Abstract
Toxin-antitoxin (TA) systems are widespread among the plasmids and genomes of bacteria and archaea. This work reports the first description of a functional TA system in Streptomyces that is identical in two species routinely used in the laboratory: Streptomyces lividans and S. coelicolor. The described system belongs to the YefM/YoeB family and has a considerable similarity to Escherichia coli YefM/YoeB (about 53% identity and 73% similarity). Lethal effect of the S. lividans putative toxin (YoeBsl) was observed when expressed alone in E. coli SC36 (MG1655 ΔyefM-yoeB). However, no toxicity was obtained when co-expression of the antitoxin and toxin (YefM/YoeBsl) was carried out. The toxic effect was also observed when the yoeBsl was cloned in multicopy in the wild-type S. lividans or in a single copy in a S. lividans mutant, in which this TA system had been deleted. The S. lividans YefM/YoeBsl complex, purified from E. coli, binds with high affinity to its own promoter region but not to other three random selected promoters from Streptomyces. In vivo experiments demonstrated that the expression of yoeBsl in E. coli blocks translation initiation processing mRNA at three bases downstream of the initiation codon after 2 minutes of induction. These results indicate that the mechanism of action is identical to that of YoeB from E. coli.
Introduction
Toxin-antitoxin (TA) modules were originally identified as plasmid maintenance or stability modules [1]. Later, such modules were described as being very abundant in the genome of different bacteria and archaea [2]. The role of these systems in the genome is not clear; however, they have been reported to act as guardians against DNA lost, serve as protection against invading DNA, and involved in stress management either through programmed cell death of a wide part of the population or contributing to the origin of persister cells by inducing a dormant stage (stasis) that permit to the cells to be highly tolerant to antibiotics [2], [3].
Regarding the nature and action of the antitoxin, three types of TA systems -class I, II, and III- have been described, class II being the most abundant [4]. This class II of TA systems comprises two small proteins, which act as a toxin-antitoxin complex in which the toxin is inactivated by the antitoxin. The efficiency of these TA systems depends on a difference in lifespan between the toxin and antitoxin. While toxins are highly resistant to proteases, the lifespan of antitoxin is shorter than the toxin owing to their high susceptibility to protease activity. When the toxins are released from the complex they produce their toxic effect through different modes of action: acting as endoribonucleases, poisoning DNA gyrase, inhibiting translation initiation or elongation or inducing defects in cell wall synthesis [5], [6]. Mainly, antitoxin counteracts toxin activity by direct protein-protein interaction and also by repressing transcription of the TA system through interaction with palindromic sequences within the promoters. In this regulation of their own promoter, toxins act as co-repressors, cooperatively improving the DNA interaction. However, in the case of three-component systems, antitoxin and DNA-binding activities are encoded by two separated proteins [7], [8], [9].
TA systems have been studied mostly in Gram-negative bacteria and, as usual, the organism studied in greatest depth is Escherichia coli, in which at least 33 TA systems have been identified [10]. The availability of a large number of genomes and the use of bioinformatics tools have permitted the identification of a huge number of putative TA systems in different microorganisms [11]; these have been completed in later studies and are accessible at some servers such as: http://genoweb.univ-rennes1.fr/duals/RASTA-Bacteria/ [12] and http://bioinfo-mml.sjtu.edu.cn/TADB/ [13]. In regards to the structure of these proteins and their activities, up to 12 toxin super-families and 20 antitoxin super-families have been described and validated [9].
Our work started after the prediction of the existence of three putative TA loci in the chromosome of Streptomyces coelicolor by Pandey and Gerdes [11]. The present study is the first experimental demonstration of the functionality of one of these systems in S. lividans, and in S. coelicolor. The orthologous genes of both organisms are identical, even in the promoter region, and correspond to an operon that encodes the proteins identified under the NCBI accession numbers ZP_06531415 and ZP_06531416 in S. lividans and to the proteins encoded by the operon formed by the SCOs2235/2236 from S. coelicolor. The system shows considerable similarity to the YefM/YoeB system from E. coli, composed of the protein YefM, which is an unstable antitoxin, and YoeB, which is a stable toxin. E. coli YefM/yoeB form a heterotrimer (toxin∶antitoxin 1∶2) that inactivates the effect of the toxin [14]. Free toxin acts by inhibiting translation initiation by associating directly with the 50S ribosome subunit. In particular, it interacts with the A site, originating mRNA cleavage and releasing the 3′-end portion of the mRNA from the ribosome [15].
Here we show that the introduction of the S. lividans toxin gene (yoeBsl) in multicopy plasmids is toxic in E. coli SC36, in S. lividans, and in S. coelicolor wild types strains and that its effect is reversed by the co-expression of the antitoxin gene (yefMsl). S. coelicolor (ΔyefM/YoeB) and S. lividans (ΔyefM/yoeB) null mutants have greater sensitivity to the toxin expression than the wild-type strains and the expression of a single copy of the corresponding gene is lethal. Purification of the S. lividans antitoxin (YefMsl), toxin (YoeBsl), and the TA complex (YefM/YoeBsl) allowed us to observe the specific binding of this complex to the promoter region of the bicistronic operon. In vivo experiments demonstrated that the toxin YoeBsl acts inhibiting translation initiation by processing the mRNA at three bases downstream of the initiation codon. The data reported constitute the first experimental demonstration of the functionality of a TA system in Streptomyces.
Results
Identification of TA systems in Streptomyces
Three TA loci were proposed in the chromosome of S. coelicolor by sequence similarity [11]. One of them was classified as a relBE type (GI: 21220706, 21220707) and the other two as phd/doc type(s) (GI: 21218953, 21218954 and GI: 21224247, 21224248). Here we experimentally characterized the first one (GI: 21220706, 21220707), encoded by the SCOs2235/2236 (these were named yefMsc and yoeBsc, respectively). Identical gene sequences were present in S. lividans, and they were designated yefMsl and yoeBsl respectively. The yefMsl gene encodes the putative antitoxin YefMsl (orthologous to YefMsc) and yoeBsl encodes the putative YoeBsl toxin (orthologous to YoeBsc). This corresponds to an operon that encodes the proteins identified under the NCBI accession numbers ZP_06531415 and ZP_06531416 respectively.
Although the system yefMsc-yoeBsc was first recognized as a relBE system, actual studies permit classify it as a hybrid system on which YefMsc antitoxin shows more sequence homology with Phd superfamily and the YoeBsc toxin belongs to the ParE/RelE toxin superfamily. These hybrid associations are more common than originally thought [9].
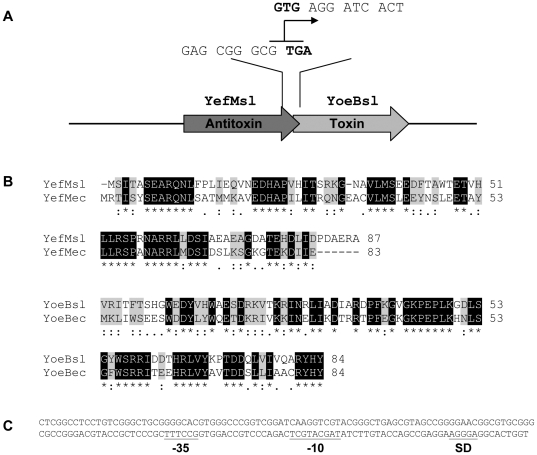
This locus is a bicistronic operon in which the upstream gene encodes the putative antitoxin of 9.7 kDa with a pI of 4.57 and the downstream one encodes the putative toxin of 9.9 kDa with a pI of 9.4. The last codon of the antitoxin gene overlaps with the first GTG codon of the toxin (Fig. 1A). Both proteins share clear identity with the YefM and YoeB proteins from E. coli (52% and 54% respectively) (Fig. 1B) and with other relBE-type TA systems from different microorganisms (data not shown). Upstream of the antitoxin gene, there is an intergenic region of 173 pb that may acts as the promoter of this system and it is identical in S. coelicolor and in S. lividans. Analysis of this region with BPROM identified the putative -35 and -10 boxes and a putative Shine-Dalgarno region (Fig. 1C).
Figure 1. A) Schematic representation of the S. lividans Antitoxin-Toxin bicistronic operon.
The overlap between the two genes is shown. The stop codon of the antitoxin and the start codon of the toxin are shown in bold. B) Clustal alignment of the predicted amino acid sequence of the putative S. lividans antitoxin (YefMsl) with E. coli YefM (YefMec) and the putative S. lividans toxin (YoeBsl) with E. coli YoeB (YoeBec). Identical amino acids are boxed in black and conservative amino acid substitutions are boxed in grey. Semi-conservative substitutions are shown with one dot. C) DNA sequence of the putative promoter of the S. lividans TA system. The putative -35 and -10 regions as well as the ribosome-binding site (SD) are underlined.
Overexpression of the S. lividans toxin gene is lethal in E. coli
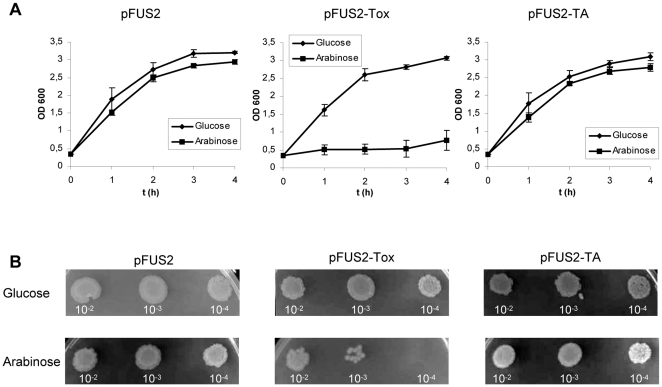
The effect of the overexpression of yoeBsl was studied in E. coli SC36 (ΔyefM-yoeB strain). PCR amplification of the yoeBsl gene from the S. lividans genome and cloning into the pFUS2 plasmid [16] provided the pFUS2-tox plasmid (Materials and Methods). In this plasmid, the S. lividans toxin gene is under the control of the arabinose-inducible PBAD promoter. Plasmid pFUS2-tox was transferred into E. coli SC36, and cell growth was tested in the presence of glucose or arabinose (repressing and inducing conditions respectively). Normal cell growth was observed when the E. coli (pFUS2-tox) cells were cultivated in the presence of glucose. However, a strong reduction in cell growth was observed when the liquid cultures were shifted to an arabinose-containing medium (Fig. 2A). A reduction in the number of viable cells was also observed when the different cultures were inoculated on LB plates one hour after protein induction (Fig. 2B). In contrast, the growth of E. coli strains containing pFUS2 (empty plasmid) or pFUS2-TA (which carries the complete operon under the control of the PBAD promoter) was normal and fairly similar in both liquid glucose- and arabinose-containing medium (Fig. 2A) and also on LB plates (Fig. 2B). The same result was obtained when a carboxy-His-tagged YoeBsl (pFUS2-ToxHis6) was produced in E. coli (data not shown).
Figure 2. Effect of the overproduction of YoeBsl and YefM/YoeBsl complexes in E. coli SC36 (MG1655 ΔyefM-yoeB).
A) Cells transformed with control plasmid (pFUS2), with the plasmid carrying yoeBsl (pFUS2-Tox), or with the plasmid carrying the yefM/yoeBsl (pFUS2-TA) were grown in liquid LB medium to mid-logarithmic phase. At this time (time zero), 0.2% glucose (diamond) was added to one half of each culture and 0.2% arabinose (square) to the other half. Cell growth was monitored measuring the OD600 of the cultures at different times. The means and standard deviation of three different experiments is presented. B) 5 µl of serial dilutions of the different cultures, taken one hour after protein induction, were inoculated in LB plates and incubated overnight at 37°C. The upper plates were inoculated with cells from media with glucose (repression), and the lower plates were inoculated with cells from media with arabinose (induction).
These results demonstrated that the protein encoded by the yoeBsl gene is a potent toxin against E. coli cells and that the protein encoded by yefMsl can counteract this toxicity when it is expressed at the same time. Therefore, these observations indicate that this operon works as a typical TA locus in E. coli.
Overexpression of the toxin gene is lethal in S. lividans
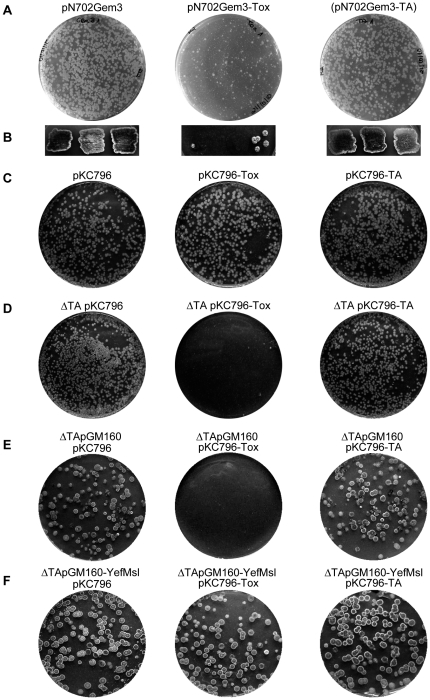
The effect of overexpression of the putative toxin encoding gene yoeBsl was also studied by transforming wild-type S. lividans protoplasts with a multicopy plasmid (a pN702Gem3 derivative) that expressed yoeBsl gene under the control of the strong Streptomyces promoter xysAp [17]. The number of transformants obtained in the S. lividans wild-type strain with the multicopy plasmid pN702Gem3-Tox was very low in comparison with the number of transformants obtained when the same protoplasts were transformed with an identical amount of the empty plasmid (pN702Gem3) or with plasmid pN702Gem3-TA, bearing the complete operon. Also, the colonies obtained with the plasmid pN702Gem3-Tox were smaller in size indicating the toxicity of the YoeBsl protein due to its overexpression in S. lividans (Fig. 3A). In addition, the few colonies carrying the plasmid pN702Gem3-Tox hardly grew when were reinoculated on patches on plates of R2YE medium supplemented with 1% xylose (xysAp-inducer) suggesting that the accumulation of the toxin in these cells make them non viable (Fig. 3B). However, when S. lividans wild type protoplasts were transformed with an integrative plasmid pKC796-Tox (a pKC796 derivative that carries the yoeBsl gene under the control of the same promoter (xysAp) [17]) the number of colonies was similar to that obtained with the pKC796 empty plasmid or with the pKC796 derivative containing the complete operon (pKC796-TA) (Fig. 3C).
Figure 3. Effect of YoeBsl and YefM/YoeBsl complexes on the viability of the wild-type S. lividans (A, B, C), and on the S. lividans .
ΔTA (ΔyefM/yoeBsl ) mutant (D, E, F). A) R2YE agar plates showing the colonies obtained in transformations with the same DNA amount of empty multicopy vector (pN702Gem3), the plasmid carrying the yoeBsl gene (pN702Gem3-Tox) or the plasmid containing yefM/yoeBsl genes (pN702Gem3-TA). B) Viability of the colonies obtained in the transformation after streaking them onto R2YE media containing 1% xylose (inducing conditions of the xysAp promoter). C) R2YE agar plates showing the colonies resulted from the transformation of the S. lividans wild type strain with the same DNA amount of empty integrative vector (pKC796) or with this plasmid carrying the yoeBsl gene (pKC796-Tox) or the plasmid carrying the yefM/yoeBsl genes (pKC796-TA). D) As in C, but using the S. lividans ΔTA (ΔyefM/yoeBsl) mutant as host. E and F) Effect of coexpression of YefMsl and YoeBsl, from different compatible plasmids, in S. lividans ΔyefM/yoeBsl mutant. Protoplasts of S. lividans ΔyefM/yoeBsl mutant carrying the empty multicopy vector pGM160 (E) or the plasmid pGM160-yefMsl (F) were transformed with the same amount of integrative plasmid pKC796 or its derivatives pKC796-Tox or pKC796-TA and inoculated in R2YE plates. The presence of pGM160-yefMsl in this strain eliminates the lethality originated by pKC796-Tox.
These results indicated that the toxicity of YoeBsl seemed to depend on the amount of protein accumulated in the cell and suggested that the expression of the endogenous single copy of the antitoxin encoding yefMsl gene in the genome could counteract the toxic effect of an extra copy introduced with pKC796-Tox, but not the higher amount originated by the multicopy plasmid pN702Gem3-Tox.
To test this hypothesis, a deletion of the yefM/yoeBsl operon was performed in S. lividans by means of REDIRECT technology (see Materials and Methods) and the resulting mutant (ΔyefM/yoeBsl) was used as a receptor for plasmids pKC796-Tox and the corresponding empty vector. The integration of a single copy of yoeBsl in this ΔTA strain (plasmid pKC796-Tox) was lethal and no transformants were obtained, while the number of colonies obtained with the control plasmids was similar to that obtained in the wild-type strain (Figure 3D). However, when the S. lividans ΔyefM/yoeBsl null mutant was transformed with a plasmid containing the antitoxin gene and then the yoeBsl gene was integrated in the genome with pKC796-Tox the number of colonies obtained was similar to that obtained with control plasmids (pKC796 and pKC796-TA). This assay demonstrates that the inhibition of colony formation induced by a single copy of the toxin is counteracted by the expression of the antitoxin gene (Figure 3E and 3F).
The same experiments were carried out with a ΔyefM/yoeBsc mutant of S. coelicolor, obtained also along this work, and identical results were obtained (data not shown).
All these results demonstrate that in fact, this TA system works as a typical TA system in Streptomyces.
The S. lividans Toxin-Antitoxin complex interacts with its TA promoter
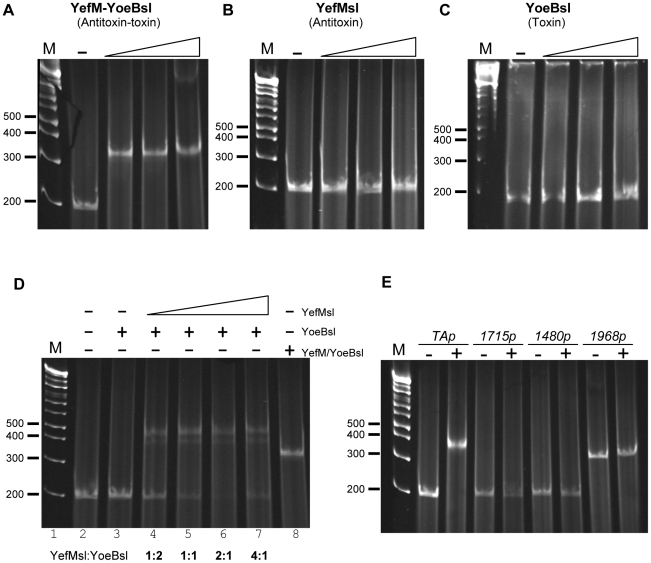
The binding of toxin-antitoxin complexes to their promoters is the main way of regulation of the TA operon [18]. To study this, the yefMsl gene or the complete yefM/yoeBsl operon, were cloned in the E. coli expression vector pET22b under the control of the T7 promoter, obtaining pET22b-Anti and pET22b-TA respectively (Materials and Methods). These plasmids were introduced into E. coli BL21 (DE3) cells, and the antitoxin and the antitoxin-toxin complexes were purified. The toxin protein alone was purified from the antitoxin-toxin complexes as indicated in materials and methods. EMSA assays were used to analyze the interactions of Streptomyces TA complexes with the 173 bp double-stranded DNA corresponding to the intergenic region upstream of the antitoxin gene of S. lividans. The purified toxin-antitoxin complexes produced a single retardation band in the migration of the DNA corresponding to the TA promoter at a concentration of 1 µM (Figure 4A). However, no band-shift was detected, under the used conditions, when only the antitoxin (concentrations of 2–8 µM) or the toxin (concentrations of 1–4 µM) was used to bind the promoter (Figure 4B and 4C respectively). EMSA assays with the in vitro reconstituted YefM-His6/YoeB-His6sl complex were performed at different antitoxin/toxin ratios, and this permitted us to observe that the best ratio for DNA retardation was 2∶1 (Figure 4D). Two retardation bands were observed, one more intense than the other, and none of them migrated to the same level as the retardation band obtained with the natural-antitoxin-toxin complex purified from E. coli cells (Figure 4D lanes 4, 5, 6 and 7 versus lane 8) (see discussion).
Figure 4. EMSA assays of the 173 bp intergenic region upstream of the yefM/yoeBsl operon with different proteins: (A) purified complex YefM/YoeB-His6sl (0, 1, 2 and 5 µM); (B) purified YefM-His6sl (0, 2, 4 and 8 µM); (C) Purified YoeB-His6sl (0, 1, 2, and 4 µM).
D) EMSA assays with in vitro reconstituted YefM-His6/YoeB-His6sl complexes. The absence or presence of YoeB-His6sl, and/or YefM-His6sl is indicated by −/+ respectively. 2 µM of YoeB-His6sl were used (lanes 3–7), and mixed with increasing amounts (1, 2, 4 and 8 µM) of YefM-His6sl (lanes 4–7). 1 µM of natural purified YefM/YoeB-His6sl complex was used as a control (lane 8). E) EMSA assays of different promoters from S. coelicolor (the SCO number is indicated) with (+) or without (−) 1 µM of the purified complex YefM/YoeB-His6 sl.
No retardation was detected when three other S. coelicolor promoters, randomly selected (Materials and Methods), were used (Figure 4E). Thus, the interaction of the YefM/YoeBsl complex with the DNA of its promoter was specific and was correlated with the typical regulation of other TA systems described in different organisms.
Purified YoeBsl inhibits protein synthesis on a Cell-free system
The effect of purified YoeB-His6sl on cell-free protein synthesis was examined over MazG. The synthesis of MazG protein from plasmid pET11a-mazG was performed at 37°C for 30 min in the absence and presence of YoeB-His6sl using an E. coli T7 S30 extract system (Promega) (Fig. 5A). MazG synthesis was almost completely blocked at YoeB-His6sl concentrations of 0.5 µM or above. Similar inhibition was observed when purified YoeBec was added [15]. We then tested the effect of YefM-His6sl antitoxin on the YoeBsl-mediated inhibition of MazG synthesis and found that the addition of YefM-His6sl recovered MazG synthesis (Fig. 5A, lane 6).
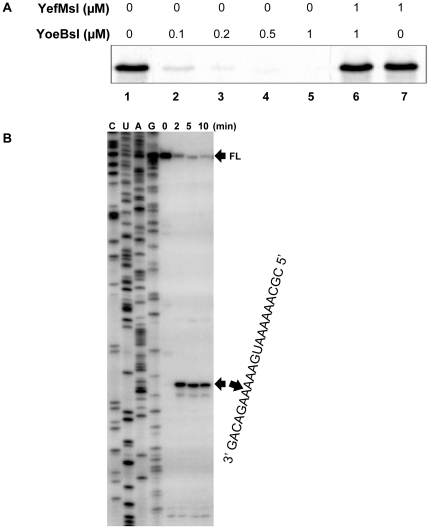
Figure 5. Effect of YoeBsl on protein synthesis in a cell-free system and Primer extension analysis of YoeBsl cleavage sites in the ompA mRNA in vivo.
A) MazG protein synthesis was carried out using E. coli T7 S30 extract system for circular DNA (Promega) with pET11a-mazG. Lane 1, without YoeB-His6sl; lanes 2 to 5, 0.1, 0.2, 0.5 and 1 µM YoeB-His6sl were added, respectively; lane 6, 1 µM YoeB-His6sl plus 1 µM YefM-His6sl; and lane 7, 1 µM YefM-His6sl was added. B) Total RNA was prepared from E. coli BL21 cells harboring pFUS2-Tox at indicated time points before and after the induction of yoeBsl expression. The major cleavage site is indicated by an arrowhead on the right side of the gel. The sequence of the major cleavage site in the ompA mRNA is also shown on the right side of the gel. The sequence ladder for ompA was obtained using pCR®2.1-TOPO-ompA as template. The full-length RNA bands (FL) are indicated with an arrow.
YoeBsl cleavages ompA mRNA in vivo
We next attempted to identify the cleavage sites of the ompA mRNA by primer extension experiments. For this purpose, total RNA was extracted from E. coli BL21(DE3) cells harboring pFUS2-Tox at different time intervals following induction of yoeBsl expression. The primer extension analyses of the ompA mRNA demonstrated that the distinct major bands exhibiting the specific cleavage sites in the mRNA appeared as early as 2 min after YoeBsl induction (Fig. 5B, lane 2). The major bands resulted from the cleavage of the mRNA at three bases downstream of the initiation codon, AUG, and most notably no other bands were observed in the regions between the 5′-end of the mRNA and the initiation codon indicating that YoeBsl may function only when it associates with the ribosomal translation machinary. These data showed that YoeBsl might primarily inhibit translation initiation, as shown with E. coli YoeBec [15].
Discussion
The putative roles of TA systems located in genomes are not clear. Some of them have been implicated in programmed cell death under different stress conditions, such as MazE/F from E. coli [19], (and references therein) or in the fruiting body formation of Myxococcus xanthus, where a MazF homologue is involved in the regulation cascade controlled by the MrpC regulator [20]. Several models have been proposed to explain the persistence of these systems in genomes, where they may contribute to the stability of the genomes and plasmids, and might act as anti-addiction modules by preventing post-segregational killing [2], [19].
The abundance of these systems in different organisms has been detected thanks to the massive sequencing and the use of bioinformatics tools, which have shown that TA systems may represent 1.5% of the coding sequences in certain free-living microorganisms. In fact, this presence may be more abundant in some obligate intracellular species such as Ricketsia bellii OSU 85–389, where they represent 2.2% of its ORFs. However, TA systems are absent in other obligate intracellular species such as Buchnera aphicola or in different species of Chlamydia, suggesting their loss due to genome reduction [9]. Horizontal gene transfer associated with mobile genetic elements has been proposed to explain the presence of highly conserved orthologous in phylogenetically different organisms [2].
Only three TA systems had been proposed in S. coelicolor [11] when we began this work. However, recently, up to 24 TAs have been proposed [13], although in no case has their functionality been demonstrated. Here, the first study of the functionality of one of these putative TA systems of Streptomyces is described, corresponding to the SCOs2235/2236 of S. coelicolor and to the ZP_06531415/ZP_06531416 sequences of S. lividans. This system is similar to the YefM/YoeB system from E. coli, sharing about 53% identity and 73% similarity in both proteins. Even, two arginine residues highly conserved in the E. coli YefM orthologs (R10 and R31) [5] that are important in the direct interaction with the operator sequence are conserved in Streptomyces antitoxin in similar positions (R9 and R31). This observation suggests a putative horizontal transfer of this TA system between the ancestors of both organisms due that other randomly taken S. coelicolor and S. lividans genes from primary metabolism share only about 20–34% identity with their E. coli orthologous genes.
BLAST analysis of the proteins studied (YefM/YoeBsl) against 16 species of Streptomyces sequenced by the Broad Institute (http://www.broadinstitute.org/annotation/genome/streptomyces_group/GenomesIndex.html) confirmed the presence of YefM/YoeBsl orthologous in 7 of them and their absence in another 9 Streptomyces species. Surprisingly, some orthologous genes from different Streptomyces species have lower identity with the system studied than the YefM/YoeB system from E. coli. It is also of interest that two strains -S. hygroscopicus ACCT53653 and Streptomyces sp. C- each have two YefM/YoeB orthologous systems in their genome that are fairly similar (90% and 79% similarity, respectively) to the TA studied here, suggesting a putative total or partial duplication of this TA system. Interestingly, one of these putative TA systems in S. hygroscopicus ACCT53652 has a putative toxin (ZP_07297953) with only 52 amino acids, while S. coelicolor and S. lividans toxin has 84 amino acids.
The organization of the yefM/yoeBsl operon is similar to other bacterial TA modules in which the first gene encodes the antitoxin and overlaps the toxin gene in three nucleotides, supporting the existence of translational coupling between both genes. Upstream of the antitoxin gene, there is an intergenic region of 173 nucleotides that may act as a bidirectional promoter region and that is identical in S. coelicolor and S. lividans. This promoter has the TA operon on one side and the glnE gene that encodes the glutamate-ammonia-ligase adenylyltransferase on the other. EMSA assays demonstrated the high capacity of the YefM/YoeBsl complexes to bind this intergenic region. A divergent promoter is also present upstream of the E. coli yefM/yoeB system, but in this case the hisL gene is present on the other side of the promoter. Long and short palindromes with the core motif 5-TGTACA-3 are present in the E. coli promoter and have been described as the binding site for the YefM/YoeBec complexes [5], [21]. In the Streptomyces yefM/yoeB promoter there is a palindromic sequence 5′-TCGTACGA-3′ overlapping the putative -10 region and a downstream almost perfect palindrome 5′-TGTACC-3′ separated by a centre-to-centre distance of 12 bp that is the same distance between the palindromic sequences described in E. coli. Preliminary results indicate that these sequences form part of the binding sites of the YefM/YoeBsl complex (data not shown).
Deletion of the complete yefM/yoeB operon from S. coelicolor and from S. lividans originated strains with a slight retardation on sporulation when cultured on MSA medium but no other phenotypes were obviously different from the corresponding wild-type strains (data not shown). This “no-effect” has been described for simultaneous deletions of several TA systems from E. coli [22]. However, more detailed studies of these E. coli strains have later revealed differences in the capacity of this TA-deleted strain to produce biofilm. In particular, those authors observed that the E. coli antitoxin YefM clearly increased biofilm formation through an as yet unknown mechanism [23]. These results point to the need for a large number of tests aimed at identifying the true role of the Streptomyces TAs under laboratory conditions.
Overproduction of YoeBsl in the ΔyefM/yoeB E. coli SC36 strain originated a strong reduction in cell viability that was reversed by the co-expression of the YefMsl antitoxin. A similar lethal effect was observed when only one copy of the toxin gene was integrated into the chromosome of the S. lividans, or S. coelicolor ΔyefM/yoeB mutants, where no transformants were obtained. However, this toxic effect was not observed in S. lividans or S. coelicolor wild-type strains when an extra copy of the toxin gene was integrated in the genome, and the toxic effect was only detected when a multicopy plasmid was used to express the toxin gene. These results suggest that in these strains the endogenous antitoxin was able to block the lethal effect of an extra copy of the toxin gene but not enough when more copies of the gene were present.
In vitro experiments demonstrated that the His-tagged YoeB-His6sl and YefM-His6sl proteins are active in experiments of inhibition of protein synthesis. However, EMSA assays, demonstrated that in vitro reconstituted YefM-His6/YoeB-His6sl complex produced two retarded bands with the cognate promoter instead of one as it was obtained with the S. lividans TA complex purified from E. coli. These results suggest a different conformation of the reconstituted complex maybe due to the extra His6-tag present in the antitoxin carboxy terminus. Similar results have been described previously with the YefM/YoeB proteins from E. coli [21].
In vivo experiments with the protein YoeBsl demonstrated that its activity is identical to YoeBec processing ompA mRNA mainly at three bases downstream of the initiation codon. However, unlike YoeBec processing, where a significant amount of full-lengh ompA mRNA remains even 30 min after induction of the E. coli toxin [15], most of the full-lengh mRNA disappears after only two minutes of yoeBsl expression. This result suggests a higher mRNA processing activity of the YoeBsl compared to YoeBec.
Materials and Methods
Bacterial strains and growth conditions
The E. coli strains used were as follows: DH5α [24] for the cloning and isolation of plasmids; BL21 (DE3) (Stratagene) to express and purify proteins, and SC36 (MG1655 ΔyefM-yoeB) [25] to evaluate toxin toxicity. E.coli BW25113 (pIJ790), ET12567 (pUZ8002), and DH5α (pBT30) were used for gene replacement [26]. All strains were grown in Luria-Bertani (LB) liquid broth or on LB agar. All manipulations in E. coli were performed following standard procedures [24].
S. coelicolor M145, S. lividans 1326 and derivatives were grown on solid R2YE medium for transformation, on MSA medium for sporulation [27], and in liquid YES medium (1% yeast extract 10.3% sucrose [pH 7.2] supplemented with 0.5% glucose, 5 mM MgCl2 and 0.5% glycine) for collecting cells to make protoplasts. Liquid cultures were carried out in baffled flasks at 28°C and 200 rpm. All manipulations in Streptomyces were done as indicated by Kieser [27].
Deletion of the yefM/yoeB operon in S. coelicolor and in S. lividans
REDIRECT PCR-targeting technology [26] was used to replace the genes of the entire yefM/yoeB operon to an apramycin (aac(3)IV gene) resistance cassette in S. coelicolor M145 and in S. lividans 1326. Mutagenic cassettes were flanked by the recognition sequence of yeast Flipase (FRT) and contained the oriT (FRT-aac(3)IV-oriT-FRT) conjugation transfer origin and were amplified with primers LS-013 and LS-014 (Table 1), using plasmid pIJ773 as template [26]. The cassettes generated were introduced into E. coli BW25113 (pIJ790), harboring the cosmid 7B11, and preinduced for λRed functions by the addition of arabinose to obtain a TA-disrupted version of the mutant cosmid. The disrupted cosmid, confirmed by restriction analysis, was isolated and transferred from E. coli ET12567 (pUZ8002) to S. coelicolor M145 and to S. lividans by conjugation. Exconjugants were selected on MSA medium containing apramycin (50 µg/mL), and the double crossover products were identified by their sensitivity to kanamycin (50 µg/mL). The antibiotic-resistant marker and the oriT region were eliminated in two steps. In the first, the corresponding disrupted cosmid was introduced into the E. coli DH5α (pBT30) strain (harboring the flipase gene, FLP), in which the recombination between both FRT mutagenesis cassette-flanking regions takes place. In this new cosmid, only 81 base pairs (SCAR) in-frame with the adjacent ORFs remained. Then, the SCAR cosmid was transferred to the Streptomyces apramycin resistance mutant strains by protoplast transformation, first selecting neomycin resistance clones (unique recombination), and then the apramycin- and neomycin-sensitive strains (double recombination). PCR assays confirmed the correct recombination in the new Streptomyces mutant strains.
Table 1. Oligonucleotides used.
| Name | Sequence 5′-3′ | Use |
| LS-001 | TTTTTTGAATTCTGTGCGGCTGCCCTTCCGCC | Forward oligonucleotide to amplify the promoter of SCO1968. The sequence recognized by EcoRI is underlined. |
| LS-002 | TTTTTTCATATGCGTACTCCTCGCGTCGAACG | Reverse oligonucleotide to amplify the promoter of SCO1968. The sequence recognized by NdeI is underlined. |
| LS-005 | TTTTTTCATATGTCCATCACCGCCAGCGAAG | Forward oligonucleotide for cloning the TA operon into pXHis1 and pET22b. The sequence recognized by NdeI is underlined. |
| LS-008 | TTTTTTCATATGAGGATCACTTTCACGTCCCAC | Forward oligonucleotide for cloning the toxin gene into pXHis1. The sequence recognized by NdeI is underlined. |
| LS-009 | TTTTTTCTCGAGTCAGTAGTGGTAGCGCGCCTGG | Reverse oligonucleotide for cloning the toxin gene and TA operon into pXHis1. The sequence recognized by XhoI is underlined. |
| LS-013 | CAGACTCGTACGATATCTTGTACCAGCCGAGGAAGGGAGGCACTGGTATGATTCCGGGGATCCGTCGACC | Forward oligonucleotide to obtain the mutagenic cassette. The sequence matching the sequence of the disruption cassette is underlined. |
| LS-014 | GCTTCGGCTTTCGCCGGTCGCGGGTGTCGTGTCCGTACCGGCGGGTGTCATGTAGGCTGGAGCTGCTTC | Reverse oligonucleotide to obtain the mutagenic cassette. The sequence matching the sequence of the disruption cassette is underlined. |
| LS-019 | TTTTTTGAATTCTCGGCCTCCTGTCGGGCTG | Forward oligonucleotide to amplify TA promoter. The sequence recognized by EcoRI is underlined. |
| LS-020 | TTTTTTCATATGACCAGTGCCTCCCTTCCTCGG | Reverse oligonucleotide to amplify TA promoter. The sequence recognized by NdeI is underlined. |
| LS-021 | TTTTTTCTCGAGGTAGTGGTAGCGCGCCTGGAC | Reverse oligonucleotide for cloning the TA operon into pET22b. The sequence recognized by XhoI is underlined. |
| LS-022 | TTTTTTCTCGAGCGCCCGCTCCGCGTCCGGG | Reverse oligonucleotide for cloning the antitoxin gene into pET22b. The sequence recognized by XhoI is underlined. |
| AY-147 | TAGAACACGGGTCCGACAGTCC | Forward oligonucleotide to amplify the promoter of SCO1715. |
| AY-148 | ATCGCTCCCTCGCAACCGATTC | Reverse oligonucleotide to amplify the promoter of SCO1715. |
| AY-159 | AGAGAGTATGTCCTAAATGTCCGG | Forward oligonucleotide to amplify the promoter of SCO1480. |
| AY-160 | GCCTACGTCACCTCGGATGTCG | Reverse oligonucleotide to amplify the promoter of SCO1480. |
Toxicity evaluation in E. coli
yoeBsl DNA was amplified by PCR from S. lividans 1326 genomic DNA using primers LS-008 and LS-009 (Table 1). The resulting fragment was digested with NdeI and XhoI and ligated into plasmid pXHis1 [28] (Table 2) digested with the same enzymes to obtain plasmid pXHis-Tox, which was used as an intermediate plasmid. Plasmid pFUS2-Tox was obtained by digesting pXHis-Tox with NdeI and HindIII and ligated into plasmid pFUS2 [16] (Table 2), digested with the same enzymes. This construction placed the ORF of the putative toxin gene under the control of the arabinose-inducible promoter PBAD and had the fdt transcriptional terminator at the 3′ end.
Table 2. Plasmids used.
| Plasmid | Characteristics | Reference |
| pIJ773 | pBluescript SK derivative containing the Apramycin resistance cassette. | [26] |
| pXHis1 | pBluescript SK derivative. Ampicillin resistance. The xysA promoter from S. halstedii controls xys1Δ expression. | [28] |
| pXHis-Tox | pXHis1 derivative. The xysA promoter from S. halstedii controls toxin expression. | This work |
| pXHis-TA | pXHis1 derivative. The xysA promoter from S. halstedii controls TA expression. | This work |
| pFUS2 | E. coli expression vector. Kanamycin resistance. PBAD promoter. | [16] |
| pFUS2-Tox | pFUS2 derivative. PBAD promoter controls toxin expression. | This work |
| pFUS2-TA | pFUS2 derivative. PBAD promoter controls TA expression. | This work |
| pN702GEM3 | E.coli/Streptomyces shuttle vector. Neomycin resistance. High-copy number. | [29] |
| pN702Gem3-Tox | pN702GEM3 derivative. The xysA promoter from S. halstedii controls toxin expression. | This work |
| pN702Gem3-TA | pN702GEM3 derivative. The xysA promoter from S. halstedii controls TA expression. | This work |
| pKC796 | E.coli/Streptomyces shuttle vector. Apramycin resistance. Integrative plasmid. | [30] |
| pKC796-Tox | pKC796 derivative. The xysA promoter from S. halstedii controls toxin expression. | This work |
| pKC796-TA | pKC796 derivative. The xysA promoter from S. halstedii controls TA expression. | This work |
| pET22b | E. coli expression vector. Ampicillin resistance. | Novagen |
| pET22b-Anti | pET22b derivative. Expressing antitoxin gene with a His6 tag at the carboxy terminal. | This work |
| pET22b-TA | pET22b derivative. Expressing the TA operon. In this construction the toxin gene has a His6 tag at the carboxy terminal. | This work |
| pGM160 | E.coli/Streptomyces shuttle vector. Thiostrepton and gentamicin resistance. | [31] |
| pGM160-YefMsl | pGM160 derivate. The xysA promoter from S. halstedii controls YefMsl expression. | This work |
A similar strategy was used to amplify the genes of the complete operon (yefM/yoeBsl), using primers LS-005 and LS-009 (Table 1). The resulting PCR fragment was digested with NdeI and XhoI and ligated into plasmid pXHis1 digested with the same enzymes to obtain plasmid pXHis-TA, which was used as an intermediate plasmid. Plasmid pFUS2-TA was obtained by digesting pXHis-TA with NdeI and HindIII and ligated into plasmid pFUS2 digested with the same enzymes. This construction placed the complete operon under the control of the arabinose-inducible promoter PBAD and had the fdt transcriptional terminator.
E. coli SC36 cells transformed with pFUS2 (control), pFUS2-Tox or pFUS2-TA were grown at 37°C on LB broth supplemented with 50 µg/mL of kanamycin to an OD600 of 0.5–0.8 and the cultures were divided into two parts. One half of each culture was grown in the presence of 0.2% glucose (repression conditions) and the other half in the presence of 0.2% arabinose (induction conditions). Culture growth was monitored measuring the OD600. In addition, samples were obtained 1 hour after induction and 5-µl drops of different dilutions of cultures were spread onto the surface of LB agar supplemented with 50 µg/mL of kanamycin. Plates were incubated overnight at 37°C.
Toxicity evaluation in Streptomyces
Multicopy plasmids were generated by cloning the yoeBsl toxin encoding gene or the complete operon into plasmid pN702Gem3 [29] (Table 1). Plasmids pXHis-Tox and pXHis-TA were digested with BglII and the DNA fragments containing the genes (Tox and TA) were ligated with pN702Gem3 (Table 2) digested with the same enzyme. The toxin and antitoxin-toxin genes of the resulting plasmids, pN702Gem3-Tox and pN702Gem3-TA, are regulated by the xylanase promoter xysAp [17]. All the constructions were flanked by two transcriptional terminators.
Integrative Streptomyces plasmids, whose gene expression was regulated by xysAp, were generated by cloning the corresponding BglII/BglII fragments into the integrative plasmid pKC796 [30] (Table 2). Plasmids pKC796-Tox and pKC796-TA were obtained respectively.
Multicopy plasmid pGM160-YefMsl was generated by cloning the antitoxin gene between BstBI and BamHI sites of pGM160 plasmid [31].
These plasmids were introduced by protoplast transformation into S. lividans 1326 wt and ΔTA (lacking the chromosomal copy of the system) and in S. coelicolor M145 wt and the corresponding ΔTA mutant. Cell viability was estimated by checking their growth in R2YE medium after incubation at 30°C.
Protein purification
S. lividans antitoxin-encoding DNA (yefMsl) and the DNA corresponding to the TA operon (yefM/yoeBsl) were amplified by PCR using primers LS005/LS022 and LS005/LS021 respectively (Table 1) and cloned between the NdeI and XhoI sites in the pET-22b vector (Novagen) to produce the antitoxin or toxin, respectively, tagged C-terminally with a hexahistidine motif, yielding pET22b-Anti and pET22b-TA respectively.
YefM-His6sl and the YefM/YoeB-His6sl complex were overproduced in E. coli BL21 (DE3) transformed with the corresponding plasmids. Five hours after induction with 1 mM IPTG, cells were harvested at 5000×g at 4°C for 10 min. The cell pellet was resuspended in lysis buffer (5 mM Na2HPO4/NaH2PO4, pH 7.5, 300 mM NaCl, 0.1%Triton X100, 5 mM imidazole), and then sonicated and centrifuged for 30 min. at 100.000×g. The supernatant was applied to a column containing 2 ml of NTA-Ni resin (Qiagen). The column was washed three times with 5 mL of washing buffer 1 (5 mM Na2HPO4/NaH2PO4, pH 7.5, 300 mM NaCl, 0.1% Triton X100, 20 mM imidazole) and twice with 5 mL of washing buffer 2 (5 mM Na2HPO4/NaH2PO4, pH 7.5, 300 mM NaCl, 0.1% Triton X100, 30 mM imidazole). Tagged proteins were eluted 3 times with 0.5 mL of elution buffer 1 (5 mM Na2HPO4/NaH2PO4, pH 7.5, 300 mM NaCl, 0.1% Triton X100, 250 mM imidazole) and twice with elution buffer 2 (5 mM Na2HPO4/NaH2PO4, pH 7.5, 300 mM NaCl, 0.1% Triton X100, 1 M imidazole). Fractions containing the highest concentrations of proteins were pooled and dialyzed with D-Tube TM Dialyzer Maxi (Novagen) for 48 h against 2 L of dialysis buffer (50 mM Tris, pH 7.5, 100 mM NaCl, 10% glycerol).
Purification of the toxin YoeB-His6sl was carried out in E. coli BL21 (DE3) cells transformed with pET22b-TA that produces the complex toxin-antitoxin. Protein production and purification were done as described previously but the second wash of the NTA-Ni resin, containing the complex antitoxin/toxin-His6, was performed with 5 mM Na2HPO4/NaH2PO4, pH 7.5, 300 mM NaCl, 0.1 Triton X100%, 20 mM imidazole, 6 M guanidine hydrochloride to denature the antitoxin protein YefMsl [21]. YoeB-His6sl was then eluted as described above. Fractions containing the highest concentrations of protein were pooled and dialyzed successively for 2h against 500 mL of dialysis buffer containing 3 M, 2 M, and 1 M urea, followed by 24 h against 2 L of dialysis buffer without urea. The concentration of TA complex was estimated assuming an antitoxin∶toxin ratio of 2∶1), as has been described for E. coli YefM-YoeB complexes [14]).
Electrophoretic mobility assays (EMSA)
The TA promoter was amplified by PCR from S. lividans 1326 genomic DNA using primers LS-019 and LS-020 (Table 1). The resulting PCR fragment (196 bp) was used in the binding reactions with the proteins. Different Streptomyces promoters unrelated to TA systems were used as controls: namely, the promoter of SCO1968 (a putative secreted hydrolase, amplified with primers LS-001 and LS-002, Table 1); SCO1715 (a putative homogentisate 1,2-dioxygenase, amplified with primers AY-147 and AY-148, Table 1), and SCO1480 a hypothetical protein, amplified with primers AY-159 and AY-160, Table 1).
The binding reactions contained 150–200 ng of DNA, 10 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 100 mM NaCl, 10% glycerol, 2 µg of salmon sperm DNA, and different concentrations of the proteins. The mixtures were incubated at 30°C for 20 min and electrophoresed at 4°C on 5% native polyacrylamide gels in TBE buffer 1× (90 mM Tris, 89 mM boric acid, 2 mM EDTA, pH 8.3). DNA was visualized by gel staining with ethidium bromide (0.5 µg/mL in TBE buffer). When YefM-His6sl and YoeB-His6sl were added separately, the two proteins were first incubated at incubation at 30°C for 20 min prior to the addition of DNA.
Assay of protein synthesis in vitro
Cell-free protein synthesis was performed with an E. coli T7 S30 Extract System for Circular DNA (Promega). The reaction mixture was prepared as described in the manufacture's protocol. Then, different amounts of YoeB-His6sl and YefM-His6sl were added in a final volume of 29 µl. The reaction was started by the addition of pET11a-mazG plasmid DNA [15], [32] and the mixture was incubated for 30 min at 37°C. Proteins were precipitated with acetone and analyzed by 15% SDS-PAGE. The dried gel was followed by autoradiography.
Primer extension analysis in vivo
For primer extension analysis of mRNA cleavage sites in vivo, total RNA was extracted from the E. coli BL21(DE3) cells containing pFUS2-Tox at different time points after YoeBsl induction. Primer extension was carried out as described previously [33].
Sequence analysis
All constructions were sequenced in both strands using a Perkin Elmer ABI Prism 377 DNA sequencer. In silico plasmids were obtained with the Gene Construction Kit software (GCK, Textco). BPROM software (http://linux1.softberry.com/berry.phtml) was used to search for conserved sequences in the putative promoter of the TA system.
Acknowledgments
Thanks are due to Drs. R. Díaz and M. Espinosa for their help in this work and Drs. P. San-Segundo and E. Vijgenboom for correction and suggestions. We thank Drs. M. Lemonnier and K. Gerdes for providing E. coli strains and plasmids. M.J. Jiménez Rufo is thanked for her excellent technical work and N. Skinner and S. Ajmal for supervising the English versions of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work has been supported by grants of the Ministerio de Ciencia e Innovación, Spain (Grants EUI2008-03631 to RIS and BFU2010-17551 to MD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Holcik M, Iyer VN. Conditionally lethal genes associated with bacterial plasmids. Microbiology. 1997;143(Pt 11):3403–3416. doi: 10.1099/00221287-143-11-3403. [DOI] [PubMed] [Google Scholar]
- 2.Van Melderen L. Toxin-antitoxin systems: why so many, what for? Curr Opin Microbiol. 2010;13:781–785. doi: 10.1016/j.mib.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 4.Makarova KS, Wolf YI, Koonin EV. Comprehensive comparative-genomic analysis of type 2 toxin-antitoxin systems and related mobile stress response systems in prokaryotes. Biol Direct. 2009;4:19. doi: 10.1186/1745-6150-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey SE, Hayes F. Influence of operator site geometry on transcriptional control by the YefM-YoeB toxin-antitoxin complex. J Bacteriol. 2009;191:762–772. doi: 10.1128/JB.01331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutschler H, Gebhardt M, Shoeman RL, Meinhart A. A novel mechanism of programmed cell death in bacteria by toxin-antitoxin systems corrupts peptidoglycan synthesis. PLoS Biol. 2011;9:e1001033. doi: 10.1371/journal.pbio.1001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Hoz AB, Ayora S, Sitkiewicz I, Fernandez S, Pankiewicz R, et al. Plasmid copy-number control and better-than-random segregation genes of pSM19035 share a common regulator. Proc Natl Acad Sci U S A. 2000;97:728–733. doi: 10.1073/pnas.97.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallez R, Geeraerts D, Sterckx Y, Mine N, Loris R, et al. New toxins homologous to ParE belonging to three-component toxin-antitoxin systems in Escherichia coli O157:H7. Mol Microbiol. 2010;76:719–732. doi: 10.1111/j.1365-2958.2010.07129.x. [DOI] [PubMed] [Google Scholar]
- 9.Leplae R, Geeraerts D, Hallez R, Guglielmini J, Dreze P, et al. Diversity of bacterial type II toxin-antitoxin systems: a comprehensive search and functional analysis of novel families. Nucleic Acids Res. 2011;39:5513–5525. doi: 10.1093/nar/gkr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi Y, Park JH, Inouye M. Toxin-antitoxin systems in bacteria and archaea. Annu Rev Genet. 2011;45:61–79. doi: 10.1146/annurev-genet-110410-132412. [DOI] [PubMed] [Google Scholar]
- 11.Pandey DP, Gerdes K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 2005;33:966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sevin EW, Barloy-Hubler F. RASTA-Bacteria: a web-based tool for identifying toxin-antitoxin loci in prokaryotes. Genome Biol. 2007;8:R155. doi: 10.1186/gb-2007-8-8-r155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao Y, Harrison EM, Bi D, Tai C, He X, et al. TADB: a web-based resource for Type 2 toxin-antitoxin loci in bacteria and archaea. Nucleic Acids Res. 2011;39:D606–611. doi: 10.1093/nar/gkq908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamada K, Hanaoka F. Conformational change in the catalytic site of the ribonuclease YoeB toxin by YefM antitoxin. Mol Cell. 2005;19:497–509. doi: 10.1016/j.molcel.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Inouye M. The inhibitory mechanism of protein synthesis by YoeB, an Escherichia coli toxin. J Biol Chem. 2009;284:6627–6638. doi: 10.1074/jbc.M808779200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemonnier M, Ziegelin G, Reick T, Munoz Gomez A, Diaz-Orejas R, et al. Bacteriophage P1 Ban protein is a hexameric DNA helicase that interacts with and substitutes for Escherichia coli DnaB. Nucleic Acids Res. 2003;31:3918–3928. doi: 10.1093/nar/gkg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez S, Santamaría RI, Fernández-Ábalos JM, Díaz M. Identification of the sequences involved in the glucose-repressed transcription of the Streptomyces halstedii JM8 xysA promoter. Gene. 2005;351:1–9. doi: 10.1016/j.gene.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Buts L, Lah J, Dao-Thi MH, Wyns L, Loris R. Toxin-antitoxin modules as bacterial metabolic stress managers. Trends Biochem Sci. 2005;30:672–679. doi: 10.1016/j.tibs.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Van Melderen L, Saavedra De Bast M. Bacterial toxin-antitoxin systems: more than selfish entities? PLoS Genet. 2009;5:e1000437. doi: 10.1371/journal.pgen.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nariya H, Inouye M. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell. 2008;132:55–66. doi: 10.1016/j.cell.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Kedzierska B, Lian LY, Hayes F. Toxin-antitoxin regulation: bimodal interaction of YefM-YoeB with paired DNA palindromes exerts transcriptional autorepression. Nucleic Acids Res. 2007;35:325–339. doi: 10.1093/nar/gkl1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsilibaris V, Maenhaut-Michel G, Mine N, Van Melderen L. What is the benefit to Escherichia coli of having multiple toxin-antitoxin systems in its genome? J Bacteriol. 2007;189:6101–6108. doi: 10.1128/JB.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Wang X, Ma Q, Zhang XS, Wood TK. Toxin-antitoxin systems in Escherichia coli influence biofilm formation through YjgK (TabA) and fimbriae. J Bacteriol. 2009;191:1258–1267. doi: 10.1128/JB.01465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanahan D. Studies on transformation of Escherichia coli with plasmids. Journal of Molecular Biology. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 25.Christensen SK, Maenhaut-Michel G, Mine N, Gottesman S, Gerdes K, et al. Overproduction of the Lon protease triggers inhibition of translation in Escherichia coli: involvement of the yefM-yoeB toxin-antitoxin system. Mol Microbiol. 2004;51:1705–1717. doi: 10.1046/j.1365-2958.2003.03941.x. [DOI] [PubMed] [Google Scholar]
- 26.Gust B, Challis GL, Fowler K, Kieser T, Chater KF. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A. 2003;100:1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieser T, Hopwood DA, Bibb JM, Chater KF, Buttner MJ. Practical Streptomyces genetics. Norwich, UK: John Innes Foundation; 2000. [Google Scholar]
- 28.Adham SA, Campelo AB, Ramos A, Gil JA. Construction of a xylanase-producing strain of Brevibacterium lactofermentum by stable integration of an engineered xysA gene from Streptomyces halstedii JM8. Appl Environ Microbiol. 2001b;67:5425–5430. doi: 10.1128/AEM.67.12.5425-5430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Abalos JM, Reviejo V, Díaz M, Rodríguez S, Leal F, et al. Posttranslational processing of the xylanase Xys1L from Streptomyces halstedii JM8 is carried out by secreted serine proteases. Microbiology. 2003;149:1623–1632. doi: 10.1099/mic.0.26113-0. [DOI] [PubMed] [Google Scholar]
- 30.Kuhstoss S, Richardson MA, Rao RN. Plasmid cloning vectors that integrate site-specifically in Streptomyces spp. Gene. 1991;97:143–146. doi: 10.1016/0378-1119(91)90022-4. [DOI] [PubMed] [Google Scholar]
- 31.Muth G, NuBbaumer B, Wohlleben W, Püler A. A vector system with temperature-sensitive replication for gene disruption and mutational cloning in Streptomyces. Mol Gen Genet. 1989;219:341–348. [Google Scholar]
- 32.Zhang J, Inouye M. MazG, a nucleoside triphosphate pyrophosphohydrolase, interacts with Era, an essential GTPase in Escherichia coli. J Bacteriol. 2002;184:5323–5329. doi: 10.1128/JB.184.19.5323-5329.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi Y, Park JH, Inouye M. MqsR, a crucial regulator for quorum sensing and biofilm formation, is a GCU-specific mRNA interferase in Escherichia coli. J Biol Chem. 2009;284:28746–28753. doi: 10.1074/jbc.M109.032904. [DOI] [PMC free article] [PubMed] [Google Scholar]