Abstract
Research on the functional meaning of EEG frequency bands during memory processing has only examined two developmental periods: infancy and from late childhood to adulthood. The purpose of this study was to examine changes in EEG power for three toddler EEG frequency bands (3–5 Hz, 6–9 Hz, 10–12 Hz) during a verbal recall task. To this end, we asked three questions: (a) Which frequency band(s) discriminate baseline from memory processing?; (b) Which frequency band(s) differentiate between memory encoding and retrieval processes?; (c) Which frequency band(s) distinguish toddlers with high and low verbal recall performance? Analysis of 2-year-olds’ (n = 79) power values revealed that all three frequency bands differentiated the retrieval and encoding phases from the baseline phase; however, the particular regions that exhibited this dissociation varied. Retrieval-related increases in 3–5 Hz (theta) power were widespread. Only the 3–5 Hz and 6–9 Hz bands distinguished encoding and retrieval processes; retrieval power values were higher than encoding power values. High and low verbal recall performers were discriminated by all frequency bands; high performers had greater power values than low performers. Thus, the 3–5 Hz (theta) and 6–9 Hz (alpha) bands were most informative about 2-year-olds’ memory processes. Theta and alpha rhythms are critical to memory processes during late childhood and adulthood, and our findings provide initial evidence that these rhythms are also intricately linked to memory processing during toddlerhood. These findings are discussed in relation to behavioral changes in memory processes.
Keywords: declarative memory, EEG power, memory encoding, memory retrieval, toddlers
1. Introduction
The encoding and retrieval of declarative memories—essential processes for normal cognitive functioning—is intricately linked to activity of the medial temporal lobe (MTL) and the prefrontal cortex (PFC; Ghetti et al., 2010; Ofen et al., 2007). Substantial behavioral research has revealed age-related changes in memory encoding and retrieval from infancy through adulthood (see Kail, 1984; Rovee-Collier and Cuevas, 2008; Schneider and Pressley, 1997, for reviews). Neuroscience research, on the other hand, has only examined these processes during two developmental periods: infancy and from late childhood through adulthood. Thus, there is a significant gap in our current understanding of the neural correlates of memory encoding and retrieval during early to middle childhood.
In the present study, we were particularly interested in the emergence of verbal recall around 2 years of age because (a) toddlerhood marks a transitional period between infancy and childhood; (b) this is the youngest age that verbal recall can be assessed; and (c) a high degree of variability in verbal recall is likely to be evident at this age, which is optimal for examining relations between psychophysiological measures and individual differences in verbal recall. To this end, we recorded electroencephalography (EEG) during memory encoding and retrieval, calculated EEG power (i.e., a measure of neural activity), and used a multiple frequency band approach to identify which toddler frequency band(s) are functionally related to memory processing. In the following sections, we first discuss the development of EEG frequency bands during infancy and toddlerhood and then review related psychophysiological memory research findings.
1.1. Development of EEG frequency bands
In general, adult EEG frequency bands are clearly defined and their functional significance is widely accepted. The same cannot be said for infant and toddler EEG frequency bands. Early longitudinal research demonstrated that infant and toddler EEG is at a much lower frequency than adult EEG (see Bell, 1998; Bell and Fox, 1994, for reviews). Consequently, developmental EEG researchers have questioned the appropriateness of using traditional adult frequency bands (Pivik et al., 1993; Stroganova and Orekhova, 2007).
For adults, the dominant EEG rhythm during quiet wakefulness is the 8–13 Hz alpha rhythm. The frequency of the alpha rhythm is postulated to increase early in development (Lindsley, 1939). Accordingly, the alpha rhythm during early childhood functions as the alpha rhythm during adulthood, just at a lower frequency. Two lines of research have suggested that the infant and toddler 6–9 Hz band is analogous to the adult alpha rhythm. Using spectral analysis to examine longitudinal baseline data, Marshall and colleagues (2002) found that 6–9 Hz was the dominant frequency across most scalp electrode sites from infancy to 4 years of age. Thus, both the infant/toddler 6–9 Hz rhythm and the adult 8–13 Hz alpha rhythm are prominent during quiet wakefulness. Additional research suggests that the infant/toddler and adult alpha rhythms exhibit similar functional dissociations. During “eyes closed” baseline, the adult alpha rhythm typically exhibits increases in amplitude over the occipital cortex. The same pattern of findings has been found for the infant 5.2–9.6 Hz rhythm during a “lights off” period that was hypothesized to be equivalent to the “eyes closed” baseline (Stroganova et al., 1999).
In line with other developmental EEG researchers (e.g., McLaughlin et al., 2010; see Stroganova and Orekhova, 2007, for a review), we consider the infant/toddler 6–9 Hz band to be analogous to the adult alpha rhythm, and use this as a reference point when interpreting the meaning of lower and higher toddler frequency bands. The adult 8– 13 Hz alpha rhythm, for instance, is centered between the lower, adjacent 4–8 Hz theta rhythm and the upper, adjacent ≈13–25 Hz beta rhythm. Accordingly, the infant/toddler 3–5 Hz and 10–12 Hz bands, which are adjacent to the 6–9 Hz band, are likely analogous to the adult theta and beta rhythms, respectively (McLaughlin et al., 2010; Orekhova et al., 2006; see Stroganova and Orekhova, 2007, for a review). In sum, the present study’s multiple frequency band analysis of 2-year-olds’ neural activity (i.e., EEG power) during memory encoding and retrieval provides the essential framework for future developmental investigations of memory processes.
1.2. Memory processes: Neural activity
Functional magnetic resonance imaging (fMRI) research with adults and older children (i.e., 8 years and older) has emphasized the role of the MTL and PFC during memory encoding and retrieval (Ghetti et al., 2010; Ofen et al., 2007). Similar fMRI research has not been completed with younger children because of the technique’s extreme sensitivity to motor artifacts. EEG, on the other hand, is a noninvasive imaging technique that is relatively resistant to motor artifacts, making it the preferred brain imaging technique during infancy and early childhood (Casey and de Haan, 2002). Although EEG does not have the spatial resolution of fMRI, it does have superior temporal resolution.
EEG research with adults and older children has revealed changes in neural activity (i.e., EEG power) during memory encoding and retrieval. Specifically, memory-related changes in EEG power (as compared to a baseline/reference interval) have been found for the theta, alpha, and beta rhythms (Babiloni et al., 2004; Klimesch et al., 1996, 1997, 1999; Krause et al., 2001, 2007; Mölle et al., 2002; Sederberg et al., 2003). Similar multiple band frequency analyses of memory processes have not been completed during infancy or early childhood. Clearly, our understanding of behavioral changes in encoding and retrieval are limited by a gap in the literature about corresponding changes in neural activity.
To our knowledge, only one infant/toddler study has examined changes in EEG power associated with declarative memory processes. Morasch and Bell (2009) analyzed 6–9 Hz EEG during the encoding and retrieval stages of a deferred imitation task (i.e., nonverbal declarative memory task). They found that 10-month-olds with high recall performance (i.e., ordered recall) exhibited baseline-to-retrieval increases in EEG power at temporal sites. No retrieval-related changes were found for low performers (i.e., no ordered recall). A similar nonsignificant pattern (i.e., high performers, p = .10) was displayed at frontal sites. However, neither high nor low performing 10-month-olds displayed changes in EEG power during encoding at frontal or temporal electrode sites. Because of the single frequency band approach, it is unknown whether these findings reflect general or frequency-specific developmental phenomena. Clearly, additional infant and toddler multi-frequency band EEG research is essential to understanding age-related changes in neural activity during memory processing.
1.3. Research questions
The present study analyzed changes in EEG power associated with 2-year-olds’ memory encoding and retrieval for three different frequency bands: 3–5 Hz (theta), 6–9 Hz (alpha), 10–12 Hz (beta). Although research with adults and older children has revealed changes in EEG power for theta, alpha, and beta rhythms during memory encoding and retrieval (Babiloni et al., 2004; Krause et al., 2001, 2007; Mölle et al., 2002; Sederberg et al., 2003) as well as differences in power between memory encoding and retrieval for theta and alpha rhythms (Babiloni et al., 2004; Krause et al., 2001, 2007), it is unknown whether 2-year-olds will exhibit similar changes in neural activity. Infant memory research has failed to reveal encoding-related changes in 6–9 Hz EEG power, and retrieval-related changes in 6–9 Hz power varied as a function of performance (Morasch and Bell, 2009). To this end, the current study addressed three questions: (a) Which frequency band(s) discriminate baseline from memory processing?; (b) Which frequency band(s) differentiate between memory encoding and retrieval processes?; (c) Which frequency band(s) distinguish toddlers with high and low verbal recall performance? The answers to these questions will enhance our understanding of the neural correlates of memory encoding and retrieval during toddlerhood.
2. Materials and methods
2.1. Participants
A total of 122 two-year-old children (62 girls, 60 boys; 5 Hispanic, 117 Non-Hispanic; 112 Caucasian, 1 African American, 9 Multi-Racial) participated in our immediate recall task as part of a longitudinal study examining cognitive development from infancy through early childhood. Children were seen between 1 year 11 months and 2 years 4 months of age (M = 2 years 0 months, SD = 23 days). All children were born within 2 weeks of their expected due dates and had no diagnosed neurological problems or developmental delays. For parents who reported educational information (120 mothers, 115 fathers), all completed a high school education (7.5 % and 4.3% technical degree; 41.7% and 34.8% bachelor’s degree; 26.7% and 32.2% graduate degree; respectively). Average maternal and paternal age at birth was 30.0 and 33.6 years (SD = 4.9 and 6.9), respectively. Children were given a small gift and parents were paid for the laboratory visit.
2.2. Procedure
2.2.1. EEG recordings
Continuous EEG recordings were collected throughout a battery of tasks in an ongoing longitudinal examination of cognitive development. Recordings made during baseline and the memory task are described and reported in the current study.
EEG was recorded during baseline and during the memory task. Recordings were made from 16 left and right scalp sites: frontal pole (Fp1, Fp2), medial frontal (F3, F4), lateral frontal (F7, F8), central (C3, C4), temporal (T7, T8), medial parietal (P3, P4), lateral parietal (P7, P8), and occipital (O1, O2). All electrode sites were referenced to Cz during recording. EEG was recorded using a stretch cap (Electro-Cap, Inc.; Eaton, OH) with electrodes in the 10/20 system pattern (Jasper, 1958; Pizzagalli, 2007). During the electrode application, a research assistant entertained and distracted the toddler by playing with age-appropriate toys. This entertainment period also served to help the toddler “warm up” to the laboratory setting. After the cap was placed on the toddler’s head, recommended procedures regarding EEG data collection with children were followed (Pivik et al., 1993). Specifically, a small amount of abrasive was placed into each recording site and the scalp gently rubbed. Following this, conductive gel was placed in each site. Electrode impedances were measured and accepted if they were below 10K ohms.
The electrical activity from each lead was amplified using separate SA Instrumentation Bioamps (San Diego, CA) and bandpassed from .1 to 100 Hz. Activity for each lead was displayed on the monitor of an acquisition computer. The EEG signal was digitized on-line at 512 samples per second for each channel so that the data were not affected by aliasing. The acquisition software was Snapshot-Snapstream (HEM Data Corp.; Southfield, MI) and the raw data were stored for later analyses.
2.2.2. EEG analysis
EEG data were examined and analyzed using EEG Analysis System software developed by James Long Company (Caroga Lake, NY). First, the data were re-referenced via software to an average reference configuration (Lehmann, 1987). Average referencing, in effect, weighted all the electrode sites equally and eliminated the need for a noncephalic reference. Active (F3, F4, etc.) to reference (Cz) electrode distances vary across the scalp. Without the re-referencing, power values at each active site may reflect interelectrode distance as much as they reflect electrical potential. The average reference configuration requires that a sufficient number of electrodes be sampled and that these electrodes be evenly distributed across the scalp. Currently, there is no agreement concerning the appropriate number of electrodes (Davidson et al., 2000; Hagemann et al., 2001; Luck, 2005), although the 10/20 configuration that we used does satisfy the requirement of even scalp distribution.
The re-referenced EEG data were artifact scored for eye blinks using Fp1 and Fp2 (Myslobodsky et al., 1989) and for gross motor movements and these artifact-scored epochs were eliminated from all subsequent analyses. The data then were analyzed with a discrete Fourier transform (DFT) using a Hanning window of 1-s width and 50% overlap. Power was computed for three frequency bands: 3–5 Hz (actually 2.5–5.5 Hz), 6–9 Hz (5.5–9.5 Hz), 10–12 Hz (9.5–12.5 Hz). The power was expressed as mean square microvolts and the data were transformed using the natural log (ln) to normalize the distribution.
2.2.3. Data available for analysis
To be included in our final sample, it was necessary for children to provide behavioral and psychophysiological data. Behavioral data were available for 122 children, EEG electrodes were accepted by 102 children, and EEG electrodes were worn for the entire experimental session by 93 toddlers. Psychophysiological data were available for 92 children (i.e., 1 equipment failure). The psychophysiological data were screened for motor artifacts, and 79 two-year-olds had sufficient artifact-free EEG (≥ 6 DFT windows) data during each experimental phase (i.e., baseline, encoding, and retrieval) for inclusion in subsequent analyses. Children with sufficient psychophysiological data (n = 79) did not differ in age, language skills (MacArthur-Bates Communicative Development Inventory (MCDI): total vocabulary and mean length utterance), or recall ability compared to children who did not contribute EEG data (n = 43; all t’s ≤ 1.40, p’s ≥ .16)1.
2.2.4. Baseline EEG
Resting physiology was recorded as the 2-year-old sat in a high chair. Mothers were instructed to not talk to toddlers during the EEG recording. The toddler watched a 60-s segment (M = 68.3 s, SD = 11.6; artifact-free DFT windows: M = 85, SD = 25) of a neutral cartoon that provided a period of physiology containing comparable eye movements and gross motor artifact to what was exhibited during the memory task. The recording of EEG continued as the memory task was administered.
2.2.5. Memory task
During the memory task, the experimenter showed the toddler a small bag, asked the toddler if he/she had ever gone grocery shopping, and told the toddler that they were going to play the grocery store game. Three common food items (i.e., juice box, single-serving box of cereal, single-serving bag of chips) were shown to toddlers during the encoding portion of the memory task. For each item, the experimenter (a) said the toddler’s name (to make sure the toddler was looking); (b) held the item so that the toddler could see it, (c) asked the toddler to name the item, and (d) named the item before placing it into the opaque bag. Once all of the items had been placed in the bag, the experimenter asked, “What’s in the bag?” (i.e., retrieval). The number of correct verbal responses (0–3) provided a measure of immediate recall memory performance. Interrater reliability was calculated for 24% of the sample and percentage agreement on number of items correctly recalled was 92%.
Event marks were placed on the physiological record so that the EEG recordings could be synchronized with the encoding (i.e., object presentations: M = 45.8 s, SD = 17.3; artifact-free DFT windows: M = 57, SD = 23) and retrieval (i.e., recall: M = 30.7 s, SD = 14.2; artifact-free DFT windows: M = 35, SD = 23) during the memory task. For each of the three items, encoding-related EEG started when the item was shown to the toddler, continued as the child attempted to name the item and the experimenter named the item, and stopped when the item was placed in the bag. The retrieval-related EEG started immediately after the experimenter asked the recall question and continued until toddlers indicated that they were finished with the retrieval phase (e.g., recalled all items, said “I don’t know/remember”). Thus, electrophysiological data not included in the analyses were those recorded when the experimenter was (a) introducing the grocery store game to the toddler and (b) saying the child’s name before displaying each item. After the memory task, the EEG cap was gently removed and the gels were washed from the toddler’s hair.
2.2.6. Language
The MCDI "Words and Sentences” form (Fenson et al., 1992) was completed by toddlers’ mothers to provide a measure of toddler verbal ability. The MCDI, designed for use with 16- to 30-month-olds, is an inventory of common words and phrases. This inventory has high internal consistency (α = .96) and strong documented test-retest reliability (α > .90 for all ages tested during the toddler period; Fenson et al., 1992). Mothers indicated their toddler’s production of the items on the inventory (words scale) as well as their toddler’s early grammatical ability, specifically, the complexity of multi-word utterances. Our measures of verbal ability were total vocabulary and mean length utterance.
3. Results
The analyses consisted of repeated-measures multivariate analysis of variance (MANOVAs) for each frequency band with region (i.e., frontal pole, medial frontal, lateral frontal, central, temporal, medial parietal, lateral parietal, occipital), hemisphere (i.e., left, right), and processing stage (i.e., baseline, encoding, retrieval) as within-subjects factors and performance group (i.e., low recall, high recall) as a between-subjects factor (Picton et al., 2000). We present performance group findings first in order to account for any interactions involving processing stage. A multivariate approach for assessing multivariate interaction effects has been suggested by Keselman (1998). For ease in examining any interactions involving processing stage or performance group, follow-up MANOVAs were performed. A Bonferroni procedure was adopted to limit the familywise Type I error rate (α = .10). We decided not to use a familywise error rate with α = .05 because this method would be too conservative for correlated variables (i.e., regional EEG power values; Yoder et al., 2004).
3.1. Performance group
Toddlers were categorized as having either low (i.e., no recall: n = 54) or high (i.e., recall ≥ one item; one item: n = 16; two items: n = 8; three items: n = 1) verbal recall performance. For 2-year-olds with MCDI data (low performers: n = 50; high performers: n = 22), independent groups t-tests confirmed that the two performance groups did not differ in age or verbal ability (i.e., total vocabulary, mean length utterance), all t’s ≤ 1.50, p’s ≥ .142.
Prior to examination of group differences in ln EEG power values, it was important to verify that the two performance groups had equal amounts of artifact-free EEG (Pivik et al., 1993). The amount of artifact-free data (i.e., the number of DFT windows) for the two performance groups was not significantly different for the baseline, t(77) = 0.15, p = .88, or encoding, t(77) = 0.83, p = .41, phases. Although there were no between-group differences in the duration of the baseline, t(77) = 0.91, p = .37, and encoding, t(77) = 0.44, p = .66, phases, the duration of the retrieval phase was longer for high performers than low performers, t(77) = 3.85, p < .001. This finding is not unexpected considering that high performers recalled more information during the retrieval phase than low performers. Consequently, high performers had more artifact-free data during the recall phase than low performers, t(77) = 1.98, p = .051.
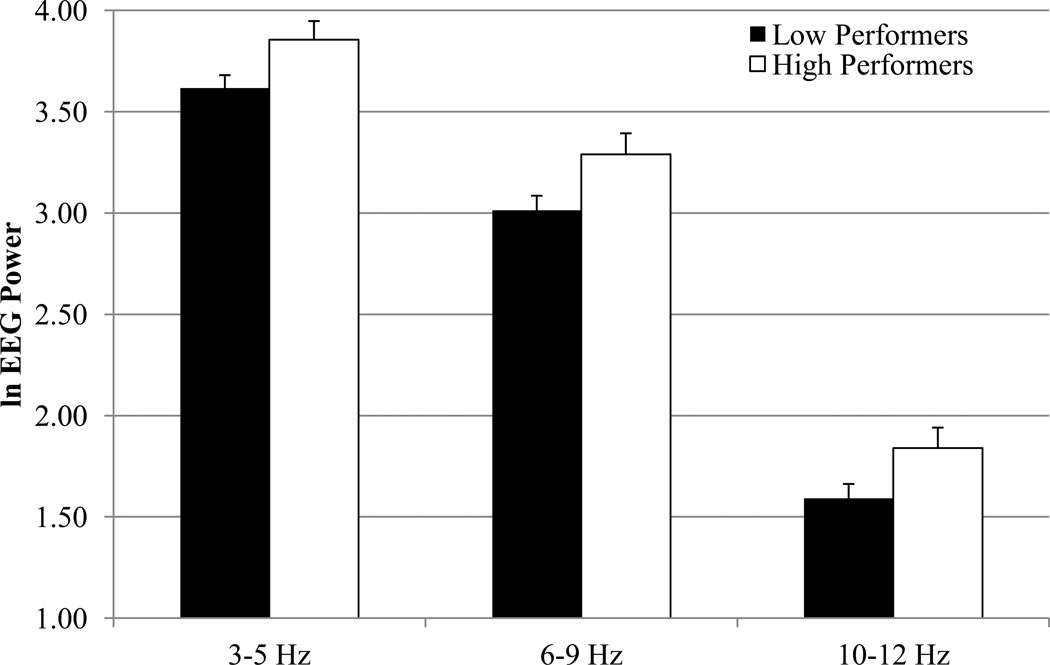
The results of the EEG power MANOVAs are displayed in Table 1. There was a main effect of performance group for the 3–5 Hz, 6–9 Hz, and 10–12 Hz bands. As can be seen in Figure 1, high verbal recall performers had greater power values than low performers. There were no interactions involving performance group for any frequency band.
Table 1.
Summary of Multivariate Analyses F Values for Memory Processing Comparisons as a Function of Performance Group.
| Group | Stage | Region | Hemi | G × S | G × R | G × H | S × R | S × H | R × H | |
|---|---|---|---|---|---|---|---|---|---|---|
| df | 1, 72 | 2, 71 | 7, 66 | 1, 72 | 2, 71 | 7, 66 | 1, 72 | 14, 59 | 2, 71 | 7, 66 |
| 3–5 Hz | 4.47* (.06) | 23.16*** (.40) | 10.48*** (.53) | 1.92* (.31) | ||||||
| 6–9 Hz | 4.70* (.06) | 6.03** (.15) | 12.58*** (.57) | 4.72*** (.53) | ||||||
| 10–12 Hz | 3.92+ (.05) | 14.04*** (.28) | 12.00*** (.56) | 2.05* (.33) |
Note.
p ≤ .001;
p ≤ .01;
p ≤ .05;
p ≤ .055.
Effect sizes (ηp2) are in parentheses. Three- and four-way interactions were not significant for any frequency band.
Figure 1.
EEG power values for low and high verbal recall performance groups in 2-year-olds at 3–5 Hz, 6–9 Hz, and 10–12 Hz frequency bands.
3.2. Processing stage
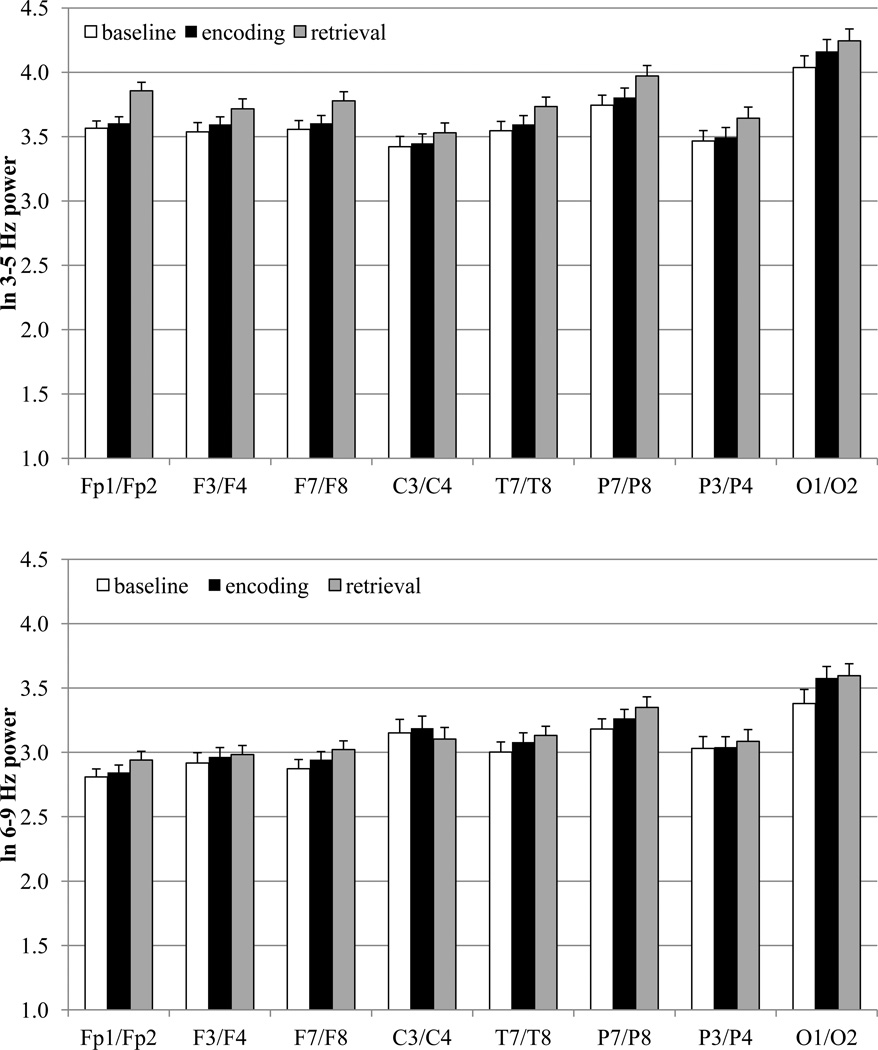
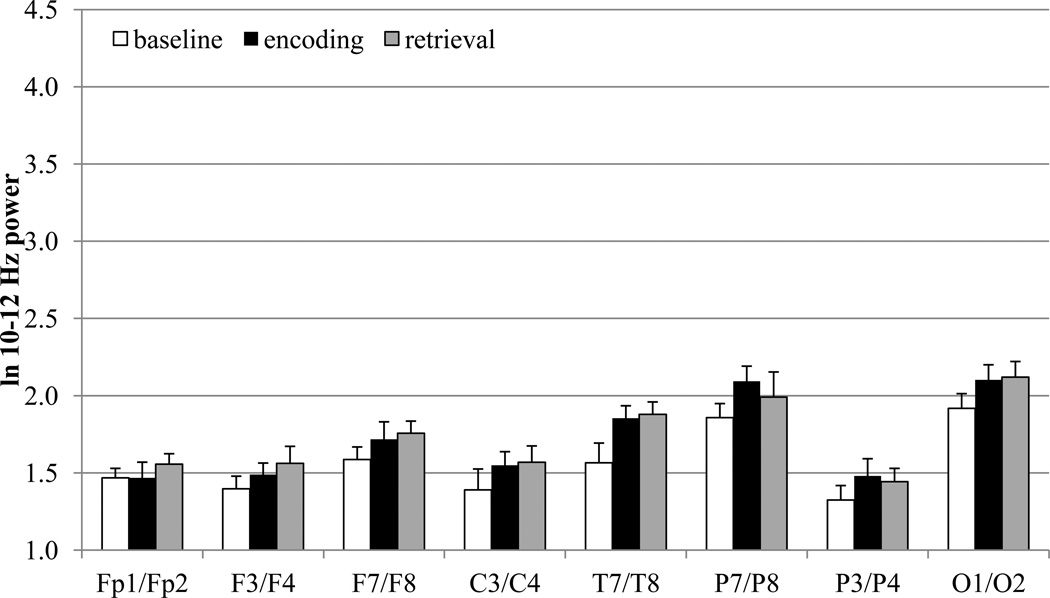
The means for EEG power during baseline, encoding, and retrieval are displayed in Figure 2 and the results of the MANOVAs are displayed in Table 1. As can be seen from Figures 1 and 2, the three frequency bands exhibit very different power values in toddlers, with the 3–5 Hz band yielding the greatest power values and the 10–12 Hz the lowest power values. The y-axes in Figures 1 and 2 are on the same scale for each frequency band to highlight these differences.
Figure 2.
EEG power values for baseline, encoding, and recall in 2-year-olds at 3–5 Hz, 6–9 Hz, and 10–12 Hz frequency bands.
There was a main effect for processing stage as well as a Stage × Region interaction for all three frequency bands. There were no other interactions involving processing stage for any frequency band. For ease in examining the Stage × Region interaction for each frequency band, we collapsed across all nonsignificant factors (i.e., hemisphere and performance group) and completed separate follow-up regional MANOVAs on the EEG power values for each of the eight electrode sites. The results of the follow-up regional MANOVAs are displayed in Table 2. For each frequency band, the adjusted p value was ≤ .01 (.10/8 = .01).
Table 2.
Summary of Regional Multivariate Analyses F Values for Memory Processing Comparisons.
| Fp1/Fp2 | F3/F4 | F7/F8 | C3/C4 | T7/T8 | P7/P8 | P3/P4 | O1/O2 | |
|---|---|---|---|---|---|---|---|---|
| df | 2, 77 | 2, 77 | 2, 77 | 2, 76 | 2, 76 | 2, 77 | 2, 75 | 2, 76 |
| 3–5 Hz | 16.39*** (.30) | 13.17*** (.26) | 14.00*** (.27) | 5.41** (.13) | 11.60*** (.23) | 18.16*** (.32) | 10.17*** (.21) | 9.67*** (.20) |
| 6–9 Hz | 3.34* (.08) | 7.16*** (.16) | 3.86* (.09) | 4.49* (.11) | 8.80*** (.19) | 12.37*** (.25) | ||
| 10–12 Hz | 3.45* (.08) | 8.75*** (.19) | 3.37* (.08) | 7.15*** (.16) | 6.36** (.14) | 5.24** (.12) | 3.42* (.08) |
Note.
p ≤ .001;
p ≤ .01;
p ≤ .05.
Effect sizes (ηp2) are in parentheses.
For the 3–5 Hz band, there was a main effect for processing stage for all electrode sites. Pairwise comparisons for these electrode sites were accomplished after adopting a Bonferroni procedure to control the overall level of significance (eight electrodes with three comparisons for each electrode site; p = .10/24 = .004). EEG power values were higher during the retrieval (i.e., recall) phase than during the baseline (all electrode sites) and encoding phases (all electrode sites except occipital sites; also central sites, p = .008, failed to reach the adjusted level of significance). Baseline and encoding power values were significantly different at only one region. For occipital sites, encoding power values were higher than baseline power values.
For the 6–9 Hz band, there was a main effect for processing stage for lateral frontal, lateral parietal, and occipital electrode sites. Pairwise comparisons for these electrode sites were accomplished after adopting a Bonferroni procedure to control the overall level of significance (three electrodes with three comparisons for each electrode site; p = .10/9 = .01). For all three electrode pairs, EEG power values were higher during the retrieval phase as compared to the baseline phase. Although encoding power values were higher than baseline values for all three electrode sites, only occipital electrode sites reached the adjusted level of significance (lateral frontal: p = .023; lateral parietal: p = .047). For lateral frontal and lateral parietal sites, retrieval power values were higher than encoding power values.
For the 10–12 Hz band, there was a main effect for processing stage for lateral frontal, temporal, lateral parietal, and medial parietal electrode sites. Pairwise comparisons for these electrode sites were accomplished after adopting a Bonferroni procedure to control the overall level of significance (four electrodes with three comparisons for each electrode site; p = .10/12 = .008). Power values were higher during the encoding phase, as compared to the baseline phase, for temporal and lateral parietal electrode sites. For lateral frontal, temporal, and medial parietal sites, retrieval power values were also higher than baseline power values. For to 10–12 Hz band, there were no significant differences between power values during the retrieval and encoding phases.
3.3. Summary of findings
All frequency bands differentiated the retrieval phase from the baseline phase, but the particular regions that exhibited this dissociation varied (3–5 Hz: all electrodes; 6–9 Hz: lateral frontal, lateral parietal, and occipital electrodes; 10–12 Hz: lateral frontal, temporal, and medial parietal electrodes). Furthermore, all bands discriminated the encoding phase from the baseline phase: 3–5 Hz (occipital electrodes), 6–9 Hz (occipital electrodes), 10–12 Hz (temporal and lateral parietal electrodes). Encoding and retrieval processes were distinguished by the 3–5 Hz (all electrode sites except central and occipital) and the 6–9 Hz (lateral frontal and lateral parietal electrodes) bands. The 3–5 Hz, 6–9 Hz, and 10–12 Hz bands discriminated high from low performers.
4. Discussion
The present study provides a unique contribution to the memory processes literature. This is the first multiple frequency band analysis of changes in neural activity (i.e., EEG power) during memory encoding and recall at 2 years of age. Our findings reveal valuable information concerning the functional meaning of three different toddler frequency bands during memory processing.
4.1. Which frequency band(s) discriminate baseline from memory processing?
At 2 years of age, all three frequency bands discriminated memory processing (i.e., encoding and retrieval) from baseline activation. Retrieval-related increases in EEG power were widespread for the 3–5 Hz (theta; all electrodes) frequency band, and more localized for the 6–9 Hz (alpha; lateral frontal, lateral parietal, and occipital electrodes), and 10–12 Hz (beta; lateral frontal, temporal, and medial parietal electrodes). Encoding-related increases in EEG power, on the other hand, were localized for all frequency bands: 3–5 Hz (occipital electrodes), 6–9 Hz (occipital electrodes), 10–12 Hz (temporal and lateral parietal electrodes).
In general, we found increases in 2-year-olds’ EEG power during memory processing for all frequency bands. A more complex pattern of frequency band-dependent increases and decreases is exhibited by adults and older children. Our results parallel evidence of encoding- and retrieval-related increases in theta EEG power in adults and/or older children (Babiloni et al., 2004; Krause et al., 2001, 2007).
Widespread increases in theta power during memory retrieval are also exhibited during late childhood (Krause et al., 2001, 2007). The hippocampus plays an essential role in learning and memory; theta oscillations in the hippocampus of non-human animals induce synaptic plasticity (i.e., long-term potentiation; Pavlides et al., 1988). Scalp theta rhythms are posited (a) to be related to hippocampal theta oscillations in non-human animals and (b) to detect activity within the hippocampocortical feedback loop (Babiloni et al., 2004; Klimesch et al., 1997). Klimesch and colleagues (1994; 1997) have found evidence that the theta rhythm is related to episodic (e.g., declarative) memory processes. Thus, the 3–5 Hz toddler frequency band potentially reflects theta oscillations which are likely integral to episodic memory processes.
Our 6–9 Hz findings during toddlerhood reveal potential changes in the alpha band response during memory processes between infancy and adulthood. During a declarative memory task with 10-month-olds, Morasch and Bell (2009) found retrieval-related increases in 6–9 Hz power evident with successful performance; however, infants failed to display encoding-related increases in 6–9 Hz power. Our findings reveal that by 2 years of age, encoding- and retrieval-related increases in 6–9 Hz power are found regardless of task performance. Changes in the 6–9 Hz band response between infancy and toddlerhood likely coincide with more efficient encoding and retrieval processes (see Rovee-Collier and Cuevas, 2008, for a review). Research with adults and older children has revealed that the alpha band generally exhibits encoding-related increases in EEG power as well as retrieval-related decreases in power, although different patterns have been found for lower and upper alpha rhythms (Babiloni et al., 2004; Klimesch et al., 1999; Krause et al., 2001, 2007; Mölle et al., 2002). Alpha rhythms are posited to reflect activity within thalamocortical feedback loop as well as memory processes (Babiloni et al. 2004; Klimesch et al., 1999).
Less is known, however, about the role of the beta rhythm during memory processing. There is evidence of encoding-related increases (Mölle et al., 2002) and decreases in beta rhythms as well as retrieval-related decreases (Krause et al., 2007). Working memory research with 8-month-old infants has also noted memory-related increases in power for 3–5 Hz, 6–9 Hz, and 10–12 Hz bands (Bell, 2002). It is plausible that the direction of retrieval-related changes in alpha and beta power might switch between toddlerhood and late childhood, and that these changes potentially coincide with milestones in memory development, such as childhood amnesia. Clearly, additional developmental psychophysiological research on memory processes that captures important developmental milestones is essential to creating a comprehensive understanding of early memory processes.
4.2. Which frequency band(s) differentiate between memory encoding and retrieval processes?
In contrast to our baseline comparisons, only two frequency bands differentiated 2-year-olds’ encoding and retrieval processes: 3–5 Hz (all electrode sites except central and occipital), 6–9 Hz (lateral frontal and lateral parietal electrodes). Specifically, EEG power was higher during memory retrieval as compared to memory encoding. We interpret these findings to reflect increased cognitive demands during the “intentional” retrieval phase as compared to our “incidental” encoding phase (i.e., toddlers were not informed of the subsequent recall phase at the time of encoding). Similar patterns have been found for theta and alpha rhythms during late childhood and/or adulthood (Babiloni et al., 2004; Krause et al., 2001, 2007).
4.3. Which frequency band(s) distinguish toddlers with high and low verbal recall performance?
In the present study, 2-year-olds with high and low verbal recall performance were distinguished by the 3–5 Hz, 6–9 Hz, and 10–12 Hz bands. For all three frequency bands, high performers had greater EEG power values than low performers. Infant and toddler EEG researchers have considered increasing EEG power values as a function of age to be indicative of brain maturation (Marshall et al., 2002; see Bell, 1998; Bell and Fox, 1994, for reviews). Accordingly, the brain electrical activity of high performers is potentially more mature than that of low performers. Importantly, analyses of age, language abilities, and parental-ratings of temperament failed to reveal any group differences (but see Footnote 2 in regards to ECBQ). Thus, our memory performance findings are likely related to verbal recall ability, as opposed to age, language abilities, or temperament. It is plausible, however, that some other variable (e.g., motivation) could be related to our performance findings. Our findings provide initial insight into the psychophysiological difference of toddlers who exhibit high and low verbal recall performance.
Previous deferred imitation research with 10-month-olds has found retrieval-related increases in 6–9 Hz power only for high performers (Morasch and Bell, 2009). Likewise, Klimesch and colleagues (1996) have noted different patterns of change in lower and upper alpha power as a function of task performance. Together, these findings suggest a role for alpha rhythms in differentiating high and low memory performers. Surprisingly, we found no significant group by memory processing stage interactions at 2 years of age.
Our behavioral data revealed that our verbal recall task was challenging for many 2-year-olds although the task demands were relatively simple (i.e., three related encoding items, encoding items were named and presented visually, recall was assessed immediately). Given that prior studies have also found that 2-year-old children exhibit poor verbal recall memory (Perlmutter and Myers, 1979; Simcock and Hayne, 2003), our findings are not surprising. We chose to examine verbal recall at 2 years of age because it is the youngest age that verbal recall can be assessed and the variability in verbal recall performance is optimal for examining individual differences in brain-behavior relations. It is plausible, however, that we failed to find different memory-related patterns of change in EEG power as a function of performance because our verbal recall task was challenging for both high and low performers. Toddlers typically perform better on nonverbal (e.g., behavioral reenactment, recognition) than verbal memory measures (Simcock and Hayne, 2002, 2003), and the majority of research assessing infant and toddler recall memory has used nonverbal tasks (e.g., deferred imitation, elicited imitation; Hayne and Simcock, 2009; Rose et al., 2005). Future toddler EEG research should include both verbal and nonverbal measures of declarative memory to examine whether different patterns of findings are revealed as a function of performance measure.
Although we are not familiar with any between-subjects memory performance comparisons of other EEG rhythms, within-subjects memory performance comparisons have revealed changes in theta, alpha, and beta rhythms (Klimesch et al., 1997; Sederberg et al., 2003). Thus, evidence of verbal recall performance dissociations in EEG power for 3–5 Hz, 6–9 Hz, and 10–12 Hz during toddlerhood is consistent with previous findings.
4.4. Potential Limitations
In the present study, we adapted the verbal recall memory procedures used in psychophysiological examinations with older children and adults for use with 2-year-olds. For instance, instead of having participants memorize a set of written words or auditorily presented pre-recorded words, an experimenter presented the encoding of words as part of a grocery store game. This modification was essential to ensuring that toddlers remained in the experimental situation and were engaged in the encoding of each item. There is evidence, however, that infants and children exhibit changes in EEG while gazing at the face of a speaking adult using infant-directed speech (infants) or telling a story (children; Orekhova et al., 2006). From a memory perspective, infants and children in Orekhova et al.’s study were not only engaged in a social interaction with an adult; they were also encoding visual and auditory information.
Although the memory task in the present study is very different than the conditions used in Orekhova et al.’s (2006) examination of infant- and child-directed speech, it is plausible that social interaction and speech during our memory task contributed to our findings. A comparison of the two studies, however, reveals different patterns of findings for the middle and upper frequency bands. Orekhova et al. found frequency-dependent power increases and decreases for infants and children. Specifically, infants (who are closest in age to our sample) exhibited increases in 3.2–5.6 Hz power and decreases in 6–7.6 Hz and 9.2–10 Hz power at multiple electrode sites. In the present study, 2-year-olds exhibited only increases in EEG power during memory encoding and retrieval for the 3–5 Hz, 6–9 Hz, and 10–12 Hz frequency bands, which is consistent with previous psychophysiological research with adults and older children (Babiloni et al., 2004; Krause et al., 2001, 2007). Thus, we interpret the discrepancies between Orekhova et al.’s findings and our findings to reflect the primary recruitment of memory processes, as opposed to social processes, during our memory task. However, the inability to separate the possible contribution of social interaction and speech to our memory findings is a potential limitation of the current study. Clearly, additional toddler research with other verbal and nonverbal memory tasks as well as research with 3- to 8-year-olds with more “adult-like” memory tasks is essential to our understanding of the psychophysiological correlates of memory processes during early and middle childhood.
Finally, although we refer to the toddler 3–5 Hz and 6–9 Hz bands as theta and alpha, respectively, it is likely that the frequency of the theta rhythm (adult 4–8 Hz) increases between infancy and toddlerhood. Thus, caution must be used when comparing our 6–9 Hz findings to adult alpha findings, as our 6–9 Hz findings may include some upper theta activity. An examination of the functional properties of the alpha band, as completed by Stroganova and colleagues with infants (1999), is necessary to determine the precise boundaries of the alpha band during toddlerhood. However, 6–9 Hz is the prominent rhythm during quiet wakefulness during toddlerhood (Marshall et al., 2002), which is analogous to the adult 8–13 Hz alpha rhythm.
4.5. Conclusions
At 2 years of age, the 3–5 Hz (theta) and 6–9 Hz (alpha) bands were most informative about memory processes. Although all three frequency bands (including 10–12 Hz) distinguished memory processes from baseline, the 3–5 Hz and 6–9 Hz bands were the only bands to both differentiate encoding and retrieval processes as well as discriminate high from low verbal recall performers. Theta and alpha rhythms are critical to memory processes during late childhood and adulthood, and our findings provide initial evidence that these rhythms are also intricately linked to memory processing during toddlerhood.
Research Highlights.
Cuevas, K, Raj, V., & Bell, M. A.: A Frequency Band Analysis of Two-Year-Olds’ Memory Processes
Two-year-olds participated in a three-item immediate verbal recall task.
Toddlers exhibited encoding-related increases in 3–5, 6–9, and 10–12 Hz EEG power.
Toddlers exhibited retrieval-related increases in 3–5, 6–9, and 10–12 Hz EEG power.
The 3–5 and 6–9 Hz bands differentiated toddlers’ encoding and retrieval processes.
The 3–5, 6–9, and 10–12 Hz toddler bands discriminated high/low memory performers.
Acknowledgments
This research was supported by grant HD049878 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our research as well as the assistance of Annie Cardell, Anjolii Diaz, Katherine Morasch, Morgan Hubble, and numerous undergraduate research assistants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There were significant differences in parental ratings on several subscales of the Early Childhood Behavior Questionnaire (Putnam et al., 2006) for children with and without usable EEG data. Children without EEG data had higher ratings on the activity level, frustration, high intensity pleasure, and sociability subscales than children with EEG data, all t’s ≥ 1.98, p’s ≤ .05. Likewise, children with EEG data had higher ratings of inhibitory control than children without EEG data, t(111) = 2.27, p = .025. Toddlerhood is a particularly challenging point in development to obtain usable EEG data. It is not surprising that the aforementioned temperamental characteristics were associated with children who did not accept EEG electrodes, removed EEG electrodes during the experimental session, or had too many motor artifacts for EEG analysis. However, we did not perform a Bonferroni-correction for the number of analyses (e.g., p = .10/18 = .0056), and it is plausible that these between-group differences occurred by chance. Toddlers with and without EEG data did not differ on any of the 13 other subscales of the ECBQ, all t’s ≤ 1.75, p’s ≥ .083. Importantly, there were no differences between the two groups in our primary measures of interest (i.e., language, recall).
Toddlers with high and low verbal recall performance differed in parental ratings on the activity level, t(70) = 2.09, p = .04, and fear, t(70) = 2.21, p = .03, subscales of the ECBQ. The high performance group had higher ratings on the activity level and fear subscales than the low performance group. These findings were unexpected and in the opposite direction than would be predicted based on verbal recall performance. However, we did not perform a Bonferroni-correction for the number of analyses (e.g., p = .10/18 = .0056), and it is likely that these two between-group differences occurred by chance. Toddlers in the high and low recall groups did not differ on any of the other 16 ECBQ subscale, all t’s ≤ 1.69, p’s ≥ .096.
References
- Babiloni C, Babiloni F, Carducci F, Cappa S, Cincotti F, Del Percio C, Rossini PM. Human cortical EEG rhythms during long-term episodic memory task. A high-resolution EEG study of the HERA model. NeuroImage. 2004;21:1576–1584. doi: 10.1016/j.neuroimage.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Bell MA. The ontogeny of the EEG during infancy and childhood: Implications for cognitive development. In: Garreau B, editor. Neuroimaging in Child Neuropsychiatric Disorders. Berlin: Springer-Verlag; 1998. pp. 97–111. [Google Scholar]
- Bell MA. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology. 2002;39:450–458. doi: 10.1017.S0048577201393174. [DOI] [PubMed] [Google Scholar]
- Bell MA, Fox NA. Brain development over the first year of life: Relations between electroencephalographic frequency and coherence and cognitive and affective behaviors. In: Dawson G, Fischer KW, editors. Human Behavior and the Developing Brain. New York: Guilford; 1994. pp. 314–345. [Google Scholar]
- Casey BJ, de Haan M. Introduction: new methods in developmental science. Dev. Sci. 2002;5:265–267. [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2nd ed. Cambridge, UK: Cambridge University Press; 2000. pp. 27–52. [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Thal D, Bates E, Hartung JP, Pethick S, Reilly JS. MacArthur Communicative Development Inventories. Baltimore, MD: Paul H. Brooks Publishing Co.; 1992. [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, Bunge SA. Developmental differences in medial temporal lobe function during memory encoding. J. Neurosci. 2010;30:9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF. The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology. 2001;38:847–857. [PubMed] [Google Scholar]
- Hayne H, Simcock G. Memory development in toddlers. In: Courage ML, Cowan N, editors. The Development of Memory in Infancy and Childhood. Hove, UK: Psychology Press; 2009. pp. 43–68. [Google Scholar]
- Jasper HH. The 10/20 international electrode system. Electroencephalogr. Clin. Neurophysiol. 1958;10:371–375. [Google Scholar]
- Kail R. The Development of Memory in Children. New York: W.H. Freeman; 1984. [Google Scholar]
- Keselman HJ. Testing treatment effects in repeated measures designs: An update for psychophysiological researchers. Psychophysiology. 1998;35:470–478. [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr. Clin. Neurophysiol. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Doppelmayr M, Ripper B, Schwaiger J, Pfurtscheller G. Event-related desynchronization (ERD) and the Dm effect: Does alpha desynchronization during encoding predict later recall performance? Int. J. Psychophysiol. 1996;24:47–60. doi: 10.1016/s0167-8760(96)00054-2. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997;34:169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler Th. ‘Paradoxical alpha synchronization in a memory task. Cogn. Brain Res. 1999;7:493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Krause CM, Salminen P-A, Sillanmäki L, Holopainen IE. Event-related desynchronization and synchronization during a memory task in children. Clin. Neurophysiol. 2001;112:2233–2240. doi: 10.1016/s1388-2457(01)00684-8. [DOI] [PubMed] [Google Scholar]
- Krause CM, Pesonen M, Hämäläinen H. Brain oscillatory responses during the different stages of an auditory memory search task in children. NeuroReport. 2007;18:213–216. doi: 10.1097/WNR.0b013e3280148ea0. [DOI] [PubMed] [Google Scholar]
- Lehmann D. Principles of spatial analysis. In: Gevins AS, Remond A, editors. Handbook of Electroencephalography and Clinical Neuropsychology, rev. ser. Vol. 1: Methods of Analysis of Brain Electrical and Magnetic Signals. Amsterdam: Elsevier; 1987. pp. 309–354. [Google Scholar]
- Lindsley DB. A longitudinal study of the occipital alpha rhythm in normal children: Frequency and amplitude standards. J. Gen. Psychol. 1939;55:197–213. [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. p. 388. [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, Sheridan MA, Marshall P, Nelson CA. Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;68:329–336. doi: 10.1016/j.biopsych.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Fehm HL, Born J. EEG theta synchronization conjoined with alpha desynchronization indicate intentional encoding. Eur. J. Neurosci. 2002;15:923–928. doi: 10.1046/j.1460-9568.2002.01921.x. [DOI] [PubMed] [Google Scholar]
- Morasch KC, Bell MA. Patterns of brain-electrical activity during declarative memory performance in 10-month-old infants. Brain Cogn. 2009;71:215–222. doi: 10.1016/j.bandc.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Myslobodsky MS, Coppola R, Bar-Ziv J, Karson C, Daniel D, van Praag H, Weinberger DR. EEG asymmetries may be affected by cranial and brain parenchymal asymmetries. Brain Topogr. 1989;1:221–228. doi: 10.1007/BF01129599. [DOI] [PubMed] [Google Scholar]
- Ofen N, Kao Y-C, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JDE. Development of the declarative memory system in the human brain. Nat. Neurosci. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN, Elam M. EEG theta rhythm in infants and preschool children. Clin. Neurophysiol. 2006;117:1047–1062. doi: 10.1016/j.clinph.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Greenstein YJ, Grudman M, Winson J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase o theta-rhythm. Brain Res. 1988;439:383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- Perlmutter M, Myers NA. Development of recall in 2- to 4-year-old children. Dev. Psychol. 1979;15:73–83. [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Taylor MJ. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox NA, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3rd ed. Cambridge, UK: Cambridge University Press; 2007. pp. 56–84. [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The Early Childhood Behavior Questionnaire. Infant Behav. Dev. 2006;29:386–401. doi: 10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Recall memory in the first three years of life: a longitudinal study of preterm and term children. Dev. Med. Child Neurol. 2005;47:653–659. doi: 10.1017/S0012162205001349. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier C, Cuevas K. Infant memory. In: Roediger HL III, editor; Byrne J, editor. Cognitive psychology of memory. Vol. 2: Learning and Memory: A Comprehensive Reference, 4 vols. Oxford: Elsevier; 2008. pp. 687–714. [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J. Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Pressley M. Memory Development Between Two and Twenty. Mahwah, NJ: Erlbaum; 1997. [Google Scholar]
- Simcock G, Hayne H. Breaking the barrier? Children fail to translate their preverbal memories into language. Psychol. Sci. 2002;13:225–231. doi: 10.1111/1467-9280.00442. [DOI] [PubMed] [Google Scholar]
- Simcock G, Hayne H. Age-related changes in verbal and nonverbal memory during early childhood. Dev. Psychol. 2003;39:805–814. doi: 10.1037/0012-1649.39.5.805. [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV. EEG and infant states. In: de Haan M, editor. Infant EEG and Event-Related Potentials. New York: Psychology Press; 2007. pp. 251–287. [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clin. Neurophysiol. 1999;110:997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Yoder PJ, Blackford JU, Waller NG, Kim G. Enhancing power while controlling for family-wise error: An illustration of the issues using electrocortical studies. J. Clin. Exp. Neuropsychol. 2004;26:320–331. doi: 10.1080/13803390490510040. [DOI] [PubMed] [Google Scholar]