Abstract
While human embryonic stem cells (hESCs) may one day facilitate the treatment of degenerative diseases requiring cell replacement therapy, the success of regenerative medicine is predicated on overcoming the rejection of replacement tissues. Given the role played by dendritic cells (DCs) in the establishment of immunological tolerance, we have proposed that DC, rendered tolerogenic during their differentiation from hESC, might predispose recipients to accept replacement tissues. As a first step towards this goal, we demonstrate that DC differentiated from H1 hESCs (H1-DCs) are particularly responsive to the immunosuppressive agent rapamycin compared to monocyte-derived DC (moDC). While rapamycin had only modest impact on the phenotype and function of moDC, H1-DC failed to upregulate CD40 upon maturation and displayed reduced immunostimulatory capacity. Furthermore, coculture of naïve allogeneic T cells with rapamycin-treated H1-DC promoted an increased appearance of CD25hi Foxp3+ regulatory T cells, compared to moDC. Our findings suggest that conditioning of hESC-derived DC with rapamycin favours a tolerogenic phenotype.
1. Introduction
Human embryonic stem cells (hESCs) derived under conditions compliant with their downstream clinical application, serve as a renewable source of cell types that may one day enable the replacement of tissues whose function has become compromised by chronic or degenerative disease [1]. Nevertheless, the routine implementation of cell replacement therapy (CRT) requires strategies to address the immunological barriers encountered by the use of hESC of allogeneic origin [2]. While conventional immunosuppression offers a potential solution to the immunogenicity of hESC-derived tissues, the risks inherent in its protracted use make the induction of transplantation tolerance an attractive alternative.
Dendritic cells (DCs) play a critical role in determining the outcome of antigen presentation to naive T cells, either promoting their activation and subsequent immunity, or favouring the induction of tolerance [3]. The delivery of foreign antigen to DC in the steady state by conjugation to monoclonal antibodies (mAbs) specific for the surface receptor CD205, was, for instance, found to render recipient mice specifically tolerant to the antigen upon subsequent immunization [4]. Such findings have been extended to a transplantation setting by demonstrating how administration of immature donor DC to mice across a minor histocompatibility barrier is sufficient to secure the indefinite survival of donor skin grafts. In this model, the resulting tolerance could be attributed to the polarisation of responding T cells towards a regulatory phenotype, characterised by upregulation of the transcription factor Foxp3 [5]. Such findings, together with early success at inducing tolerance in healthy human volunteers by the administration of immature antigen-pulsed monocyte-derived DC (moDC) [6], augur well for the future use of DC as a conditioning regime in the context of CRT. Indeed, the recent description of protocols for the differentiation of DC from hESC under conditions substantially free of animal products paves the way for such an approach: given that this source of DC would share with the replacement tissue the very alloantigens to which tolerance must be established, their administration in advance of CRT might be anticipated to condition the recipient to accept the transplanted tissue, providing the DC have first been rendered stably tolerogenic [7]. Accordingly, Senju et al. generated DC expressing the inhibitory receptor programmed death ligand 1 (PD-L1) by genetic modification of the parent hESC line [8], a similar approach in the mouse having successfully yielded DC capable of preventing the onset of experimental autoimmune encephalomyelitis by induction of tolerance to myelin antigens [9]. While such a strategy is clearly promising, the administration of genetically modified cells to patients poses additional regulatory barriers, suggesting that exposure of DC to pharmacological agents, known to promote a tolerogenic phenotype, may prove to be a more pragmatic approach [10].
The macrocyclic triene antibiotic, rapamycin, displays potent immunosuppressive properties that are routinely employed to facilitate whole-organ transplantation. In addition to its systemic use, however, rapamycin has been shown to render DC profoundly protolerogenic through inhibition of mammalian target of rapamycin (mTOR) signalling pathways. In the mouse, rapamycin-treated DC display profoundly suppressed allostimulatory capacity in vitro and enhanced propensity for the induction of Foxp3+ regulatory T (Treg) cells [11]. Furthermore, exposure to rapamycin, unlike other immunosuppressive agents, leads to the upregulation of CCR7 by both mouse and human DC and a commensurate increase in responsiveness to CCL19, compatible with their trafficking in vivo to regional lymph nodes [12, 13]. Furthermore, the administration of rapamycin-treated recipient DC pulsed with donor alloantigens has secured the indefinite survival of tissue allografts in various animal models [14–16], the resulting tolerance having been demonstrated to rely on the expansion of antigen-specific Treg cells [17]. Nevertheless, despite its compelling credentials, rapamycin has been reported to exert quite distinct effects on human DC, depending on the source and subset involved [18]. We have, therefore, investigated the compatibility of protocols for the differentiation of DC from the H1 hESC line (H1-DC) with the use of rapamycin. Here we report that H1-DCs are peculiarly sensitive to the immunomodulatory effects of rapamycin, compared with conventional moDC, as evidenced by the specific loss of immunogenicity and enhanced capacity to polarise responding T cells towards a regulatory phenotype. Our findings provide an important first step towards the use of DC differentiated from hESC in the establishment of tolerance to replacement tissues, providing a proof of concept for their future application in regenerative medicine.
2. Materials and Methods
2.1. Isolation of Primary Cells
Monocytes and naïve T cells were isolated from peripheral blood mononuclear cells (PBMCs) of buffy coats (NHS Blood Transfusion Service) or from blood provided by volunteers under informed consent using CD14-coated beads or naïve CD4+ T cell selection kit (Miltenyi Biotec). Cell populations were positively selected or depleted from PBMC using AutoMACS separation according to the manufacturer's instructions.
2.2. Culture of hESC
H1 ESCs were cultured in X-VIVO-10 medium (without gentamycin or phenol red, Lonza) supplemented with nonessential amino acids (PAA Laboratories GmbH), 2 mM L-glutamine (PAA Laboratories GmbH), 50 μM 2-mercaptoethanol (Sigma), 0.5 ng/mL recombinant human transforming growth factor β (TGF-β, R&D Systems), and 80 ng/mL recombinant human basic fibroblast growth factor (bFGF, R&D Systems) on 6-well plates, previously coated with Matrigel (phenol red-free, growth factor reduced, BD Biosciences) diluted 1 : 30 using ice-cold knockout Dulbecco's Modified Eagle's Medium (KO-DMEM, Invitrogen). Supplemented X-VIVO-10 medium was replaced daily except the day following passaging.
Human ESCs were routinely passaged as cell clusters of about 0.5 mm diameter every 4–6 days. For passaging, colonies were incubated in filter-sterilised warm collagenase IV (Invitrogen) until detachment of the stromal cells. Stromal cells were removed by washing with Dulbecco's Phosphate-Buffered Saline (DPBS) and hESC were scraped off into supplemented X-VIVO-10 Medium for 1 : 5 passaging. All cell cultures were incubated in a humidified incubator at 37°C and 5% CO2.
2.3. Differentiation of hESC
H1 hESCs were plated at 3 × 106 per well of 6-well ultralow attachment (ULA) plates (Costar) in a total volume of 4 mL of X-VIVO-15 medium (Lonza), supplemented with 1 mM sodium pyruvate, nonessential amino acids, 2 mM L-glutamine (all PAA Laboratories GmbH) and 5 μM 2-mercaptoethanol (Sigma). The following growth factors were added: 50 ng/mL recombinant human bone morphogenetic protein-4 (BMP-4, R&D Systems), 50 ng/mL recombinant human vascular endothelial growth factor (VEGF, R&D Systems), 20 ng/mL recombinant human stem cell factor (SCF, R&D Systems), and 50 ng/mL recombinant human granulocyte macrophage-colony stimulating factor (GM-CSF, R&D Systems). After 2-3 days, the medium was topped up with 2 mL of fresh supplemented X-VIVO-15 medium to produce a total volume of 6 mL. Subsequent feeding was performed every 2-3 days by replacing 2-3 mL of old medium with new supplemented X-VIVO-15 medium from which every 5 days a growth factor was removed starting with BMP-4 at day 5, followed by VEGF at day 10 and SCF at day 15 of differentiation [19]. Once macrophage-like cells were observed, 25 ng/mL of IL-4 (Peprotech) was added, which was increased stepwise to 100 ng/mL.
On days 30–35, monocytes were harvested by gentle pipetting, leaving adherent macrophages in the culture dish. The cell suspension was passed through a 70 μm cell strainer (BD Falcon) to remove cellular debris, washed with DPBS and plated at 1−1.5 × 106 monocytes per well of a 6-well Cellbind plate (Corning) in X-VIVO-15 supplemented with 50 ng/mL GM-CSF and 100 ng/mL IL-4.
2.4. Derivation of DC from Human Monocytes
Monocytes were cultured in RPMI 1640 (Invitrogen) supplemented with 2 mM L-glutamine (PAA laboratories GmbH), 50 U/mL penicillin (PAA laboratories GmbH), 50 μg/mL streptomycin (PAA laboratories GmbH), 10% heat-inactivated and filter-sterilised fetal bovine serum (FBS), 50 ng/mL GM-CSF, and 100 ng/mL IL-4 on 6-well Cellbind plates for 6–8 days.
2.5. DC Maturation and Rapamycin Treatment
Two days after monocytes were plated, monocyte-derived and hESC-derived immature DC were treated with 10 ng/mL and 5–7 ng/mL of rapamycin (Sigma), respectively. On day 5, DCs were matured for 48 hr using a maturation cocktail consisting of 50 ng/mL of GM-CSF (R&D Systems), 100 ng/mL IL-4 (R&D Systems), 20 ng/mL IFNγ (R&D Systems), 50 ng/mL TNFα (R&D Systems), 10 ng/mL of IL-1β (R&D Systems), and 1 μg/mL PGE2 (Sigma). On day 6-7, DCs were harvested by gentle pipetting, passed through a 70 μm cell strainer, centrifuged, and resuspended prior to their use in experiments.
2.6. Allogeneic Mixed Leukocyte Reaction (MLR)
DCs were incubated in 10 μg/mL mitomycin C (Sigma) in supplemented RPMI 1640 at 37°C for 30 minutes. Cells were washed, resuspended in supplemented RPMI 1640, and plated in triplicate to give either 2.5 × 103 cells, 5 × 103 cells, or 1 × 104 cells in a total volume of 100 μl per well using 96-well round-bottom plates (Corning). Naïve CD4+ T cells were plated at 5 × 104 cells per well to yield a stimulator to responder ratio of 1 : 5, 1 : 10, and 1 : 20 and a total volume of 200 μl/well. Wells containing T cells and mitomycin C-treated DC alone were included as controls for background proliferation of either cell type. Cells were incubated for 5 days at 37°C, after which T cells were pulsed with 0.5 μCi of [3H]-thymidine per well for 18 hr before harvesting.
2.7. DC-T-Cell Cocultures
DC (2 × 105) and 1 × 106 T cells were cocultured in supplemented RPMI 1640 using 24-well Cellbind plates (Corning). After 7 days of coculture, cells were harvested and stained for CD4, CD25, and Foxp3 and analysed by flow cytometry as described below.
2.8. Flow Cytometry
Cells were incubated for 15 min in blocking solution (5% normal rat serum, 0.5% bovine serum albumin, and 0.1% NaN3 in DPBS) on ice. Cells were washed with DPBS containing 1% FBS and 0.1% NaN3 and resuspended in this solution together with one or several of the following fluorescently labelled antibodies: SSEA-4 (clone: MC-813-70, R&D Systems), eZFluor™ anti-human CD4-FITC and either CD25-APC or CD25-AF488 Cocktail (eBioscience), CD83 (HB15e, AbD Serotec), CD86 (BU63, AbD Serotec), CD40 (LOB7/6, AbD Serotec), PD-L1 (AbD Serotec), CD127 (40131, R&D Systems), CTLA-4 (BNI3, BD Pharmingen), MHC II HLA-DR/DQ/DP (WR18, AbD Serotec), CD80 (MEM-233, AbD Serotec), CD45 (15.2, AbD Serotec), CD14 (MEM18, AbD Serotec), CD11c (BU15, AbD Serotec), and CD13 (AbD Serotec). Cells were incubated at 4°C in the dark for 30–60 minutes. For the last 10 minutes, 250 ng/mL 7-AAD was added. Cells were washed, fixed in 2% formaldehyde, and analysed by flow cytometry.
Intracellular staining was performed according to the manufacturer's instructions using permeabilisation and fixation buffers (eBioscience) and antibodies specific for Oct-4 (240408, R&DSystems) or Foxp3 (eBioscience).
3. Results
3.1. Differentiation and Characterisation of DC from the H1 hESC Line
In order to investigate whether protocols we have established previously for the differentiation of DC from hESC might be compatible with the use of rapamycin, we made use of the well-characterised H1 hESC line. In keeping with its downstream clinical application, H1 was maintained in serum-free medium devoid of animal products and feeder cells, as described previously [20, 21]. Under these conditions, H1 formed compact colonies with clearly defined boarders (Figure 1(a)), the individual cells displaying a high nucleus : cytoplasm ratio and prominent heterochromatin. Flow cytometric analysis revealed expression of the transcription factor Oct-4 and stage-specific embryonic antigen 4 (SSEA-4), both of which are known to strongly correlate with pluripotency (Figure 1(b)).
Figure 1.
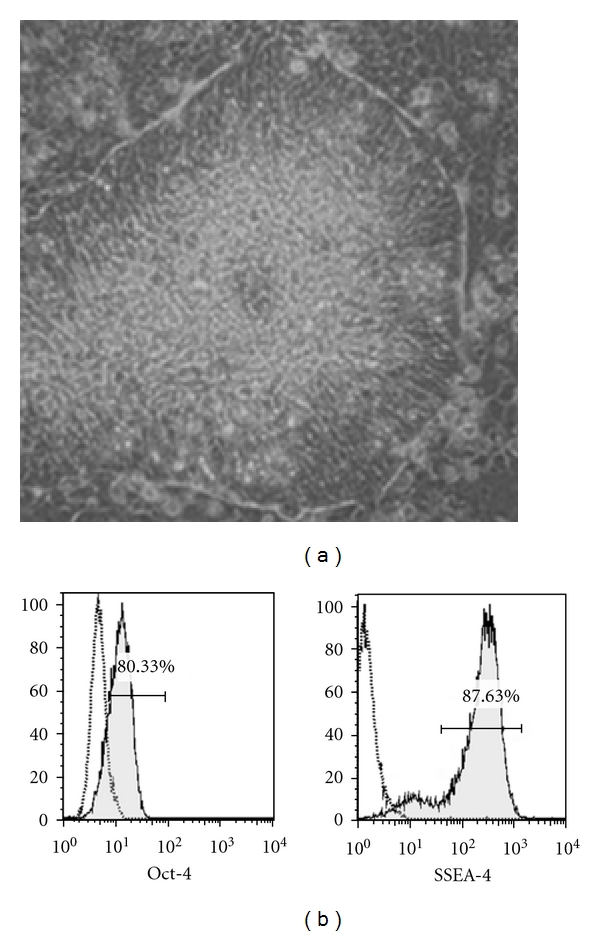
Maintenance of the H1 hESC line. (a) Colony of H1 hESC showing the morphology typical of pluripotent stem cells, including prominent boarders (×20 magnification). (b) Expression by H1 hESC of the transcription factor Oct-4 and the surface marker SSEA-4, both of which correlate with pluripotency. Dead cells were removed from flow cytometric analysis using 7-AAD staining. Open histograms represent appropriate isotype controls.
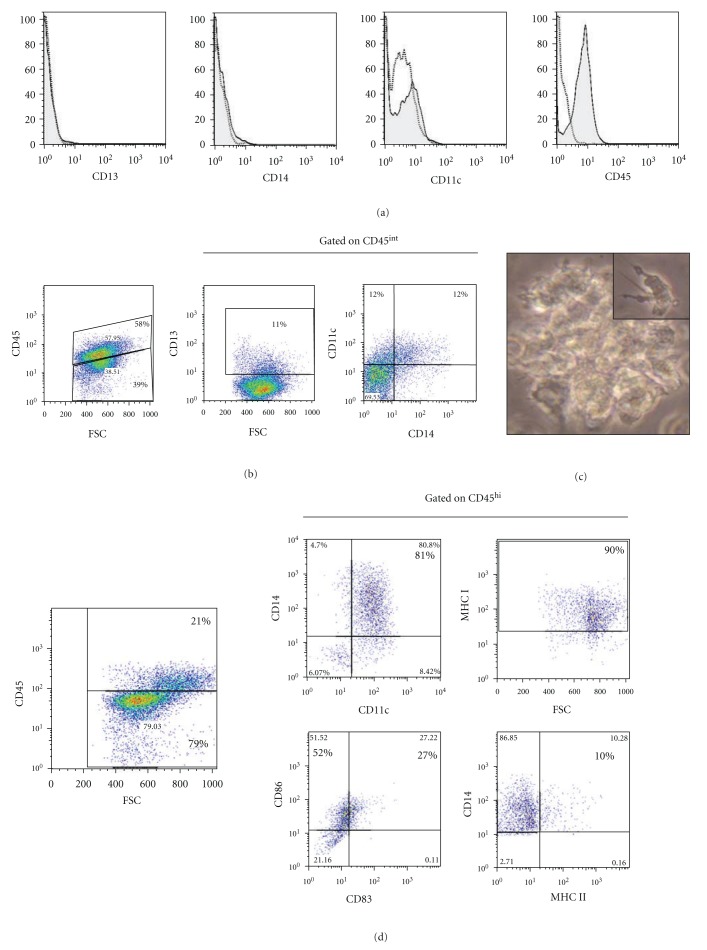
The differentiation of H1 was directed along the DC lineage in ultralow attachment plates by exposure to a cocktail of growth factors consisting of BMP-4, VEGF, SCF, and GM-CSF, as described previously [19]. The initiation of hematopoiesis was apparent by day 20 of culture, as evidenced by the appearance of CD45+ cells, although the lack of expression of CD13, CD14, and CD11c suggested that commitment to the myeloid lineage had yet to occur (Figure 2(a)). In contrast, by day 27 of culture, a small proportion of cells, residing within a population expressing intermediate levels of CD45, had upregulated these markers, consistent with their progressive commitment to the myeloid lineage (Figure 2(b)). Indeed, from day 28 of culture onwards, cells with the characteristic morphology of human DC could be identified within cultures, either as clusters with prominent veils of cytoplasm or individual cells with long dendrites (Figure 2(c)).
Figure 2.
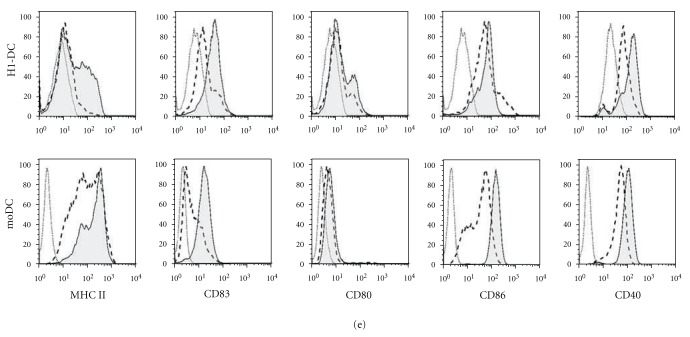
Time course of DC differentiation from H1 hESC. Cells were harvested from cultures at various time points and analysed by flow cytometry for the onset of hematopoiesis and the appearance of DC. (a) Cells harvested at day 20 of culture showing expressing of CD45 but lack of myeloid commitment, as evidenced by staining for CD13, CD14, and CD11c. Open histograms show levels of background staining using isotype-matched control antibodies. (b) Appearance of CD45int cells at day 27 of culture, accompanied by the upregulation of myeloid-specific markers. (c) Photomicrograph, taken at day 28 of culture, showing the morphology of DC, including veils of cytoplasm and long dendrites (inset) (×40 magnification). (d) Cells harvested at day 33 of culture, showing the appearance of a CD45hi population containing predominantly DC progenitors expressing CD14, CD11c, CD86 and MHC class I. (e) Phenotype of immature and mature H1-DCs compared with human moDC. DCs were cultured either in medium alone or medium supplemented with the maturation cocktail and stained for MHC class II, the maturation marker CD83 and classical costimulatory molecules. Dead cells were excluded from the analysis using 7-AAD. Dashed histograms show the phenotype of immature DCs while the filled histograms represent mature DCs. Open histograms depict background staining using isotype-matched controls.
By day 33 of culture, up to 21% of cells had adopted a CD45hi phenotype, the majority of which were CD11c+ (Figure 2(d)). Whereas these cells predominantly expressed MHC class I and CD86, CD83, and MHC class II expression were low, consistent with the phenotype of immature DC. Culture of H1-DC for 2 days in a cocktail of cytokines consisting of GM-CSF, IL-4, IFNγ, TNFα, IL-1β, and PGE2 induced their maturation, as evidenced by the upregulation of CD83, similar to moDC (Figure 2(e)). Although, as previously described, MHC class II was not upregulated by H1-DC to the same extent as their monocyte-derived counterparts [19], surface expression of the costimulatory molecules CD40, CD80, and CD86 was consistent with our previous reports of the ability of this novel source of DC to stimulate proliferative responses among naïve allogeneic T cells [19].
3.2. Rapamycin Reduces the Immunogenicity of H1-DC
We next investigated whether the exposure of H1-DC to rapamycin could promote the acquisition of a protolerogenic phenotype, similar to that described for other populations of mouse and human DC [11–13]. Accordingly, we cultured H1-DC with rapamycin for 3 days prior to inducing their maturation with proinflammatory cytokines and assessed their surface phenotype and immunostimulatory capacity in the allogeneic MLR. Whereas the addition of 10 ng/mL of rapamycin to moDC had only a modest impact on their viability, H1-DC proved especially sensitive to its toxicity, undergoing significant levels of apoptosis at concentrations greater than 7 ng/mL, as described in other studies [22]. Nevertheless, careful titration of the compound revealed that exposure of H1-DC to concentrations between 5 and 7 ng/mL exerted immunomodulatory effects without compromising their viability. Interestingly, conditioning of H1-DC with rapamycin did not appear to inhibit their maturation since they upregulated CD83 and CD86 and maintained surface expression of MHC class II and the inhibitory receptor PD-L1 (Figure 3(a)), strongly implicated in the polarisation of naïve T cells towards a Treg phenotype [23]. Significantly, however, H1-DC consistently failed to up-regulate CD40 following exposure to rapamycin, even though higher concentrations of the pharmacological agent had little impact on CD40 expression by moDC (Figure 3(b)). Consistent with their reduced levels of CD40 expression, the immunostimulatory capacity of rapamycin-treated H1-DC was significantly reduced in cocultures with naïve allogeneic T cells (Figure 3(c)). In contrast, 10 ng/mL of rapamycin exerted only modest inhibitory effects on the capacity of moDC to stimulate proliferative responses among naïve allogeneic T cells (Figure 3(c)).
Figure 3.
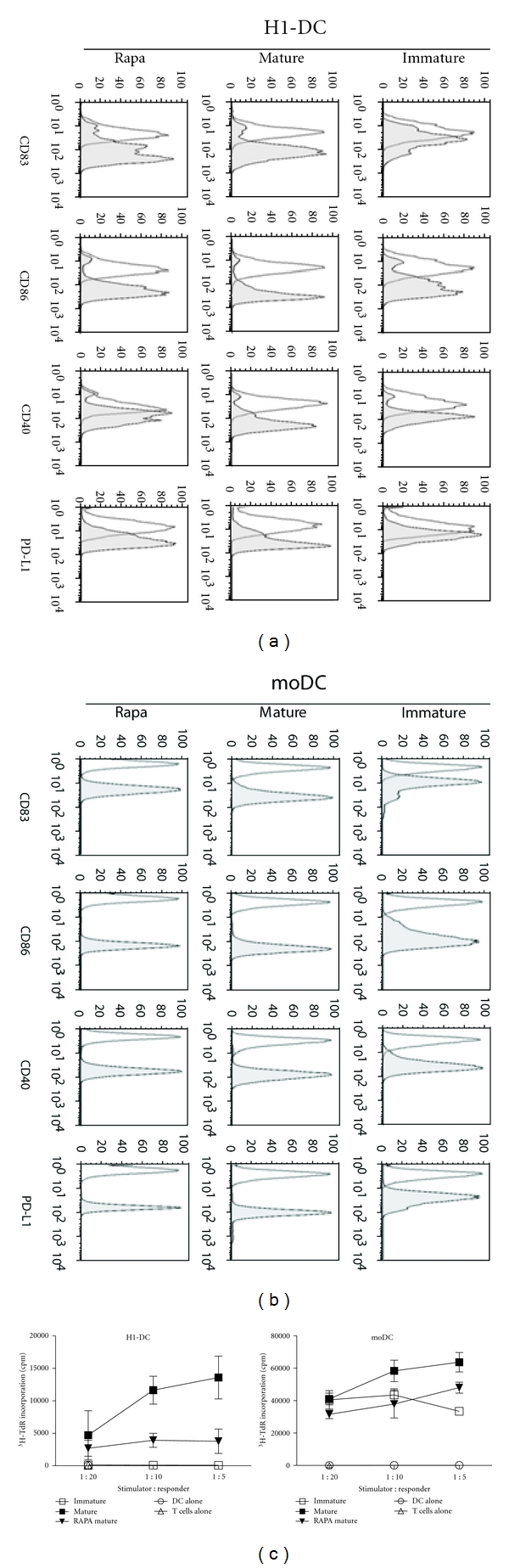
Effect of rapamycin (Rapa) on the phenotype and function of H1-DC. DCs were either untreated, matured in response to the maturation cocktail or treated with Rapa for 3 days prior to maturation. (a) H1-DC stained for the expression of the maturation marker CD83, the costimulatory molecules CD86 and CD40, as well as the inhibitory receptor PD-L1. Dead cells were excluded from analysis using 7-AAD. Open histograms represent the level of background staining using appropriate isotype-matched controls. Data from one of 3 independent experiments are shown. (b) Phenotypic analysis of control populations of moDC treated and stained in parallel with rapamycin. (c) Effect of rapamycin on the allostimulatory capacity of DC in the allogeneic MLR. DCs were mitotically-inactivated using mitomycin C and plated in triplicate at a top dose of 104 cells per well of a 96-well round-bottomed plate; naïve CD4+ T cells were plated at 5 × 104 cells/well. Cells were incubated for 5 days before pulsing with 3H-thymidine overnight. Graphs show the mean of triplicate cultures ± S.D. Data are shown from one experiment, representative of 3 independent experiments.
3.3. Rapamycin-Treated H1-DC Polarise Naïve T Cells towards a Regulatory Phenotype
Given the reduced immunostimulatory capacity of rapamycin-treated H1-DC and their acquisition of a CD40lo PD-L1+ phenotype, we next investigated whether their coculture with naïve CD4+ T cells might favour the induction of Treg cells, defined as CD4+CD25hi cells with persistent expression of Foxp3. Although at the outset, T cells enriched for CD4+ cells were predominantly Foxp3− (Figure 4(a)), coculture with immature H1-DC for 7 days, resulted in up to 8.5% of CD4+CD25hi cells retaining Foxp3 expression by the end of the culture period (Figure 4(b)). When the H1-DC had been matured prior to coculture with naïve allogeneic T cells, the proportion of cells committed to the Treg cell lineage increased marginally to 12.5%. However, the use of H1-DC, which had been induced to mature following exposure to rapamycin, consistently resulted in a significant increase in the induction of Treg cells which represented approximately 26.5% of CD4+CD25hi cells, similar results being obtained in four independent experiments. By contrast, rapamycin conditioning of moDC exerted only a marginal effect on the ability of the cells to polarise responding T cells towards a regulatory phenotype (Figure 4(b)).
Figure 4.
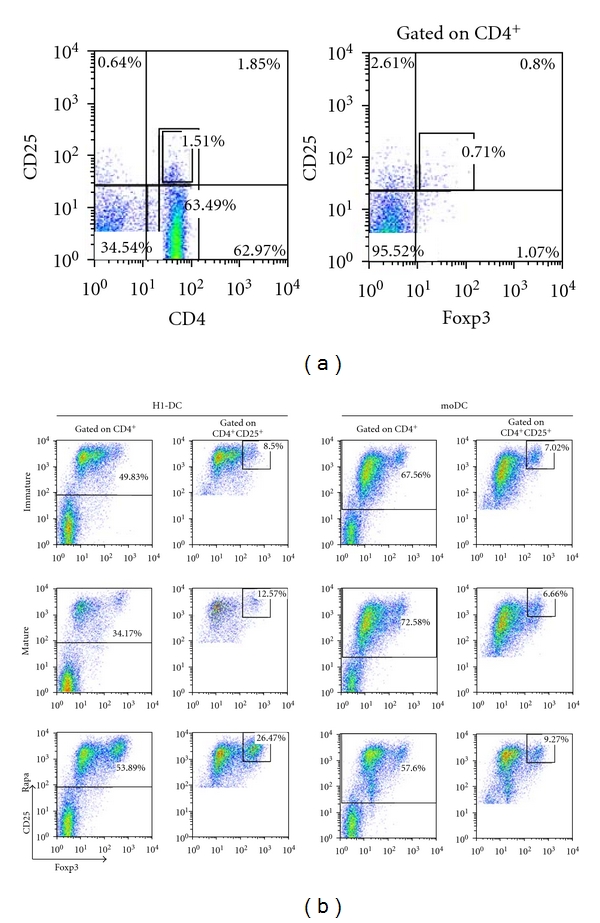
Enhanced capacity of rapamycin-treated H1-DC to promote Treg induction. (a) The starting population of naive CD4+ T cells was analysed by flow cytometry for the expression of CD25 and Foxp3, markers associated with commitment of T cells to the regulatory T cell lineage. (b) Rapamycin enhances the capacity of H1-DC to induce Treg cells compared to moDC. DCs were either untreated, matured with the maturation cocktail or treated with rapamycin for 3 days prior to maturation. DCs were harvested, washed, and plated at 2 × 105 per well with 106 naive CD4+ T cells per well of a 24-well plate to yield a ratio of DC : T cells of 1 : 5. On day 7, cocultures were stained for CD4, CD25, and Foxp3 and analysed. Dead cells were excluded from the analysis using 7-AAD staining. Data from one experiment representative of 4 independent experiments are shown.
Given that the identification of bona fide human Treg cells is confounded by the universal upregulation of CD25 by activated T cells and their transient expression of Foxp3, irrespective of final lineage commitment, we investigated whether CD25hi Foxp3+ cells appearing in such cultures displayed other known phenotypic features of Treg cells. Cells co-expressing CD25 and Foxp3 were found to express CTLA-4 (Figure 5(a)), while lacking expression of the α subunit of the IL-7R, CD127 (Figure 5(b)), such a phenotype being strongly suggestive of a regulatory function [24, 25].
Figure 5.
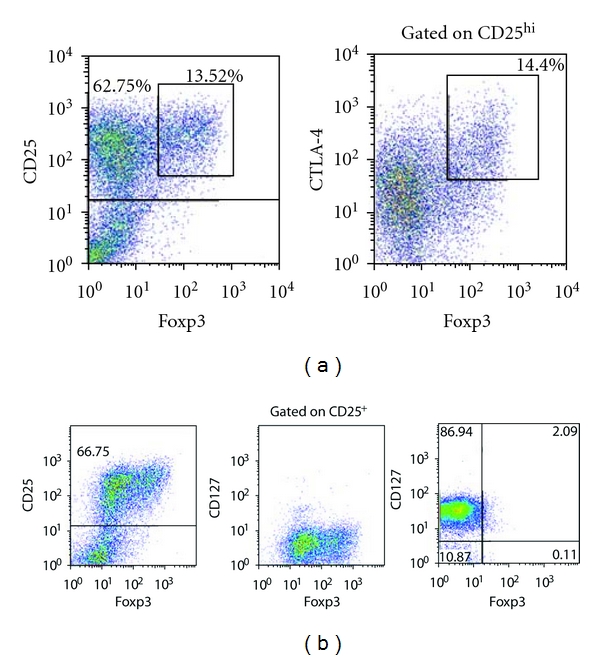
Phenotypic characterisation of putative CD25hi Foxp3+ Treg cells from cocultures of DC and naïve T cells. The CD25hi Foxp3+ population expresses CTLA-4 (a) but lacks expression of CD127 in comparison with control Foxp3− T cells (b), consistent with the reported phenotype of bona fide Treg cells.
4. Discussion
The development of robust protocols for the differentiation of DC from hESC lines, derived under cGMP conditions, offers a potentially unlimited source of cells with little variability between batches, which may be subjected to rigorous quality control. The potent immunostimulatory capacity of DC differentiated in this way has suggested that they will find a likely application in the presentation of tumour associated antigens to the T-cell repertoire, thereby overcoming many of the limitations inherent in the use of moDC for cancer immunotherapy [19]. Nevertheless, given the accumulation of evidence in favour of an additional role played by DC in the establishment and ongoing maintenance of immunological tolerance [3], the availability of DC differentiated from hESC suggests they may enjoy a broader remit. We have, for instance, proposed that hESC-derived DC might be exploited to induce tolerance to the alloantigens they express, thereby conditioning recipients to accept replacement tissues differentiated from the same parent cell line [7]. This prospect is, however, contingent on the development of clinically compliant strategies to ensure the stable tolerogenicity of DC generated in this way. While the introduction of transgenes, such as PD-L1, at the ESC stage might confer on the resulting DC an immunomodulatory function [8], the additional regulatory hurdles encountered by the administration to patients of genetically modified cells, has fuelled attempts to identify approved pharmacological agents that coerce DC to adopt a protolerogenic phenotype [10].
Rapamycin is one such agent routinely exploited for its immunosuppressive properties in the treatment of allograft rejection but which has been shown to exert a profound effect on the function of individual components of the immune system, including DC. Indeed, treatment of DC with rapamycin in vitro has been demonstrated to arrest them in an immature or semimature state rendering them tolerogenic [11–13]. Accordingly, in various preclinical transplantation models, administration of rapamycin-treated recipient DC, pulsed with a source of donor alloantigens, secured the long-term survival of organ allografts [14–16]. If such a conditioning regime could be applied to DC differentiated from hESC, it may prove feasible to establish operational tolerance to the alloantigens they endogenously express, in advance of CRT. As a first step towards this goal, we have demonstrated the sensitivity of H1-DC to rapamycin which significantly reduces their immunostimulatory properties in the allogeneic MLR (Figure 3(b)), an in vitro correlate of the direct pathway of alloantigen presentation. Furthermore, rapamycin substantially augments their ability to polarise responding CD4+ T cells towards a regulatory phenotype (Figure 4(b)), as determined by their sustained expression of Foxp3 and adoption of a CTLA4+CD127− phenotype. Furthermore, our preliminary results indicate that, while maturation of H1-DC induces secretion of high levels of the inflammatory cytokine IL-6 [19], prior exposure to rapamycin significantly reduces IL-6 production, possibly guiding responding T cells away from Th1/Th17 commitment towards a Treg phenotype. These results strongly suggest, therefore, that rapamycin may have the desirable properties of preventing activation of alloreactive T cells through both the direct and indirect pathways of alloantigen presentation, the induction of Treg cells potentially modulating responsiveness to indirectly presented alloantigens that have been reprocessed by endogenous recipient DC. In contrast to our findings with H1-DC, rapamycin-treatment of moDC had only a modest impact on their immunostimulatory capacity and little effect on their surface phenotype. Although our results are contrary to some other reports [12, 18], many studies have typically used higher concentrations of rapamycin and regimes for the maturation of moDC involving exposure to bacterial products, such as lipopolysaccharide, which target different intracellular signalling pathways from those solicited upon culture with the cocktail of proinflammatory cytokines used in these studies.
Despite the profound effect that rapamycin exerts on the functional potential of H1-DC, phenotypic analysis of cells treated with the compound was largely unremarkable, with the exception that upregulation of the costimulatory molecule CD40 upon maturation was prevented by prior exposure to the compound. The significance of these findings may lie in the growing appreciation of the role played by CD40 as the fulcrum on which the balance between tolerance and immunity has been shown to pivot. For instance, the administration to mice of CD40−/− DC laden with foreign antigen was shown to induce profound antigen-specific tolerance upon subsequent immunization, results which are consistent with the induction of a repertoire of Treg cells [26]. Furthermore, in mice receiving foreign antigen chemically conjugated to CD205-specific mAb as a way of delivering antigen to DC in the steady state, the induction of tolerance could be abrogated in favour of systemic immunity by the concomitant administration of agonistic antibodies specific for CD40 [4].
It is the central role played by CD40 in a transplantation setting that underlies the success of strategies for intervening in allograft rejection based on the blockade of CD40-CD154 interactions [27]. Although unanticipated complications associated with the use of mAb specific for CD154 have hindered the application of such a strategy to the clinic; the long-term acceptance of allografts in mice was found to bear the distinctive features of regulation, including linked suppression and infectious tolerance [28, 29]. A conditioning regime that limits the delivery of CD40 signalling by donor DC might, therefore, be anticipated to predispose recipients towards tolerance based on the generation of a repertoire of alloantigen-specific Treg cells. Indeed, the level of expression of CD40 has been shown to be critical in determining the outcome of antigen recognition in a model of Leishmania donovani infection, high levels of expression inducing effector T cells, low levels favouring polarisation towards a Treg phenotype [30]. Furthermore, blockade of the CD40-CD154 axis in combination with rapamycin was shown to achieve tolerance even across a xenogeneic barrier [31]. Although the mechanisms involved were not specifically elucidated in this study, it may be significant in our own experiments that PD-L1 expression by H1-DC was unaffected by rapamycin treatment, being upregulated in response to maturation stimuli, irrespective of prior exposure to the compound. Given the essential role described for PD-L1 in polarisation of naïve T cells towards a Treg cell phenotype [23], it is tempting to speculate that it is by altering the critical balance between costimulatory and inhibitory signals delivered by H1-DC, that rapamycin treatment strongly favours a tolerogenic profile.
5. Conclusions
We have demonstrated previously that hESC may be differentiated into populations of immunogenic DC whose properties may be exploited in regimes of cancer immunotherapy. Here, we extend this paradigm by showing that our protocols are fully compliant with the use of rapamycin which favours a protolerogenic phenotype of the resulting DC. Our results pave the way for the future use of rapamycin-conditioned hESC-derived DC in regimes for the induction of tolerance, as a prelude to CRT.
Conflict of Interests
A. Reddy and K. P. Nishimoto declare a potential financial conflict of interests as employees of Geron Corporation.
Acknowledgments
The authors are grateful to Tim Davies, Naoki Ichiryu, and Simon Hackett for helpful discussions and to Lara Whitmore for administrative assistance. A. J. Leishman is the recipient of an MRC capacity building studentship, awarded to the Oxford Stem Cell Institute. This work was performed under a sponsored research agreement between the University of Oxford and Geron Corporation, with additional support from the Medical Research Council UK, in the form of Grant G0802538 awarded to P. J. Fairchild.
References
- 1.Klimanskaya I, Rosenthal N, Lanza R. Derive and conquer: sourcing and differentiating stem cells for therapeutic applications. Nature Reviews Drug Discovery. 2008;7(2):131–142. doi: 10.1038/nrd2403. [DOI] [PubMed] [Google Scholar]
- 2.Fairchild PJ, Robertson NJ, Minger SL, Waldmann H. Embryonic stem cells: protecting pluripotency from alloreactivity. Current Opinion in Immunology. 2007;19(5):596–602. doi: 10.1016/j.coi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 4.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. Journal of Experimental Medicine. 2001;194(6):769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yates SF, Paterson AM, Nolan KF, et al. Induction of regulatory T cells and dominant tolerance by dendritic cells incapable of full activation. Journal of Immunology. 2007;179(2):967–976. doi: 10.4049/jimmunol.179.2.967. [DOI] [PubMed] [Google Scholar]
- 6.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. Journal of Experimental Medicine. 2001;193(2):233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairchild PJ, Cartland S, Nolan KF, Waldmann H. Embryonic stem cells and the challenge of transplantation tolerance. Trends in Immunology. 2004;25(9):465–470. doi: 10.1016/j.it.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Senju S, Suemori H, Zembutsu H, et al. Genetically manipulated human embryonic stem cell-derived dendritic cells with immune regulatory function. Stem Cells. 2007;25(11):2720–2729. doi: 10.1634/stemcells.2007-0321. [DOI] [PubMed] [Google Scholar]
- 9.Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand 1. Journal of Immunology. 2005;174(4):1888–1897. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- 10.Leishman AJ, Silk KM, Fairchild PJ. Pharmacological manipulation of dendritic cells in the pursuit of transplantation tolerance. Current Opinion in Organ Transplantation. 2011;16(4):372–378. doi: 10.1097/MOT.0b013e3283484b42. [DOI] [PubMed] [Google Scholar]
- 11.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. Journal of Immunology. 2007;178(11):7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 12.Sordi V, Bianchi G, Buracchi C, et al. Differential effects of immunosuppressive drugs on chemokine receptor CCR7 in human monocyte-derived dendritic cells: selective upregulation by rapamycin. Transplantation. 2006;82(6):826–834. doi: 10.1097/01.tp.0000235433.03554.4f. [DOI] [PubMed] [Google Scholar]
- 13.Reichardt W, Dürr C, Von Elverfeldt D, et al. Impact of mammalian target of rapamycin inhibition on lymphoid homing and tolerogenic function of nanoparticle-labeled dendritic cells following allogeneic hematopoietic cell transplantation. Journal of Immunology. 2008;181(7):4770–4779. doi: 10.4049/jimmunol.181.7.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks JM, Kuo YR, Taieb A, et al. Prolongation of composite tissue allograft survival by immature recipient dendritic cells pulsed with donor antigen and transient low-dose immunosuppression. Plastic and Reconstructive Surgery. 2008;121(1):37–49. doi: 10.1097/01.prs.0000293754.55706.7f. [DOI] [PubMed] [Google Scholar]
- 15.Ikeguchi R, Sacks JM, Unadkat JV, et al. Long-term survival of limb allografts induced by pharmacologically conditioned, donor alloantigen-pulsed dendritic cells without maintenance immunosuppression. Transplantation. 2008;85(2):237–246. doi: 10.1097/TP.0b013e31815e870e. [DOI] [PubMed] [Google Scholar]
- 16.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce Ag-specific T cell regulation and prolong graft survival. American Journal of Transplantation. 2005;5(2):228–236. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 17.Horibe EK, Sacks J, Unadkat J, et al. Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transplant Immunology. 2008;18(4):307–318. doi: 10.1016/j.trim.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Haidinger M, Poglitsch M, Geyeregger R, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. Journal of Immunology. 2010;185(7):3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 19.Tseng SY, Nishimoto KP, Silk KM, et al. Generation of immunogenic dendritic cells from human embryonic stem cells without serum and feeder cells. Regenerative Medicine. 2009;4(4):513–526. doi: 10.2217/rme.09.25. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnology. 2001;19(10):971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnology and Bioengineering. 2005;91(6):688–698. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- 22.Woltman AM, De Fijter JW, Kamerling SWA, et al. Rapamycin induces apoptosis in monocyte- and CD34-derived dendritic cells but not in monocytes and macrophages. Blood. 2001;98(1):174–180. doi: 10.1182/blood.v98.1.174. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Pino-Lagos K, De Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(27):9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. Journal of Experimental Medicine. 2006;203(7):1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Putnam AL, Xu-yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. Journal of Experimental Medicine. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochweller K, Anderton SM. Systemic administration of antigen-loaded CD40-deficient dendritic cells mimics soluble antigen administration. European Journal of Immunology. 2004;34(4):990–998. doi: 10.1002/eji.200324782. [DOI] [PubMed] [Google Scholar]
- 27.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 28.Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. Journal of Immunology. 1999;163(9):4805–4810. [PubMed] [Google Scholar]
- 29.Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. Journal of Immunology. 2000;165(9):4783–4786. doi: 10.4049/jimmunol.165.9.4783. [DOI] [PubMed] [Google Scholar]
- 30.Martin S, Agarwal R, Murugaiyan G, Saha B. CD40 expression levels modulate regulatory T cells in Leishmania donovani infection. Journal of Immunology. 2010;185(1):551–559. doi: 10.4049/jimmunol.0902206. [DOI] [PubMed] [Google Scholar]
- 31.Muller YD, Mai G, Morel P, et al. Anti-CD154 mAB and rapamycin induce T regulatory cell mediated tolerance in rat-to-mouse islet transplantation. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010352. Article ID e10352. [DOI] [PMC free article] [PubMed] [Google Scholar]