Abstract
Ethanol exposure during perinatal development can cause cognitive abnormalities including difficulties in learning, attention, and memory, as well as heightened impulsivity. The purpose of this study was to assess performance in spatial learning and impulsive choice tasks in rats subjected to an intragastric intubation model of binge ethanol exposure during human third trimester-equivalent brain development. Male and female Sprague–Dawley rat pups were intubated with ethanol (5.25 g/kg/day) on postnatal days 4–9. At adolescence (between postnatal days 35–38), these rats and sham intubated within-litter controls were trained in both spatial and cued versions of the Morris water maze. A subset of the male rats was subsequently tested on a delay-discounting task to assess impulsive choice. Ethanol-exposed rats were spatially impaired relative to controls, but performed comparably to controls on the cued version of the water maze. Ethanol-exposed rats also showed greater preference for large delayed rewards on the delay discounting task, but no evidence for altered reward sensitivity or perseverative behavior. These data demonstrate that early postnatal intermittent binge-like ethanol exposure has prolonged, detrimental, but selective effects on cognition, suggesting that even relatively brief ethanol exposure late in human pregnancy can be deleterious for cognitive function.
Keywords: alcohol, binge ethanol exposure, delay discounting, hippocampus, impulsivity, Morris water maze, rat, spatial learning
Introduction
Ethanol exposure during brain growth and maturation disrupts a variety of processes that are crucial to the development of a healthy and normally functioning nervous system, including cell differentiation and migration, axonal and dendritic growth, synaptogenesis, and signal transduction (Miller, 1993, 1996; West et al., 1994; Eckardt et al., 1998; Luo and Miller, 1998; Costa et al., 2000; Granato and Van Pelt, 2003; DuBois et al., 2004). Disruptions in these processes may account for the widely reported cognitive deficits observed in individuals exposed to ethanol prenatally, including impairments in learning and executive functions and elevations in impulsive behavior (Burd and Martsolf, 1989; Streissguth et al., 1994; Goodlett and Peterson, 1995; Goodlett and Johnson, 1997; Connor et al., 2000; Mihalick et al., 2001; Fryer et al., 2007; Olmstead et al., 2009). A number of studies in rat models indicate that binge ethanol exposure during postnatal days (PD) 4–9 alters GABAergic signaling in the medial septum, a brain region which sends robust projections to the hippocampus and which is heavily implicated in cognitive processes including learning and memory (Hsiao et al., 1998, 2001, 2004). As such, changes in septohippocampal signaling are well positioned to contribute to many of the cognitive abnormalities associated with developmental ethanol exposure. Prominent among these abnormalities are deficits in tests of spatial learning and memory, which have been reported in a variety of developmental ethanol exposure models (Goodlett et al., 1987; Gianoulakis, 1990; Goodlett and Peterson, 1995; Goodlett and Johnson, 1997; Kim et al., 1997; Girard et al., 2000; Johnson and Goodlett, 2002; Dubois et al., 2006). Notably, however, ethanol exposure also has deleterious effects on other neuronal populations (such as cerebellar Purkinje cells) that could also influence motor function and motivation (Goodlett et al., 1998; Lee et al., 2008), and thus confound some widely used performance measures in spatial learning assessments (e.g. latency to reach the escape platform in the Morris water maze).
In addition to effects on spatial learning and memory, developmental ethanol exposure can affect other aspects of neurobehavioral function. Impulsivity is commonly associated with prenatal ethanol exposure in humans (Connor et al., 2000; Mihalick et al., 2001; Fryer et al., 2007), and there is some evidence from animal studies that developmental ethanol exposure can increase impulsive action [‘motoric’ impulsivity, or the inability to withhold a prepotent motor response (Olmstead et al., 2009)]. To our knowledge, however, there has been little investigation of the effects of developmental ethanol exposure on impulsive choice (Pupe et al., 2011).
The purpose of this study was two-fold: first, in order to dissociate impairments in learning and memory from impairments in sensorimotor function, we conducted a large-scale study of male and female rats exposed to a third trimester binge ethanol model, using carefully selected performance measures in the Morris water maze. Second, to determine how this binge ethanol exposure regimen affects choice impulsivity, we assessed performance of a subset of the male rats from the water maze study in a delay discounting task (Evenden and Ryan, 1996; Cardinal et al., 2000; Simon et al., 2007; Simon et al., 2010).
Methods
Subjects
Timed-pregnant Sprague–Dawley rat dams were housed at the College of Medicine Animal Care Facility in accord with the rules and regulations of the Texas A&M University Laboratory Animal Care Committee. Dams were maintained under controlled (22–25°C) conditions on a 12 : 12 h light/dark cycle (lights on 07:00–19:00 h) and were fed standard rat chow (Harlan Laboratories, Indianapolis, Indiana, USA) with free access to water. Gestational day 22 was designated as PD 0. On PD 2, litters were adjusted to eight pups, with equal numbers of males and females of similar size when available. Pups were subjected to binge ethanol exposure during PD 4–9. This postnatal period is a time at which considerable brain growth and maturation occur in rats and is analogous to the third trimester of human prenatal development (Dobbing and Sands, 1979). At PD 26, litters were transferred to the Texas A&M University Psychology Department vivarium (AALAC-accredited) for water maze assessment. After transfer, rats (n = 81, 36 females, 45 males) were individually housed with a regular 12 : 12 h light/dark cycle and climate control at 25°C before beginning behavioral testing. Rats were provided with food and water ad libitum and allowed to adjust to their new environment for 5 days. After 5 days, rats were marked with black hair dye to provide the contrast necessary for tracking their position in the Morris water maze. Rats were then handled daily in the water maze testing room for 5 days. Water maze assessment began between PD 35 and 38.
Binge ethanol exposure
The ethanol exposure procedure has been used extensively in our laboratory to investigate effects of developmental ethanol exposure on basal forebrain physiology (Hsiao et al., 1998, 2001, 2004; DuBois et al., 2004, 2006). This procedure is considered to model binge drinking because on six separate days, blood ethanol concentrations (BECs) are increased from zero to high levels (over 250 mg/100 ml blood) and return to zero before once again being rapidly increased on each of the 6 days. This pattern and magnitude of blood ethanol levels meets the Centers for Disease Control definition of binge drinking, which is drinking that is sufficient to increase BECs to at least 0.08% (80 mg/100 ml blood) (Centers for Disease Control and Prevention, 2010). As previously described (Hsiao et al., 2001), on the morning of PD 4, pups within each litter were assigned to either ‘ethanol-intubated’ or ‘control’ treatment groups with equal numbers of males and females of similar size in each treatment within a litter. Ethanol-containing milk was made fresh daily by diluting 95% (w/v) ethanol USP in Enfamil (concentrate with Lipil and iron; Mead Johnson, Glenview, Illinois, USA). Ethanol-intubated pups received the ethanol-containing milk through manual oral gastric intubation with a PE-10 plastic tube tipped with a short segment of soft silastic tubing (0.64 mm outer diameter). Ethanol (5.25 g/kg/day; 11.9% v/v; 27.8 ml/kg; divided into two doses administered 2 h apart) was administered on PD 4–9, during the middle of the light cycle. Two hours after the second ethanol feeding, additional ‘milk alone’ feeding was given to ethanol-treated pups to offset reduced nursing during intoxication. Control pups, littermates of the ethanol-treated group, were also intubated with the same intragastric tube under the same schedule, but not given milk (as their ability to nurse was not impaired by ethanol intoxication, and because of concern that the added calories could exaggerate differences in nutritional intake between the groups). Each intubation procedure took less than 1 min per pup. During an intubation session (~ 15 min), individual litters were kept on a cloth-covered heating pad (~ 35°C) and then immediately returned to their dam. On PD 9, all pups were tattooed with nontoxic permanent black India ink for future identification. Pups stayed with the dam until weaned on PD 25, when they received a subcutaneous microchip (Avid Identification Systems, Norco, California, USA). BECs were determined using gas chromatography (Maier et al., 1999). Blood samples (10 μl from the tip of the tail) were collected in heparinized Unipets (Becton Dickinson, Franklin Lakes, New Jersey, USA) on PD 6 from all pups. Samples were collected 1.5 h after the second ethanol treatment, a time point previously shown to represent a peak BEC level in the artificial rearing (pup-in-a-cup) and intragastric intubation models (Hsiao et al., 2001). Control and ethanol-exposed peak BECs, body weight, and percent growth were compared using two-tailed independent t-tests for unequal variance.
Water maze
Apparatus
Spatial learning abilities were assessed on the Morris water maze task using a modified protocol described previously (Mendez et al., 2008; Bizon et al., 2009). The maze, a white circular tank measuring 183 cm in diameter with a wall height of 58 cm, was filled with water (27°C) made opaque with the addition of nontoxic white tempera paint. A retractable white escape platform (12 cm diameter, HVS Image, UK) was submerged 2 cm below the water surface near the center of the southwest quadrant of the maze. Black curtains, on which were affixed large white geometric shapes (extramaze cues), surrounded the maze. Data were analyzed using a computer-based video tracking system (Water 2020; HVS Image).
Spatial reference memory (hidden platform) task
Spatial reference memory was assessed as described previously (Mendez et al., 2008; Bizon et al., 2009). Briefly, rats received three daily training trials with a 30 s intertrial interval (ITI) over six consecutive days. In each trial, rats were placed into the water facing the wall of the maze at one of four equally spaced start positions (north, south, east, or west). The start positions were varied in a pseudorandom manner, such that all rats started from each of the locations approximately the same number of times. Once in the water, rats were allowed to swim until they found the hidden platform or until 90 s elapsed, at which time the rats were guided to the escape platform by the experimenter. Rats remained on the platform for 30 s and then were placed in a holding chamber for 30 s before the next trial. Every sixth trial was a probe trial in which the platform was lowered to the bottom of the maze for the first 30 s of the trial, after which it was raised to allow the rats to escape.
Cued (visible platform) task
After spatial reference memory training, rats were given a single session with six trials of cue training. For cue training, rats were trained to escape to a visible platform (painted black and protruding 2 cm above the water surface). Both the start position and platform location were varied in each trial, making the extra-maze cues explicitly irrelevant to the platform location. In each trial, rats were allowed to search for the platform for 90 s, and then were allowed to remain there for 30 s before a 30-s ITI.
Behavioral and statistical analyses
For each task, data files were created by the Water 2020 software and were exported to Microsoft Excel and SPSS (version 16.0; IBM, Armonk, New York, USA) for analysis. In all statistical comparisons, P values less than 0.05 were considered significant. Training trial data were averaged into three blocks consisting of the five trials preceding each probe trial, and performance was analyzed using both path length and cumulative search error measures. Path length is the total distance traveled from the start position to the platform and is reported in centimeters. To calculate cumulative search error, the rat's distance from the platform was sampled 10 times per second and these distances were averaged into 1s bins. Cumulative search error is the sum of these 1s bins minus the optimal path from the start location to the platform (Gallagher et al., 1993; Mendez et al., 2008; Bizon et al., 2009). Thigmotaxis, which is the percent time spent in the maze periphery, was assessed as the time the rat spent swimming in the outer 10% of the maze (Montgomery et al., 2008). Additional measures of performance [e.g. latency, defined as the time (s) it took the rat to reach the platform, and swim speed, defined as the rat's swim speed (cm/s) averaged across the entire trial] also were recorded. Interpolated probe-trial data (i.e. every sixth trial) were analyzed using the mean search error. This measure was derived by dividing the cumulative search error in these trials by the duration of the probe trial (30 s). Comparisons between control and ethanol-exposed groups in both training trial blocks and probe trials were conducted using three factor analyses of variance (ANOVAs) (treatment × sex × training trial block or probe trial).
Delay discounting task
Subjects
Following water maze testing, a subset of the male rats (n = 8 ethanol exposed and n = 8 control) was randomly selected for further testing in the delay discounting task (female rats were not included in this portion of the study because of space constraints). Beginning between PD 42 and 45, rats were food-restricted to 85% of their free-feeding weight over the course of 1 week, and were maintained as such throughout the duration of the delay discounting task by limiting their home cage food intake to approximately 15 g/day (in addition to food earned in the tasks). Water was freely available at all times.
Apparatus
Testing in the delay discounting and control tasks was conducted in eight identical standard rat behavioral test chambers (30.5 × 25.4 × 30.5 cm, Coulbourn Instruments, Whitehall, Pennsylvania, USA) with metal front and back walls, transparent Plexiglas side walls, and a floor composed of steel rods (0.4 cm in diameter) spaced 1.1 cm apart. Each test chamber was housed in a sound attenuating cubicle, and equipped with a recessed food pellet delivery trough fitted with a photobeam to detect head entries and a 1.12 W lamp to illuminate the food trough. This trough, into which the 45 mg grain-based food pellet rewards (PJAI; Test Diet, Richmond, Indiana, USA) were delivered, was located 2 cm above the floor in the center of the front wall. Two retractable levers were located to the left and right of the food delivery trough, 11 cm above the floor. Experiments were controlled and data were collected by a computer interfaced with the behavioral test chambers and equipped with Graphic State 3.01 software (Coulbourn Instruments).
Procedures
The procedures for the delay discounting task were similar to those described previously (Evenden and Ryan, 1996; Simon et al., 2007). On the day before the start of behavioral testing, each rat was given five 45 mg food pellets in its home cage to reduce neophobia to the food reward used in the task. The task began with a 64-min session of magazine training consisting of 38 deliveries of a single food pellet with an ITI of 100 ± 40 s. In the following session, rats were shaped to press a single lever (either left or right, counterbalanced across groups; the other was retracted during this phase of training) in order to receive a single food pellet. Once they reached a criterion of 50 lever presses during a 30-min session, they were shaped to press the opposite lever using the same schedule and criterion. Following completion of lever press shaping, both levers were retracted, and rats were shaped to nose poke into the food trough during simultaneous illumination of the trough light and a 1.12 W house light. When a nose poke occurred, a single lever was extended, and a lever press resulted in immediate delivery of a single food pellet. Immediately following the lever press, the house and trough lights were extinguished and the lever was retracted. The left and right levers were presented an equal number of times, with no more than two consecutive presentations of the same lever. Rats were trained to a criterion of at least 60 successful trials in an hour with an ITI of 40 ± 10 s, after which testing on the delay discounting task began.
Each session in the delay discounting task consisted of five blocks of 12 trials each. Within each session, each of the 60 s trials began with a 10 s illumination of the food trough and house lights. A nose poke into the food trough during this time extinguished the food trough light and triggered extension of either a single lever (forced choice trials) or both levers simultaneously (free choice trials). Trials in which rats failed to nose poke during this time window were scored as omissions. A press on one lever (either left or right, counterbalanced across groups) resulted in delivery of a single food pellet immediately following the lever press. A press on the other lever resulted in delivery of three food pellets after varying delays. Levers remained extended for 10 s, and failures to press either lever within this window were scored as omissions. Once either lever was pressed, both levers were retracted and the house light was extinguished until food delivery. Food delivery was accompanied by reillumination of both lights, which were again extinguished upon entry to the food trough for food collection or after 10 s, whichever occurred sooner. Each of the five 12-trial blocks in a session began with two forced choice trials in which only one lever was extended (one for each lever). This was followed by 10 free choice trials in which both levers were extended. During the first 12-trial block, the delay to the large reward was set at 0 s. In subsequent 12-trial blocks, the delay to the large reward increased to 4, 8, 16, and 32 s. Because trials were of fixed duration, reward choice did not influence the rapidity of progress through the trials (i.e. choice of the small immediate reward did not result in more or sooner choice opportunities). Thus, choice of the large delayed reward was, from an objective perspective, an ‘optimal’ choice, as it resulted in more food delivery over the course of a session. The percentage of free choice trials in which rats chose the large reward lever (number of large reward lever choices/total responses) was calculated for each block as an indicator of reward preference (Simon et al., 2007, 2010). Rats were tested in the delay discounting task for 40 daily sessions, at which point stable performance was observed across the final five sessions (36–40 – see Data analysis for description of stable performance).
Equal rewards condition
To test for the ability to detect and respond to delays to reward delivery, the amount of food associated with each of the levers was equalized (i.e. one food pellet for either choice) while the delays remained the same as in the delay discounting task. Rats were tested under these conditions for 10 sessions, at which point stable performance was achieved using the criteria described below.
No delays condition
To test for the ability to detect and respond to differences in reward magnitude, the amounts of food associated with each lever were restored to their initial conditions (one food pellet vs. three food pellets) and the delays preceding the large reward were eliminated (thus rendering the large reward lever the optimal choice in all blocks). Rats were tested under these conditions for 10 sessions, at which point stable performance was achieved.
Within-session perseveration condition
To determine whether ethanol exposure caused perseverative behavior (continued choice of a response option even after that option becomes disadvantageous), the conditions were set to match the ‘no delays’ condition in trial blocks 1 and 2 (i.e. one food pellet vs. three food pellets, with no delays to either reward), and in the third block, the reward magnitudes associated with each lever reversed (such that the lever that previously produced three food pellets now produced one pellet, and vice versa) and remained in this reversed condition for the remaining blocks 4 and 5. This design was chosen to elicit a shift in behavior over the course of a single session, analogous to the shift observed in the delay discounting task. Rats were tested under these conditions for 12 sessions, at which point stable performance was achieved.
Data analysis
Raw data files were exported from Graphic State software and compiled using a custom macro written for Microsoft Excel (Dr Jonathan Lifshitz, University of Kentucky). Statistical analysis was conducted in SPSS 18.0. Analyses of stable performance in the decision-making tasks were conducted using a two-factor repeated measures ANOVA (trial block × test session). Stable performance was defined as a main effect of trial block in the absence of main effects or interactions involving test session (Simon et al., 2007). Comparisons between groups in the decision-making tasks were conducted using two-factor repeated measures ANOVA (exposure condition × trial block). For all analyses, P-values less than 0.05 were considered significant.
Results
Ethanol exposure
Blood ethanol samples collected on PD 6 from male and female pups showed substantial intoxication 90 min after the ethanol treatments. BECs (mean ± SEM; females = 342 ± 7 mg/dl, males = 345 ± 9 mg/dl; P > 0.05) were similar for males and females and consistent with values found previously at this time point, which reflect peak BECs observed following ethanol intubation (Hsiao et al., 2001, 2004). Over the 6 days of intubation treatment, both control and ethanol-exposed pups essentially doubled their body weights (mean ± SEM; control PD 4 = 10.4 ± 0.2 g, PD 10 = 24.5 ± 0.3 g; ethanol PD 4 = 10.7 ± 0.2 g, PD 10 = 20.9 ± 0.4 g). However, relative weight gains were slightly smaller for both male and female animals following ethanol exposure (mean ± SEM, PD 10/PD 4 wt: female control = 237 ± 3%, ethanol = 198 ± 3%, P < 0.05; male control = 234 ± 2%, ethanol = 193 ± 3%, P < 0.05). The slower growth for the ethanol group was likely because of an intoxication-induced reduction in nursing, because these pups appeared less active for several hours after intubation.
Water maze
Swim speed
Swim speed differences were assessed during training in the visible platform task. A two-factor ANOVA (ethanol exposure × sex) revealed a main effect of ethanol exposure on mean swim speed, such that ethanol-exposed rats swam significantly faster than controls [F(1,77) = 22.72, P < 0.001].
Cued (visible platform) task
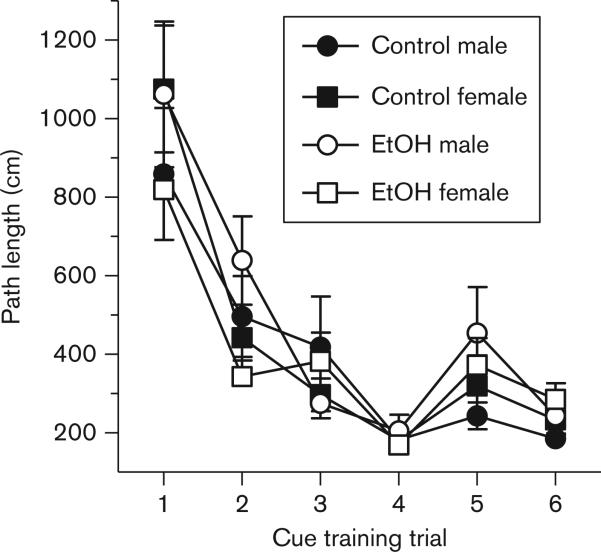
In the visible platform task, a three factor ANOVA (ethanol exposure × sex × training trial) revealed that all groups performed comparably over the six cue training trials as assessed by the path length measure. As illustrated in Figure 1, all groups decreased their path length to the visible platform across trials [F(5,385) = 46.30, P < 0.001]; however, there were no significant main effects or interactions involving ethanol exposure or sex on path length to reach the platform.
Fig. 1.
Cue (visible platform) training performance in control male (filled circles), control female (filled squares), ethanol-exposed (EtOH) male (open circles), and EtOH female (open squares) rats (data points represent means ± SEM). All groups decreased their path length to the visible platform across trials, and there were no effects of EtOH exposure or sex on path length.
Spatial reference memory task
Training trials
In order to identify any pretraining differences in the rats’ ability to ascertain the position of the hidden platform, cumulative search error and path length measures were assessed in the first training trial of the hidden platform (spatial) water maze task. As expected, neither cumulative search error nor path length in the first trial differed as a function of ethanol exposure (F's < 0.37, NS).
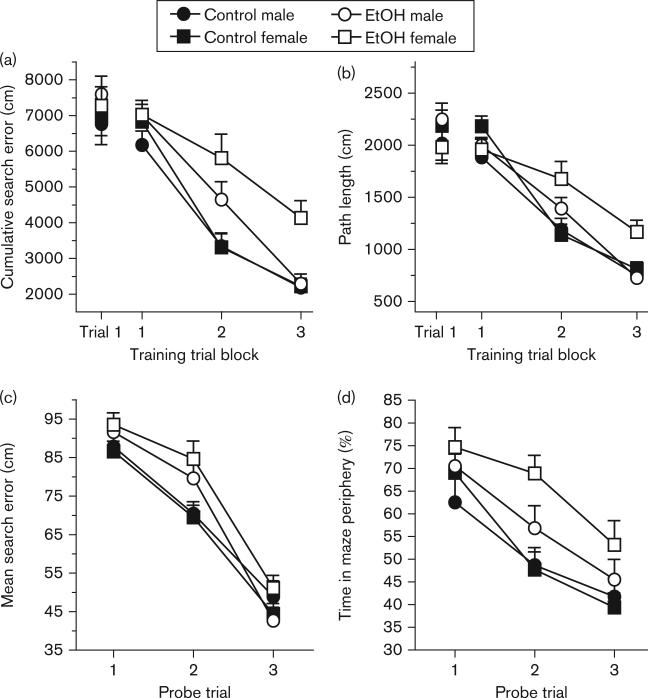
Across all training trials, a three-factor ANOVA (ethanol exposure × sex × trial block) performed on both cumulative search error and path length revealed that, across groups, all rats improved over the course of training [cumulative search error: F(2,154) = 170.50, P < 0.001; path length: F(2,154) = 178.70, P < 0.001] (Fig. 2). There was also a significant main effect of ethanol exposure [cumulative search error: F(1,77) = 19.86, P < 0.001; path length [F(1,77) = 7.41, P < 0.01], such that ethanol-exposed rats performed worse than controls, as well as a significant main effect of sex [cumulative search error: F(1,77) = 3.98, P < 0.05; path length F(1,77) = 5.65, P < 0.05] such that female rats performed worse than males. In addition, there was a significant interaction between ethanol exposure and trial block (cumulative search error: F(2,154) = 5.95, P < 0.01; path length [F(2,154) = 7.31, P < 0.01], such that the performance of control rats improved more rapidly than ethanol-exposed rats. Finally, there was a significant three-way interaction between ethanol exposure, sex, and trial block (cumulative search error [F(2,154) = 3.72, P < 0.05]; path length [F(2,154) = 4.93, P < 0.05], suggesting that ethanol-exposed males improved to control levels across training trials, whereas the performance of ethanol-exposed females did not reach levels displayed by control females.
Fig. 2.
Spatial reference memory performance on water maze training and probe trials. The cumulative search error (a) and path length (b) of control male (filled circles), control female (filled squares), ethanol (EtOH) male (open circles), and EtOH female (open squares) rats did not significantly differ in the first training trial. (a) The figure shows that over the course of training trial blocks there were significant main effects of EtOH exposure and of sex on cumulative search error. There was a significant interaction between EtOH exposure and trial blocks such that EtOH-exposed rats had higher cumulative search error over the course of training compared with controls. A significant three-way interaction between EtOH exposure, sex, and trial block was also found, such that EtOH-exposed females improved at a slower rate than EtOH male rats. (b) Mean (± SEM) path length across three training trial blocks, confirming the water maze performance as assessed by cumulative search error (a). (c) The mean (± SEM) search error of all groups decreased over the probe trials, indicating that all participants demonstrated improved spatial performance over time. There was a significant main effect of EtOH exposure on mean search error such that EtOH-exposed rats had significantly higher mean search error compared with control rats. (d) Rats’ percent time in the maze periphery (thigmotaxis) during interpolated probe trials. EtOH-exposed rats spent significantly more time in the maze periphery compared with control rats.
Probe trials
Spatial learning performance was also assessed by evaluating rats’ mean proximity to the platform (mean search error) and the percent time spent in the outer 10% of maze (thigmotaxis) across the three probe trials interpolated throughout the training protocol (Fig. 2). A three-factor ANOVA (ethanol exposure × sex × probe trial) confirmed, as observed during training trials, that all rats demonstrated improved spatial performance across the probe trials, as evidenced by reduced mean search error [F(2,154) = 165.18, P < 0.001] and reduced thigmotaxis [F(2,154) = 44.73, P < 0.001]. There was a significant main effect of ethanol exposure on both mean search error [F(1,67) = 9.80, P < 0.01] and thigmotaxis [F(1,67) = 7.61, P < 0.01], such that ethanol-exposed rats had significantly greater mean search error and spent significantly more time in the maze periphery compared with control rats. Also, there was a significant interaction between ethanol exposure and trial block (mean search error: F(2,154) = 4.58, P < 0.05; thigmotaxis F(2,154) = 3.62, P < 0.05], such that the performance of control rats improved more rapidly than that of ethanol-exposed rats. However, there were neither significant main effects nor interactions involving sex and the two other factors.
Delay discounting task
There were no differences between control and ethanol-exposed rats in the number of sessions required to complete the shaping procedures [t(14) = 1.13, NS], indicating that both groups were able to acquire the task procedures at the same rate (control mean, 4.13; SEM = 0.13; ethanol exposed mean, 4.38; SEM = 0.18). Following shaping, rats were tested for 40 sessions in the delay discounting task.
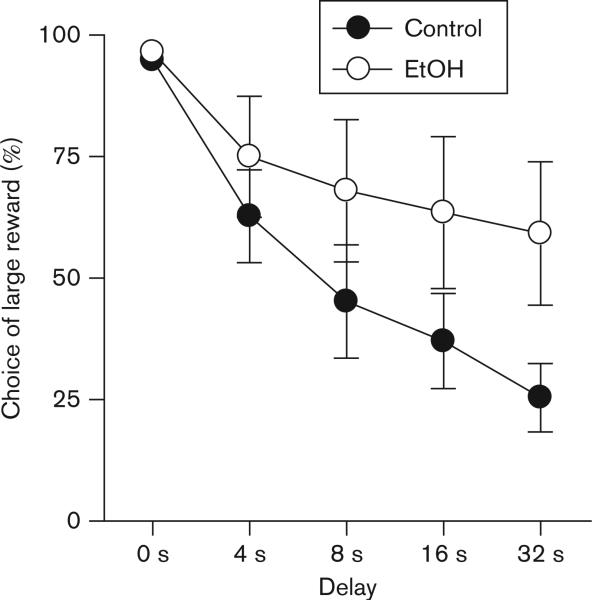
As shown in Figure 3, all rats decreased their choice of the large reward as the delay to large reward delivery increased across the course of the test sessions. A two-factor ANOVA conducted on averaged data from the last five test sessions (delay × ethanol exposure) revealed a significant main effect of delay [F(4,56) = 27.82, P < 0.001], indicating that, as a group, all rats discounted the large reward as the delay to its delivery increased. There was no significant main effect of ethanol exposure, but, importantly, there was a significant interaction between delay and ethanol exposure [F(4,56) = 2.55, P < 0.05] demonstrating that discounting of delayed rewards was attenuated in ethanol-exposed rats compared with control rats (i.e. decreased impulsive choice). There was no significant difference in the percentage of omitted trials between ethanol-exposed and control groups [t(14) = 0.93, NS: control mean = 2.2%, SEM = 1.93; ethanol exposed mean = 0.4%, SEM = 0.15].
Fig. 3.
Delay discounting in control and ethanol (EtOH)-exposed rats. The vertical axis indicates mean (± SEM) percent choice of the large reward. All rats discounted the value of the large reward (as shown by decreased choice of the large reward) as delays increased. However, EtOH-exposed rats showed less discounting of delayed rewards than controls (significant interaction between EtOH exposure and delay).
Equal-rewards control
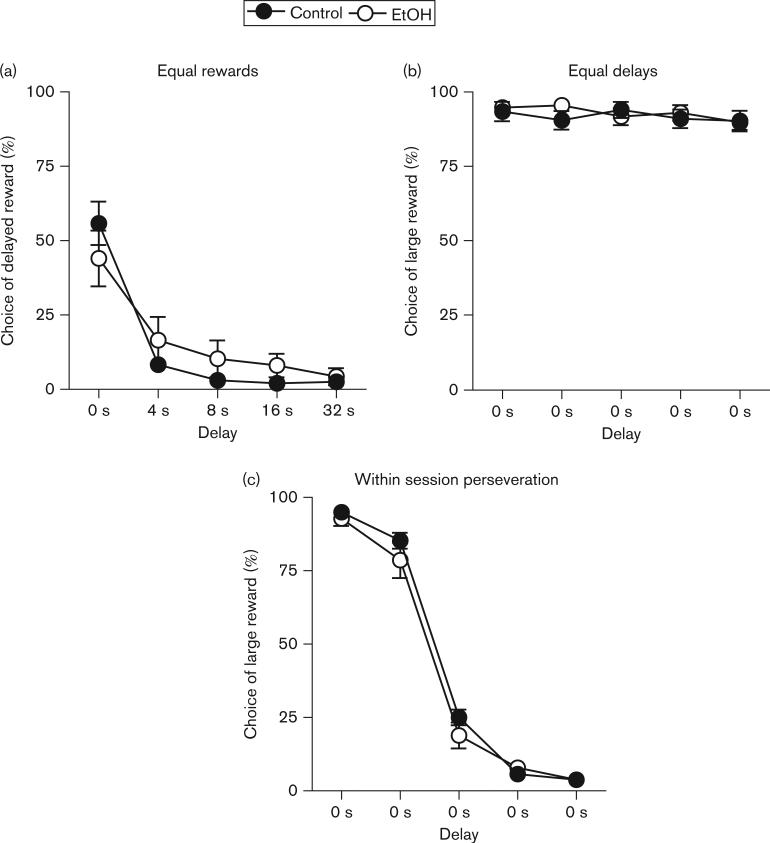
To determine whether ethanol-exposed and control rats differed in their ability to perceive and respond to delays to reward delivery, the task contingencies were modified such that both immediate and delayed reward levers resulted in only a single food pellet. This resulted in a shift in the pattern of choice performance, such that rats showed a robust preference for the immediate over the delayed lever in trial blocks 2–5, in which a delay was present (Fig. 4a). Analysis of the mean percentage of choices of the delayed reward in the last five sessions, using a two-factor ANOVA (delay × ethanol exposure), revealed a significant main effect of delay [F(4,56) = 69.38, P < 0.001] as well as a significant interaction between delay and exposure condition [F(4,56) = 3.09, P < 0.05], suggesting that ethanol exposure may have altered rats’ sensitivity to changes in delay duration.
Fig. 4.
Performance on delay discounting control tasks. (a) Performance on the equal rewards task (choices between immediate and delayed rewards of equal magnitude), on which there was a significant interaction between ethanol (EtOH) exposure and delay. (b) Performance on the equal delays task (choices between small immediate and large immediate rewards). (c) Performance on the within-session perseveration task (choices between small immediate and large immediate rewards in blocks 1 and 2, followed by reversal of the lever-outcome contingencies in blocks 3–5. All data points represent means ± SEM.
Equal-delays control
To determine whether ethanol-exposed and control rats differed in their ability to perceive and respond to different reward magnitudes, task contingencies were modified such that the reward magnitudes were returned to their original condition (one food pellet vs. three food pellets), but the delays to the large reward were eliminated. This resulted in both control and ethanol-exposed groups switching their choice preference almost exclusively to the large reward lever (Fig. 4b). Analysis of the mean percentage of choices of the large reward in the last five sessions using a two-factor ANOVA (trial block × ethanol exposure) revealed a significant main effect of trial block [F(4,56) = 3.06, P < 0.05], but no significant main effect or interaction involving ethanol exposure condition (F < 2.43, NS) These data strongly indicate that both control and ethanol-exposed rats in this study were similarly sensitive to the different reward magnitudes used in the task.
Within-session perseveration control
To determine whether the differences between control and ethanol-exposed rats in the delay discounting task could be accounted for by perseverative responding on the large reward lever, the task contingencies were modified to induce a robust shift in lever preference. In blocks 1 and 2, the contingencies were identical to those used in the equal delays control; however, in blocks 3 through 5, the rewards produced by each lever were switched (such that the large reward lever now produced the small reward, and vice versa). These contingencies resulted in robust choice of the large reward lever in blocks 1 and 2, and an equally robust switch to choice of the new large reward lever in blocks 3–5 (Fig. 4c). Analysis of the mean percentage of choices of the large reward in the last five stable sessions, using a two-factor ANOVA (trial block × ethanol exposure), revealed a significant main effect of block [F(4,56) = 613.92, P < 0.001], but no significant main effect or interactions involving ethanol exposure (F < 1.22, NS). These data indicate that ethanol-exposed rats were able to detect within-session changes in reward magnitude normally, and adjust their lever choice accordingly.
Comparisons between delay discounting and water maze performance
There is some evidence that the same brain systems (basal forebrain, hippocampus) may be involved in both water maze and delay discounting task performance. Hence, we used both bivariate and partial (factoring out ethanol exposure condition) correlations to determine if performance in the delay discounting task (mean percent choice of the large reward during stable performance) was related to any of the measures of water maze performance (training trial search error and path length, as well as probe trial mean search error and thigmotaxis). There were no significant correlations between delay discounting and any measure of water maze performance (r < 0.26, NS).
Discussion
The data reported here show that binge ethanol exposure in rats during PD 4–9 (a model of third trimester human exposure) produces sex-specific impairments in spatial learning and memory in the Morris water maze during adolescence that are not attributable to concomitant motor impairments in the task. The same rats also showed an unexpected decrease in impulsive choice in a delay discounting task in adulthood (increased preference for large delayed rewards), which was not because of alterations in reward magnitude discrimination or response perseveration. These data show that even short regimens of developmental ethanol exposure can have dramatic and long-lasting effects on different aspects of cognitive function.
Spatial learning and memory
Teratogenic effects of ethanol exposure during brain development can impair spatial memory. In humans, adolescent males diagnosed with fetal alcohol syndrome perform significantly worse than age-matched control subjects on a virtual water maze task used to test place learning (Hamilton et al., 2003). In animals, a number of studies have described effects of prenatal and perinatal ethanol exposure on spatial cognitive abilities (Blanchard et al., 1987; Goodlett et al., 1987; Gianoulakis, 1990; Goodlett and Peterson, 1995; Minetti et al., 1996). There is a vast literature focusing on the effects of protracted ethanol exposure at early gestational ages in rats; however, as the importance of the brain growth spurt period has been realized, the effects of ethanol on the late gestation–early postnatal period have become of significant interest (Goodlett et al., 1987; Girard et al., 2000; Johnson and Goodlett, 2002; Hamilton et al., 2003). In one of the first studies to investigate effects of ethanol exposure during this period of development, exposure to high-concentration but short-duration ethanol produced more pronounced impairments in spatial learning than less-concentrated but longer-duration exposure (Goodlett et al., 1987). These data, together with several other, more recent reports (including the results reported here), show that relatively brief ethanol exposure, even late in development, can have long-term cognitive consequences.
In this study, differences in swim speed were found during the cued visible platform task, such that ethanol-exposed rats swam significantly faster than controls. These results may be because of locomotor hyperactivity often reported in children and in preweaning and juvenile rats after perinatal ethanol exposure (Bond and Di Giusto, 1977; Osborne et al., 1980; Abel, 1982; Leonard, 1988; Abel and Reddy, 1997; Tran et al., 2000; Mattson et al., 2001). Given these observed swim speed differences, care was taken to use water maze performance measures that were minimally confounded by alterations in motor function. Specifically, cumulative search error, a measure shown to be very sensitive to spatial memory deficits (Gallagher et al., 1993; LaSarge et al., 2007; Bizon et al., 2009), and path length (total distance navigated to reach the escape platform) were used in lieu of latency (time to reach platform) to assess training trial performance, as latency measures are confounded by swim speed. Probe-trial performance was assessed by mean search error, a proximity measure that is independent of swim speed because probe trials are of a fixed duration (30 s). Thigmotaxis, a measure of time spent in the outer 10% (periphery) of the maze, was used as an additional measure of the effects of ethanol and sex on probe-trial performance (Montgomery et al., 2008; Bizon et al., 2009).
Ethanol-exposed rats were significantly impaired in water maze performance compared with controls. Although all rats improved their performance across training trials, ethanol-exposed rats demonstrated less accurate and longer swim paths to reach the platform. Moreover, during probe trials, ethanol-exposed rats had significantly reduced spatial bias relative to controls. Ethanol-exposed rats also exhibited greater thigmotaxis, spending significantly more time navigating the periphery of the maze, a behavior which is prevalent in this task in rats with poor spatial memory. During the visible platform task, there were no differences between ethanol-exposed and control rats in path length to reach the platform, demonstrating that sensorimotor or motivational deficits were not responsible for the robust spatial learning deficits observed here. These data indicate a clear and pronounced spatial learning deficit which is independent of motoric abnormalities associated with ethanol exposure.
Another important finding from the current study was that adolescent female rats appeared more susceptible than males to ethanol-induced spatial learning impairments, despite PD 6 BEC data which were consistent with similar levels of ethanol exposure in females and males when measured shortly after dosing. Although ethanol-exposed males took longer to learn the platform location than control males, they did reach control levels of performance by the completion of training (Fig. 2). In contrast, ethanol-exposed females still performed markedly worse than control females even after 6 days of training. These data are consistent with the results of some studies (Kelly et al., 1988; Minetti et al., 1996), but they contrast with others that report no sex differences or even the opposite (males more affected than females) in the effects of ethanol on cognition (Blanchard et al., 1987; Johnson and Goodlett, 2002). These differing results may be because of the differences in the parameters of ethanol administration, as there is evidence that the time and dose of ethanol exposure, as well as the time of testing, can contribute to sexually dimorphic effects of ethanol on spatial learning (Kelly et al., 1989, 1998; Goodlett and Peterson, 1995; Johnson and Goodlett, 2002). For example, Goodlett and Peterson (1995) reported that, using a comparable ethanol exposure regimen (PD 4–9), both male and female juvenile rats displayed deficits in spatial navigation. However, males needed only 3 days of exposure (PD 4–6 or PD 7–9) to induce spatial learning deficits, whereas females required the full exposure period (PD 4–9) to induce comparable deficits. Clearly, more work is needed to understand how prenatal ethanol exposure differentially affects cognition across sexes.
Delay discounting
Prenatal ethanol exposure in humans is associated with elevations in impulsive behavior, although these reports have relied on observational, rather than laboratory measures of impulsivity (Connor et al., 2000; Mihalick et al., 2001; Fryer et al., 2007). Consistent with these reports, Olmstead et al. (2009) found evidence for increased motor impulsivity in a go/no-go task in prenatal ethanol-exposed guinea pigs. In contrast, the current study found evidence for the opposite pattern of results (decreased impulsivity) using a delay (temporal) discounting task. There are a number of possible explanations for this discrepancy, including differences in species, ethanol dosing regimen, and, importantly, the type of impulsivity assessed. Ethanol-exposed guinea pigs in the Olmstead et al. study showed an increase in motor responding in an inappropriate context (‘motor impulsivity’), which can be distinguished from the ‘choice impulsivity’ assessed by the delay discounting task (Evenden, 1999). Although there is some overlap between these two forms of impulsivity (and they tend to be comorbid in human subjects), there is also evidence that they have dissociable neural substrates (Winstanley et al., 2006), which could account for the differences in the effects of ethanol exposure. In addition, it should be noted that in contrast to the results presented here, a recently published study found no effects of prenatal ethanol exposure on delay discounting in rats (Pupe et al., 2011). It is likely that the difference in the ethanol exposure regimens used (prenatal vs. postnatal) accounts for the different outcomes of the two studies, as the task design used by Pupe et al. (2011) was nearly identical to that used here.
The effects of ethanol exposure on impulsive choice may be due to alterations in brain systems involving the prefrontal cortex. Developmental ethanol exposure can cause reductions in cell number and alterations in dendritic spine formation in the prefrontal cortex (Mihalick et al., 2001; Hamilton et al., 2010), and this region has been strongly implicated in decision-making and regulation of impulsive choice (Floresco et al., 2008; Dalley et al., 2011). Damage to orbital prefrontal cortex in particular can cause a decrease in impulsive choice similar to that observed here (Winstanley et al., 2004), and a similar pattern of decreased impulsive choice is observed in aged rats, which may result from decreased sensitivity to changes in the delay to reward delivery (Simon et al., 2010). The fact that ethanol-exposed rats showed somewhat greater preference for the delayed reward than controls when reward magnitudes were equal is consistent with this hypothesis, and suggests that ethanol exposure may have caused rats to place less ‘value’ on the costs of elapsed time.
The idea that developmental ethanol exposure alters the valuation of reward costs is similar to that proposed recently by Nasrallah et al. (2011) who report that ethanol consumption in adolescent rats (from PD 30 to 49) caused a lasting increase in preference for large risky over small guaranteed rewards in a probability discounting task in which large rewards were associated with varying probabilities of omission. In this study, ethanol consumption also altered transient nucleus accumbens dopamine responses to risky rewards, which Nasrallah et al. (2011) interpreted as evidence for altered neural encoding of reward costs. Hence, even though ethanol-exposed rats in the present study increased their preference for the large delayed reward (which in the context of the task could be interpreted as ‘supraoptimal’ performance, as it resulted in greater food delivery), this pattern of performance may actually reflect an impairment in the ability to encode or apply information about reward costs (in this case the costs of delayed reward delivery) to guide effective decision making. Importantly, the effects of ethanol exposure in the present study were likely not due to gross deficits in perception of differences in reward magnitude, as ethanol-exposed rats were no different from controls in their preference for large over small rewards in the absence of delays. This finding is also consistent with the findings of Nasrallah et al. (2011), who found no changes in transient dopamine responses to guaranteed rewards in ethanol-exposed rats. These similarities suggest that both early postnatal and later adolescent exposure to ethanol may have similar effects on the neural basis of cost-benefit decision making. In addition, although there is some evidence that ethanol exposure can induce perseverative behavior (Olmstead et al., 2009), the decreased impulsive choice observed here was likely not due to a gross inability to switch levers when one became disadvantageous (i.e. perseveration), as ethanol-exposed rats were no different from controls in the perseveration control task.
The results of this study clearly confirm previous reports that have demonstrated ethanol-induced spatial learning impairments in rat models of human third trimester binge ethanol exposure. Importantly, ethanol-exposed rats displayed faster swim speeds than controls, highlighting the importance of choosing water maze measures that are not confounded by alterations in motor function. Using these measures, ethanol-exposed rats had significant mnemonic impairments, which affected females more profoundly than males. Ethanol exposure also caused an increased preference for large delayed over small immediate rewards (decreased impulsive choice), which was associated with altered sensitivity to delays, possibly reflecting a deficit in accounting for reward costs. Future studies directly linking neurobiological outcomes with the behavioral alterations observed here will be necessary to fully elucidate the deleterious effects of developmental ethanol exposure.
Acknowledgements
This work was supported in part by Public Health Service Grants AA 12386 (G.D.F.) and AG 029421 (J.L.B.). Preliminary reports of this work were presented in abstract form at the Annual Meeting of the Society for Neuroscience in 2009.
Footnotes
Conflicts of interest There are no conflicts of interest.
References
- Abel EL. In utero alcohol exposure and developmental delay of response inhibition. Alcohol Clin Exp Res. 1982;6:369–376. doi: 10.1111/j.1530-0277.1982.tb04993.x. [DOI] [PubMed] [Google Scholar]
- Abel EL, Reddy PP. Prenatal high saturated fat diet modifies behavioral effects of prenatal alcohol exposure in rats. Alcohol. 1997;14:25–29. doi: 10.1016/s0741-8329(96)00081-x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Riley EP, Hannigan JH. Deficits on a spatial navigation task following prenatal exposure to ethanol. Neurotoxicol Teratol. 1987;9:253–258. doi: 10.1016/0892-0362(87)90010-9. [DOI] [PubMed] [Google Scholar]
- Bond NW, Di Giusto EL. Prenatal alcohol consumption and open-field behaviour in rats: effects of age at time of testing. Psychopharmacology (Berl) 1977;52:311–312. doi: 10.1007/BF00426717. [DOI] [PubMed] [Google Scholar]
- Burd L, Martsolf JT. Fetal alcohol syndrome: diagnosis and syndromal variability. Physiol Behav. 1989;46:39–43. doi: 10.1016/0031-9384(89)90318-1. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of D-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Alcohol and public health binge drinking fact sheet. Alcohol and Public Health. [31 August 2011];2010 Available at http://www.cdc.gov/alcohol/fact-sheets/binge-drinking.htm.
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol. 2000;18:331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Costa ET, Savage DD, Valenzuela CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res. 2000;24:706–715. [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dubois C, Naassila M, Daoust M, Pierrefiche O. Early chronic ethanol exposure in rats disturbs respiratory network activity and increases sensitivity to ethanol. J Physiol. 2006;576(Pt 1):297–307. doi: 10.1113/jphysiol.2006.111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois DW, Parrish AR, Trzeciakowski JP, Frye GD. Binge ethanol exposure delays development of GABAergic miniature postsynaptic currents in septal neurons. Brain Res Dev Brain Res. 2004;152:199–212. doi: 10.1016/j.devbrainres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- DuBois DW, Trzeciakowski JP, Parrish AR, Frye GD. GABAergic miniature postsynaptic currents in septal neurons show differential allosteric sensitivity after binge-like ethanol exposure. Brain Res. 2006;1089:101–115. doi: 10.1016/j.brainres.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Floresco SB St, Onge JR, Ghods-Sharifi S, Winstanley CA. Corticolimbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;31:1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Rats exposed prenatally to alcohol exhibit impairment in spatial navigation test. Behav Brain Res. 1990;36:217–228. doi: 10.1016/0166-4328(90)90060-r. [DOI] [PubMed] [Google Scholar]
- Girard TA, Xing HC, Ward GR, Wainwright PE. Early postnatal ethanol exposure has long-term effects on the performance of male rats in a delayed matching-to-place task in the Morris water maze. Alcohol Clin Exp Res. 2000;24:300–306. [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Kelly SJ, West JR. Early postnatal alcohol exposure that produces high blood alcohol levels impairs development of spatial navigation learning. Psychobiology. 1987;15:64–74. [Google Scholar]
- Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64:265–275. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Pearlman AD, Lundahl KR. Binge neonatal alcohol intubations induce dose-dependent loss of Purkinje cells. Neurotoxicol Teratol. 1998;20:285–292. doi: 10.1016/s0892-0362(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Granato A, Van Pelt J. Effects of early ethanol exposure on dendrite growth of cortical pyramidal neurons: inferences from a computational model. Brain Res Dev Brain Res. 2003;142:223–227. doi: 10.1016/s0165-3806(03)00094-4. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with fetal alcohol syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hamilton GF, Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC Layer II/III pyramidal neurons. Synapse. 2010;64:127–135. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao SH, Mahoney JC, West JR, Frye GD. Development of GABAA receptors on medial septum/diagonal band (MS/DB) neurons after postnatal ethanol exposure. Brain Res. 1998;810(1–2):100–113. doi: 10.1016/s0006-8993(98)00891-9. [DOI] [PubMed] [Google Scholar]
- Hsiao SH, Acevedo JL, DuBois DW, Smith KR, West JR, Frye GD. Early postnatal ethanol intubation blunts GABA(A) receptor up-regulation and modifies 3alpha-hydroxy-5alpha-pregnan-20-one sensitivity in rat MS/DB neurons. Brain Res Dev Brain Res. 2001;130:25–40. doi: 10.1016/s0165-3806(01)00194-8. [DOI] [PubMed] [Google Scholar]
- Hsiao SH, DuBois DW, Miranda RC, Frye GD. Critically timed ethanol exposure reduces GABAAR function on septal neurons developing in vivo but not in vitro. Brain Res. 2004;1008:69–80. doi: 10.1016/j.brainres.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Johnson TB, Goodlett CR. Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male rats. Alcohol Clin Exp Res. 2002;26:83–93. [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hulsether SA, West JR. Impaired spatial navigation in adult female but not adult male rats exposed to alcohol during the brain growth spurt. Behav Brain Res. 1988;27:247–257. doi: 10.1016/0166-4328(88)90121-0. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Black AC, Jr, West JR. Changes in the muscarinic cholinergic receptors in the hippocampus of rats exposed to ethyl alcohol during the brain growth spurt. J Pharmacol Exp Ther. 1989;249:798–804. [PubMed] [Google Scholar]
- Kim CK, Kalynchuk LE, Kornecook TJ, Mumby DG, Dadgar NA, Pinel JP, et al. Object-recognition and spatial learning and memory in rats prenatally exposed to ethanol. Behav Neurosci. 1997;111:985–995. doi: 10.1037//0735-7044.111.5.985. [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, et al. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiol Aging. 2007;28:928–936. doi: 10.1016/j.neurobiolaging.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Lee Y, Rowe J, Eskue K, West JR, Maier SE. Alcohol exposure on postnatal day 5 induces Purkinje cell loss and evidence of Purkinje cell degradation in lobule I of rat cerebellum. Alcohol. 2008;42:295–302. doi: 10.1016/j.alcohol.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Leonard BE. Alcohol as a social teratogen. Prog Brain Res. 1988;73:305–317. doi: 10.1016/S0079-6123(08)60512-9. [DOI] [PubMed] [Google Scholar]
- Luo J, Miller MW. Growth factor-mediated neural proliferation: target of ethanol toxicity. Brain Res Brain Res Rev. 1998;27:157–167. doi: 10.1016/s0165-0173(98)00009-5. [DOI] [PubMed] [Google Scholar]
- Maier SE, Miller JA, Blackwell JM, West JR. Fetal alcohol exposure and temporal vulnerability: regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exp Res. 1999;23:726–734. doi: 10.1111/j.1530-0277.1999.tb04176.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Montgomery KS, LaSarge CL, Simon NW, Bizon JL, Setlow B. Long-term effects of prior cocaine exposure on Morris water maze performance. Neurobiol Learn Mem. 2008;89:185–191. doi: 10.1016/j.nlm.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalick SM, Crandall JE, Langlois JC, Krienke JD, Dube WV. Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicol Teratol. 2001;23:453–462. doi: 10.1016/s0892-0362(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Miller MW. Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol Clin Exp Res. 1993;17:304–314. doi: 10.1111/j.1530-0277.1993.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Miller MW. Limited ethanol exposure selectively alters the proliferation of precursor cells in the cerebral cortex. Alcohol Clin Exp Res. 1996;20:139–143. doi: 10.1111/j.1530-0277.1996.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Minetti A, Arolfo MP, Virgolini MB, Brioni JD, Fulginiti S. Spatial learning in rats exposed to acute ethanol intoxication on gestational day 8. Pharmacol Biochem Behav. 1996;53:361–367. doi: 10.1016/0091-3057(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Montgomery KS, Mackey J, Thuett K, Ginestra S, Bizon JL, Abbott LC. Chronic, low-dose prenatal exposure to methylmercury impairs motor and mnemonic function in adult C57/B6 mice. Behav Brain Res. 2008;191:55–61. doi: 10.1016/j.bbr.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL. Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. Proc Natl Acad Sci USA. 2011;108:5466–5471. doi: 10.1073/pnas.1017732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Martin A, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure increases disinhibition and perseverative responding in the adult guinea pig. Behav Pharmacol. 2009;20(5–6):554–557. doi: 10.1097/FBP.0b013e3283305e27. [DOI] [PubMed] [Google Scholar]
- Osborne GL, Caul WF, Fernandez K. Behavioral effects of prenatal ethanol exposure and differential early experience in rats. Pharmacol Biochem Behav. 1980;12:393–401. doi: 10.1016/0091-3057(80)90043-x. [DOI] [PubMed] [Google Scholar]
- Pupe S, Brys II, Asherson PJE, Bizarro L. Prenatal alcohol exposure did not affect impulsivity in rats that perfromed delay or probability discounting tasks. Psychol Neurosci. 2011;4:123–130. [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, et al. Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol Aging. 2010;31:853–862. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, et al. Maternal drinking during pregnancy: attention and short-term memory in 14-year-old offspring – a longitudinal prospective study. Alcohol Clin Exp Res. 1994;18:202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Tran TD, Cronise K, Marino MD, Jenkins WJ, Kelly SJ. Critical periods for the effects of alcohol exposure on brain weight, body weight, activity and investigation. Behav Brain Res. 2000;116:99–110. doi: 10.1016/s0166-4328(00)00263-1. [DOI] [PubMed] [Google Scholar]
- West JR, Chen WJ, Pantazis NJ. Fetal alcohol syndrome: the vulnerability of the developing brain and possible mechanisms of damage. Metab Brain Dis. 1994;9:291–322. doi: 10.1007/BF02098878. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]