Abstract
Background. All-trans-retinoic acid (atRA) is effective for many proliferative diseases. We investigated the protective effects of atRA against atherosclerosis. Methods. Rabbits were randomly allocated to receive basal diet or an HFD for 4 weeks. HFD group then received rosuvastatin (3 mg/day), atRA (5 mg/kg/day), or the same volume of vehicle, respectively, for next 8 weeks. Results. HFD group showed increases in plasma lipids and aortic plaque formation. P-selectin expression and fibrinogen binding on platelets or deposition on the intima of the aorta also increased significantly as did the levels of TNF-α, IL-6, and fibrinogen in plasma. After 8 weeks of treatment with atRA, there was a significant decrease in plasma lipids and improvement in aortic lesions. AtRA also inhibited the expression of P-selectin and fibrinogen binding on platelets and deposition on the intima of the aorta. Conclusion. AtRA can ameliorate HFD-induced AS in rabbits by inhibiting platelet activation and inflammation.
1. Introduction
All-trans-retinoic acid (atRA) is a family of signalling molecules that are chemically related to vitamin A (retinol). AtRA has potent in vitro effects on a number of processes involved in vascular injury and repair, such as modulating smooth muscle cell (SMC) proliferation and inducing SMC differentiation [1–4]. Recently, atRA was shown to limit restenosis after balloon angioplasty in a rabbit model [5], as well as favourable effects in reducing neointimal mass and eliciting geometric remodelling in injured rat carotid artery [6]. However, to date there are no reports concerning its role in high-fat diet- (HFD-) induced atherosclerosis (AS).
AS is a widespread and one of the most dangerous cardiovascular diseases which cause considerable threat to human health worldwide. The formation and development of AS is a long-term process [7–9] which usually lasts for decades and results from complicated environmental factors. The accumulation of low-density lipoproteins (LDLs) in the walls of arteries is thought to be an initiator of AS, which in turn induces the migration of mononuclear cells and development of inflammation [7]. Eventually, the extracellular matrix interacts with migrating cells to form plaques, which constrict the arteries making them less flexible and more likely to impede blood flow [10, 11]. Recently, the activation of platelets and P-selectin are thought to play a central role in homeostasis and thrombosis, which also contribute to the formation of AS, as they have been shown to actively promote atherosclerotic lesion development [12, 13]. It has been shown that P-selectin is expressed in mononuclear phagocytes, endothelial cells, and SMCs from atheroma [11, 12]. Intracellular P-selectin also has the potential to translocate rapidly to the cell surface following stimulation by adenosine diphosphate (ADP), thrombin, collagen, and other inflammatory stimuli [14, 15]. On the other hand, fibrinogen has been implicated in the development of inflammation, thrombosis, and AS and has been shown to promote P-selectin expression on the surface of platelets [16, 17].
The present study was undertaken to investigate the possible therapeutic effects of atRA on HFD-induced AS, with a special focus on its potential to inhibit platelet activation, prevent inflammation, and lower blood lipids and reduce aortic lesions, while rosuvastatin was used as a positive control.
2. Methods
2.1. Animal Procedures
New Zealand male rabbits (ordinary class) were purchased from an experimental animal facility in Nanjing, China. The rabbits (2.0 ± 0.2 kg) were maintained in individual cages under moderate temperature and changing light (12-hour light, 12-hour dark) conditions with access to food and clean water. After a 3-day acclimation period, the rabbits were randomly divided into two groups. The control group (n = 6) was given a basal diet, while another group was given HFD comprising 5% lard and 1% cholesterol. Four weeks later, animals in HFD group were divided into three groups, and received either rosuvastatin calcium (3 mg/day), atRA (5 mg/kg/day), or the same volume of vehicle by intragastric administration for 8 weeks (n = 6/group). Rosuvastatin and atRA were dissolved with water.
At the end of the experiment, the rabbits were fasted for 8 hours prior to anaesthesia (3% pentobarbital w/v administered at 1 mL/kg). Blood was collected from the common carotid artery. The rabbits were sacrificed, and the aortas were carefully removed and cut open. The tissue was observed and then placed in 10% (w/v) neutral formalin for 24 hours. Animal experimental procedures were conducted in accordance with the Internal Animal Care and Use Committee of the Anhui Medical University and complied with the Guide for the Care and Use of Laboratory.
2.2. Reagents
Rosuvastatin calcium was purchased from AstraZeneca; atRA and ADP were purchased from Sigma. Thrombin was purchased from Pharmaceutical Co., Ltd., Hunan. Anti-rabbit CD62-P FITC-conjugated antibody was purchased from Bioss, Beijing. Chicken polyclonal anti-Fibrinogen (FITC) antibody was purchased from Abcam. SP-9000/9001/9002 Histostain-Plus Kits were purchased from ZYMED.
2.3. The Blood Collection and the Platelet Preparation
Blood samples were collected in tubes containing 3.8% sodium citrate (1/9 v/v) for biochemical measurements and flow cytometry. Blood sample was centrifuged at 1000 g for 10 minutes to collect the plasma. Platelet-rich plasma (PRP) was obtained by centrifugation at 300 g for 10 minutes. Platelets were then isolated from the PRP using PBS to wash twice as previously described [13, 17].
2.4. Detection of the Platelet P-Selectin (CD62-P) Expression and the Fibrinogen Binding by Flow Cytometry
Resting platelets and platelets activated by thrombin (1 U/mL) or ADP (final concentration is 20 uM) were incubated with FITC-conjugated anti-rabbit CD62P or fibrinogen for 30 minutes at room temperature in the dark. PBS (0.5 mL) was added to the sample immediately before acquisition of data. All samples were analyzed by flow cytometry by FC500 (Beckman Coulter), using 10,000 events per sample with light scatter and fluorescence channels set at a logarithmic gain. The platelet population was analyzed for mean fluorescence intensity (MFI).
2.5. Biochemical Measurement
The concentrations of plasma lipids were determined using diagnostic enzyme assay kits. Plasma fibrinogen, IL-6, and TNF-α were measured using a Quantikine rabbit fibrinogen, IL-6, and TNF-α enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems).
2.6. Morphology and Immunohistochemistry
The root of the aorta from sacrificed animals was longitudinally cut and fixed in 4% paraformaldehyde overnight. Then, the tissue specimens were cut at 5 μm thickness for subsequent hematoxylin-eosin (HE) staining or immunohistochemistry analysis. The method for HE staining of aortic tissues was conducted according to the previous report [18]. Immunohistochemistry analysis was performed according to the method [19]. Briefly, sections of aortic tissues were deparaffinised, rehydrated, and fixed with methanol-0.3% H2O2 solution for 30 min at room temperature. The antigen was prepared by quick cooling 3 min after highly compressed heating. The primary antibody against CD62-P or fibrinogen was added and incubated overnight. Then, the sections of aortic tissues were incubated with biotinylated antibody (anti-rat IgG) and horseradish streptavidin for 30 min at 37°C, respectively. Finally, the samples were incubated with diaminobenzidine (DAB) for coloration and counterstained with hematoxylin for 2 min to stain the nuclei bluish. The positively stained cells showed brown color; photographs were taken using the Image-Pro Plus 5.1 image operation system. The aortic intima-media thickness and vascular wall thickness were measured by JD-801 pathological image analysis system with total 3 slices, each slice was randomly selected five view, and then took a mean value (μm).
2.7. Statistical Analysis
All the statistical analyses were undertaken using the SPSS program, version 13.0, for Windows. All data are expressed as mean ± standard deviation (SD). Comparisons between groups were carried out using one-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) method. Values of P < 0.05 were regarded as statistically significant.
3. Results
3.1. atRA Improved Plasma Lipids in HFD-Induced AS Rabbits
Compared with control group, HFD-induced significant increases in plasma lipids, including total cholesterol (Total-C), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). Oral administration of rosuvastatin or atRA both significantly reduced Total-C and LDL-C, and both significantly increased HDL-C under HFD feeding (Table 1). These findings suggest that atRA intervention may help to restore the lipid imbalance induced by HFD.
Table 1.
Levels of plasma lipids in different groups (mmol/L).
| Group | Total-C | LDL-C | HDL-C | TG |
|---|---|---|---|---|
| Control | 1.51 ± 0.56 | 0.70 ± 0.33 | 0.66 ± 0.36 | 0.23 ± 0.11 |
| HFD | 29.37 ± 4.36* | 24.57 ± 4.57* | 2.05 ± 1.39* | 2.72 ± 2.42* |
| Rosuvastatin | 16.27 ± 2.40▲ | 9.21 ± 1.27▲ | 5.75 ± 2.32▲ | 1.39 ± 0.98▲ |
| atRA | 17.24 ± 4.44▲ | 12.92 ± 3.19▲ | 3.35 ± 1.55▲ | 0.90 ± 0.78▲ |
*P < 0.05 compared to control group; ▲P < 0.05 compared to HFD group.
3.2. atRA Ameliorated Arterial Plaques in HFD-Induced AS Rabbits
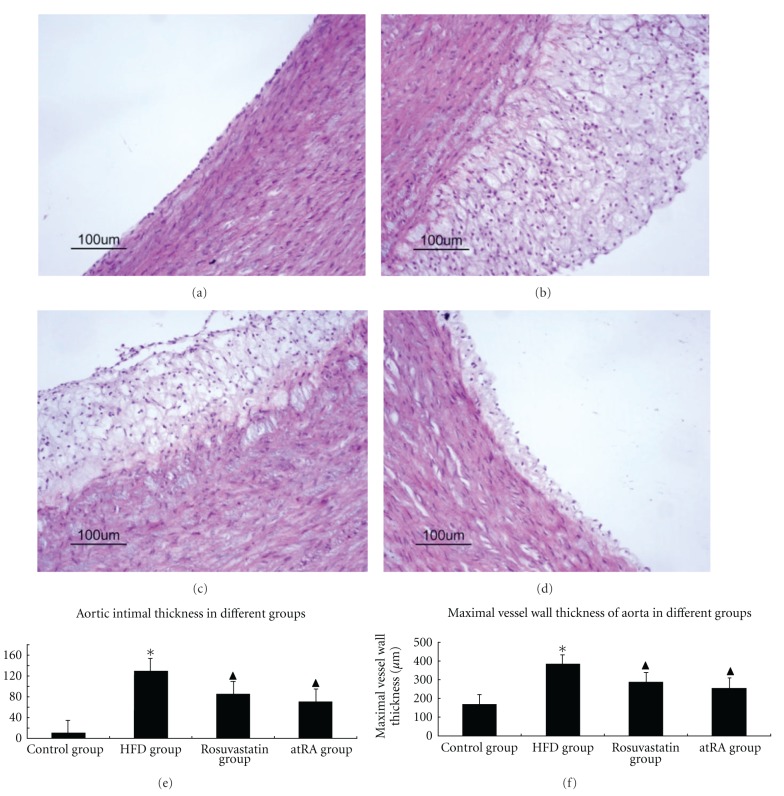
The characteristics of arterial lesions were examined by pathological section HE staining using light microscopy. In control group, the vessel walls were round with even thicknesses. The inner and the outer elastic plates were clear and complete. The endothelial cell core was stained and evenly arranged. No SMCs were seen underneath the endothelium (Figure 1(a)).
Figure 1.
Characteristics of arterial lesions. Pathological sections of arterial lesions were examined by HE staining in different groups. In the control group (a), the vessel walls were thin and smooth with even thicknesses; in the HFD group (b), there were more foam cells and necrotic substances in the intima. Compared with the HFD group, there were less foam cells, necrotic substances in the intima from rosuvastatin group (c) and atRA group (d) (magnification ×200). Statistical results of aortic intimal thickness (e) and maximal vessel wall thickness (f) among different groups; *P < 0.05 compared to control group, ▲P < 0.05 compared to HFD group.
In HFD group, the vessel walls were uneven, and significant hyperplasia of intima was present. There was evidence of foam cells and necrotic substances, where cholesterol crystals and a few inflammatory cells were observed. The inner elastic plates were broken, and a large proportion of SMCs with spindle-shape cores were arranged in disorder (Figure 1(b)). Treatment with rosuvastatin and atRA resulted in more even blood vessels, smoother intima, fewer foam cells and inflammatory cells, and less necrotic substances (Figures 1(c) and 1(d)). Moreover, we measured the aortic intimal thickness and maximal vessel wall thickness in this study. We observed that HFD feeding resulted in transparent increases in either aortic intimal thickness and maximal vessel wall thickness, which were significantly decreased in either atRA or rosuvastatin-treated groups (Figures 1(e) and 1(f)).
3.3. atRA Inhibited Expression of CD62-P and Fibrinogen Binding in Platelets
Platelet activation was investigated by checking platelet CD62-P expression and fibrinogen binding by flow cytometry. As shown in Figures 2 and 3, the percentage and MFI of fibrinogen and CD62-P were significantly lower in resting platelets from all groups compared with findings in thrombin and ADP-stimulated platelets. Platelets from the control group (in either resting or activated states) had lower fibrinogen and CD62-P expression compared with that seen in the HFD, rosuvastatin, and atRA groups. However, when compared to HFD group, platelets from rosuvastatin and atRA groups had significantly lower expression of fibrinogen and CD62-P on platelet surfaces, suggesting that atRA suppressed platelet activation.
Figure 2.
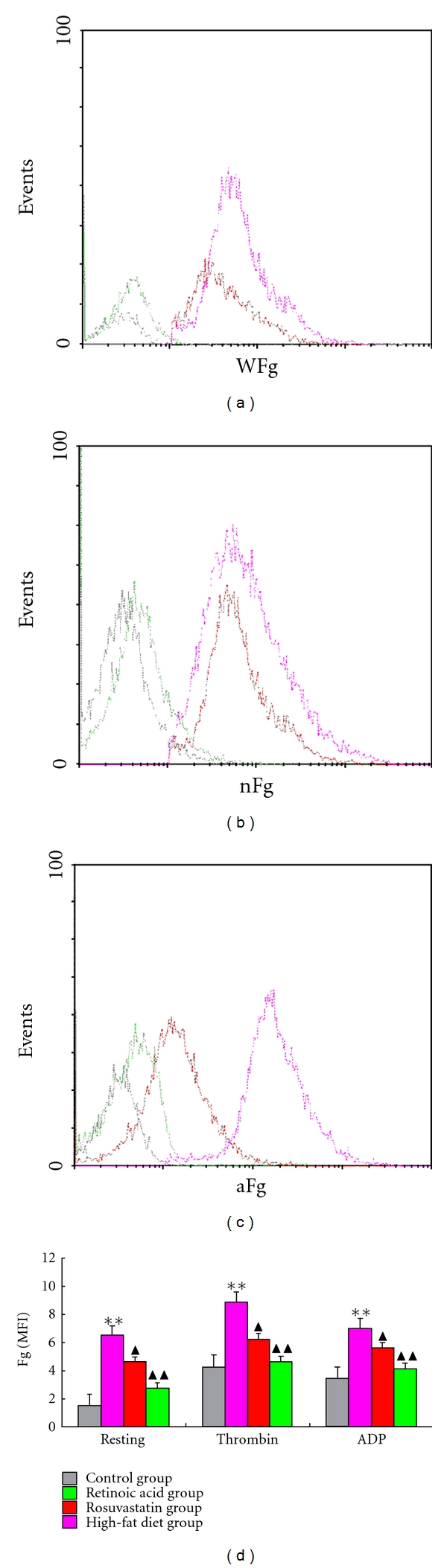
Platelet fibrinogen binding in different groups. Platelets isolated from rabbits were incubated with FITC-labelled anti-fibrinogen antibody and analyzed with flow cytometry to examine the platelets surface change. Representative histograms are shown. (a) The binding of fibrinogen on the resting platelets surface from different groups. (b) The binding of fibrinogen on the thrombin-activated platelets from different groups. (c) The binding of fibrinogen on the ADP-activated platelets from different groups. (d) Mean fluorescence intensity (MFI) of fibrinogen binding on platelets surface in different groups. Data are mean ± standard deviation (SD). Comparisons between groups were carried out using one-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) method (n = 6). **P < 0.01 compared to control group, ▲P < 0.05, ▲▲P < 0.01 compared to HFD group.
Figure 3.
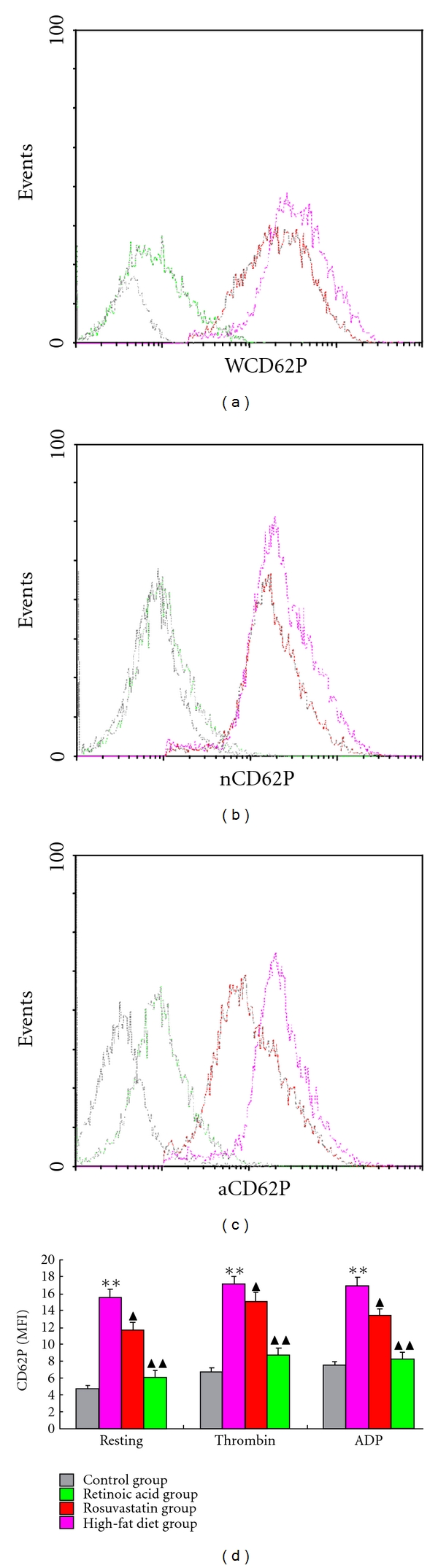
Platelet CD62-P expressions in different groups. Isolated platelets were incubated with FITC-labelled anti-CD62P antibody, and surface CD62-P changes were monitored by flow cytometry. Representative histograms from every group are shown. (a) The expression of CD62-P on the resting platelets surface from different groups. (b) The expression of CD62-P on the thrombin-activated platelets from different groups. (c) The expression of CD62-P on the ADP-activated platelets from different groups. (d) Mean fluorescence intensity (MFI) of CD62-P expression on platelets surface in different groups. Data are mean ± standard deviation (SD). Comparisons between groups were carried out using one-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) method (n = 6). **P < 0.01 compared to control group, ▲P < 0.05, ▲▲P < 0.01 compared to HFD group.
3.4. atRA Repressed Deposition of Fibrinogen and Expression of CD62-P on the Arteries
As shown in Figures 4(a) and 5(a), respectively, a very low level of fibrinogen deposition and CD62-P expression was found in the intima from the control group, in comparison with that in the HFD group (Figures 4(b) and 5(b)). But rosuvastatin or atRA treatment resulted in improvements in fibrinogen deposition and CD62-P expression in the intima of arteries (Figures 4(c) and 4(d)) and (Figures 5(c) and 5(d)). Lesions from HFD group contained extensive foam cell accumulation which was not obvious in atRA treatment group. The presence of significantly larger lesions in HFD group than control group suggested that fibrinogen and CD62-P may play a role in atherosclerotic lesion growth.
Figure 4.
Fibrinogen deposition in arteries from different groups with immunohistochemistry. Areas of positive fibrinogen deposition are shown in brown. The representative photographs of immunohistochemistry from different groups are shown. In the control group (a), the deposition of fibrinogen in the intima was minimum, while in the HFD group (b), there was much more fibrinogen deposition in the intima. In the rosuvastatin group (c), there was less fibrinogen deposition in the intima compared with HFD group. In the atRA group (d), the fibrinogen deposition was significantly less in comparison with either HFD or rosuvastatin group (magnification ×400).
Figure 5.
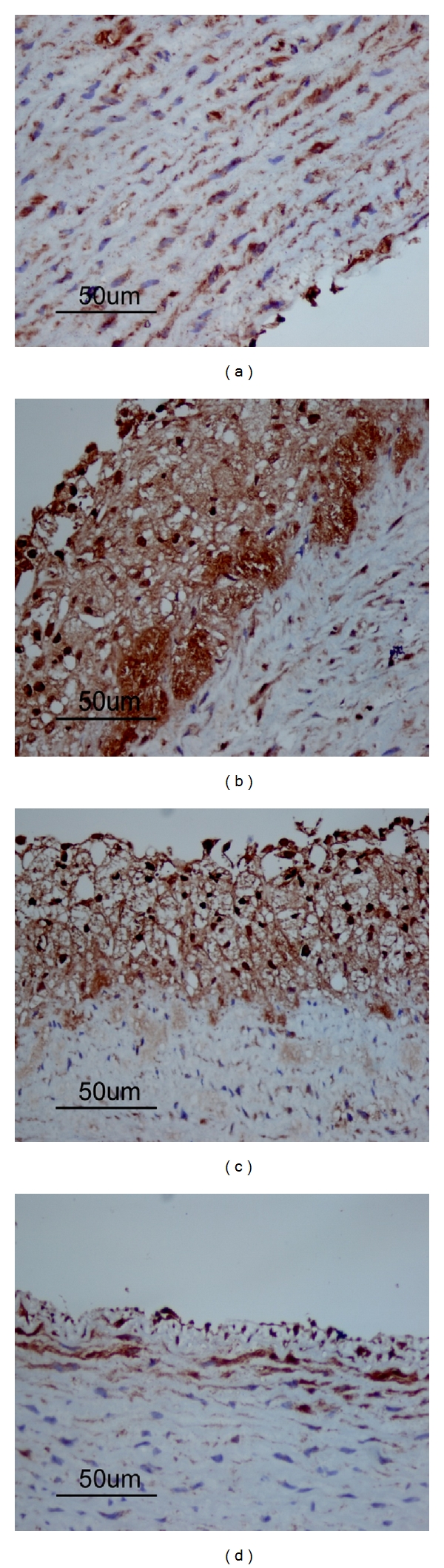
CD62-P expressions in atherosclerotic lesion from different groups with immunohistochemistry. Representative photographs of immunohistochemistry from different groups are shown. Positive CD62-P expression is shown in brown. In the control group (a), the expression of CD62-P in the intima was minimum. In the HFD group (b), there was significantly increased CD62-P expression in the intima. In the rosuvastatin group (c), there was less but considerable CD62-P expression in the intima. Compared to the HFD group, there was significantly reduced CD62-P expression in the intima from atRA group (d) (magnification ×400).
3.5. atRA Lowered Levels of Inflammatory Cytokines and Fibrinogen in Plasma
Beside IL-6 and TNF-α, plasma fibrinogen is also an important marker of inflammation [20]. Levels of fibrinogen, TNF-α, and IL-6 were significantly higher in the HFD group than in the control group (Table 2), while they were significantly lowered by both rosuvastatin and atRA treatment. Moreover, the effect was numerically more marked with atRA than with rosuvastatin.
Table 2.
Levels of fibrinogen and cytokines in different groups.
| Group | Fibrinogen (g/L) | TNF-α (pg/mL) | IL-6 (pg/mL) |
|---|---|---|---|
| Control | 2.42 ± 0.29 | 79.54 ± 12.53 | 38.76 ± 6.36 |
| HFD | 4.03 ± 0.36* | 142.12 ± 14.57* | 62.04 ± 6.39* |
| Rosuvastatin | 3.69 ± 0.24 | 101.88 ± 16.78▲ | 49.97 ± 7.32▲ |
| atRA | 2.87 ± 0.30▲ | 95 ± 11.89▲ | 43.82 ± 5.71▲ |
*P < 0.05 compared to control group; ▲P < 0.05 compared to HFD group.
4. Discussion
AtRA is an active metabolite of vitamin A that inhibits cell migration, regulates extrinsic coagulation, and promotes cell differentiation [1–3, 21, 22]. Although earlier studies had focused on its antitumor effects [21–23], it has been shown that atRA limits restenosis after balloon angioplasty in a rabbit model [5], suggesting its potential role in other AS models. In current study, we found that oral administration of atRA improved characteristics of AS and lowered expressions of P-selectin and fibrinogen and concentrations of inflammatory cytokines in AS rabbits induced by HFD. Rosuvastatin, a classical lipid-lowering drug of statins in clinic [10, 24], was used as a positive control, and comparable effect was found between atRA - and rosuvastatin- the treated rabbits in current study.
The initial stage of AS involves the adherence of monocytes to the surface of the injured endothelium, a process facilitated by adhesion molecules [7]. Then, activated platelets cause the Weibel-Palade body release leading to CD62-P mediated leukocyte rolling, suggesting that platelet CD62-P promotes development of inflammation and deterioration of AS [25–27]. On the other hand, fibrinogen deposition stimulates chemokine secretion and facilitates a neutrophil-endothelial interaction in septic shock [16, 28]. We have previously shown that fibrinogen enhances platelet intracellular CD62-P levels and affects CD62-P expression on the surface of platelets [13, 17, 27, 29]. These findings support a role of fibrinogen in AS and suggest that CD62-P may contribute to the formation of AS.
Although both rosuvastatin and atRA treatments resulted in effective amelioration of AS in rabbits, much more improvement could be found in the atRA group than rosuvastatin-treated rabbits, including less aortic plaques, greater inhibition on fibrinogen deposition, and expression of P-selectin on platelets and intima of aorta. Moreover, we found that the lipids-lowering effect between rosuvastatin and atRA showed a little difference, For example the extents of lowering of LDL-C and increasing of HDL-C were greater in the rosuvastatin group than atRA. These findings suggest that atRA might reduce AS formation through a different mechanism to rosuvastatin. One possibility is that atRA might inhibit PDGF-induced cell proliferation and induce apoptosis in aortic SMCs [1–3]. Secondly, a more important possibility is that atRA might prevent the development of AS by greater inhibition on the expression of CD62-P and secretion of fibrinogen, in addition to its lipids-lowering effect, because P-selectin has been demonstrated to be a therapeutic target for AS [30].
Clinical and experimental studies support the idea that chronic inflammation plays a major role in the development of AS [11]. In our study rosuvastatin and atRA were both shown to counteract the increases in inflammatory cytokines such as IL-6 and TNF-α resulting from HFD. These findings are in agreement with previous studies [31, 32] which highlight the role of anti-inflammatory properties of rosuvastatin in preventing the development of AS, including inhibition of endothelial cell adhesion and monocyte adherence, reduction in tissue plasminogen activation, and suppression of TNF-α and MCP-1 in the vessel wall [33–35]. Fibrinogen is not only implicated in development of cardiovascular disease but is also an inflammatory marker [20]. The inhibition of fibrinogen and inflammatory cytokines by atRA underscores the anti-inflammatory effect by atRA in AS model [36]. However, it has also been proposed that atRA may increase foam cell formation and atherosclerotic lesion via upregulation of CD36 and MCP-1 in vitro [37], suggesting that the biological functions of atRA in AS are inclusive which need further investigations.
Taken together, the results of present study warrant the further investigation of atRA as a potential agent for preventing and treating AS and restenosis. Since the underlying signal transduction and mechanisms that result in inhibition of fibrinogen and CD62-P expression by atRA remain to be elucidated, further studies should be carried out to investigate the underlying mechanisms of the inhibition of fibrinogen and CD62-P expression by atRA, as well as the optimal dosage and route of administration, so that its protective effects can be maximized and side effects be reduced.
Conflict of Interests
The authors declare that there are no conflict of interests.
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (no. 30971226), Doctoral Fund of Ministry of Education of China (20103420110001), the Intercollegiate Key Project of Nature Science of Anhui Province (no. KJ2011A158), the Natural Science Foundation of Anhui Province (no. 090413269X), and the Youth Research Program of Anhui Provincial Health Department (no. 09B106). The authors would like to thank Dr. Heyu Ni for his advice during the experiments.
References
- 1.Axel DI, Frigge A, Dittmann J, et al. All-trans retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells. Cardiovascular Research. 2001;49(4):851–862. doi: 10.1016/s0008-6363(00)00312-6. [DOI] [PubMed] [Google Scholar]
- 2.Miano JM, Topouzis S, Majesky MW, Olson EN. Retinoid receptor expression and all-trans retinoic acid-mediated growth inhibition in vascular smooth muscle cells. Circulation. 1996;93(10):1886–1895. doi: 10.1161/01.cir.93.10.1886. [DOI] [PubMed] [Google Scholar]
- 3.Ou H, Haendeler J, Aebly MR, et al. Retinoic acid-induced tissue transglutaminase and apoptosis in vascular smooth muscle cells. Circulation Research. 2000;87(10):881–887. doi: 10.1161/01.res.87.10.881. [DOI] [PubMed] [Google Scholar]
- 4.Wiegman PJ, Barry WL, McPherson JA, et al. All-trans-retinoic acid limits restenosis after balloon angioplasty in the focally atherosclerotic rabbit: a favorable effect on vessel remodeling. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(1):89–95. doi: 10.1161/01.atv.20.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Herdeg C, Oberhoff M, Baumbach A, et al. Effects of local all-trans-retinoic acid delivery on experimental atherosclerosis in the rabbit carotid artery. Cardiovascular Research. 2003;57(2):544–553. doi: 10.1016/s0008-6363(02)00709-5. [DOI] [PubMed] [Google Scholar]
- 6.Miano JM, Kelly LA, Artacho CA, Nuckolls TA, Piantedosi R, Blaner WS. all-Trans-retinoic acid reduces neointimal formation and promotes favorable geometric remodeling of the rat carotid artery after balloon withdrawal injury. Circulation. 1998;98(12):1219–1227. doi: 10.1161/01.cir.98.12.1219. [DOI] [PubMed] [Google Scholar]
- 7.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray CJL, Lopez AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. The Lancet. 1997;349(9063):1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 9.Riba R, Nicolaou A, Troxler M, Homer-Vaniasinkam S, Naseem KM. Altered platelet reactivity in peripheral vascular disease complicated with elevated plasma homocysteine levels. Atherosclerosis. 2004;175(1):69–75. doi: 10.1016/j.atherosclerosis.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Hu MY, Li YL, Jiang CH, Liu ZQ, Qu SL, Huang YM. Comparison of lycopene and fluvastatin effects on atherosclerosis induced by a high-fat diet in rabbits. Nutrition. 2008;24(10):1030–1038. doi: 10.1016/j.nut.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 12.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101(7):2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 13.Zhai Z, Wu J, Xu X, et al. Fibrinogen controls human platelet fibronectin internalization and cell-surface retention. Journal of Thrombosis and Haemostasis. 2007;5(8):1740–1746. doi: 10.1111/j.1538-7836.2007.02625.x. [DOI] [PubMed] [Google Scholar]
- 14.George JN, Lyons RM, Morgan RK. Membrane changes associated with platelet activation. Exposure of actin on the platelet surface after thrombin-induced secretion. Journal of Clinical Investigation. 1980;66(1):1–9. doi: 10.1172/JCI109821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Öckinger J, Stridh P, Beyeen AD, et al. Genetic variants of CC chemokine genes in experimental autoimmune encephalomyelitis, multiple sclerosis and rheumatoid arthritis. Genes and Immunity. 2010;11(2):142–154. doi: 10.1038/gene.2009.82. [DOI] [PubMed] [Google Scholar]
- 16.Rezaee F, Maas A, De Maat MPM, Verheijen JH, Koopman J. Effect of genetic background and diet on plasma fibrinogen in mice. Possible relation with susceptibility to atherosclerosis. Atherosclerosis. 2002;164(1):37–44. doi: 10.1016/s0021-9150(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Lang S, Zhai Z, et al. Fibrinogen is required for maintenance of platelet intracellular and cell-surface P-selectin expression. Blood. 2009;114(2):425–436. doi: 10.1182/blood-2008-03-145821. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Dai M, Jia W. Paeonol attenuates high-fat-diet-induced atherosclerosis in rabbits by anti-inflammatory activity. Planta Medica. 2009;75(1):7–11. doi: 10.1055/s-0028-1088332. [DOI] [PubMed] [Google Scholar]
- 19.Park K, Lee DG, Kim SW, Paick JS. Dimethylarginine dimethylaminohydrolase in rat penile tissue: reduced enzyme activity is responsible for erectile dysfunction in a rat model of atherosclerosis. International Journal of Impotence Research. 2009;21(4):228–234. doi: 10.1038/ijir.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papageorgiou N, Tousoulis D, Siasos G, Stefanadis C. Is fibrinogen a marker of inflammation in coronary artery disease? Hellenic Journal of Cardiology. 2010;51(1):1–9. [PubMed] [Google Scholar]
- 21.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. Journal of Experimental Medicine. 2007;204(8):1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. New England Journal of Medicine. 1997;337(15):1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 23.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100(13):4298–4302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- 24.Tehrani S, Mobarrez F, Antovic A, et al. Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thrombosis Research. 2010;126(3):e225–e231. doi: 10.1016/j.thromres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Ramström S, Öberg KV, Åkerström F, Enström C, Lindahl TL. Platelet PAR1 receptor density-correlation to platelet activation response and changes in exposure after platelet activation. Thrombosis Research. 2008;121(5):681–688. doi: 10.1016/j.thromres.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Schwertz H, Zimmerman GA, Weyrich AS. Fibrinogen selects selectins. Blood. 2009;114(2):p. 234. doi: 10.1182/blood-2009-04-216135. [DOI] [PubMed] [Google Scholar]
- 27.Zhai Z, Wu J, Xu X, et al. From mice to men: lessons from intravital microscopy thrombosis model and hypofibrinogenemia patients-roles of vWF, fibrinogen, and fibronectin in thrombus formation. National Research Forum for Young Investigators in Circulatory and Respiratory Health May. 2004:6–9. [Google Scholar]
- 28.Keating FK, Dauerman HL, Whitaker DA, Sobel BE, Schneider DJ. Increased expression of platelet P-selectin and formation of platelet-leukocyte aggregates in blood from patients treated with unfractionated heparin plus eptifibatide compared with bivalirudin. Thrombosis Research. 2006;118(3):361–369. doi: 10.1016/j.thromres.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Wu J, Zhai Z, et al. A novel fibrinogen Bβ chain framshift mutation in a patient with severe congenital hypofibrinogenaemia. Thrombosis and Haemostasis. 2006;95(6):931–935. doi: 10.1160/TH06-01-0020. [DOI] [PubMed] [Google Scholar]
- 30.Woollard KJ, Chin-Dusting J. Therapeutic targeting of P-selectin in atherosclerosis. Inflammation and Allergy. 2007;6(1):69–74. doi: 10.2174/187152807780077345. [DOI] [PubMed] [Google Scholar]
- 31.Tailleux A, Gozzo A, Torpier G, et al. Increased susceptibility of low-density lipoprotein to ex vivo oxidation in mice transgenic for human apolipoprotein B treated with 1 melatonin-related compound is not associated with atherosclerosis progression. Journal of Cardiovascular Pharmacology. 2005;46(3):241–249. doi: 10.1097/01.fjc.0000175232.11079.7e. [DOI] [PubMed] [Google Scholar]
- 32.Witt-Enderby PA, Radio NM, Doctor JS, Davis VL. Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. Journal of Pineal Research. 2006;41(4):297–305. doi: 10.1111/j.1600-079X.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 33.Barreto AC, Maeda NY, Soares RPS, Cícero C, Lopes AA. Rosuvastatin and vascular dysfunction markers in pulmonary arterial hypertension: a placebo-controlled study. Brazilian Journal of Medical and Biological Research. 2008;41(8):657–663. doi: 10.1590/s0100-879x2008000800003. [DOI] [PubMed] [Google Scholar]
- 34.Kleemann R, Princen HMG, Emeis JJ, et al. Rosuvastatin reduces atherosclerosis development beyond and independent of its plasma cholesterol-lowering effect in APOE∗3-Leiden transgenic mice: evidence for antiinflammatory effects of rosuvastatin. Circulation. 2003;108(11):1368–1374. doi: 10.1161/01.CIR.0000086460.55494.AF. [DOI] [PubMed] [Google Scholar]
- 35.Stalker TJ, Lefer AM, Scalia R. A new HMG-CoA reductase inhibitor, rosuvastatin, exerts anti-inflammatory effects on the microvascular endothelium: the role of mevalonic acid. British Journal of Pharmacology. 2001;133(3):406–412. doi: 10.1038/sj.bjp.0704070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang ZS, Zeng CL, Zhu LJ, Jiang L, Li N, Hu H. Salvianolic acid A inhibits platelet activation and arterial thrombosis via inhibition of phosphoinositide 3-kinase. Journal of Thrombosis and Haemostasis. 2010;8(6):1383–1393. doi: 10.1111/j.1538-7836.2010.03859.x. [DOI] [PubMed] [Google Scholar]
- 37.Wuttge DM, Romert A, Eriksson U, Törmä H, Hansson GK, Sirsjö A. Induction of CD36 by all-trans retinoic acid: retinoic acid receptor signaling in the pathogenesis of atherosclerosis. The FASEB Journal. 2001;15(7):1221–1223. doi: 10.1096/fj.00-0488fje. [DOI] [PubMed] [Google Scholar]