Abstract
Background
Mitochondria are both the cellular powerhouse and the major source of reactive oxygen species. Coenzyme Q10 plays a key role in mitochondrial energy production and is recognized as a powerful antioxidant. For these reasons it can be argued that higher mitochondrial ubiquinone levels may enhance the energy state and protect from oxidative stress. Despite the large number of clinical studies on the effect of CoQ10 supplementation, there are very few experimental data about the mitochondrial ubiquinone content and the cellular bioenergetic state after supplementation. Controversial clinical and in vitro results are mainly due to the high hydrophobicity of this compound, which reduces its bioavailability.
Principal Findings
We measured the cellular and mitochondrial ubiquinone content in two cell lines (T67 and H9c2) after supplementation with a hydrophilic CoQ10 formulation (Qter®) and native CoQ10. Our results show that the water soluble formulation is more efficient in increasing ubiquinone levels. We have evaluated the bioenergetics effect of ubiquinone treatment, demonstrating that intracellular CoQ10 content after Qter supplementation positively correlates with an improved mitochondrial functionality (increased oxygen consumption rate, transmembrane potential, ATP synthesis) and resistance to oxidative stress.
Conclusions
The improved cellular energy metabolism related to increased CoQ10 content represents a strong rationale for the clinical use of coenzyme Q10 and highlights the biological effects of Qter®, that make it the eligible CoQ10 formulation for the ubiquinone supplementation.
Introduction
Coenzyme Q10 (CoQ10), also known as ubiquinone, is the predominant form of coenzyme Q in humans. It is a lipid-soluble molecule composed of a redox active quinone ring and a hydrophobic tail. In the mitochondrial respiratory chain it acts as a mobile electron transporter and is a cofactor of uncoupling proteins [1]. When reduced, it is a powerful antioxidant that prevents oxidative damage by free radicals, including oxidation of lipids within the mitochondrial membrane [2]. There is evidence that CoQ10 affects the expression of hundreds of human genes involved in cell signaling, metabolism and nutrient transport [3] and it may have anti-inflammatory effects via gene expression modification [4]. Heart, kidney, brain and liver tissues show the highest concentration of CoQ10, which is endogenously synthesized and in small part assimilated from the diet [5].
The fundamental role of ubiquinone in mitochondrial function and cellular bioenergetics should make it the main dietary supplement in situations where its production is inadequate [6] or in pathological conditions where alterations of mitochondrial enzymes involved in CoQ10 redox mechanisms occur [7] such as cardiovascular disease [8], metabolic diseases [9], oxidative stress and aging [10].
The rationale for CoQ10 therapy is supported by the evidence of decreasing CoQ10 levels with age in human and animal tissues, further suggesting a potential therapeutic role in age-related neurodegenerative disorders [11], [12], [13].
Despite these potential beneficial effects on disorders related to mitochondrial dysfunction, clinical studies showed controversial results. The use of CoQ10 in neurodegenerative disorders failed to demonstrate any positive result in patients with Huntington's [14] and Parkinson's diseases [15] or amyotrophic lateral sclerosis [16]. Controversial results were observed in primary hypertension and statin induced myalgia [17] as well.
Therapeutic applications of CoQ10 are greatly limited by its poor bio-availability, due to its lack of solubility in aqueous media. A recent study demonstrated that, in rats, only 3% of orally administered CoQ10 can be absorbed [18]. Several advancements have been made to enhance the bioavailability of CoQ10 using various approaches like size reduction, solubility enhancement (by solid dispersion, prodrug, complexation, ionization) and use of novel drug carriers such as liposomes, microspheres, nanoparticles, nanoemulsions and self-emulsifying systems [19], [20]. For an updated review see: Villalba et al. [21].
The goal of the present study was to increase the mitochondrial content of CoQ10 in cultured cells (T67 and H9c2 cell lines), in order to improve their bioenergetics parameters. For this purpose we supplemented cultured cells with a water-soluble CoQ10 formulation Qter®, obtained by terclatration of native CoQ10. Mitochondrial respiration rate supported by different substrates (glucose, glutamate/malate and succinate/glycerol 3-phosphate), cellular ATP and protein content were analyzed to describe the energy state of the cells. The antioxidant properties of CoQ10 and Qter® were assessed by means of fluorogenic probes (DCFDA and MitoSOX red). Moreover, we wanted to highlight the importance of a correct ubiquinone insertion into the mitochondrial membrane that depends mainly on its bioavailability, rather than on the administered amount.
Results
Titration of Coenzyme Q10 uptake
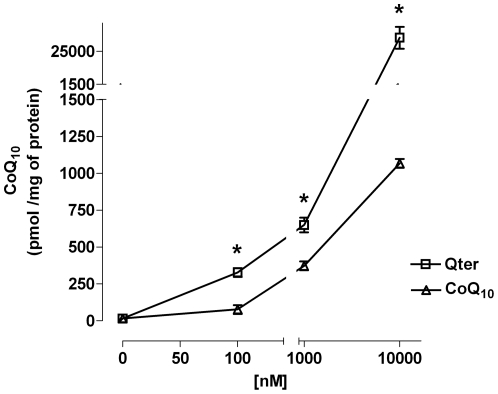
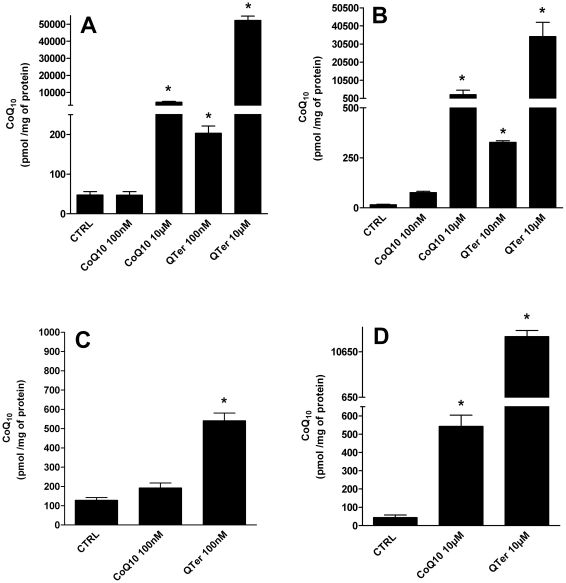
Preliminary experiments were designed to establish the adequate concentration of ubiquinone, and to evaluate the effects of the pharmaceutically inactive matrix used to terclatrate CoQ10. Treatment for 24 hours with CoQ10 concentrations ranging from 10 nM to 10 µM, or vehicle, did not significantly alter the cell viability as confirmed by trypan blue exclusion method (data not shown). As shown in Figure 1 CoQ10 uptake was constantly more efficient in Qter® treated cells compared to native CoQ10. In particular 100 nM Qter® appeared to be sufficient to significantly increase CoQ10 content, while micro molar concentrations of native CoQ10 had to be administered to achieve a similar concentration. In addition, we found that mitochondrial CoQ10 content in cells treated with 100 nM Qter® was similar to that measured in cells treated with 10 µM native CoQ10 (Fig. 2C and 2D).
Figure 1. Titration of CoQ10 uptake in H9c2 cells.
H9c2 cells were treated with different concentrations of native CoQ10 or Qter® dissolved in colture medium. After 24 hours cells were carefully washed with PBS and CoQ10 was extracted with exane/ethanol 5∶2 from whole cells and its concentration was determined by HPLC analysis. Data are normalized on total cellular protein content. Values are means ± S.D., n = 3, * p<0.001 vs. native CoQ10 treated samples.
Figure 2. HPLC determination of CoQ10 cellular content.
HPLC determination of CoQ10 cellular content in T67 cells (A) and H9c2 (B) treated for 24 hours with native CoQ10 or Qter® at different concentrations (100 nM and 10 µM). Coenzyme Q10 content was measured also in isolated mitochondria from T67 (C) and H9c2 (D) cells. Data were normalized on total cellular and mitochondrial protein content. Values are means ± S.D., n = 3, * p<0.001 vs. control.
Cellular and mitochondrial distribution of CoQ10
Figure 2A and 2B show the CoQ10 intracellular concentrations following treatment with different formulations and concentrations of ubiquinone. At the lowest concentration tested (100 nM) native CoQ10 was not able to significantly increase the cellular amount of CoQ10 in both cell lines, but only with a higher concentration (10 µM) it was possible to achieve significant results (p≤0.001). On the other hand the increase of cellular CoQ10 amount is at least 4 fold higher with Qter® compared to native CoQ10 and significant effects (p≤0.001) are already visible at the lower concentration (100 nM), that has no effect for native CoQ10. In human astrocytoma mitochondria 100 nM Qter® treatment showed a 3 fold increase of mitochondrial CoQ10 content compared to the same concentration of native CoQ10 (Figure 2C). A similar result was observed in isolated mitochondria from embryonic rat heart cells (Figure 2D) where mitochondrial CoQ10 content was 20-fold higher with 10 µM of Qter® compared to the same amount of native CoQ10 (p≤0.001).
Mitochondrial respiration and cellular ATP content
We tested the effects of CoQ10 supplementation on cellular respiration (Table 1).
Table 1. Respiratory rates of intact H9c2 and T67 cells treated for 24 hours with 100 nM native CoQ10 or Qter®.
| EndogenousRespirationnmoles O2 min−1/106cells | UncoupledRespirationnmoles O2 min−1/106cells | |
| T67 | ||
| Control | 3.49±0.42 | 4.27±0.19 |
| CoQ10 | 3.84±0.19 | 4.26±0.55 |
| Qter | 3.60±0.28 | 4.92±0.41* |
| H9c2 | ||
| Control | 6.75±1.34 | 19.23±2.60 |
| CoQ10 | 7.40±0.35 | 17.05±0.36 |
| Qter | 7.35±0.63 | 25.85±3.28* |
Respirometric analyses were performed under endogenous and uncoupled conditions. The maximal uncoupled respiration was measured in the presence of 500 nM FCCP. Respiratory rates are expressed as nmoles O2 min−1/106cells ± S.D. from at least three independent experiments. *p<0.05 vs. control.
Glucose supported oxygen consumption rates (OCR) were measured in intact cells in the presence and absence of 500 nM of the uncoupler FCCP. No effect on endogenous cell respiration was observed when both cell lines were treated with 100 nM native CoQ10 or Qter®, while the uncoupled OCR were significantly increased only by 100 nM Qter® in the two cell lines tested. A similar trend could be observed in permeabilized cells (Table 2). The state 3 respiration sustained by glutamate/malate was increased by treatment with Qter® and not with native CoQ10 in H9c2 cells (p<0.05 vs. control). Succinate/glycerol 3-phosphate supported oxygen consumption was significantly increased in both cell lines only after Qter® supplementation (p<0.001 vs. control). To test whether similar effects could be achieved raising the amount of CoQ10 administered, we treated the cells with higher amounts of ubiquinone (10 µM) but we couldn't observe any improvement in the endogenous cellular respiration rate even in presence of FCCP, as reported in Table 3. In these conditions native CoQ10 treatment decreased the rate of uncoupled respiration.
Table 2. Respiratory rates of permeabilized H9c2 and T67 cells treated for 24 hours with 100 nM native CoQ10 or Qter®.
| Glutamate/Malatenmoles O2 min−1/106cells | Succinate/Glycerol 3-phosphatenmoles O2 min−1/106cells | |
| T67 | ||
| Control | 3.92±1.02 | 5.26±1.31 |
| CoQ10 | 3.26±1.05 | 6.25±0.92 |
| QTer | 4.3±1.14 | 7.85±0.07 * |
| H9c2 | ||
| Control | 8.81±0.16 | 12.60±0.70 |
| CoQ10 | 9.20±0.14 | 13.76±0.28 |
| QTer | 10.32±0.66* | 16.95±0.21** |
Respirometric analyses were performed in the presence of 5 mM Glutamate/Malate or 12,5 mM Succinate/Glycerol 3-phosphate. Respiratory rates are expressed as nmoles O2 min−1/106 cells ± S.D. from at least three independent experiments, *p<0.05 vs. control. **p<0.001 vs. control.
Table 3. Percentage of respiratory rates measured in T67 cells treated for 24 hours with 10 µM native CoQ10 or Qter®.
| % of endogenous respiration rate | ||
| Cell treatment | - FCCP | +500 nM FCCP |
| No treatment | 100±7.2 | 131±13 |
| 10 µM CoQ10 | 100±8.6 | 84±8,6 |
| 10 µM Qter | 100±1.1 | 102±14 |
Respirometric analyses were performed under endogenous and uncoupled conditions (500 nM FCCP). Respiratory rates in the presence of FCCP are expressed as percentage of oxygen consumption respect to the endogenous respiration ± S.D.
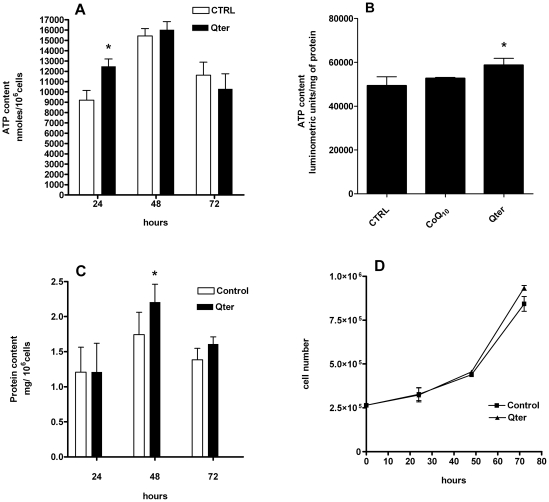
Effects on ATP and protein content and cellular growth were analyzed at 24, 48 and 72 hours after 100 nM Qter® supplementation (Fig. 3). HPLC analysis showed that the ATP content was significantly higher in H9c2 cells treated with 100 nM Qter® for 24 h compared to the control, while no differences were observable at later time points (Figure 3A). ATP increase was confirmed also by luminometric assay in cells treated with the same amount of Qter® while native CoQ10 failed to show any effect (Figure 3B). Interestingly, cellular protein content was normal at 24 hours, and after 48 h cells treated with 100 nM Qter® showed higher protein content (Figure 3C). Qter® administration has no effect on cell growth (Figure 3D).
Figure 3. Effect of Qter® treatment on ATP, protein content and cell growth in H9c2 cells.
H9c2 cells were treated up to 72 hours with 100 nM Qter® and the ATP content was measured at 24, 48 and 72 hours by HPLC analysis (A). Panel B shows the intracellular ATP content after 24 hours treatment with 100 nM Qter® or native CoQ10, measured using luminescence ATP detection assay. Data are reported as arbitrary luminometric units and normalized on total protein content. (Values are means ± S.D.,n = 5, * p≤0.01 vs control). H9c2 cells treated with 100 nM Qter up to 72 hours were assayed for protein content at 24, 48 and 72 hours. Protein content was evaluated by Lowry method (C), (Values are means ± S.D., n = 5, * p≤0.05 vs. control). Cell growth was assessed by trypan blue exclusion method (D).
Oxidative stress
It is well known that mitochondrial impairment is the principal source of ROS in the cell, moreover ROS production may be stimulated by treatment with radicals initiators such as tert-butyl hydroperoxide (TBH). To assess the ROS levels in biological samples we utilized two fluorescent probes: DCFDA and MitoSOX Red.
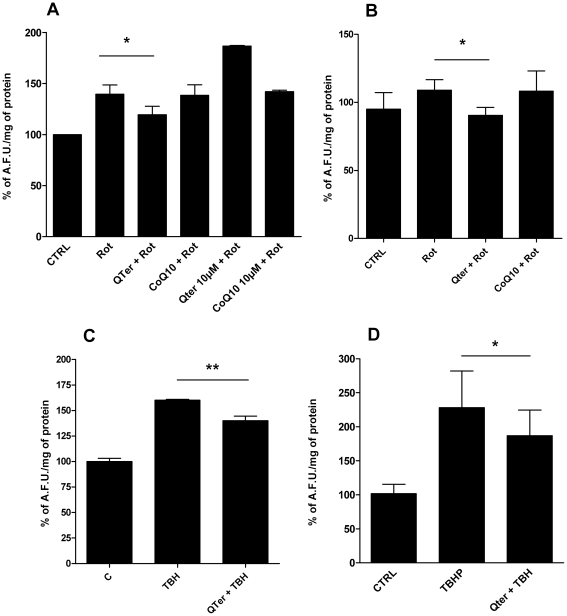
Figure 4 shows the protective effect of Qter® against oxidative stress induced by 100 nM Rotenone (a specific Complex I inhibitor) in T67 cells (Fig. 4A) and H9c2 (Fig. 4B) or 100 µM TBH in T67 cells (Fig. 4C) and H9c2 (Fig. 4D).
Figure 4. Effects of CoQ10 supplementation on oxidative stress induced by Rotenone and t-Butyl hydroperoxide (TBH).
ROS were detected following DCFDA fluorescence in cells treated for 24 hours with 100 nM or 10 uM native CoQ10 or Qter®. ROS were induced by 48 hours treatment with 100 nM Rotenone in T67 (A) and H9c2 cells (B) or by 30 minutes exposure to 100 µM TBH in T67 cells (C) and H9c2 cells (D). Data are the mean ± S.D. of at least three different determinations and are expressed as arbitrary fluorescence units (A.F.U.) normalized on protein content. Protein content was evaluated by Lowry method. Asterisks refer to the statistically significant decrease of ROS production in Rotenone/TBH treated samples supplemented with quinones (n = 5, * p≤0.05); ** p≤0.001).
Pre-treating cells for 24 hours with 100 nM Qter® reduces the total amounts of cellular ROS (Fig. 4A, 4B, 4C, 4D), whereas in the same conditions native CoQ10 is less efficient. Moreover, cellular pre-treatment with higher Qter® concentration (10 µM), not only failed to improve protection against ROS production, but increased the oxidative stress. (Fig. 4A)
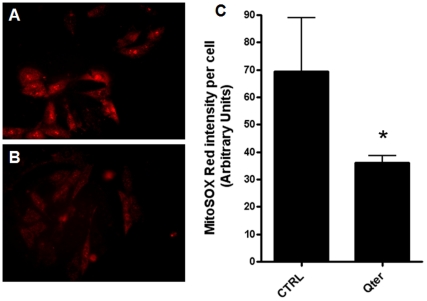
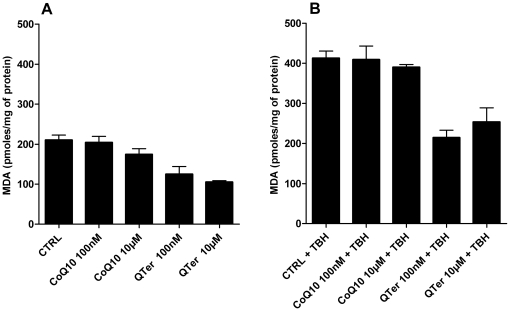
Figure 5A and 5B report the MitoSOX Red staining of H9c2 cells without (Fig. 5A) and with (Fig. 5B) 100 nM Qter® pre-treatment for 24 hours: the lower staining observed in Figure 5B suggests that cellular CoQ10 supplementation reduces the ROS level also in absence of an oxidative insult. In Figure 5C is reported the MitoSOX Red fluorescence intensity obtained by Image J software analysis. ROS damage can be evaluated by measuring the presence of oxidative products such as malondialdehyde (MDA) and conjugated dienes. We observed that Qter treatment caused a statistically significant reduction of all lipid oxidation markers. Figure 6A and 6B show the MDA levels in T67 cells, both in absence (Fig. 6A) and in presence (Fig. 6B) of an oxidative insult, induced by treatment with 100 µM TBH. Even in this case it is possible to appreciate the higher efficiency of Qter® supplementation with respect to native CoQ10.
Figure 5. Analysis of physiological mitochondrial superoxide production using MitoSOX Red.
The representative fluorescence images showed the oxidized MitoSOX fluorescence signal in control H9c2 cells (A) and H9c2 cells following 24 treatment with 100 nM Qter® (B). The fluorescence intensity reported in panel C was quantified by Image J software. Values are presented as means ± SD; n = 20. * p≤0.001.
Figure 6. Malondialdehyde (MDA) levels in T67 cells treated with native CoQ10 or Qter.
Cells were pre-treated for 24 hours with native CoQ10 and QTer (100 nM and 10 µM). Panel A shows the MDA levels in the absence of external oxidative stress. Panel B shows the MDA levels after 30 minutes exposure to 100 µM TBH. Data are the mean of two different experimental determinations and are normalized on total protein content.
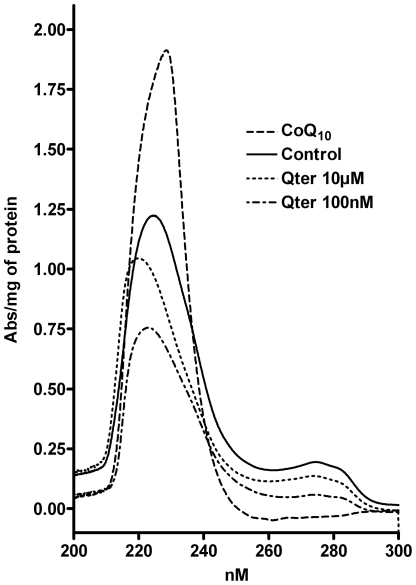
Figure 7 reports the differential absorption spectra of conjugated dienes extracted from T67 cells pre-treated with CoQ10 after 100 µM TBH exposure. The spectrum of cells treated with 100 nM Qter showed the lowest absorbance and the peak was shifted towards shorter wavelengths according to the presence of a lower conjugation status. On the other hand, the spectrum obtained by cells treated with 10 µM of native CoQ10 showed a higher absorbance and the peak was red shifted, indicating a high amount of conjugated dienes.
Figure 7. UV Spectra of conjugated dienes.
Membrane lipids were extracted from T67 cells treated for 24 hours with Qter (100 nM and 10 µM) and CoQ10 (10 µM) after 30 minutes exposure to 100 µM TBH. Each spectrum was obtained as a difference spectra between TBH treated and TBH untreated samples. Spectra are normalized on total protein content and are representative of three different sets of experiments.
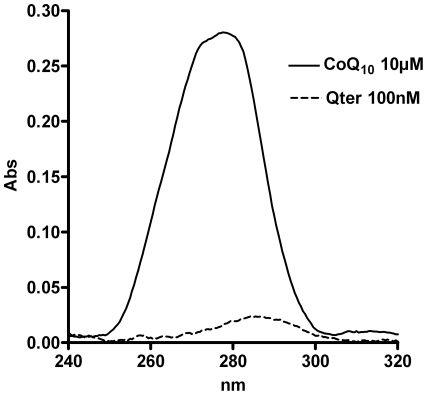
The oxidative stress observed in cells treated with high amounts of native CoQ10 can be due to an incomplete reduction of the supplemented quinone. Figure 8 shows the absorption spectra of CoQ10 extracted from T67 cells treated with 10 µM native CoQ10 or 100 nM Qter. The spectrum obtained from 100 nM Qter treated cells showed a maximum close to 290 nm, indicating that ubiquinone is mainly present in the reduced form. When cells were treated with 10 µM of native CoQ10, the spectrum was broad and the maximum was shifted towards 275 nm, indicating the contemporary presence of oxidized and reduced quinone forms.
Figure 8. UV spectra of oxidized and reduced ubiquinone.
T67 cells were treated for 24 hours with 100 nM Qter or 10 µM native CoQ10, then ubiquinone was immediately extracted, from an equal number of cells, with isopropyl alcohol (for further details see Materials and methods) and the UV spectra were recorded between 320 and 240 nm. The ubiquinone extracted from 100 nM Qter treated sample appears to be completely reduced with a maximum absorption peak at 290 nm, while the ubiquinone extracted from 10 µM native CoQ10 treated sample has a maximum absorption peak shifted towards 275 nm, indicating the presence of the ubiquinone oxidized form. Spectra are representative of three different experiments.
Mitochondrial membrane potential
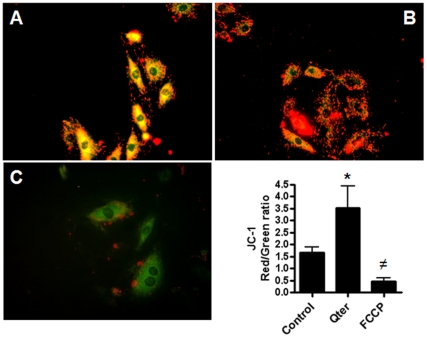
To assess whether Qter® administration could alter mitochondrial membrane potential, JC-1 fluorescence assay was performed in H9c2 cells. In control cells with normal mitochondrial membrane potential, JC-1 accumulates in mitochondria as aggregates with a red fluorescence emission while the monomeric form is prevalent in the cytoplasm with a green fluorescence emission (Figure 9A).
Figure 9. Assessment of mitochondrial potential by JC-1 staining.
Representative images show JC-1 fluorescence in control H9c2 cells (A), H9c2 cells treated for 24 hours with 100 nM Qter® (B) and H9c2 cells treated with 500 nM of the uncoupler FCCP (C). Panel D shows quantitative analysis of Red/Green fluorescence ratio measured by ImageJ software. (n = 20, * p≤0.001 vs. control; ≠p≤0.001 vs. control.).
In Qter treated cells, JC-1 probe was mainly in the aggregated state resulting in a higher red/green fluorescence ratio, suggesting a higher incorporation of the probe into mitochondria as a consequence of a higher membrane potential(Figure 9B). Treatment with 500 nM FCCP prevent JC-1 mitochondrial incorporation, resulting in a pronounced green fluorescence due to the complete loss of mitochondrial membrane potential (Figure 9C). The red/green fluorescence ratios are summarized in Figure 9D.
Discussion
Coenzyme Q10 is a lipid-soluble compound mainly found in mitochondria. It is mostly endogenously produced within cells though small amounts can be provided by food intake. Analysis of CoQ10 subcellular distribution shows that a large portion of CoQ10 (40–50%) is localized in the mitochondrial inner membrane, with smaller amounts in the other organelles and in the cytosol. The high concentration of CoQ10 in the mitochondria reflects its important role in electron transport chain: age-related decrease in mitochondrial CoQ10 content is responsible for oxygen consumption decline).
From a physiological point of view, tissue CoQ10 content is subject to regulation by several factors including oxidative stress and aging [1], [22].
Supplementation with CoQ10 has been thought to be beneficial, especially for situations in which adequate CoQ10 production is adversely affected [6]. A large number of clinical studies have evaluated the effects of CoQ10 supplementation on oxidative stress, both in physiological or pathological conditions.
The results obtained in vivo about CoQ10 tissue distribution are quite controversial. Ibrahim et al. (Ibrahim et al., 2000) [23] observed that CoQ10 oral administration did not alter the levels of this compound in the heart. Furthermore there is no evidence so far showing that dietary CoQ10, which is found to increase the CoQ10 content in lipoproteins and in the liver, is taken up by other tissues under normal conditions. These uncertain results could be partially attributed to the poor water solubility of CoQ10 that impairs its intestinal absorption, tissue distribution and mitochondrial incorporation.
The aim of the present study is to evaluate the in vitro efficacy of CoQ10 supplementation in improving mitochondrial function and protection against oxidative stress. Cells supplemented with CoQ10 do not often show any improvement in their bioenergetics status.
These negligible effects can be explained by several factors, first of all its strong lipophilic nature that results in its accumulation in extra-mitochondrial membranes [24], [25] while only a small portion (∼11%) can reach the mitochondria [26], [27]. The exogenous CoQ10 found in mitochondria is likely to be localized in the outer membrane, thus it is not available to the respiratory chain [28], [29].
Our data showed that Qter® has a better cellular uptake and mitochondrial incorporation compared to native CoQ10: from 10 to 100 fold lower concentrations are required to achieve similar cellular and mitochondrial CoQ10 amount.
A better uptake is the first step to proper CoQ10 insertion into biological membranes and in particular for a significant incorporation in the inner mitochondrial membrane (IMM). We can assume that Qter® promotes a correct CoQ10 insertion into the IMM since an increase in mitochondrial respiration, ATP production, mitochondrial membrane potential and protein synthesis are observed in the cell lines tested. In an interesting paper by Somayajulu et al. similar results were reported using a different water soluble CoQ10 formulation in human neuroblastoma cells (SH-SY5Y) and teratocarcinoma cells (NT2); in particular the authors described a protective effect of CoQ10 treatment on mitochondrial potential, ATP levels and oxidative stress after hydrogen peroxide exposure [30].
The great importance of a correct insertion is well explained by our data: cells treated with 10 µM of native CoQ10 present a mitochondrial ubiquinone concentration close to the one observed in cells treated with 100 nM Qter®, but the bioenergetic effects are quite different.
Moreover lipid peroxidation induced by an oxidative insult is reduced in 100 nM Qter® treated cells as shown in Figure 6B and Figure 7.
For this reason the chemical formulation of CoQ10 may play a crucial role in determining the correct integration of the molecule in the mitochondrial membrane. Nevertheless an increased content of CoQ10 in the mitochondrial membrane does not necessarily imply an automatic increase in mitochondrial function (Table 3). It is well known that respiration rate and ATP synthesis are highly regulated processes that are affected by many factors, primarily cell energy requirements.
Respiration data reported in Table 2 suggest that the mitochondrial ubiquinone content can affect the oxygen consumption under high energy requirement conditions (e.g. high ADP content). In this condition the oxygen consumption rate increases and the CoQ10 content could became the rate limiting factor. A similar behavior is observed in intact cells under FCCP uncoupled condition; in fact, the reported values of endogenous respiration rate were most likely due to an intermediate respiration state (state4/state 3 mixed state) in which the rate-limiting step was not affected by CoQ10 addition (Table 1).
Our data show that a high respiration rate is positively correlated with the increased amount of mitochondrial CoQ10, suggesting that its supplementation can play an important role in diseases related to CoQ10 deficiency (aging, Parkinson Disease, Alzheimer and mitochondrial myophaties). These data correlate with increased NADH-Cyt.c and Succinate-Cyt.c reductase activity observed in Hl-60 cells treated with CoQ10 reported by Navas and co-workers [31].
The mitochondrial respiratory chain organization could play an important role in the increase of respiratory activity. Currently, two models have been proposed: the random collision model [32] and a supercomplex organization called Respirasome [33]. In the first model the electron transfer through the respiratory chain is assured by free diffusion of each component within the IMM. In this scenario, CoQ10 forms a pool used by all the CoQ-dependent respiratory Complexes (mainly Complex I, II and III). On the other hand, the Respirasome requires a solid state organization in which only bound CoQ10 is involved in electron transfer. This last hypothesis seems to be in contrast with a dose dependent effect of CoQ10 addition on the respiratory rate. However, it may be possible that the bound ubiquinone should be in equilibrium with the pool. This hypothesis could explain the beneficial effect of exogenous CoQ10 supplementation [34].
Nevertheless, treatment with high doses of ubiquinone, despite of its formulation, induces a loss of sensitivity to uncoupling agents and increases oxidative stress. We can argue that an excessive incorporation of CoQ10 may perturb the lipid environment of cellular membranes while oxidative stress may be due to the excess of ubiquinone that remains in its oxidized form. For this reason it is not recommended to treat patients with high doses of coenzyme Q10. In particular, our results show that it is necessary to use from 10 to 100 fold concentrations of native CoQ10 to achieve comparable amounts of ubiquinone respectively in whole cells or isolated mitochondria.
The antioxidant role of the CoQ10 reduced form is well known. It localizes in cellular membranes where it acts as a ROS scavenger together with vitamin E. Cells treated with 100 nM Qter® appear to be more resistant to oxidative stress; in fact, Figure 8 shows that in this condition the quinone is mainly in the reduced form
The higher Qter® efficiency is mainly due to its greater water solubility. In fact, compared with native CoQ10, Qter® is about 200 times more soluble in water, while retaining its antioxidant capacity [35], [36].
Conclusion
Our in vitro study underlines important issues regarding CoQ10 treatment. Present results demonstrate that adequate channeling of CoQ10 is important to ensure proper cellular uptake. The vehicle used to terclatrate CoQ10 maintains it in a monomeric form, that results in a correct insertion into membranes, in particular in the inner mitochondrial membrane. The improved bioavailability allows treatments with low doses of ubiquinone that prevent unspecific accumulation with deleterious effects on cell viability.
Although CoQ10 supplementation has shown beneficial effect in many physiopathological alterations, there are few experimental evidences of a direct improvement of mitochondrial functions after CoQ10 treatment. Some interesting papers by Somayajulu and McCarthy describe the protective effect of a water soluble CoQ10 in in vitro and in vivo studies [30], [37], [38].
We demonstrated that increased mitochondrial ubiquinone content results in a general improvement of bioenergetic parameters, like oxygen consumption, ATP content, mitochondrial potential and protein synthesis.
Recently, the beneficial effect of terclatrated CoQ10 supplementation in vivo, both in animal models [39], [40] and humans [41] has been reported. Thus, this work represents a strong rationale for the clinical use of Coenzyme Q10 and highlights the enhanced biological effects of Qter® that make it the eligible CoQ10 formulation for ubiquinone supplementation in patients.
Materials and Methods
Reagents
All chemicals used throughout the present study were of the highest analytical grade, purchased from Sigma-Aldrich, unless otherwise specified. Dulbecco's modified Eagle's medium, trypsin, penicillin, streptomycin and fetal bovine serum were purchased from Invitrogen. Qter® was supplied by Scharper Therapeutics S.r.l. (Milan, Italy). Native CoQ10 was from Kaneka, Japan.
Drug preparation
Qter® is described in the patent number WO/2003/097012 by Actimex S.r.l. CoQ10 is 10% (w/w) of Qter® formulation. Qter® concentration refers to the CoQ10 amount into the multicomposite material. Qter solution was freshly prepared dissolving Qter in DMEM at 100 nM or 10 µM CoQ10 final concentration.
Native CoQ10 stock solution was prepared in ethanol at 5 mM concentration and diluted with DMEM to final concentration of 100 nM or 10 µM prior to use.
Cell culture
The T67 human glioma cell line was derived by Lauro et al. [42] from a World Health Organization (WHO) Grade III gemistocytic astrocytoma. H9c2 embryonal rat heart-derived cells were obtained from European Collection of Cell Cultures, ECACC. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 100 UI/ml penicillin, 100 µg/ml streptomycin, and 40 µg/ml gentamycin, in a 5% CO2 atmosphere at 37°C, with saturating humidity. Cell viability and number were measured by trypan blue exclusion method [43].
Preparation of Mitochondria Fractions
Mitochondria were isolated according to procedures previously described [44].
Extraction and quantification of Coenzyme Q
Treated cells were carefully washed with PBS before extraction procedures. Extraction of coenzyme Q from cells and isolated mitochondria was performed as described by Takada et al. [45]. Quantification of CoQ10 was performed by HPLC analysis. 50–100 µl of ethanolic extract was chromatographed on a C18 column (Kinetex, Phenomenex, 2.6 µm, 100×4.6 mm), using a mobile phase consisting of ethanol: water (97∶3, v/v) at a flow rate of 0.6 ml/min. The concentrations of CoQ10 were obtained by comparison of the peak areas with those of standard solutions. Data are reported as the mean ± standard deviation of at least three independent experiments.
To evaluate the reduction state of ubiquinone, cells were treated for 24 hours with 10 µM of native CoQ10 or 100 nM Qter. After this time, T67 adherent cells were washed twice with PBS, then detached by trypsin-EDTA and centrifuged at 300×g for 3 min; then the pellet was resuspended in PBS, and centrifuged again. The pellet was resuspended in cold isopropyl alcohol and vortexed for 30 seconds, then centrifuged at 15000 g at 4°C for 5 minutes. The organic phase was transferred in a quartz cuvette and the UV spectrum was collected between 240 and 320 nm with a Jasco V-550 spectrophotometer.
Cell permeabilization
Cells were permeabilized with digitonin according to Chomyn A. [39] and immediately used for polarographic assay [44], [46]. Cell number and permeabilization was measured by trypan blue exclusion method.
Oxygen consumption
Intact cells
T67 and H9c2 cell lines were treated for 24 h at 37°C in 5% CO2 with 100 nM CoQ10 or Qter® in DMEM plus FBS. Intact cells (1×106 cells) were assayed for glucose supported oxygen consumption at 30°C in DMEM using a thermostatically controlled oxygraph (Instech Mod.203).
Permeabilized cells
Cells were treated as above and assayed for oxygen consumption in respiration buffer (250 mM sucrose, 20 mM HEPES, 10 mM MgCl2, 1 mM ADP, 2 mM KH2PO4, pH 7.4) after permeabilization. Mitochondrial respiration (state 3 respiration) from complex I was started by adding 5 mM glutamate/malate (G/M) and then stopped with 2.5 µM Rotenone. Subsequently 12.5 mM succinate/glycerol-3-phosphate (S/G3P) was added to restart the respiration. In all experiments maximal respiration rate (uncoupled respiration) was achieved by adding 500 nM FCCP and oxygen consumption was completely inhibited by adding 4 µM Antimycin A at the end of the experiments.
Reactive oxygen species (ROS) detection
H9c2 and T67 cells were seeded in 24-well plates at 4×104 cells/well. After 24 h incubation at 37°C in 5% CO2 in culture medium supplemented with 100 nM CoQ10 or Qter®, cells were washed with phosphate buffered saline (PBS) and treated for 48 h with 100 nM Rotenone. Alternatively cells were treated for 30 minutes with 100 µM tert-butyl hydroperoxide (TBH) in PBS. Subsequently, cells were washed with PBS and treated with 10 µM DCFDA (2′,7′-dichlorofluorescein diacetate, DCFH-DA) in DMEM for 30 minutes, then washed again with PBS and the fluorescence increase in each well was measured (λexc = 485 nm; λem = 535 nm) with a plate reader (Wallac Victor, Perkin-Elmer, USA). Data are reported as the mean ± standard deviation of at least three independent experiments. In a separate set of experiments, basal oxidative stress in H9c2 cells was measured using the mitochondrial superoxide indicator MitoSOX Red. Cells were treated with 100 nM Qter® for 24 hours at 37°C in 5% CO2, then 5 µM MitoSOX was added. After 20 minutes of incubation, cells were washed twice with PBS and images were obtained using an IX50 inverted fluorescence microscope (Olympus, Tokyo) at 20× magnification. Fluorescence intensity was quantified by Image J software (NIH).
ATP content
Intracellular ATP level was measured using luminescence ATP detection assay (ATPlite, PerkinElmer, USA) according to manufacturer's instructions. Data were reported as arbitrary luminometric units, measured with the microplate reader Wallac Victor multilabel counter and normalized to total protein content, determined by Lowry method [47]. Alternatively, intracellular ATP was measured by HPLC method. ATP was extracted essentially as described by Strehler et al. [48]. Distilled water (180 µl) preheated to 100°C was added to cellular samples in Eppendorf tubes and boiled for 5 min with occasional vortexing. Tubes were transferred to ice until HPLC analysis. A mobile phase containing 100 mM K2HPO4 (pH 5.75), 0.1% TBAF (tetrabutylammonium fluoride) and 2% acetonitrile was pumped through a Kinetex C18 (Phenomenex) column at ambient temperature at a flow rate of 0,6 ml/min [49]. Absorbance at 254 nm was monitored by a photodiode array detector (Waters 996). ATP peak was identified by its retention time and by using co-chromatography with standard.
Thiobarbituric acid assay of malondialdehyde and lipid-conjugated dienes assay
T67 cells were treated for 24 h at 37°C in 5% CO2 with native CoQ10 (10 µM and 100 nM) or Qter® (10 µM or 100 nM) in DMEM plus FBS and washed with PBS before treating for 30 minutes with 100 µM tert-butyl hydroperoxide (TBH) in PBS.
After TBH treatment, adherent cells were washed twice with PBS, then detached by trypsin-EDTA and centrifuged at 300×g for 3 min; then the pellet was resuspended in PBS and centrifuged again.
Quantification of thiobarbituric acid reactive substances (TBARS) was carried out as described by Buege and Aust [50].
The formation of conjugated dienes, in T67 cells treated as above was assayed according to Buege and Aust [50].
JC-1 stain for mitochondrial membrane potential (Δψm) measurement
The fluorescent probe JC-1 (5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazol carbocyanine iodide) was used to measure the mitochondrial membrane potential (Δψ) of H9c2 cells. JC-1 is a cationic dye that is accumulated in mitochondria following membrane potential.
At low concentrations the probe is present in monomeric form, with green fluorescence emission (525±10 nm), but at higher concentrations it forms J-aggregates after accumulation in the mitochondrion, with red fluorescence emission (590±10 nm).
After incubation with 10 µM JC-1 at 37°C for 10 min, the culture medium containing JC-1 was removed. Cells were washed twice with PBS, and analyzed by IX50 fluorescence microscope (Olympus, Tokyo).) at 20× magnification. Fluorescence intensity was quantified by Image J software (NIH). Mitochondrial depolarization was achieved by treating cells with 500 nM FCCP, indicated by a decrease in the red/green fluorescence intensity ratio
Statistical analysis
Statistical analysis of data was performed with Student's t-test using GraphPad Prism software.
Acknowledgments
The authors thank Dr. G. Manini and Dr. P. Arzuffi for critical reading of the manuscript.
Footnotes
Competing Interests: AS is currently employed in Scharper Therapeutics as a Medical Affairs Assistant and contributed to the data analysis and to the critical reading of the manuscript. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: The authors have no support or funding to report.
References
- 1.Spindler M, Beal MF, Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr Dis Treat. 2009;5:597–610. doi: 10.2147/ndt.s5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geromel V, Rotig A, Munnich A, Rustin P. Coenzyme Q10 depletion is comparatively less detrimental to human cultured skin fibroblasts than respiratory chain complex deficiencies. Free Radic Res. 2002;36:375–379. doi: 10.1080/10715760290021216. [DOI] [PubMed] [Google Scholar]
- 3.Groneberg DA, Kindermann B, Althammer M, Klapper M, Vormann J, et al. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells. Int J Biochem Cell Biol. 2005;37:1208–1218. doi: 10.1016/j.biocel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, et al. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors. 2008;32:179–183. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Aberg F, Appelkvist EL, Dallner G, Ernster L. Uptake of dietary coenzyme Q supplement is limited in rats. J Nutr. 1995;125:446–453. doi: 10.1093/jn/125.3.446. [DOI] [PubMed] [Google Scholar]
- 6.Silver MA, Langsjoen PH, Szabo S, Patil H, Zelinger A. Effect of atorvastatin on left ventricular diastolic function and ability of coenzyme Q10 to reverse that dysfunction. Am J Cardiol. 2004;94:1306–1310. doi: 10.1016/j.amjcard.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 7.Di Giovanni S, Mirabella M, Spinazzola A, Crociani P, Silvestri G, et al. Coenzyme Q10 reverses pathological phenotype and reduces apoptosis in familial CoQ10 deficiency. Neurology. 2001;57:515–518. doi: 10.1212/wnl.57.3.515. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Kaur H, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol Ther. 2009;124:259–268. doi: 10.1016/j.pharmthera.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32:810–812. doi: 10.2337/dc08-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Kheradpezhou M, Shavali S, El Refaey H, Eken J, et al. Neuroprotective actions of coenzyme Q10 in Parkinson's disease. Methods Enzymol. 2004;382:488–509. doi: 10.1016/S0076-6879(04)82027-5. [DOI] [PubMed] [Google Scholar]
- 12.Shults CW, Haas R. Clinical trials of coenzyme Q10 in neurological disorders. Biofactors. 2005;25:117–126. doi: 10.1002/biof.5520250113. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Dai G, Li G, Yang ES. Coenzyme Q10 reduces beta-amyloid plaque in an APP/PS1 transgenic mouse model of Alzheimer's disease. J Mol Neurosci. 2009;41:110–113. doi: 10.1007/s12031-009-9297-1. [DOI] [PubMed] [Google Scholar]
- 14.Huntington Study Group. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Neurology. 2001;57:397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]
- 15.Storch A, Jost WH, Vieregge P, Spiegel J, Greulich W, et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol. 2007;64:938–944. doi: 10.1001/archneur.64.7.nct60005. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann P, Thompson JL, Levy G, Buchsbaum R, Shefner J, et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann Neurol. 2009;66:235–244. doi: 10.1002/ana.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young JM, Florkowski CM, Molyneux SL, McEwan RG, Frampton CM, et al. Effect of coenzyme Q(10) supplementation on simvastatin-induced myalgia. Am J Cardiol. 2007;100:1400–1403. doi: 10.1016/j.amjcard.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Bhagavan HN, Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;7(Suppl):S78–88. doi: 10.1016/j.mito.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Beg S, Javed S, Kohli K. Bioavailability enhancement of coenzyme Q10: an extensive review of patents. Recent Pat Drug Deliv Formul. 2010;4:245–255. doi: 10.2174/187221110793237565. [DOI] [PubMed] [Google Scholar]
- 20.Balakrishnan P, Lee BJ, Oh DH, Kim JO, Lee YI, et al. Enhanced oral bioavailability of Coenzyme Q10 by self-emulsifying drug delivery systems. Int J Pharm. 2009;374:66–72. doi: 10.1016/j.ijpharm.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Villalba JM, Parrado C, Santos-Gonzalez M, Alcain FJ. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin Investig Drugs. 2010;19:535–554. doi: 10.1517/13543781003727495. [DOI] [PubMed] [Google Scholar]
- 22.Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, et al. Coenzyme Q10 in health and disease. Eur J Clin Nutr. 1999;53:764–770. doi: 10.1038/sj.ejcn.1600880. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim WH, Bhagavan HN, Chopra RK, Chow CK. Dietary coenzyme Q10 and vitamin E alter the status of these compounds in rat tissues and mitochondria. J Nutr. 2000;130:2343–2348. doi: 10.1093/jn/130.9.2343. [DOI] [PubMed] [Google Scholar]
- 24.Lenaz G, Samori B, Fato R, Battino M, Parenti Castelli G, et al. Localization and preferred orientations of ubiquinone homologs in model bilayers. Biochem Cell Biol. 1992;70:504–514. doi: 10.1139/o92-078. [DOI] [PubMed] [Google Scholar]
- 25.Cornell BA, Keniry MA, Post A, Robertson RN, Weir LE, et al. Location and activity of ubiquinone 10 and ubiquinone analogues in model and biological membranes. Biochemistry. 1987;26:7702–7707. doi: 10.1021/bi00398a025. [DOI] [PubMed] [Google Scholar]
- 26.Bentinger M, Dallner G, Chojnacki T, Swiezewska E. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic Biol Med. 2003;34:563–575. doi: 10.1016/s0891-5849(02)01357-6. [DOI] [PubMed] [Google Scholar]
- 27.Santos-Ocana C, Do TQ, Padilla S, Navas P, Clarke CF. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J Biol Chem. 2002;277:10973–10981. doi: 10.1074/jbc.M112222200. [DOI] [PubMed] [Google Scholar]
- 28.Geromel V, Darin N, Chretien D, Benit P, DeLonlay P, et al. Coenzyme Q(10) and idebenone in the therapy of respiratory chain diseases: rationale and comparative benefits. Mol Genet Metab. 2002;77:21–30. doi: 10.1016/s1096-7192(02)00145-2. [DOI] [PubMed] [Google Scholar]
- 29.Lopez LC, Quinzii CM, Area E, Naini A, Rahman S, et al. Treatment of CoQ(10) deficient fibroblasts with ubiquinone, CoQ analogs, and vitamin C: time- and compound-dependent effects. PLoS One. 2010;5:e11897. doi: 10.1371/journal.pone.0011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somayajulu M, McCarthy S, Hung M, Sikorska M, Borowy-Borowski H, et al. Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by Coenzyme Q10. Neurobiol Dis. 2005;18:618–627. doi: 10.1016/j.nbd.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Ayala DJ, Lopez-Lluch G, Garcia-Valdes M, Arroyo A, Navas P. Specificity of coenzyme Q10 for a balanced function of respiratory chain and endogenous ubiquinone biosynthesis in human cells. Biochim Biophys Acta. 2005;1706:174–183. doi: 10.1016/j.bbabio.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Hackenbrock CR, Chazotte B, Gupte SS. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr. 1986;18:331–368. doi: 10.1007/BF00743010. [DOI] [PubMed] [Google Scholar]
- 33.Schagger H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim Biophys Acta. 2002;1555:154–159. doi: 10.1016/s0005-2728(02)00271-2. [DOI] [PubMed] [Google Scholar]
- 34.Lenaz G, Genova ML. Mobility and function of coenzyme Q (ubiquinone) in the mitochondrial respiratory chain. Biochim Biophys Acta. 2009;1787:563–573. doi: 10.1016/j.bbabio.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Carli F, Corvi Mora P, Canal T. Co-grinding process for the preparation of a ternary composition. 2003. European patent office. Wipo website. Available: http://www.wipo.int/patentscope/search/en/WO2003097012. Accessed 2012 Feb 23.
- 36.Corvi Mora P, Canal T, Ruzzier F. Composition containing micronutrients with improved anti-oxidant activity and the use thereof. 2008. Wipo website. Available: http://www.wipo.int/patentscope/search/en/WO2007009997. Accessed 2012 Feb 23.
- 37.Somayajulu-Nitu M, Sandhu JK, Cohen J, Sikorska M, Sridhar TS, et al. Paraquat induces oxidative stress, neuronal loss in substantia nigra region and parkinsonism in adult rats: neuroprotection and amelioration of symptoms by water-soluble formulation of coenzyme Q10. BMC Neurosci. 2009;10:88. doi: 10.1186/1471-2202-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol. 2004;201:21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Fetoni AR, Piacentini R, Fiorita A, Paludetti G, Troiani D. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL). Brain Res. 2009;1257:108–116. doi: 10.1016/j.brainres.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Seo AY, Vorobyeva DA, Carter CS, Anton SD, et al. Beneficial effects of a Q-ter based nutritional mixture on functional performance, mitochondrial function, and oxidative stress in rats. PLoS One. 2010;5:e10572. doi: 10.1371/journal.pone.0010572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fumagalli S, Fattirolli F, Guarducci L, Cellai T, Baldasseroni S, et al. Coenzyme Q10 terclatrate and creatine in chronic heart failure: a randomized, placebo-controlled, double-blind study. Clin Cardiol. 2011;34:211–217. doi: 10.1002/clc.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauro GM, Di Lorenzo N, Grossi M, Maleci A, Guidetti B. Prostaglandin E2 as an immunomodulating factor released in vitro by human glioma cells. Acta Neuropathol. 1986;69:278–282. doi: 10.1007/BF00688305. [DOI] [PubMed] [Google Scholar]
- 43.Gorman A, McCarthy J, Finucane D, Reville W. Cotter TG, Martin SJ, editors. Morphological assessment of apoptosis. Techniques in Apoptosis, A Users Guide. 1996. pp. 6–7.
- 44.Chomyn A. Platelet-mediated transformation of human mitochondrial DNA-less cells. Methods Enzymol. 1996;264:334–339. doi: 10.1016/s0076-6879(96)64031-2. [DOI] [PubMed] [Google Scholar]
- 45.Takada M, Ikenoya S, Yuzuriha T, Katayama K. Simultaneous determination of reduced and oxidized ubiquinones. Methods Enzymol. 1984;105:147–155. doi: 10.1016/s0076-6879(84)05020-5. [DOI] [PubMed] [Google Scholar]
- 46.Chomyn A. In vivo labeling and analysis of human mitochondrial translation products. Methods Enzymol. 1996;264:197–211. doi: 10.1016/s0076-6879(96)64020-8. [DOI] [PubMed] [Google Scholar]
- 47.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 48.Strehler BL. Adenosine-5V-triphosphate and creatine phosphate, determination with luciferase. Methods of enzymatic analysis. 1963:559–572. [Google Scholar]
- 49.Napolitano MJ, Shain DH. Quantitating adenylate nucleotides in diverse organisms. J Biochem Biophys Methods. 2005;63:69–77. doi: 10.1016/j.jbbm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]