Abstract
Progesterone is being utilized as a therapeutic means to ameliorate neuron loss and cognitive dysfunction following traumatic brain injury Although there have been numerous attempts to determine the means by which progesterone exerts neuroprotective effects, studies describing the underlying molecular mechanisms are lacking What has become clear, however, is the notion that progesterone can thwart several physiological processes that are detrimental to neuron function and survival, including inflammation, edema, demyelination and excitotoxicity One clue regarding the means by which progesterone has restorative value comes from the notion that these aforementioned biological processes all share the common theme of eliciting pronounced increases in intracellular calcium. Thus, we propose the hypothesis that progesterone regulation of calcium signaling underlies its ability to mitigate these cellular insults, ultimately leading to neuroprotection. Further, we describe recent findings that indicate neuroprotection is achieved via progesterone block of voltage-gated calcium channels, although additional outcomes may arise from blockade of various other ion channels and neurotransmitter receptors.
Keywords: Progesterone, Brain Injury, Neuroprotection, Ischemia, Excitotoxicity, Calcium
1. Introduction
The neuroprotective effects of progesterone have been thoroughly examined and well documented in both in vitro and in vivo paradigms. Despite the extensive evidence for the beneficial effects of progesterone following insults such as traumatic brain injury, stroke, and excitotoxicity, the exact mechanism(s) by which progesterone exerts these neuroprotective effects remain elusive. The search for an underlying source of progesterone action in these models is complicated by the sheer magnitude of cellular events that occur following neuronal insult, thus providing few limits on putative progesterone targets. Moreover, progesterone is known to affect many of the cellular events that follow injury or toxicity including inflammation, edema, myelin degradation, excitotoxicity, and intracellular signaling. Thus, an emerging hypothesis is that progesterone exerts its neuroprotective effects by regulating many of these cellular events in parallel. Indeed, one clue as to the identity of a putative molecular target of progesterone is that ion channel signaling is linked to many of the events that occur following neuronal injury. Work from our lab and others support the hypothesis that progesterone regulation of neuronal calcium signaling via its regulation of voltage-gated calcium channels may underlie the neuroprotective effects of therapeutic concentrations of progesterone. This review will address this hypothesis in the context of two classes of studies: 1) those in which progesterone-mediated neuroprotection is established in models such as stroke injury, traumatic brain injury, and excitotoxicity, and 2) those that address the underlying modulation of calcium signaling, which may account for the observed neuroprotective effects.
2. Neuroprotective Effects of Progesterone
While there are many in vivo reports of the neuroprotective effects of progesterone, the most popular paradigm for studying this phenomenon is a stroke model in which a focal ischemia is induced by middle cerebral artery occlusion (MCAO) followed by reperfusion and subsequent assessment of neuronal death. One of the earliest reports of progesterone neuroprotection in this model was a qualitative 1990 study by Betz et al. [1] demonstrating that pretreatment with progesterone decreased MCAO-induced edema. A later study quantitatively documented that progesterone decreased the ischemic area following MCAO [2]. Offering the first insights as to the specific mechanism of action were studies that, somewhat surprisingly, discovered that progesterone need not be administered prior to artery occlusion, but that it was efficacious even when administered up to 24 hours post-injury. This finding has been corroborated by other studies in models of both focal and global (four-vessel occlusion) ischemia, with progesterone administration occurring anywhere between one-half hour to two hours post-occlusion [3; 4; 5; 6; 7; 8; 9; 10; 11; 12]. Importantly, in addition to preventing cell death, progesterone also preserved neuronal function, as assayed by rodent behavioral tests [5; 7; 8; 9; 10; 12], thus highlighting the potential therapeutic utility of progesterone as a treatment for human brain injury.
The neuroprotective effects of progesterone have also been demonstrated in rodent models of traumatic brain injury (TBI). These models often involve neuronal survival assays following controlled cortical contusion injury (CCI). Roof et al. first suggested a link between progesterone and neuroprotection in these models of brain injury with their observation that pseudopregnant rats, which have high levels of circulating progesterone, were nearly spared from injury-induced cognitive deficits [13]. The same group later found that progesterone treatment could be delayed up to 24 hours post-injury without losing effectiveness [14]. Several other studies have corroborated the finding that post-injury treatment with progesterone is neuroprotective in terms of reducing neuron loss [15; 16; 17; 18; 19; 20] and in preserving cognitive outcome [16; 17; 18; 19; 20; 21; 22]. Together these data suggest that the window of opportunity for treatment with progesterone is relatively large (i.e. on the order of hours), and thus it need not be administered immediately following a brain insult.
Paralleling the effects of progesterone neuroprotection in whole animal models of injury, are results from in vitro neuronal models of glutamate-induced excitotoxicity. Glutamate overload is one of the key neurotoxic effects thought to occur following in vivo brain injury, and thus in vitro excitotoxicity is a tractable preparation for investigating the molecular mechanisms of neuronal death following injury. Indeed, progesterone is protective against excitotoxicity in dissociated cultured neurons [23; 24; 25; 26; 27; 28] as well as in cerebral cortical slice culture [29; 30]. Furthermore, progesterone exerts protective effects in alternative models of toxicity in which trophic starvation, MPTP, oxidative stress, and β-amyloid treatment induce neuronal death [25; 31; 32], suggesting that progesterone may directly influence an upstream process common to these various forms of neuronal insult. These data have also been used to suggest that progesterone may be useful as a therapy in neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease [25].
In initial findings, the neuroprotective effects of progesterone in human injury cases parallel the results from animal models. For instance, progesterone significantly reduced cognitive impairments in human patients with TBI [33; 34; 35]. To date, the administration of progesterone has been the safest and most effective therapeutic strategy for treatment following traumatic brain injury. Thus, the use of progesterone as a therapy following TBI is now in phase III clinical trials. Although there are reports of females exhibiting better recovery from brain injury compared to males [36], this enhanced efficacy of progesterone therapy is thought to be due to the higher basal level of circulating progesterone in women, as opposed to differences in progesterone efficacy between the sexes [37; 38]. Consistent with this lack of sex-specificity, the studies cited above have utilized concentrations of progesterone that far exceed normal circulating levels of progesterone (except those associated with late-term pregnancy), and that are in excess of those needed to activate that classical progesterone receptor. While the brain does engage in de novo progesterone synthesis [39; 40], it is currently unknown whether it achieves concentrations that contribute to neuroprotection. Furthemore, The notion that progesterone could synergize with additional neuroprotective steroid hormones that act via receptor-dependent mechanisms, such as estrogen (either endogenous or therapeutically administered), is intriguing. This possibility may depend on the specific paradigm in question as progesterone and estrogen have been shown to have both synergistic [41] and antagonistic interactions in neuroprotective paradigms [42; 43].
Based on the widespread effectiveness of progesterone in numerous traumatic brain injury paradigms, the list of putative cellular and molecular targets of progesterone action is overwhelmingly large. As mentioned above, one method of simplifying the experimental and theoretical analysis of these targets is to separately group and catalog those neuroprotective effects of progesterone that may share a common upstream mechanism. For example, at least a subset of the beneficial effects of progesterone discussed above could be accounted for by direct progesterone modulation of ion channel signaling. In this spirit, the remainder of this review will briefly summarize the neuroprotective effects of progesterone that have been shown to be ion channel-dependent, and to discuss recent data from our laboratory that directly demonstrates progesterone inhibition of neuronal ion channel signaling.
3. Mechanisms for progesterone-dependent neuroprotection
3.1 Inflammation and edema
Inflammation is often triggered by stroke or TBI, and subsequently induces cerebral edema, which can lead to substantial cell death. Calcium signaling is an upstream mediator of inflammation and edema both in peripheral tissues and in the brain. As such, calcium channel blockers (specifically those that preferentially block L-type calcium channels) have been widely used to treat cardiopulmonary disease due to their ability to inhibit inflammation. Diltiazem, a non-dihydropyridine L-type calcium channel blocker, inhibits cardiopulmonary bypass induced inflammation in humans by reducing cytokine production [44]. Additionally, lercanidipine, a dihydropyridine sensitive L-type calcium-channel blocker, inhibits inflammation in patients with hypertension [45]. Finally, studies from rats have shown that dihydropyridines reduce formalin-induced inflammation in the rat paw [46].
Studies in the brain parallel these results from peripheral tissue in which numerous calcium channel blockers have been shown to reduce and/or prevent edema. For instance, rats treated with the dihydropyridine benidipine showed reduced edema [47], while the dihydropyridine calcium channel blockers, azelnidipin or amlodipine, reduced edema and neuronal loss following focal ischemia in rats [48]. The novel blockers of both calcium and sodium channels, NS-1 and T-477, were found to be neuroprotective and edema-reducing in rats that underwent focal ischemia, suggesting that inhibition of sodium channels may also play an important role [49; 50]. Finally, (S)-emopamil, a phenylalkylamine-based calcium channel blocker, reduces both edema and memory dysfunction following focal brain injury in rats, again suggesting that blocking calcium signaling during/following injury may have cognitive benefits [51].
Given the overwhelming evidence for the fact that progesterone decreases edema and inflammation in the brain [15; 20; 52; 53; 54; 55], and the fact that inflammation and subsequent edema has been shown to depend on calcium channel signaling in both peripheral tissues as well as the brain, it is reasonable to suggest that progesterone inhibition of calcium signaling may be a mechanism by which progesterone reduces inflammation and subsequent edema following injury. Consistent with this hypothesis, progesterone-mediated inhibition of inflammation and edema occur via a progesterone-dependent reduction of cytokine and inflammatory metabolite production [11; 56; 57; 58; 59], which themselves depend on calcium signaling [44]. This does not, however, rule out the hypothesis that progesterone may inhibit cytokine production in a calcium-independent manner as well.
3.2 Preservation of myelination
Severe nervous system insults often result in the degradation of myelin, the protective sheath that protects the axoplasm and facilitates salutatory conduction along axons. Progesterone has been shown to preserve myelination following spinal cord injury [60; 61; 62; 63]. Consistent with the hypothesis that progesterone influences many cellular processes via inhibition of ion channel signaling, one report [64] found that blockers of voltage-gated calcium channels prevented the loss of myelination in the spinal cords of mice with inflammatory demyelinating disease. Similarly, Winkler et al. [65] showed that treatment with the dihydropyridine nimodipine significantly reduced the degradation of myelin following spinal cord injury in rats. Although these findings collectively suggest that progesterone may prevent demyelination through inhibition of calcium channels, this hypothesis requires further testing.
3.3 Excitotoxicity
Glutamate-induced excitotoxicity, whereby injured neurons release and are inundated by massive amounts of the neurotransmitter glutamate, is widely accepted as being a key event in the initiation of neuron death following many types of neuronal insult [23; 66; 67]. Excitotoxic death of neurons is a complex process that has been shown to occur through a variety of mechanisms that are not yet fully understood. However, it is thought that release of this neurotransmitter into the extracellular space occurs sometime shortly after insult, leading to the activation of both ionotropic and metabotropic glutamate receptor-mediated signaling pathways. Subsequent intracellular calcium elevations and calcium-dependent signaling pathways induced by this sharp increase in extracellular glutamate is a major cause of injury-induced neuron death [68; 69; 70; 71]. While progesterone is protective against excitotoxicity in dissociated cultured neurons [23; 24; 25; 26; 27; 28] as well as in cerebral cortical slice culture [29; 30], there is a lack of consensus surrounding the molecular mechanisms by which this occurs. Since excitotoxicity often leads to calcium-dependent death [72] and progesterone is neuroprotective, it has been well reasoned that progesterone prevents excitotoxic neuronal death by preventing the resulting calcium overload.
4. Progesterone inhibition of calcium signaling
The influence of progesterone on calcium signaling (progesterone-calcium signaling hypothesis) may account for some of the therapeutic benefits of progesterone, including reducing inflammation and edema, preventing myelin degradation, and reducing excitotoxic neuronal death. A recent report from our laboratory directly tested the hypothesis that progesterone influence of calcium signaling underlies progesterone inhibition of excitotoxic neuron death [28]. In neurons, excitotoxic death occurs when glutamate overload sufficiently depolarizes the neuronal membrane potential to activate voltage-gated calcium channels (See Figure 1). Subsequent calcium influx activates a cascade of intracellular signaling pathways and transcription factors that initiate neuronal apoptosis. Thus, progesterone inhibition of calcium signaling and this subsequent neuronal death could occur via progesterone interaction with primary (glutamate receptor) or secondary (calcium channel) sources of calcium influx. To experimentally isolate these mechanisms, we utilized cultured striatal neurons, which lack glutamatergic input but are sensitive to external glutamate application. Additionally, progesterone has been shown to be neuroprotective in the intact striatum [73].
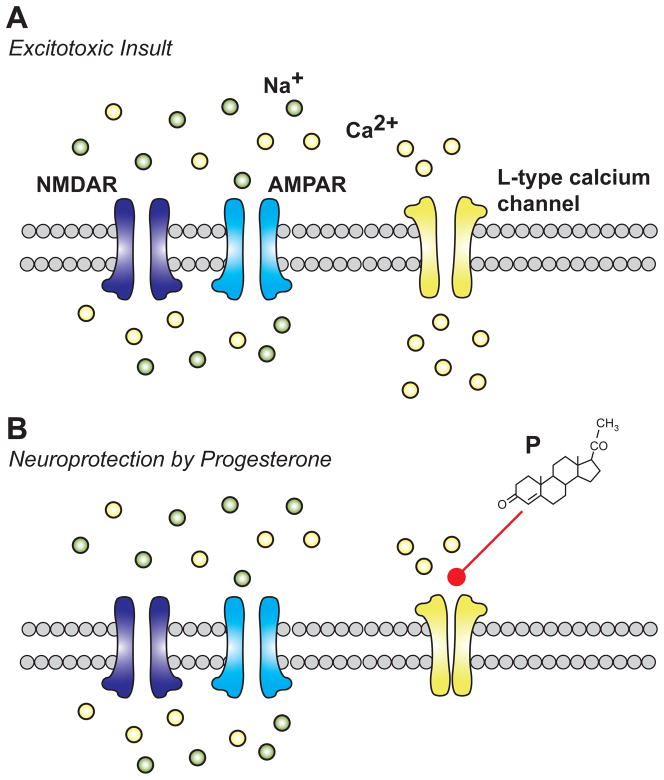
Figure 1. Putative mechanism for progesterone mediated neuroprotection.
A) Excitotoxic insult is characterized by the influx of both sodium and calcium-ions through glutamate receptors leading to depolarization-induced opening of L-type calcium channels. Calcium flux through L-type calcium channels contributes to calcium overload and subsequent activation of various calcium-mediated death pathways. B) Through an unknown mechanism, extracellular application of progesterone (P) inhibits the influx of calcium ions through L-type calcium channels, protecting the neuron from excitotoxic death.
In support of the progesterone-calcium signaling hypothesis, we found that in cultured striatal neurons progesterone completely blocked voltage-gated calcium channel-mediated neuronal death (See Figure 1). Calcium imaging and electrophysiological approaches revealed that this progesterone-mediated neuroprotection occurs via direct inhibition of voltage-gated calcium channel function. These data are consistent with studies that have implicated calcium channels in glutamate-induced cell death [74; 75; 76]. Since the concentrations of progesterone needed to achieve inhibition of L-type channels (IC50 = 27 μM) are far in excess of those needed to activate the progestin receptor [77], expression of the receptor is likely not necessary for these observed effects of progesterone. Receptor-independent actions of progesterone are consistent with the idea that progesterone neuroprotection solely depends on progesterone concentration, rather than receptor availability. Importantly, progesterone was without effect on both glutamate-induced calcium influx, as well as glutamate receptor-mediated ionic currents, demonstrating that progesterone inhibits excitotoxic death exclusively via direct inhibition of voltage-gated calcium channels. The fact that progesterone also inhibited voltage-gated (L-type) calcium channel-mediated activation of the transcription factors CREB and NFAT sheds light onto the downstream mechanisms that may contribute to excitotoxic neuronal death.
In all of the above experiments, neither the progesterone precursor pregnenolone nor the progesterone metabolite allopregnanolone exerted any inhibitory effects. Interestingly, these neurosteroids have been shown to be neuroprotective in other paradigms [78; 79; 80; 81; 82; 83], suggesting the need for further study.
While characterizing progesterone block of L-type calcium channels, it became clear that micromolar concentrations of the hormone could also influence the conductance of other voltage-gated calcium channels. For example, dose-response experiments indicated that at 100 μM, progesterone completely abolished the whole-cell calcium current. While this concentration was higher than that required for neuroprotection (i.e. 50 μM), there was no clear delineation among the progesterone concentrations used that appeared to isolate one calcium channel conductance from another. Thus, it remains unclear whether progesterone inhibition of non L-type calcium channels is neuroprotective, or represents a “supplementary” inhibition.
5. Progesterone-dependent modulation of additional ion-channels
Recent work from our laboratory suggests that this supplementary inhibition extends beyond voltage-gated calcium channels to voltage-gated potassium channels, voltage-gated sodium channels, and GABAA receptors [84]. While these additional effects may underlie the anesthetic properties of progesterone [85; 86], it is currently unclear how inhibition of these ion channels contributes to neuroprotection. There is, however, precedent in the literature for neuroprotective drugs indiscriminately influencing multiple voltage-gated ion channels. As mentioned, NS-1 or T-477, which blocks both calcium and sodium channels, reduced edema and neuron loss following ischemia [49; 50]. These results are in accordance with additional studies demonstrating that specific sodium channel blockers (tetrodotoxin, riluzole, mexiletine, or phenytoin) reduce ischemia-induced edema and cell death [87; 88], and that broad-spectrum inhibitor of cation channels reduces lesion volume in rats with MCAO-induced ischemia [89].
The ability of micromolar range concentrations of progesterone to inhibit multiple classes of ion channels parallel the effects of similarly high concentrations of nifedipine, a dihydropyridine sensitive L-type calcium channel blocker that also non-selectively inhibits voltage-gated sodium channels, potassium channels, and GABAA [84; 90; 91; 92]. The results from several other studies corroborate the finding that in addition to L-type calcium channels, dihydropyridines inhibit voltage-gated sodium channels, voltage-gated potassium channels, and GABAA receptors [90; 91; 92; 93]. Since progesterone and dihydrophyridines share neuroprotective [94; 95] and ion channel modulatory properties, they may share a similar mechanism of receptor binding and inhibition. Given these striking similarities, scientists and clinicians utilizing progesterone for therapeutic purposes would be wise to keep in mind that some neuroprotective concentrations of dihydropyridines result in unpleasant and undesired side effects [96; 97]. Thus, a comprehensive molecular understanding of the specific concentrations of and mechanisms by which progesterone inhibits specific ion channels can inform an efficacious and productive analysis of progesterone treatment for TBI.
Conclusion
While progesterone has been shown to be a highly effective means of treating TBI in both the laboratory and clinic, the molecular mechanisms by which this occurs remain elusive. In fact, progesterone has been shown to influence a variety of neuronal parameters that occur as a result of traumatic injury or toxicity including inflammation, edema, myelin degradation, excitotoxicity, and intracellular signaling. In this review, we have developed the hypothesis that progesterone influence of calcium signaling via direct inhibition of voltage-gated calcium channels underlies the neuroprotective effects of progesterone. Furthermore, we presented recent data from our lab demonstrating these inhibitory actions are not limited to calcium channels, but also occur at several voltage- and ligand-gated ion channels, similar to the actions of equimolar concentrations of dihydropyridines. Thus, future investigators are challenged to elucidate the specific conditions and mechanisms by which progesterone exerts these inhibitory effects so that we may fully exploit the therapeutic potential of progesterone.
Table 1. Neuroprotective effects of progesterone: A summary of key findings.
Key studies that demonstrate the neuroprotective effects of progesterone are organized here by the condition or disease studied. Further organization by the method of neuron insult and the animal model used; middle cerebral artery occlusion (MCAO), four-vessel occlusion (FVO), traumatic brain injury (TBI), controlled cortical impact (CCI), spinal cord contussion (SCC), amyloid-beta (Aβ), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
| Disease or Condition Studied | Insult | Model | Reference |
|---|---|---|---|
| Stroke | MCAO | Rat | 1,3,6,7,9,10,11,3,6,7,9,10,12 |
| FVO | Rat | 2,4 | |
| MCAO | Mouse | 5,11 | |
| Traumatic Injury | TBI | Human | 33,34,35 |
| CCI | Rat | 15,16,17,19,21 | |
| CCI | Mouse | 20 | |
| SCC | Rat | 22 | |
| Toxicity | Glutmate | Rat Neurons-Dissociated Culture | 23,24,25,26,27,28, |
| Glutamate | Mouse Organitypic Slice Culture | 29,30 | |
| Chronic Depolarization | Rat Neurons-Dissociated Culture | 28 | |
| Aβ or FeSO4 | Rat Neurons-Dissociated Culture | 25 | |
| Trophic Deprivation MPTP | PC12 Cultured Neurons | 31 | |
| Mouse Neurons-Dissociated Culture | 32 | ||
Acknowledgments
This work is supported by the National Institutes of Health NS41302 (PGM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Betz AL, Coester HC. Effect of steroids on edema and sodium uptake of the brain during focal ischemia in rats. Stroke. 1990;21:1199–204. doi: 10.1161/01.str.21.8.1199. [DOI] [PubMed] [Google Scholar]
- 2.Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–7. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- 3.Murphy SJ, Littleton-Kearney MT, Hurn PD. Progesterone administration during reperfusion, but not preischemia alone, reduces injury in ovariectomized rats. J Cereb Blood Flow Metab. 2002;22:1181–8. doi: 10.1097/01.WCB.0000037990.07114.07. [DOI] [PubMed] [Google Scholar]
- 4.Morali G, Letechipia-Vallejo G, Lopez-Loeza E, Montes P, Hernandez-Morales L, Cervantes M. Post-ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci Lett. 2005;382:286–90. doi: 10.1016/j.neulet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 5.Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. Journal of Cerebral Blood Flow & Metabolism. 2004;24:805–13. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- 6.Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Annals of emergency medicine. 2006;47:381–9. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Chopp M, Li Y. Neuroprotective effects of progesterone after transient middle cerebral artery occlusion in rat. J Neurol Sci. 1999;171:24–30. doi: 10.1016/s0022-510x(99)00247-6. [DOI] [PubMed] [Google Scholar]
- 8.Kumon Y, Kim SC, Tompkins P, Stevens A, Sakaki S, Loftus CM. Neuroprotective effect of postischemic administration of progesterone in spontaneously hypertensive rats with focal cerebral ischemia. J Neurosurg. 2000;92:848–52. doi: 10.3171/jns.2000.92.5.0848. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Jiang C, Liu C, Li X, Chen N, Hao Y. Neuroprotective effects of progesterone following stroke in aged rats. Behav Brain Res. 2010;209:119–22. doi: 10.1016/j.bbr.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci. 2007;25:151–9. [PubMed] [Google Scholar]
- 11.Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp Neurol. 2005;193:522–30. doi: 10.1016/j.expneurol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607:333–6. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- 14.Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp Neurol. 1996;138:246–51. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- 15.Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–9. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- 16.Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- 17.Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–59. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor CA, Cernak I, Johnson F, Vink R. Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp Neurol. 2007;205:145–53. doi: 10.1016/j.expneurol.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–18. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- 20.Jones NC, Constantin D, Prior MJ, Morris PG, Marsden CA, Murphy S. The neuroprotective effect of progesterone after traumatic brain injury in male mice is independent of both the inflammatory response and growth factor expression. Eur J Neurosci. 2005;21:1547–54. doi: 10.1111/j.1460-9568.2005.03995.x. [DOI] [PubMed] [Google Scholar]
- 21.Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharmacol Biochem Behav. 2003;76:231–42. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Thomas AJ, Nockels RP, Pan HQ, Shaffrey CI, Chopp M. Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine (Phila Pa 1976) 1999;24:2134–8. doi: 10.1097/00007632-199910150-00013. [DOI] [PubMed] [Google Scholar]
- 23.Atif F, Sayeed I, Ishrat T, Stein DG. Progesterone with vitamin D affords better neuroprotection against excitotoxicity in cultured cortical neurons than progesterone alone. J Mol Med. 2009;15:328–36. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogata T, Nakamura Y, Tsuji K, Shibata T, Kataoka K. Steroid hormones protect spinal cord neurons from glutamate toxicity. Neuroscience. 1993;55:445–9. doi: 10.1016/0306-4522(93)90513-f. [DOI] [PubMed] [Google Scholar]
- 25.Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–44. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 26.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–11. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mannella P, Sanchez AM, Giretti MS, Genazzani AR, Simoncini T. Oestrogen and progestins differently prevent glutamate toxicity in cortical neurons depending on prior hormonal exposure via the induction of neural nitric oxide synthase. Steroids. 2009;74:650–6. doi: 10.1016/j.steroids.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Luoma JI, Kelley BG, Mermelstein PG. Progesterone inhibition of voltage-gated calcium channels is a potential neuroprotective mechanism against excitotoxicity. Steroids. 2011 doi: 10.1016/j.steroids.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jodhka PK, Kaur P, Underwood W, Lydon JP, Singh M. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain-derived neurotrophic factor expression. Endocrinology. 2009;150:3162–8. doi: 10.1210/en.2008-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85:2441–9. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coughlan T, Gibson C, Murphy S. Progesterone, BDNF and neuroprotection in the injured CNS. Int J Neurosci. 2009;119:1718–40. doi: 10.1080/00207450903116430. [DOI] [PubMed] [Google Scholar]
- 32.Callier S, Morissette M, Grandbois M, Pelaprat D, Di Paolo T. Neuroprotective properties of 17beta-estradiol, progesterone, and raloxifene in MPTP C57Bl/6 mice. Synapse. 2001;41:131–8. doi: 10.1002/syn.1067. [DOI] [PubMed] [Google Scholar]
- 33.Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. 402, e1–2. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- 34.Xiao GM, Wei J, Wu ZH, Wang WM, Jiang QZ, Cheng J, Lu F, Wu JY, Xu HS, Fang R. Clinical study on the therapeutic effects and mechanism of progesterone in the treatment for acute severe head injury. Zhonghua Wai Ke Za Zhi. 2007;45:106–8. [PubMed] [Google Scholar]
- 35.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groswasser Z, Cohen M, Keren O. Female TBI patients recover better than males. Brain Inj. 1998;12:805–8. doi: 10.1080/026990598122197. [DOI] [PubMed] [Google Scholar]
- 37.Roof RL, Duvdevani R, Stein D. Progesterone treatment attenuates brain edema following contusion injury in male and female rats. Restorative neurology and neuroscience. 1992;4:425–427. doi: 10.3233/RNN-1992-4608. [DOI] [PubMed] [Google Scholar]
- 38.Hall ED, Gibson TR, Pavel KM. Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J Neurotrauma. 2005;22:669–79. doi: 10.1089/neu.2005.22.669. [DOI] [PubMed] [Google Scholar]
- 39.Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci. 2003;25:343–8. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- 40.Jung-Testas I, Hu ZY, Baulieu EE, Robel P. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology. 1989;125:2083–91. doi: 10.1210/endo-125-4-2083. [DOI] [PubMed] [Google Scholar]
- 41.Lorenz L, Dang J, Misiak M, Tameh Abolfazl A, Beyer C, Kipp M. Combined 17beta-oestradiol and progesterone treatment prevents neuronal cell injury in cortical but not midbrain neurones or neuroblastoma cells. J Neuroendocrinol. 2009;21:841–9. doi: 10.1111/j.1365-2826.2009.01903.x. [DOI] [PubMed] [Google Scholar]
- 42.Yao J, Chen S, Cadenas E, Brinton RD. Estrogen protection against mitochondrial toxin-induced cell death in hippocampal neurons: antagonism by progesterone. Brain Res. 1379:2–10. doi: 10.1016/j.brainres.2010.11.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aguirre C, Jayaraman A, Pike C, Baudry M. Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-beta. J Neurochem. 2010;115:1277–87. doi: 10.1111/j.1471-4159.2010.07038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fansa I, Gol M, Nisanoglu V, Yavas S, Iscan Z, Tasdemir O. Does diltiazem inhibit the inflammatory response in cardiopulmonary bypass? Med Sci Monit. 2003;9:PI30–6. [PubMed] [Google Scholar]
- 45.Farah R, Shurtz-Swirski R. The combined effect of calcium channel blocker Lercanidipine and antioxidants on low-grade systemic inflammation parameters in essential hypertension patients. Minerva Cardioangiol. 2008;56:467–76. [PubMed] [Google Scholar]
- 46.Gurdal H, Sara Y, Tulunay FC. Effects of calcium channel blockers on formalin-induced nociception and inflammation in rats. Pharmacology. 1992;44:290–6. doi: 10.1159/000138932. [DOI] [PubMed] [Google Scholar]
- 47.Shirakura S, Sano J, Karasawa A, Kubo K. Protective effect of benidipine against the development of glomerular sclerosis in experimental nephrotic syndrome. Jpn J Pharmacol. 1992;59:461–7. doi: 10.1254/jjp.59.461. [DOI] [PubMed] [Google Scholar]
- 48.Lukic-Panin V, Kamiya T, Zhang H, Hayashi T, Tsuchiya A, Sehara Y, Deguchi K, Yamashita T, Abe K. Prevention of neuronal damage by calcium channel blockers with antioxidative effects after transient focal ischemia in rats. Brain Res. 2007;1176:143–50. doi: 10.1016/j.brainres.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 49.Aoki Y, Tamura M, Itoh Y, Ukai Y. Cerebroprotective action of a Na+/Ca2+ channel blocker NS-7. I. Effect on the cerebral infarction and edema at the acute stage of permanent middle cerebral artery occlusion in rats. Brain Res. 2001;890:162–9. doi: 10.1016/s0006-8993(00)03167-x. [DOI] [PubMed] [Google Scholar]
- 50.Okuyama K, Kiuchi S, Okamoto M, Narita H, Kudo Y. A novel Na+ and Ca2+ channel blocker, T-477, prevents brain edema following microsphere-induced permanent occlusion of cerebral arterioles in rats. Jpn J Pharmacol. 1999;81:170–5. doi: 10.1254/jjp.81.170. [DOI] [PubMed] [Google Scholar]
- 51.Okiyama K, Smith DH, Thomas MJ, McIntosh TK. Evaluation of a novel calcium channel blocker, (S)-emopamil, on regional cerebral edema and neurobehavioral function after experimental brain injury. J Neurosurg. 1992;77:607–15. doi: 10.3171/jns.1992.77.4.0607. [DOI] [PubMed] [Google Scholar]
- 52.Li DL, Zhao HG, Wang DX, Ding YF. Effect of progesterone on cerebral cortex edema in rats exposed to focal ischemia/reperfusion. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2001;17:327–9. [PubMed] [Google Scholar]
- 53.Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198:469–78. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 54.O’Connor CA, Cernak I, Vink R. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 2005;1062:171–4. doi: 10.1016/j.brainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Wright DW, Bauer ME, Hoffman SW, Stein DG. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J Neurotrauma. 2001;18:901–9. doi: 10.1089/089771501750451820. [DOI] [PubMed] [Google Scholar]
- 56.He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404–12. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049:112–9. doi: 10.1016/j.brainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Pan DS, Liu WG, Yang XF, Cao F. Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury. Biomed Environ Sci. 2007;20:432–8. [PubMed] [Google Scholar]
- 59.Jiang C, Wang J, Li X, Liu C, Chen N, Hao Y. Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflamm Res. 2009;58:619–24. doi: 10.1007/s00011-009-0032-8. [DOI] [PubMed] [Google Scholar]
- 60.Labombarda F, Gonzalez S, Gonzalez Deniselle MC, Garay L, Guennoun R, Schumacher M, De Nicola AF. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J Neurotrauma. 2006;23:181–92. doi: 10.1089/neu.2006.23.181. [DOI] [PubMed] [Google Scholar]
- 61.De Nicola AF, Gonzalez SL, Labombarda F, Deniselle MC, Garay L, Guennoun R, Schumacher M. Progesterone treatment of spinal cord injury: Effects on receptors, neurotrophins, and myelination. J Mol Neurosci. 2006;28:3–15. doi: 10.1385/jmn:28:1:3. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez SL, Labombarda F, Deniselle MC, Mougel A, Guennoun R, Schumacher M, De Nicola AF. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J Steroid Biochem Mol Biol. 2005;94:143–9. doi: 10.1016/j.jsbmb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 63.Labombarda F, Gonzalez SL, Lima A, Roig P, Guennoun R, Schumacher M, de Nicola AF. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia. 2009;57:884–97. doi: 10.1002/glia.20814. [DOI] [PubMed] [Google Scholar]
- 64.Brand-Schieber E, Werner P. Calcium channel blockers ameliorate disease in a mouse model of multiple sclerosis. Exp Neurol. 2004;189:5–9. doi: 10.1016/j.expneurol.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 65.Winkler T, Sharma HS, Stalberg E, Badgaiyan RD, Gordh T, Westman J. An L-type calcium channel blocker, nimodipine influences trauma induced spinal cord conduction and axonal injury in the rat. Acta Neurochir Suppl. 2003;86:425–32. doi: 10.1007/978-3-7091-0651-8_88. [DOI] [PubMed] [Google Scholar]
- 66.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–68. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem. 1995;65:1704–11. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- 68.Bender C, Rassetto M, Olmos JS, Olmos SD, Lorenzo A. Involvement of AMPA/kainate-excitotoxicity in MK801-induced neuronal death in the retrosplenial cortex. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neuroscience Letters. 1985;58:293–7. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- 70.Tymianski M, Tator CH. Normal and abnormal calcium homeostasis in neurons: a basis for the pathophysiology of traumatic and ischemic central nervous system injury. Neurosurgery. 1996;38:1176–95. doi: 10.1097/00006123-199606000-00028. [DOI] [PubMed] [Google Scholar]
- 71.Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–43. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–9. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Ozacmak VH, Sayan H. The effects of 17beta estradiol, 17alpha estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiol Res. 2009;58:909–12. doi: 10.33549/physiolres.931647. [DOI] [PubMed] [Google Scholar]
- 74.Miyazaki H, Tanaka S, Fujii Y, Shimizu K, Nagashima K, Kamibayashi M, Uehara T, Okuma Y, Nomura Y. Neuroprotective effects of a dihydropyridine derivative, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarbox ylic acid methyl 6-(5-phenyl-3-pyrazolyloxy)hexyl ester (CV-159), on rat ischemic brain injury. Life Sci. 1999;64:869–78. doi: 10.1016/s0024-3205(99)00011-9. [DOI] [PubMed] [Google Scholar]
- 75.Sribnick EA, Del Re AM, Ray SK, Woodward JJ, Banik NL. Estrogen attenuates glutamate-induced cell death by inhibiting Ca2+ influx through L-type voltage-gated Ca2+ channels. Brain Res. 2009;1276:159–70. doi: 10.1016/j.brainres.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vallazza-Deschamps G, Fuchs C, Cia D, Tessier LH, Sahel JA, Dreyfus H, Picaud S. Diltiazem-induced neuroprotection in glutamate excitotoxicity and ischemic insult of retinal neurons. Doc Ophthalmol. 2005;110:25–35. doi: 10.1007/s10633-005-7341-1. [DOI] [PubMed] [Google Scholar]
- 77.Hurd C, Moudgil VK. Characterization of R5020 and RU486 binding to progesterone receptor from calf uterus. Biochemistry. 1988;27:3618–23. doi: 10.1021/bi00410a014. [DOI] [PubMed] [Google Scholar]
- 78.Akan P, Kizildag S, Ormen M, Genc S, Oktem MA, Fadiloglu M. Pregnenolone protects the PC-12 cell line against amyloid beta peptide toxicity but its sulfate ester does not. Chem Biol Interact. 2009;177:65–70. doi: 10.1016/j.cbi.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 79.Shirakawa H, Katsuki H, Kume T, Kaneko S, Akaike A. Pregnenolone sulphate attenuates AMPA cytotoxicity on rat cortical neurons. Eur J Neurosci. 2005;21:2329–35. doi: 10.1111/j.1460-9568.2005.04079.x. [DOI] [PubMed] [Google Scholar]
- 80.Guarneri P, Russo D, Cascio C, De Leo G, Piccoli T, Sciuto V, Piccoli F, Guarneri R. Pregnenolone sulfate modulates NMDA receptors, inducing and potentiating acute excitotoxicity in isolated retina. J Neurosci Res. 1998;54:787–97. doi: 10.1002/(SICI)1097-4547(19981215)54:6<787::AID-JNR6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 81.Morali G, Montes P, Hernandez-Morales L, Monfil T, Espinosa-Garcia C, Cervantes M. Neuroprotective effects of progesterone and allopregnanolone on long-term cognitive outcome after global cerebral ischemia. Restor Neurol Neurosci. 2011;29:1–15. doi: 10.3233/RNN-2011-0571. [DOI] [PubMed] [Google Scholar]
- 82.Singh S, Hota D, Prakash A, Khanduja KL, Arora SK, Chakrabarti A. Allopregnanolone, the active metabolite of progesterone protects against neuronal damage in picrotoxin-induced seizure model in mice. Pharmacol Biochem Behav. 2010;94:416–22. doi: 10.1016/j.pbb.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Kelley MH, Taguchi N, Ardeshiri A, Kuroiwa M, Hurn PD, Traystman RJ, Herson PS. Ischemic insult to cerebellar Purkinje cells causes diminished GABAA receptor function and allopregnanolone neuroprotection is associated with GABAA receptor stabilization. J Neurochem. 2008;107:668–78. doi: 10.1111/j.1471-4159.2008.05617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelley BG, Mermelstein PG. Progesterone blocks multiple routes of ion flux. Mol Cell Neurosci. 2011;48:137–41. doi: 10.1016/j.mcn.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Selye H, Masson G. Additional Steroids with Luteoid Activity. Science. 1942;96:358. doi: 10.1126/science.96.2494.358. [DOI] [PubMed] [Google Scholar]
- 86.Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009;15:119–38. doi: 10.1093/humupd/dmn044. [DOI] [PubMed] [Google Scholar]
- 87.Xie Y, Dengler K, Zacharias E, Wilffert B, Tegtmeier F. Effects of the sodium channel blocker tetrodotoxin (TTX) on cellular ion homeostasis in rat brain subjected to complete ischemia. Brain Res. 1994;652:216–24. doi: 10.1016/0006-8993(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 88.Ates O, Cayli SR, Gurses I, Karabulut AB, Yucel N, Kocak A, Cakir CO, Yologlu S. Do sodium channel blockers have neuroprotective effect after onset of ischemic insult? Neurol Res. 2007;29:317–23. doi: 10.1179/016164107X159225. [DOI] [PubMed] [Google Scholar]
- 89.Tatlisumak T, Carano RA, Takano K, Meiler MR, Li F, Sotak CH, Arndts D, Pschorn U, Fisher M. Broad-spectrum cation channel inhibition by LOE 908 MS reduces infarct volume in vivo and postmortem in focal cerebral ischemia in the rat. J Neurol Sci. 2000;178:107–13. doi: 10.1016/s0022-510x(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 90.Zhang X, Anderson JW, Fedida D. Characterization of nifedipine block of the human heart delayed rectifier, hKv1.5. J Pharmacol Exp Ther. 1997;281:1247–56. [PubMed] [Google Scholar]
- 91.Zhang XL, Gold MS. Dihydropyridine block of voltage-dependent K+ currents in rat dorsal root ganglion neurons. Neuroscience. 2009;161:184–94. doi: 10.1016/j.neuroscience.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yatani A, Brown AM. The calcium channel blocker nitrendipine blocks sodium channels in neonatal rat cardiac myocytes. Circ Res. 1985;56:868–75. doi: 10.1161/01.res.56.6.868. [DOI] [PubMed] [Google Scholar]
- 93.Das P, Bell-Horner CL, Huang RQ, Raut A, Gonzales EB, Chen ZL, Covey DF, Dillon GH. Inhibition of type A GABA receptors by L-type calcium channel blockers. Neuroscience. 2004;124:195–206. doi: 10.1016/j.neuroscience.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 94.Kittaka M, Giannotta SL, Zelman V, Correale JD, DeGiorgio CM, Weiss MH, Zlokovic BV. Attenuation of brain injury and reduction of neuron-specific enolase by nicardipine in systemic circulation following focal ischemia and reperfusion in a rat model. J Neurosurg. 1997;87:731–7. doi: 10.3171/jns.1997.87.5.0731. [DOI] [PubMed] [Google Scholar]
- 95.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–89. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Myers MG. Dihydropyridine calcium antagonists and the trough: peak ratio: focus on adverse effects. J Hypertens. 1994;(Suppl 12):S73–6. discussion S76–7. [PubMed] [Google Scholar]
- 97.Pedrinelli R, Dell’Omo G, Mariani M. Calcium channel blockers, postural vasoconstriction and dependent oedema in essential hypertension. J Hum Hypertens. 2001;15:455–61. doi: 10.1038/sj.jhh.1001201. [DOI] [PubMed] [Google Scholar]