Abstract
The demographics of ageing are changing dramatically such that there will be many more older adults in the near future. This setting likely will produce a new “boomer-driven” epidemic of physiological dysfunction, disability and risk of chronic degenerative disorders, including cardiovascular diseases (CVD). Standing out against this dreary biomedical forecast are Masters athletes, a group of middle-aged and older adults who engage in regular vigorous physical training and competitive sport. Compared with their sedentary/less active (untrained) peers, Masters athletes who perform endurance training-based activities demonstrate a more favorable arterial function-structure phenotype, including lower large elastic artery stiffness, enhanced vascular endothelial function and less arterial wall hypertrophy. As such, they may represent an exemplary model of healthy or “successful” vascular ageing. In contrast, Masters athletes engaged primarily/exclusively in intensive resistance training exhibit less favorable arterial function-structure than their endurance-trained peers and, in some instances, untrained adults. These different arterial properties likely are explained in large part by the different intravascular mechanical forces generated during endurance vs. resistance exercise-related training activities. The more favorable arterial function-structure profile of Masters endurance athletes may contribute to their low risk of clinical CVD.
Keywords: arterial stiffness, endothelium
The world is ageing--the number of older adults is on the rise. This phenomenon comes with serious physiological and health implications including increases in cardiovascular dysfunction and disease (CVD). Indeed, it has been projected that without effective intervention 40% of all U.S. adults will have at least one form of CVD by 2030, with a tripling of attendant medical costs due largely to the ageing of the population (Heidenreich et al., 2011).
In the midst of this impending epidemic of age-associated dysfunction and disease stands a physiologically exceptional group of middle-aged and older adults referred to as “Masters athletes”. These individuals exercise vigorously for most, if not all, days of the week, often engaging in athletic competitions and demonstrating enhanced age-normalized physical function and remarkable sports performance (Tanaka & Seals, 2008). Importantly, at least for those performing aerobic exercise-related training and competitions, Masters athletes have greater cardiovascular capacity (e.g., maximal cardiac output and oxygen consumption) (Tanaka & Seals, 2008) and a lower risk of CVD (Laure & Binsinger, 2009) compared with their more sedentary peers.
Many physiological and/or pathophysiological changes likely contribute to declines in cardiovascular function and increases in CVD risk with ageing. Among the most important are changes to the arterial system including stiffening of the large elastic arteries (aorta and carotid arteries), development of endothelial dysfunction and wall thickening (Lakatta & Levy, 2003). Here we summarize and update recent discussions (Seals et al., 2008; Seals et al., 2009) of evidence suggesting that these adverse vascular changes may be less manifest (or even absent) in certain subgroups of Masters athletes and, therefore, might help explain their more favorable cardiovascular capacity and health.
Large Elastic Artery Stiffness
Large elastic artery stiffness, most commonly assessed by aortic pulse wave velocity (aPWV) or the local compliance of the carotid artery (via ultrasound and tonometry), has emerged as a major independent risk factor for CVD in older adults and is linked to a greater risk of systolic hypertension, left ventricular hypertrophy and other disorders of ageing such as cognitive impairment (Lakatta & Levy, 2003; Mitchell et al., 2010). As reflected by increased aPWV and decreased carotid compliance, large elastic artery stiffness increases with age even in non-hypertensive adults free of clinical CVD (Tanaka et al., 1998; Lakatta & Levy, 2003).
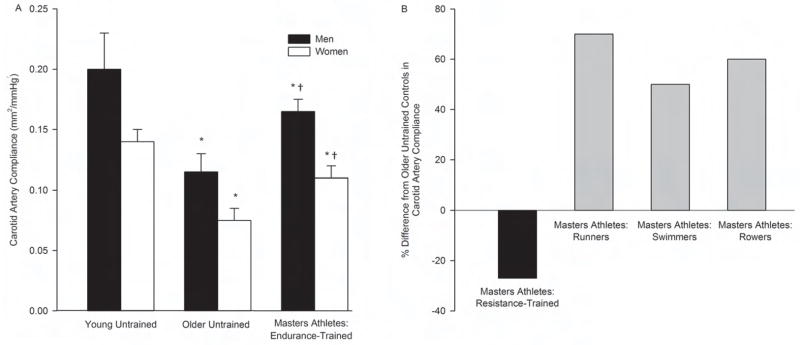
Middle-aged and older male and female Masters endurance athletes (triathletes, cyclists, runners, swimmers) demonstrate lower aPWV (Vaitkevicius et al., 1993; Tanaka et al., 1998) and greater carotid artery compliance (Tanaka et al., 2000; Monahan et al., 2001; Moreau et al., 2003; Moreau et al., 2006a; Nualnim et al., 2011) compared with their non-exercise-trained or sedentary (herein referred to as “untrained”) peers (Figure 1). aPWV in these Masters athletes is similar to those in trained and/or untrained young adults (Vaitkevicius et al., 1993; Tanaka et al., 1998), whereas carotid compliance is lower than that observed in young adult controls (Tanaka et al., 2000; Moreau et al., 2003). The lower large elastic artery stiffness in Masters endurance athletes compared with middle-aged/older untrained adults is associated with other cardiovascular benefits including lower 24-hour systolic and pulse pressures (Seals et al., 1999) and enhanced baroreflex sensitivity (Monahan et al., 2001; Nualnim et al., 2011). Little is known as to the mechanisms by which these Masters athletes maintain lower large elastic artery stiffness with age, but less oxidative stress-related suppression of arterial compliance may play an important role (Moreau et al., 2006a). A lower “subclinical” CVD risk factor burden in the Masters endurance athletes also could contribute, although subjects with major risk factors were excluded in the aforementioned studies.
Figure 1.
A) Carotid artery compliance of young untrained adults, older untrained adults and Masters athletes (adapted from Tanaka et al., 2000 and Moreau et al., 2003), B) percent difference in carotid artery compliance from study-specific older untrained controls in Masters athletes of different sports (data compiled from Cook et al., 2006, Miyachi et al., 2003 and Nualnim et al., 2011). Values are means ± SEM. *P<0.05 vs. young untrained of same sex; †P<0.05 vs. older untrained of same sex.
In contrast to their peers performing endurance training/competitions, Masters athletes engaged in sports requiring intensive resistance training have greater large elastic artery stiffness than untrained adults, as indicated by lower carotid artery compliance (Miyachi et al., 2003). Interestingly, Masters rowers, a group of athletes that perform both intensive resistance and endurance training, demonstrate enhanced carotid artery compliance compared with untrained controls (Cook et al., 2006), suggesting that even some element of endurance training can offset the apparent negative consequences of intensive resistance training. No differences in peripheral large (femoral) artery compliance have been observed among groups of Masters athletes and untrained healthy adults (Cook et al., 2006; Nualnim et al., 2011), suggesting age- and training-specific influences on large elastic arteries.
Vascular Endothelial Function
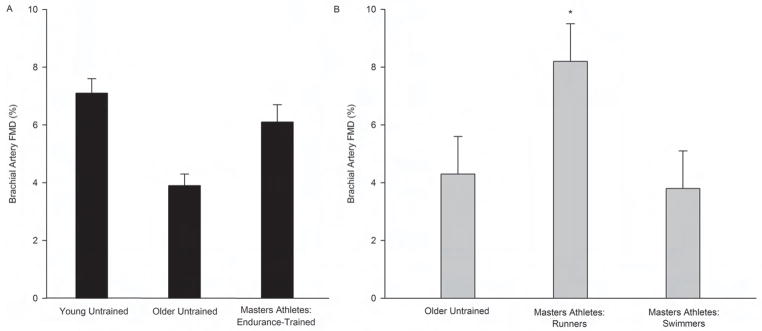
Vascular endothelial function is most commonly assessed in humans by measuring endothelium-dependent dilation (EDD) using either brachial artery flow-mediated dilation (FMD) or the forearm blood flow responses to brachial artery-infused acetylcholine (Seals et al., 2011). EDD is reduced with advancing age in untrained adults, even in the absence of CVD risk factors/disease (Seals et al., 2011). Unlike their untrained peers, however, male Masters endurance athletes have largely or completely preserved EDD with ageing (Figure 2) (DeSouza et al., 2000; Taddei et al., 2000; Eskurza et al., 2004; Eskurza et al., 2005; Franzoni et al., 2005; Black et al., 2009; Pierce et al., 2011a). These athletes also appear to be at least partially protected from impairments in EDD in response to acute ischemia/reperfusion injury (DeVan et al., 2011).
Figure 2.
Brachial artery flow-mediated dilation (FMD) of A) young untrained and older untrained men and male Masters athletes (mean data for each group compiled from Eskurza et al., 2004, Eskurza et al., 2005, Franzoni et al., 2005 and Pierce et al., 2011a), B) older untrained adults and Masters athletes of different sports (adapted from Nualnim et al., 2011). Values are means ± SEM. *P<0.05 vs. older untrained and swimmers.
Reduced vascular oxidative stress is a key mechanism by which EDD is preserved with age in male Masters athletes (Taddei et al., 2000; Eskurza et al., 2004; Franzoni et al., 2005). Indeed, there is now direct evidence of reduced oxidant stress in the vascular endothelial cells of these athletes compared with untrained controls, and this is associated with reduced endothelial cell expression of the oxidant enzyme NADPH oxidase and redox-sensitive transcription factor nuclear factor κ B, as well as increases in the expression the antioxidant enzyme manganese (mitochondrial) superoxide dismutase (SOD) and activity of endothelium-bound SOD (Pierce et al., 2011a). Reduced endothelial oxidative stress in these Masters athletes causes less destruction/greater bioavailability of the endothelium-dependent dilating molecule, nitric oxide (NO), resulting in a greater NO-mediated EDD (Taddei et al., 2000). Greater bioavailability of the critical co-factor for NO production, tetrahydrobiopterin (BH4), also plays an important role in the maintenance of EDD in these athletes (Eskurza et al., 2005). This could be due to less oxidation of BH4, increased endogenous BH4 synthesis or both. Basal NO production also is preserved in male Masters endurance athletes (Seals et al., 2008), perhaps also a result of reduced oxidative stress and enhanced BH4 bioavailability.
The mechanisms for this endothelial-protective phenotype of male Masters athletes remain to be established, though it is not clearly or consistently related to differences in clinical characteristics (Seals et al., 2008; Seals et al., 2009; Seals et al., 2011). Rather, training-induced increases in intravascular laminar shear (via increases in systemic and active limb blood flow), differences in one or more presently unidentified (protective) circulating humoral factors and/or greater resistance to a given level of potentially endothelium-damageing factors (e.g., plasma LDL cholesterol or glucose) all have been proposed (Seals et al., 2008; Seals et al., 2009; Seals et al., 2011).
In comparison to men, far fewer data are available on vascular endothelial function in female Masters endurance athletes and all of it is based on brachial artery FMD. Initial reports on small groups of women suggested greater EDD in female Masters endurance athletes compared with untrained age-matched controls (Hagmar et al., 2006; Black et al., 2009). A recent study of a much larger sample found no differences brachial FMD in endurance-trained and untrained postmenopausal women, while confirming past observations in men (Pierce et al., 2011b). Extensive analysis revealed no obvious physical or clinical characteristics that could explain the sex-specific differences. However, all of the women were estrogen-deficient and it is possible that a certain critical level of estrogen bioavailability is necessary for exercise-generated physiological signals to modulate vascular endothelial function in this group.
Finally, among Masters endurance athletes, it is possible that vascular endothelial function is influenced by the type of activity performed. A recent investigation found that brachial artery FMD was greater in middle-aged and older Masters runners compared with age- and sex-balanced groups of Masters endurance swimmers and untrained controls (Nualnim et al., 2011). To our knowledge, no cross-sectional studies are available on vascular endothelial function in primarily/exclusively resistance-trained Masters athletes.
Arterial Wall Thickness
Carotid and femoral artery intima-media thickness (IMT) are independent predictors of CVD and increase 2- to 3-fold with adult ageing in the absence of major risk factors or clinical diseases (Lakatta & Levy, 2003; Seals et al., 2008). This large artery wall thickening with age is mediated by hypertrophy of both the intimal and medial layers and likely represents one aspect of a vascular remodeling process in response to changes in intravascular mechanical forces with ageing (Seals et al., 2008). Age-associated increases in IMT also may reflect the development of subclinical or clinical-grade atherosclerotic plaques, although the latter is less likely in healthy adults.
The carotid IMT of male and female Masters endurance athletes does not differ from untrained age- and sex-equivalent untrained adults, nor are the age-related differences in carotid IMT different in endurance athletes compared with untrained adults (Moreau et al., 2002; Tanaka et al., 2002; Moreau et al., 2003). This also is the case in resistance exercise-trained Masters athletes (Miyachi et al., 2003). The absence of an effect likely is explained by the fact that “central” (e.g., carotid artery) blood pressure, a key determinant of IMT among healthy adults, does not differ in Masters athletes and untrained controls.
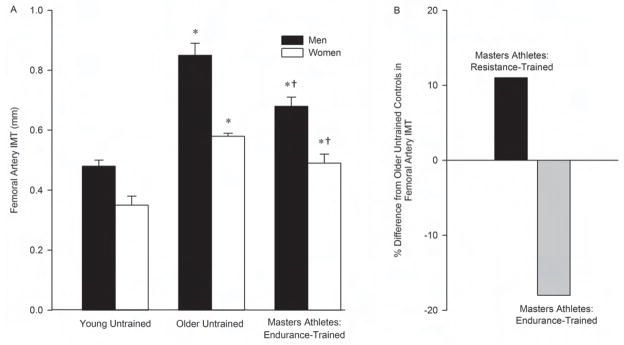
In contrast, femoral artery IMT is smaller in male and female Masters endurance athletes compared with age- and sex-matched untrained controls, and the age-associated difference is smaller in endurance-trained athletes compared with untrained adults (Figure 3) (Dinenno et al., 2001; Moreau et al., 2002; Moreau et al., 2006b). The smaller femoral IMT and accompanying increase in lumen diameter in Masters endurance athletes are features of “expansive arterial remodeling”, a process presumably aimed at normalizing wall stress in response to exercise-evoked increases in femoral blood flow required to meet the demands of the active muscles in the legs (Dinenno et al., 2001). Rather than smaller, femoral IMT is greater in resistance-trained male Masters athletes compared with untrained age-matched controls (Miyachi et al., 2005). This may be the result of the different intravascular mechanical forces generated in the systemic circulation during resistance compared with endurance training, particularly the marked increases in arterial pressure during weight lifting maneuvers.
Figure 3.
A) Femoral artery intima-media thickness (IMT) of young untrained adults, older untrained adults and Masters athletes (adapted from Moreau et al., 2006b), B) percent difference in femoral artery IMT from study-specific older untrained controls in resistance-trained male and endurance-trained Masters athletes (adapted from Miyachi et al., 2005 and Moreau et al., 2006b). Values are means ± SEM. *P<0.001 vs. young untrained of same sex; †P<0.001 vs. older untrained of same sex.
Summary and Conclusions
Large elastic artery stiffness, vascular endothelial function and large artery wall thickness are major indicators of arterial health and risk of age-associated CVD (Lakatta & Levy, 2003). Overall, Masters endurance athletes demonstrate a more favorable arterial phenotype compared with untrained middle-aged and older adults, which may explain, at least in part, their greater cardiovascular functional capacity and lower risk of CVD. As such, the Masters endurance athlete may be viewed as a model of “exceptional vascular ageing”. In contrast, Masters athletes for whom training and competitive sport require primarily or exclusively intensive resistance muscle activities exhibit a less favorable arterial function-structure profile than their endurance-trained peers and, in some cases, compared with untrained adults. The differences in arterial properties between Masters athletes engageing in endurance vs. resistance training-requiring sports likely are explained by differences in the intravascular mechanical forces generated during these activities.
Acknowledgments
Our thanks to all of the students, postdoctoral fellows and staff who contributed to the work in our laboratory. Supported by NIH R37 AG013038, T32 AG000279 and UL1 RR025780
References
- Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. 2009;297:H1109–1116. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JN, DeVan AE, Schleifer JL, Anton MM, Cortez-Cooper MY, Tanaka H. Arterial compliance of rowers: implications for combined aerobic and strength training on arterial elasticity. Am J Physiol Heart Circ Physiol. 2006;290:H1596–1600. doi: 10.1152/ajpheart.01054.2005. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- DeVan AE, Umpierre D, Harrison ML, Lin HF, Tarumi T, Renzi CP, Dhindsa M, Hunter SD, Tanaka H. Endothelial ischemia-reperfusion injury in humans: association with age and habitual exercise. Am J Physiol Heart Circ Physiol. 2011;300:H813–819. doi: 10.1152/ajpheart.00845.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534:287–295. doi: 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens. 2005;18:510–516. doi: 10.1016/j.amjhyper.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Hagmar M, Eriksson MJ, Lindholm C, Schenck-Gustafsson K, Hirschberg AL. Endothelial function in post-menopausal former elite athletes. Clin J Sport Med. 2006;16:247–252. doi: 10.1097/00042752-200605000-00011. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Laure P, Binsinger C. Chronic diseases and elite athletes: an epidemiological review. Medicina Sportiva. 2009;13:245–250. [Google Scholar]
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi M, Donato AJ, Yamamoto K, Takahashi K, Gates PE, Moreau KL, Tanaka H. Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension. 2003;41:130–135. doi: 10.1161/01.hyp.0000047649.62181.88. [DOI] [PubMed] [Google Scholar]
- Miyachi M, Tanaka H, Kawano H, Okajima M, Tabata I. Lack of age-related decreases in basal whole leg blood flow in resistance-trained men. J Appl Physiol. 2005;99:1384–1390. doi: 10.1152/japplphysiol.00061.2005. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation. 2001;104:1627–1632. doi: 10.1161/hc3901.096670. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003;57:861–868. doi: 10.1016/s0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, Dinenno FA, Blackett SD, Hoetzer GL, Desouza CA, Tanaka H. Arterial intima-media thickness: site-specific associations with HRT and habitual exercise. Am J Physiol Heart Circ Physiol. 2002;283:H1409–1417. doi: 10.1152/ajpheart.00035.2002. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Gavin KM, Plum AE, Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause. 2006a;13:951–958. doi: 10.1097/01.gme.0000243575.09065.48. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Silver AE, Dinenno FA, Seals DR. Habitual aerobic exercise is associated with smaller femoral artery intima-media thickness with age in healthy men and women. Eur J Cardiovasc Prev Rehabil. 2006b;13:805–811. doi: 10.1097/01.hjr.0000230103.55653.42. [DOI] [PubMed] [Google Scholar]
- Nualnim N, Barnes JN, Tarumi T, Renzi CP, Tanaka H. Comparison of central artery elasticity in swimmers, runners, and the sedentary. Am J Cardiol. 2011;107:783–787. doi: 10.1016/j.amjcard.2010.10.062. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Donato AJ, Larocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011a;10:1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci. 2011b;120:13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Stevenson ET, Jones PP, DeSouza CA, Tanaka H. Lack of age-associated elevations in 24-h systolic and pulse pressures in women who exercise regularly. Am J Physiol Heart Circ Physiol. 1999;277:H947–955. doi: 10.1152/ajpheart.1999.277.3.H947. [DOI] [PubMed] [Google Scholar]
- Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009;587:5541–5549. doi: 10.1113/jphysiol.2009.178822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–132. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Seals DR. Endurance exercise performance in Masters athletes: age-associated changes and underlying physiological mechanisms. J Physiol. 2008;586:55–63. doi: 10.1113/jphysiol.2007.141879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Seals DR, Monahan KD, Clevenger CM, DeSouza CA, Dinenno FA. Regular aerobic exercise and the age-related increase in carotid artery intima-media thickness in healthy men. J Appl Physiol. 2002;92:1458–1464. doi: 10.1152/japplphysiol.00824.2001. [DOI] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]