]. The food images were subdivided into high-calorie and low-calorie classes based on the energy content obtained from the United States Department of Agriculture (www.nal.usda.gov/fnic) and the Swiss nutritional database (released by the Swiss Federal Office of Health and the Swiss Federal Institute of Technology Zurich). The energy content of the low-calorie and high-calorie group ranged from 12–151 kcal/100 g (mean = 66.18, SEM = 6.50) and 160–717 kcal/100 g (mean = 352.50, SEM = 17.32), respectively. The caloric content of the high-calorie foods was significantly greater than that of the low-calorie foods (t2,98 = 15.48, p<0.001).
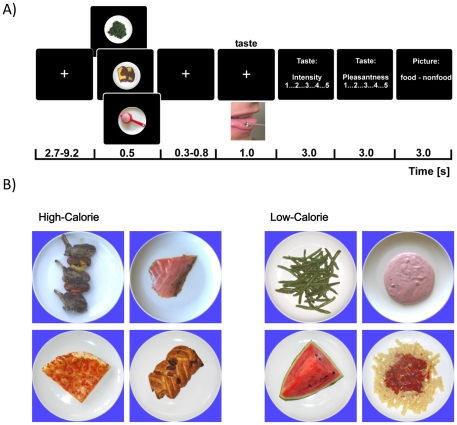
The taste stimuli were single square wave anodal pulses of 1000 ms duration applied via a stainless steel electrode (approx. 5 mm in diameter) connected to the RION TR-06 gustometer (Sensonics, Inc., NJ, USA). The electrode (anode) was placed on the anterior edge of the tongue surface; the cathode was placed on the left upper arm. Participants held the electrode gently between their teeth while keeping the mouth closed. The electrode remained on the tongue throughout experimental blocks to minimize thermal and tactile effects from the placement [23]. The taste intensity was set for each participant to the individual threshold and kept constant throughout the experiment. Taste detection thresholds were assessed via a computer-controlled ascending staircase procedure. For this, the intensity was increased in steps of 2 db until the first correct trial. Reversal points were determined by three correct responses at a given concentration when moving up the staircase and a single incorrect response when moving down the staircase. After five reversals or a maximum of 40 trials, the threshold was defined. Only participants whose thresholds could be unequivocally determined and who reported a clear taste sensation, using an established list of descriptors [32], were included in the EEG study. A schematic view of a trial is shown in Figure 1. Participants viewed a central white fixation cross on a black background presented on a 17″ TFT screen at a 80 cm distance. Fixation was followed by an image of either of three image categories (high-calorie foods, low-calorie foods or non-food) that was presented for 500 ms. Following a randomized inter-stimulus interval of 300–800 ms, the electric taste stimulus at individual threshold intensity was delivered for 1000 ms. Each taste stimulus was evaluated for its intensity and pleasantness on a 5-point Likert scale ranging from 1 to 5 immediately after stimulus offset. Participants were blind to the fact that all taste stimuli were identical throughout the experiment. On each trial, participants had to categorize images into food vs. non-food objects. Thus, participants remained naïve with respect to the implicit high- vs. low-calorie subcategory of food images. The taste onset asynchrony varied between 14–20 s. The experiment was separated by breaks during which participants ingested 30 ml of mineral water (Nestlé Aquarel) to minimize effects of varying oral moisturization. Furthermore, participants refrained from eating and drinking (only water was allowed) two hours before the testing and additionally rinsed their mouth before the start of the experiment.
Figure 1. Experimental design.
(A) Each trial started with the presentation of a fixation cross followed by an image of either a high-calorie or a low-calorie food or a non-food item. Following a variable inter-stimulus interval, a neutral taste stimulus was presented. After the offset of the taste, participants had to first rate the taste intensity and pleasantness and then categorize the image preceding the taste into food vs. non-food items. They were naïve as to the task-irrelevant, i.e. high- and low-calorie image categories. (B) To illustrate the variety of high-calorie stimuli (160–717 kcal/100 g) and low-calorie stimuli (12–151 kcal/100 g), four pictures of each category are presented. High-calorie: Lamb-chops, Salmon, Pizza, Pastry. Low-calorie: Beans, Water melon, Yoghurt, Pasta w. Tomato Sauce.
Data acquisition and pre-processing
The experiment was conducted in an electrically shielded and sound-attenuated recording booth. EEG was continuously recorded with a BioSemi Active-Two amplifier system (BioSemi, Amsterdam, The Netherlands) using 64 Ag/AgCl active electrodes mounted in an elastic cap and placed according to the international extended 10–20 system. During the recordings, the signals were referenced to CMS (common mode sense), while DRL (driven right leg) served as ground (for details see http://www.biosemi.com/faq/cms&drl.htm). Data were recorded with a sampling rate of 512 Hz and analog filtered (0.06 and 200 Hz). The continuous EEG signal was stored on hard disk for off-line analysis. EEG data were processed using EEGLAB [33] running under MATLAB (Mathworks, Natick, MA, USA) and Cartool (http://sites.google.com/site/fbmlab/cartool). Data were segmented into epochs from −500 to 1000 ms relative to the onset of the taste stimulus. Epochs with unique, non-stereotypic artifacts were manually rejected. Then, extended infomax ICA, as implemented in EEGLAB, was applied to the remaining concatenated single trials. Independent components representing common EEG artifacts such as eye blinks were visually identified and removed along with those components representing electrical stimulation artifacts [23]. Back-projected single trials were again screened for residual artifacts. On average, 4% of all trials were rejected. Data were then re-referenced to the average reference of all channels, the baseline (−200 to 0 ms) was subtracted, and a 30 Hz low-pass filter was applied.
EEG data analyses
We used a global data-driven topographic neuroimaging approach [34] to examine the spatio-temporal brain mechanisms contributing to effects of high- vs. low-calorie food viewing on subsequent taste processing. All EEG results reported are relative to the physically identical taste stimulation preceded by food images.
ERP analyses
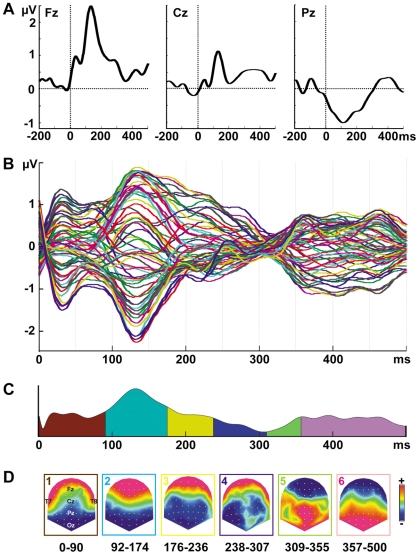
In a first step, event-related potentials (ERPs) were computed for single electrodes and plotted, averaged across experimental conditions and participants, to visualize the ERP waveform data (Fig. 2A and B).
Figure 2. Event-related responses.
(A) Exemplary group-averaged ERP waveforms from electrode Fz, Cz, and Pz. Taste onset is at time 0 ms. (B) Time course of group-averaged ERP waveforms from 64 electrodes to the taste stimuli (taste onset = at 0 ms) averaged across all conditions. (C) The temporal extent of the topographic map series is indicated as colored segments under the global field power curve. (D) Topographic maps (scaled to the maximum; top view) correspond to the temporal succession of segments in (C). Hot and cold colors reflect positive and negative ERP amplitudes, respectively.
Topographic cluster analysis
Grand-averaged ERP waveforms from 0–500 ms after taste onset were clustered into periods of common topography to identify the predominant topographic maps and their temporal sequence using a topographic atomize and agglomerate hierarchical cluster (T-AAHC) analysis [35] as implemented in the Cartool software. Model parameters were set such that clusters spatially correlating above 95% were merged. We further applied the constraint that each topographic template map has to be observable for at least 20 ms in the group-averaged data. The optimal number of topographic template maps was determined using a modified Krzanowski-Lai criterion [36]. The cluster analysis provides a data-driven means to summarize ERP data by a limited number of topographic maps and their temporal occurrence.
Source estimation (LAURA)
The stable ERP periods as obtained from the topographic cluster analysis were used to define the time windows of interest for statistical comparisons in source space. Relevant statistical comparisons were computed between taste stimuli preceded by high-calorie vs. low-calorie cues. Neural sources of the taste EEG responses were estimated using the local autoregressive average (LAURA) distributed linear inverse solution [37]. The lead field was calculated on a spherical head model with anatomical constraints (SMAC model) [38]; it contained 3005 solution points equally distributed within the gray matter of the cerebral cortex and limbic structures of the Montreal Neurological Institute's (MNI) 152 average brain. The inverse solution was first estimated for each of the 3005 nodes for each time window of interest determined by the topographic cluster analysis, so that scalar values (indicating activation strength in µV/mm3) could be extracted from each node. Then, paired Student t-tests were calculated at each source space node using the variance across time points within the period of interest. In order to correct for multiple comparisons, effects were considered significant only when a minimum of 12 neighboring nodes was observed with p≤0.001 (t1,13≥5.5). The results of the source estimations were then averaged across time points of the respective period of interest and the average was rendered on the MNI brain with the Talairach and Tournoux coordinates of the maximal statistical differences indicated [39].
Pearson's correlation coefficients were used to test for associations between the changes in brain activation induced by high- vs. low-calorie food viewing on subsequent taste perception and changes in participant's taste ratings between these conditions. The analysis was spatially focused on the key brain areas that have been previously associated with taste valuation, namely the OFC [20] and the insula [22]. Activations within the insula/opercula and OFC/ACC were observed only during the 92–174 ms period (left and right insula), during the 176–236 ms period (OFC/ACC), and during the 357–500 ms period (right insula). The maximum neural source strength was thus extracted for each participant from these areas during the time windows identified. In other words, the source node exhibiting the maximum activity for taste preceded by high- and low-calorie image viewing within these regions and during these time periods was selected for each participant. Then, the difference in source activation between taste stimuli preceded by high- and low-calorie food cues was calculated. Finally, the correlation between the difference in pleasantness ratings and the difference in the source strength was computed for each of the four combinations of area and time periods of interest. The α level was a priori set to 0.05.
Results
Behavioral data
The mean taste detection threshold was 7.1 db (range: −6 to 12 db), which is within the normal range [40]. In line with our previous findings [23], [41], participants reported predominantly metallic (32%), sour (16%) and rusty (11%) tastes during the threshold assessment. Participants' ratings for taste pleasantness and intensity during the EEG sessions were compared using Wilcoxon signed-rank tests. Overall, subjects reported a neutral, slightly pleasant, experience in response to taste stimuli with mean pleasantness scores above 2.5 on the 5-point rating scales. Taste stimuli were perceived significantly more pleasant (Z = −2.43, p = 0.01; Wilcoxon singed-rank test, 2-tailed) when preceded by images of high-calorie food (mean = 2.9, SEM = 0.15) than images of low-calorie food (mean = 2.75, SEM = 0.14). Perceived taste intensity was marginally augmented when preceded by high-calorie vs. low-calorie food pictures but this augmentation failed to reach significance (Z = −1.73, p = 0.09; Wilcoxon singed-rank test, 2-tailed).
ERP analyses
Figure 2A depicts the group-averaged ERP waveforms evoked by the taste at 64 electrodes. Six ERP map clusters accounting for 96.5% of the variance were identified (Fig. 2C). The temporal extent of each topographic map is indicated as colored segments under the global field power (GFP) curve. The topographic maps corresponding to each segment are displayed in Figure 2D. Map 1 was observed from 0–90 ms relative to taste onset and was characterized by a centro-posterior negativity and a fronto-lateral positivity. During the period of Map 2 (92–174 ms), a pronounced deflection with a maximum over frontal and a minimum over posterior electrodes constituted the P1 ERP component. The P1 was followed by a negative component, corresponding to the N1, with a minimum over posterior and central electrodes (Map 3, 176–236 ms). After two transition maps between 238–355 ms, a late positive complex (LPC) was observed with the maximum over posterior and parietal electrodes (Map 6, 357–500 ms).
Effects of high-calorie versus low-calorie food cues on taste processing
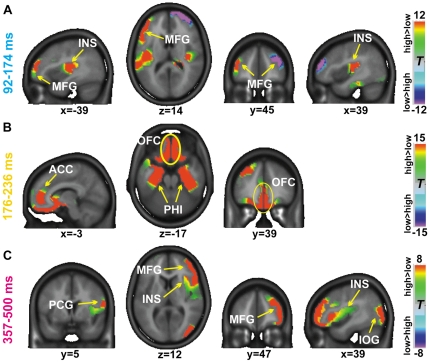
To identify brain areas that reveal differential activation as a function of the image category preceding the taste, we contrasted the neural source activity during taste perception following the viewing of high-calorie food as opposed to low-calorie food images. For this purpose, the obtained stable topographic cluster periods (cf. Figure 2) served as input for the neural source estimation algorithm (LAURA). Significant differences between taste perception following high-calorie and low-calorie food viewing were obtained over the three time intervals of interest, i.e. from 92–174 ms, from 176–236 ms and 357–500 ms. Figure 3 illustrates these differences with the Talairach coordinates of activation difference maxima indicated. Positive t-values in Figure 3 evince higher neural source activity when the taste stimuli were preceded by images depicting high-calorie food as compared to low-calorie food images and vice versa.
Figure 3. LAURA source estimations.
Statistical contrasts of LAURA source estimations computed for taste stimuli when preceded by high-calorie versus low-calorie images. Time periods were obtained from the topographical cluster analysis. Color scales represent t-values with positive values indicating stronger activity during taste perception preceded by high-calorie than low-calorie images and negative values indicating increased activity when taste was preceded by low- as compared with high-calorie images. MFG = middle frontal gyrus, INS = insula, ACC = anterior cingulate cortex, OFC = orbitofrontal cortex, PHI = parahippocampal gyrus, PCG = postcentral gyrus, IOG = inferior occipital gyrus.
Over the time interval of Map 2 presence (92–174 ms; cf. Figure 2), we observed higher activity in the transition between the right insular gyrus and FOP transition (maximum x,y,z coordinates: 35, −22, 15; t = 13.92), as well as in the left FOP (−44, −16, 14; t = 13.88) when high-calorie as compared to low-calorie images preceded the taste. Furthermore, the left middle frontal gyrus (−47, 32, 19; t = 17.07) and the right parahippocampal gyrus (0, −34, −9; t = 14.04) yielded stronger activation to taste preceded by high-calorie viewing. In contrast, the right middle frontal gyrus (22, 63, 14; t = −18.91) responded stronger to taste stimuli preceded by low calorie images than high calorie images.
Over the consecutive time period of Map 3 presence (176–236 ms), prominent differential activity was observed in the medial orbitofrontal gyrus (−3, 39, −13; t = 19.75) and the adjacent ACC. Moreover, the left superior and middle frontal gyrus (−27, 49, 26; t = 19.65) exhibited stronger activation when following high-calorie as opposed to low-calorie food images preceded the taste stimulus. In both hemispheres, the parahippocampal and fusiform area (left: −26, −6, −25; t = 16.02; right: 26, −6, −25; t = 13.55) and adjacent uncus yielded significantly stronger activation when high- vs. low-calorie food was presented prior to the taste.
Between 357–500 ms (interval of Map 6 presence), major clusters of activation differences were observed within the right middle frontal gyrus (49, 45, 3; t = 12.35), the right insula (35, 9, 9; t = 7.40) and the parietal operculum and postcentral gyrus (63, −21, 30; t = 10.30), as well as in the right inferior occipital gyrus (43, −79, 12; t = 11.17). In all these regions, neural source strength was higher when high-calorie foods were viewed before taste stimulation as opposed to low-calorie foods.
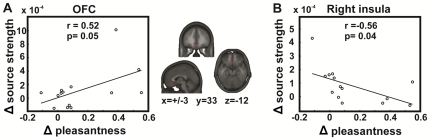
Associations between activation differences to taste preceded by high- vs. low-calorie food cues in regions of interest (insula/FOP and medial OFC) and respective alterations in taste pleasantness ratings were determined over the 92–174 ms (bilateral insula/FOP), the 176–236 ms (medial OFC), and the 357–500 ms (right insula/FOP) time intervals. Initial food cue-induced activation differences in the right and left insula (92–174 ms) were not significantly correlated with the differences in taste pleasantness. However, the activation differences observed in the medial OFC over the successive time period from 176–236 ms correlated positively with changes in taste pleasantness (Figure 4; r = 0.52, p = 0.05). The activation maxima converged across participants at the Talairach coordinates x = +/−3, y = 33, z = −12. Moreover, over the 357–500 ms interval, the source strength differences derived from individuals' activation maxima within the right insula/FOP correlated negatively with changes in taste pleasantness (r = −0.55, p = 0.04).
Figure 4. Correlations between changes in source strength and taste pleasantness ratings.
Correlations were determined between changes in brain activation (i.e. high-calorie minus low-calorie) in the OFC and right anterior insula and changes in participant's taste ratings (i.e. high-calorie minus low-calorie). The source strength was determined at the voxel exhibiting the maximum activity within the region of interest for each participant during the 176–236 ms period for the OFC and during the 357–500 ms period for the right insula.
Discussion
Our results show that the viewing of high-calorie foods increases the pleasantness and related cortical activation of subsequent hedonically neutral taste, relative to low-calorie food items. Most importantly this was observed with constant physical characteristics of taste stimulation throughout the experiment. The changes in neural activation encompassed the insula and FOP, ventral prefrontal cortex (including OFC), and ACC, i.e. regions previously associated with the hedonic evaluation of food. In particular, high-calorie cues induced an early modulation of the taste response in the insula/FOP within ∼100 ms, followed by alterations in the OFC at ∼180 ms, and in the insula/FOP around 360 ms, always relative to taste onset. Within 180 ms after taste onset, increases in pleasantness ratings of the taste preceded by high-calorie versus low-calorie cues were positively correlated with changes in activation strength in the OFC. Increased pleasantness was furthermore inversely related to activity in the right insula/FOP from 357–500 ms, possibly indicating a revaluation of the taste percept. These findings provide novel and temporally fine-grained information on the involvement of particularly the insula and OFC in the modulation of taste perception and evaluation by visual calorie cues.
Hemodynamic imaging studies have linked insular activation predominantly with subjective taste intensity while modulations in the OFC have been mostly related to taste pleasantness [13], [42]. Moreover, evidence suggests that both the insula and OFC serve as convergence zones for the representation and integration of information across senses. For example, the insula has been associated with bodily interoception based on multimodal cues [43]–[45]. On the other hand, the OFC has been directly related to the integration of visual and gustatory information [46] as well as food valuation and selection [47], [48].
Our current results show a dynamic and fast interplay of the insula, orbitofrontal, and anterior cingulate regions pointing toward an interactive integration of visual energy cues and taste. Given the established anatomical connectivity between the insula, ACC, and frontal and superior temporal cortex in primates [49], [50] and between the insula and ACC in humans [51] such finding seems reasonable. Despite the need to further elaborate on the anatomical connections, functional studies agree that the insula, OFC, and ACC are involved in reward-related processing including food and taste.
The differences in initial insular activation (92–174 ms) observed here were not correlated with changes in taste pleasantness and thus possibly reflect the formation of a precursor for valuation rather than valuation itself. In line with our hypothesis, research has demonstrated that taste representations can already be modulated by contextual cues during early levels of encoding [21]. Unexpectedly, the early effect in our study occurred without concomitant changes in taste intensity although previous studies have linked modulations of insular activity with perceived taste intensity [13], [52]. Most likely, differences between studies are due to the threshold approach taken to detect activations in statistical parametric maps. However, it has also been suggested that separate representations of the sensory and hedonic aspects of taste are hosted in the insula [46], [53]. As highlighted, the taste in our study was physically constant and the early insular activation differences changed as a function of the calorie content of the visual food cue without an association with changes in perceived taste intensity and/or taste pleasantness. Thus, this effect might also be related to expectations about the imminent taste, elicited by the visual cue. This alternative explanation of our findings is corroborated by a recent observation from functional neuroimaging that expectations about taste modulate activity in the insula [54]. We further propose that the differential activations in the left middle frontal cortex over the same time interval designate integration [55] of the visual energy cue and the taste stimulus, mediated by anatomical connections between the frontal and the anterior cingulate cortex.
Over the time interval from 176–236 ms, greatest activation differences for taste sensations altered by visual food cues were found in the OFC and adjacent ACC. These areas are involved in the hedonic evaluation of taste [16], [18], [56]. In addition, Grabenhorst and co-workers (2008) reported activation in the OFC but not in the insula, when taste pleasantness increased after the presentation of a positive word label [20]. In line with this, the taste activation differences in our study were associated with augmentations in taste pleasantness between taste stimulation preceded by high- vs. low-calorie food cues, and can be interpreted as the first level of processing that directly relates to the augmented hedonic experience.
Previously, it has been shown that the insula and adjacent opercula discriminate not only taste qualities but also tastes of different hedonic value [54], [56], [57]. Our findings especially evince that changes in pleasantness for tastes preceded by high- vs. low-calorie food cues co-vary with smaller changes in right insula/FOP activation (357–500 ms). This is consistent with the data by Nitschke and co-workers (2006) showing greater activations in the right insula when an aversive taste was perceived as more unpleasant than it actually was due to modulations of expectancy by non-verbal visual cues. In other words, the authors found that the right insula responds to aversive and unpleasant tastes, and that an increase in pleasantness, i.e. a decrease in unpleasantness in Nitschke's study (2006), of physically identical tastes induced by visual context cues leads to decreased activation in this region. In contrast, Nitschke could not show that pleasant tastes that were perceived as more pleasant following a visual cue yielded changes in insular activations. The authors attributed the lack of result to their study design, i.e. the randomized presentation of pleasant and unpleasant tastes. In our study, increases in pleasantness of a neutral, slightly positive, taste following high-calorie visual cues coincide with dampened activation in the right insula that, in turn, reduces the source strength differences to tastes preceded by high-calorie as opposed to low-calorie cues. We speculate that this effect reflects a revaluation of interoceptive taste awareness based on changes in the hedonic appraisal of the visual context cue or the diminution of aversive feelings, which is supported by findings showing that the anterior insula is implicated in coding disgust and in the experience of emotion as it is part of an emotional ‘salience’ network [45], [58].
Further prominent and sustained activations were observed in the parahippocampal gyrus (PHI), which has recently been proposed to mediate visual-contextual associations [59], [60]. Together with the observed increases in activation in the visual cortex for combinations of high-calorie cues and taste our data possibly indicate that the PHI also mediates visual-gustatory associations. However, we cannot exclude that PHI activations are due to the behavioral food – non-food discrimination task that participants had to perform after taste stimulation.
Why did we use electric taste instead of common taste qualities, e.g. a sweet solution? Electric taste is perceived as neutral in terms of its pleasantness when presented at the level of individual detection thresholds and it induces brain activations in gustatory areas (bilateral anterior insula, medial orbitofrontal cortex) [23]. While electric taste stimulation may limit the generalization of our findings to other tastes qualities, it yet provides a unique means to assess effects of visual food cues without confounds due to learned food-taste associations as they exist for basic tastes. Future studies should develop designs suitable to assess the relevance of our findings in settings in which participants interact, for example, with visual cues (e.g., advertisements) during the consumption of ecologically relevant taste stimuli or even complex foods. Such a design is hampered mainly by technical difficulties in stimulus control and the relatively high number of stimulus repetitions required for ERP analyses.
In conclusion, the present results provide evidence that high-calorie food cues enhance the hedonic evaluation of subsequently presented tastes. We suggest that the early calorie-dependent alterations in taste perception encompassing primary taste areas point to their role in taste evaluation in the presence of and/or as a consequence of visual representations of high-calorie foods. Later activation differences in the OFC and in the right insula were found to mediate subjective pleasantness likely through an enhancement of taste hedonics and revaluation of interoceptive taste awareness, respectively. The present study thus provides novel insights into cross-modal sensory interactions underlying taste and, in extension, probably also food evaluation and consumption. Future studies will have to elucidate to what extent the brain regions shown to be involved in visual-gustatory interactions could account for regulation of appetite and food intake control in real world settings.
Acknowledgments
The current address of Kathrin Ohla is at the Monell Chemical Senses Center, Philadelphia, PA, USA (kohlamonell.org). The Cartool software (http://sites.google.com/site/fbmlab/cartool) has been programmed by Denis Brunet, from the Functional Brain Mapping Laboratory, Geneva, Switzerland, and is supported by the Center for Biomedical Imaging (CIBM) of Geneva and Lausanne.
Footnotes
Competing Interests: The authors have read the journal's policy and have no conflicts. KO was and JlC and JH are employees of Nestec Ltd.. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: The present research was funded by the Nestlé Research Center, Lausanne, Switzerland, which, through the employment of KO, JlC and JH, was involved in conceiving, designing and performing the experiments, and analyzing the data. The management of Nestlé Research approved the submission and publication of this manuscript.
References
- 1.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, et al. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 2.Toepel U, Knebel JF, Hudry J, le Coutre J, Murray MM. The brain tracks the energetic value in food images. Neuroimage. 2009;44:967–974. doi: 10.1016/j.neuroimage.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Frank S, Laharnar N, Kullmann S, Veit R, Canova C, et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 4.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, et al. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- 6.Faurion A, Cerf B, Le BD, Pillias AM. fMRI study of taste cortical areas in humans. Ann N Y Acad Sci. 1998;855:535–545. doi: 10.1111/j.1749-6632.1998.tb10623.x. [DOI] [PubMed] [Google Scholar]
- 7.Frey S, Petrides M. Re-examination of the human taste region: a positron emission tomography study. Eur J Neurosci. 1999;11:2985–2988. doi: 10.1046/j.1460-9568.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- 8.Kinomura S, Kawashima R, Yamada K, Ono S, Itoh M, et al. Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Res. 1994;659:263–266. doi: 10.1016/0006-8993(94)90890-7. [DOI] [PubMed] [Google Scholar]
- 9.Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, et al. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999;10:7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- 10.Zald DH, Lee JT, Fluegel KW, Pardo JV. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998;121(Pt 6):1143–1154. doi: 10.1093/brain/121.6.1143. [DOI] [PubMed] [Google Scholar]
- 11.Schoenfeld MA, Neuer G, Tempelmann C, Schussler K, Noesselt T, et al. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience. 2004;127:347–353. doi: 10.1016/j.neuroscience.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 12.de Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guest S, Grabenhorst F, Essick G, Chen Y, Young M, et al. Human cortical representation of oral temperature. Physiol Behav. 2007;92:975–984. doi: 10.1016/j.physbeh.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham WA, Zelazo PD. Attitudes and evaluations: a social cognitive neuroscience perspective. Trends Cogn Sci. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 17.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 18.McCabe C, Rolls ET. Umami: a delicious flavor formed by convergence of taste and olfactory pathways in the human brain. Eur J Neurosci. 2007;25:1855–1864. doi: 10.1111/j.1460-9568.2007.05445.x. [DOI] [PubMed] [Google Scholar]
- 19.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 20.Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18:1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- 21.Grossman SE, Fontanini A, Wieskopf JS, Katz DB. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci. 2008;28:2864–2873. doi: 10.1523/JNEUROSCI.4063-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr, Nicolelis MA, et al. Neural ensemble coding of satiety states. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Ohla K, Hudry J, le Coutre J. The cortical chronometry of electrogustatory event-related potentials. Brain Topogr. 2009;22:73–82. doi: 10.1007/s10548-009-0076-7. [DOI] [PubMed] [Google Scholar]
- 24.Franken IH, Huijding J, Nijs IM, van Strien JW. Electrophysiology of appetitive taste and appetitive taste conditioning in humans. Biol Psychol. 2011;86:273–278. doi: 10.1016/j.biopsycho.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Hummel T, Genow A, Landis BN. Clinical assessment of human gustatory function using event related potentials. J Neurol Neurosurg Psychiatry. 2010;81:459–464. doi: 10.1136/jnnp.2009.183699. [DOI] [PubMed] [Google Scholar]
- 26.Kobal G. Gustatory evoked potentials in man. Electroencephalogr Clin Neurophysiol. 1985;62:449–454. doi: 10.1016/0168-5597(85)90055-3. [DOI] [PubMed] [Google Scholar]
- 27.Min BC, Sakamoto K. Influence of sweet suppressing agent on gustatory brain evoked potentials generated by taste stimuli. Appl Human Sci. 1998;17:9–17. doi: 10.2114/jpa.17.9. [DOI] [PubMed] [Google Scholar]
- 28.Min BC, Wada M, Sakamoto K. New apparatus stimulating the region of tongue innervated by glossopharyngeal nerve and its application to monosodium glutamate (MSG) solution. Appl Human Sci. 1998;17:67–71. doi: 10.2114/jpa.17.67. [DOI] [PubMed] [Google Scholar]
- 29.Mizoguchi C, Kobayakawa T, Saito S, Ogawa H. Gustatory evoked cortical activity in humans studied by simultaneous EEG and MEG recording. Chem Senses. 2002;27:629–634. doi: 10.1093/chemse/27.7.629. [DOI] [PubMed] [Google Scholar]
- 30.Ohla K, Busch NA, Lundström JN. Time for taste - A review of the early cerebral processing of gustatory perception. Chem Percept. 2011 doi: 10.1007/s12078-011-9106-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knebel JF, Toepel U, Hudry J, le Coutre J, Murray MM. Generating controlled image sets in cognitive neuroscience research. Brain Topogr. 2008;20:284–289. doi: 10.1007/s10548-008-0046-5. [DOI] [PubMed] [Google Scholar]
- 32.McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, et al. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44:379–387. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Murray MM, Brunet D, Michel CM. Topographic ERP analyses: a step-by-step tutorial review. Brain Topogr. 2008;20:249–264. doi: 10.1007/s10548-008-0054-5. [DOI] [PubMed] [Google Scholar]
- 35.Tibshirani R, Walther G, Botstein D, Brown P. Cluster validation by prediction strength. J Comput Graphical Stat. 2005;14:511–528. [Google Scholar]
- 36.Krzanowski W, Lai YT. A criterion for determining the number of groups in a data set using sum of square clustering. Biometrics. 1985;44:23–34. [Google Scholar]
- 37.Grave de Peralta MR, Murray MM, Michel CM, Martuzzi R, Gonzalez Andino SL. Electrical neuroimaging based on biophysical constraints. Neuroimage. 2004;21:527–539. doi: 10.1016/j.neuroimage.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 38.Spinelli L, Andino SG, Lantz G, Seeck M, Michel CM. Electromagnetic inverse solutions in anatomically constrained spherical head models. Brain Topogr. 2000;13:115–125. doi: 10.1023/a:1026607118642. [DOI] [PubMed] [Google Scholar]
- 39.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 40.Loucks CA, Doty RL. Effects of stimulation duration on electrogustometric thresholds. Physiol Behav. 2004;81:1–4. doi: 10.1016/j.physbeh.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Ohla K, Toepel U, le Coutre J, Hudry J. Electrical neuroimaging reveals intensity-dependent activation of human cortical gustatory and somatosensory areas by electric taste. Biol Psychol. 2010;85:446–455. doi: 10.1016/j.biopsycho.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Grabenhorst F, Rolls ET, Margot C, da Silva MA, Velazco MI. How pleasant and unpleasant stimuli combine in different brain regions: odor mixtures. J Neurosci. 2007;27:13532–13540. doi: 10.1523/JNEUROSCI.3337-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 44.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, et al. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- 46.Critchley HD, Rolls ET. Olfactory neuronal responses in the primate orbitofrontal cortex: analysis in an olfactory discrimination task. J Neurophysiol. 1996;75:1659–1672. doi: 10.1152/jn.1996.75.4.1659. [DOI] [PubMed] [Google Scholar]
- 47.Rolls ET. Brain mechanisms underlying flavour and appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1123–1136. doi: 10.1098/rstb.2006.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zald DH. Orbitofrontal cortex contributions to food selection and decision making. Ann Behav Med. 2009;38(Suppl 1):S18–S24. doi: 10.1007/s12160-009-9117-4. [DOI] [PubMed] [Google Scholar]
- 49.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 50.Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 51.van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grabenhorst F, Rolls ET. Selective attention to affective value alters how the brain processes taste stimuli. Eur J Neurosci. 2008;27:723–729. doi: 10.1111/j.1460-9568.2008.06033.x. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste responses of cortical neurons in freely ingesting rats. J Neurophysiol. 1989;61:1244–1258. doi: 10.1152/jn.1989.61.6.1244. [DOI] [PubMed] [Google Scholar]
- 54.Nitschke JB, Dixon GE, Sarinopoulos I, Short SJ, Cohen JD, et al. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nat Neurosci. 2006;9:435–442. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- 55.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, et al. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- 57.O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 58.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- 60.Bar M, Aminoff E, Schacter DL. Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J Neurosci. 2008;28:8539–8544. doi: 10.1523/JNEUROSCI.0987-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]