Abstract
Patellofemoral (PF) pain is a common ailment of the lower extremity. A theorized cause for pain is patellar maltracking due to vasti muscle activation imbalance, represented as large vastus lateralis:vastus medialis (VL:VM) activation ratios. However, evidence relating vasti muscle activation imbalance to patellar maltracking is limited. We investigated the relationships between VL:VM activation ratio and patellar tracking measures, patellar tilt and bisect offset, in PF pain subjects and pain-free controls. We evaluated VL:VM activation ratio and VM activation delay relative to VL activation in 39 PF pain subjects and 15 pain-free controls during walking. We classified the PF pain subjects into normal tracking and maltracking groups based on patellar tilt and bisect offset measured from weightbearing magnetic resonance imaging. Patellar tilt correlated with VL:VM activation ratio only in PF pain subjects classified as maltrackers. This suggests that a clinical intervention targeting vasti muscle activation imbalance may be effective only in PF pain subjects classified as maltrackers.
Keywords: patellofemoral pain, vastus lateralis:vastus medialis activation ratio, vastus medialis activation delay, patellar maltracking, PearlDiver database
INTRODUCTION
Patellofemoral (PF) pain is a common ailment of the lower extremity, accounting for about 1 in 4 knee injuries diagnosed in sports medicine clinics,10 with even higher incidence rates in females.9 In 2008, an estimated 971,000 patients were diagnosed with PF pain in the U.S. with an associated cost of $8.3 billion (www.pearldiverinc.com, accessed 07/12/2011). These data were based on medical records from 20 million patients and represent conservative estimates.
While several factors are associated with PF pain, patellar maltracking is considered an important contributor.15 Maltracking is typically characterized by excessive lateral alignment of the patella within the trochlear groove. Maltracking may be due to joint conformity,8 a large quadriceps angle,14 PF ligament properties,1 altered muscle recruitment at the hip with increased femoral rotation,6 and excessive foot pronation.13 However, vasti muscle activation imbalance has garnered attention from clinicians and researchers as a cause for patellar maltracking,15,31 though evidence relating this imbalance to maltracking is limited. Vasti muscle activation imbalance is generally measured as the delay in activation of the vastus medialis (VM) muscle compared to the vastus lateralis (VL) muscle,29 or as the ratio of the magnitudes of normalized muscle activations (VL:VM).27–29,31 We recently reported relationships between VM activation delay and patellar maltracking in PF pain subjects.25 It is unclear, however, if patellar tracking measures are also related to vasti activation ratio. A previous study evaluated relationships between patellar tracking measures and vasti activation ratio,27 and concluded that maltracking was not related to large vasti activation ratios. However, this study evaluated patellar tracking measures under minimal weightbearing conditions, and all PF pain subjects were grouped together. Studies highlighted the importance of evaluating patellar maltracking under weightbearing conditions,2,11 and demonstrated the need for classifying PF pain subjects into subgroups for accurate diagnosis25 and effective treatment.12 We developed a method for classifying PF pain subjects using patellar tracking measures obtained under weightbearing conditions.25 Accordingly, we investigated the relationships between VL:VM activation ratio and patellar tracking in PF pain subjects and pain-free controls. We also investigated the relationship between VL:VM activation ratio and VM activation delay in these same subjects.
METHODS
Subject Population
We recruited 54 subjects: 15 active, pain-free controls and 39 PF pain subjects (Table I). No statistical differences were found in age, height, or weight between male control and male PF pain subjects or between female control and female PF pain subjects. The PF pain subjects were diagnosed by a sports medicine physician (MF) with 20 years experience. A subject was included if he/she reported consistent PF pain for ≥3 mos (range: 3 mos to 11 yrs), and if he/she experienced reproducible anterior knee pain during ≥2 of the following activities: stair ascent/descent, kneeling, squatting, prolonged sitting, or isometric quadriceps contraction.4 For subjects with bilateral pain, the more painful knee was included. A subject was excluded if he/she had demonstrated knee ligament instability, patellar tendinopathy, joint line tenderness or knee effusion, previous knee trauma or surgery, patellar dislocation, or if signs of osteoarthritis were detected from MRI of the knee. We used the Anterior Knee Pain Score21 to evaluate subjective symptoms and functional limitations in the PF pain subjects (Table 1); a score of 100 indicated no pain or disability. Subjects were informed on all aspects of the study and provided prior consent according to the policies of our Institutional Review Board.
Table I.
Population characteristics of the pain-free controls and PF pain subjects.
| Patellofemoral pain (n = 39) | Pain-free control (n = 15) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age (years) | 28.4 | 3.8 | 22.0 – 35.0 | 30.9 | 5.8 | 50.0 – 19.0 |
| Height (meters) | 1.72 | 0.09 | 1.58 – 1.91 | 1.73 | 0.09 | 1.57 – 1.93 |
| Weight (kilograms) | 65.3 | 8.8 | 52.1 – 77.3 | 67.7 | 11.4 | 46.5 – 90.7 |
| Anterior Knee Pain Score | 72 | 14 | 42 – 97 | NA | NA | NA |
Anterior Knee Pain Score18 was used to evaluate subjective symptoms and functional limitations in the PF pain subjects.
Classification of Subjects based on Patellar Tracking Measures
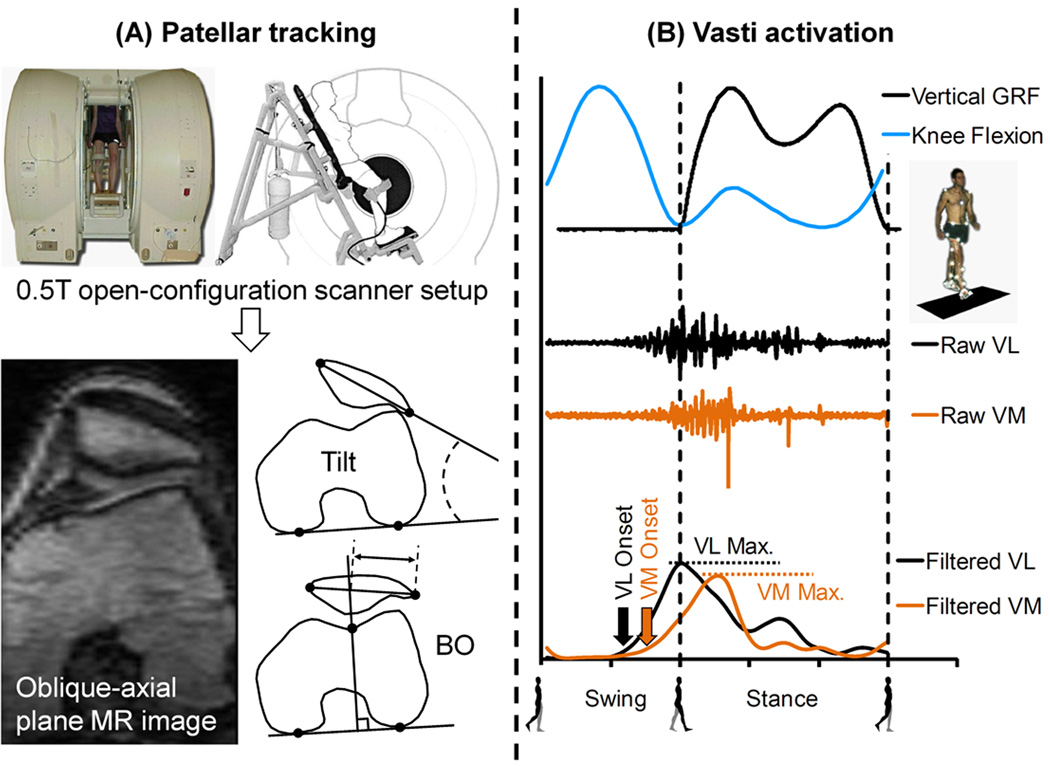
Subjects were classified into normal tracking and maltracking groups based on patellar tracking measures obtained from weightbearing MRI (Fig. 1).25 A subject’s PF joint was imaged in an upright, weightbearing posture using an open-configuration MRI scanner (0.5T SP/i, GE Healthcare, Milwaukee, WI). The subjects were requested to maintain the upright pose (at ~5° knee flexion without locking their knees) with both legs evenly loaded during scanning, and were assisted by a custom-built low-friction backrest that required a subject to support ~ 90% of his/her weight (Fig. 1A). The scan parameters were: repetition time, 33 millisecs; echo time, 9 millisecs; flip angle, 45°; matrix, 256 X 160 interpolated to 256 X 256; field of view, 20 X 20 cm; slice thickness, 2 mm. The scan time was ~2 mins; all subjects could maintain the upright position during the scan.
Figure 1.
Measurement of (A) patellar tracking, and (B) muscle EMG activity. (A) Subjects were scanned in an open-configuration MRI scanner, and oblique-axial plane images were used to calculate patellar tilt and bisect offset (BO). (B) Vertical ground reaction force (GRF), knee flexion, and raw and filtered EMG activity for vastus lateralis (VL) and vastus medialis (VM) muscles were measured during walking. Raw and filtered EMG data were synchronized with GRF and knee flexion data.
2D patellar tracking measures, patellar tilt and bisect offset, were measured from the weightbearing MRI data (Fig. 1A). An oblique-axial plane image was identified from the 3D MRI volume; this was done to maintain consistency with previous studies.11,12,25 The oblique-axial plane was prescribed to intersect the center of the patella and the most posterior points on the femoral condyles. Anatomical landmarks were identified on this image; the landmarks included the most lateral and most medial points on the patella, the most posterior points on the condyles, and the deepest point of the trochlear groove (Fig. 1A).12,25 Patellar tilt, defined as the angle between the patella and the posterior condyles, was used to measure patellar internal-external rotation relative to the femur. A more positive angle indicated greater external rotation of the patella relative to the femur. Bisect offset, defined as the percentage of the patella lateral to the midline of the femur,12 was used to measure medial-lateral position of the patella relative to the femur. A greater bisect offset percentage indicated a more lateral position of the patella. All MRI measurements were performed by a single investigator; the average intraobserver variance between measurements due to the selection of the oblique-axial plane and anatomical landmarks was 2° for patellar tilt and 4% for bisect offset.25
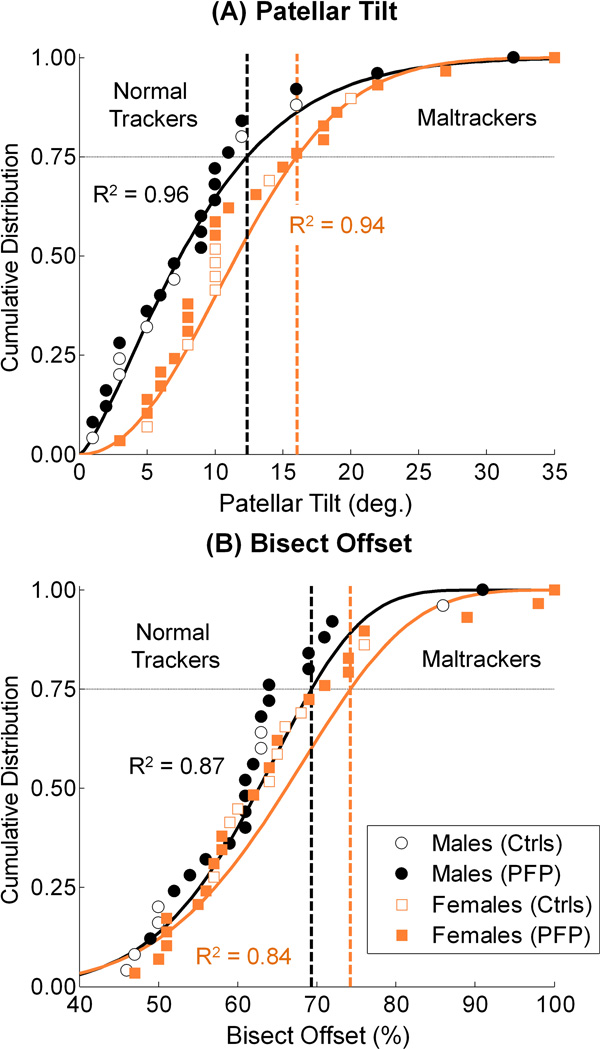
A sex-specific classification technique was developed to categorize the PF pain and pain-free subjects into normal tracking and maltracking groups (Fig. 2). A sex-specific classification was needed because of differences in patellar tracking measures between sexes.25 Due to skewness in their probability density functions, the measured patellar tilts and bisect offsets were best fit with a 2-parameter Weibull model; the coefficients of determinations (R2) were ≥ 0.84 for all cases (Fig. 2). The model includes a scale parameter, k(β) and a shape parameter, w(η).17 The shape parameter gives the distribution the flexibility to model probability distribution functions having either symmetric or skewed shapes. Because of this feature, 2-parameter Weibull distributions provided substantially better fits to our tracking measures, which we previously showed to have skewed distributions.25 The scale and shape parameter values corresponding to our distribution fits were 9.74 and 1.37 (males tilt), 13.76 and 2.14 (females tilt), 66.11 and 6.89 (males bisect offset), and 70.31 and 6.02 (females bisect offset). The Weibull distribution is commonly used to model biological and engineering phenomena. This classification is a robust method to model observed population variability and to incorporate patellar tracking data from new subjects as we recruit them in our on-going study. Sex-specific maltracking thresholds were defined as the tilt and offset values corresponding to the 75th percentile of the distributions; a subject was classified as a maltracker if his/her patellar tilt or bisect offset value was in the highest quartile of measured population data.25
Figure 2.
Sex-specific classification of pain-free controls (Ctrls) and PF pain (PFP) subjects into normal tracking and maltracking groups based on (A) patellar tilt and (B) bisect offset. The tilt and offset data were best fit with a 2-parameter Weibull model (solid lines, R2 ≥ 0.84). The values corresponding to the 75th percentile (horizontal dashed lines) were defined as the maltracking thresholds; a subject was classified as a maltracker if his/her tilt or offset value was greater than the sex-specific thresholds (vertical dashed lines).
Measurement of VL:VM Activation Ratio and VM Activation Delay
We measured VL:VM activation ratio and VM activation delay in all subjects during walking at self-selected speeds. We analyzed the symptomatic knee for the PF pain subjects or selected a knee at random for the pain-free controls. All subjects had a minimum of 3 trials during which the entire foot of the measured limb contacted a force plate . Retro-reflective markers were placed on lower limb landmarks,20 and 3D marker trajectories were measured at 60 Hz using a 6-camera motion capture system (Motion Analysis Corp., Santa Rosa, CA). Ground reaction forces were measured at 2400 Hz from a force plate (Bertec Corp., Columbus, OH). Marker trajectories were low-pass filtered using a zero-lag 4th-order Butterworth filter with a cut-off frequency of 15Hz, and lower limb joint kinematics were calculated.7 EMG signals were recorded using a multi-channel system (Motion Lab Systems, Baton Rouge, LA), and surface electrodes were placed on the VM and VL muscles.26 Subjects performed 5 trials of maximum isometric muscle contractions to elicit maximum activation of the quadriceps muscles; while seated on a chair with the knees at ~80° flexion, a subject was instructed to extend his/her symptomatic knee against the resistance of the tester. The peak EMG value from all 5 trials was assigned as a muscle’s maximum activation. Resting EMG signals were recorded with the subjects seated and relaxed. Raw EMG signals from the walking trials were high-pass filtered using a zero-lag 4th-order recursive Butterworth filter at 30Hz, and then full-wave rectified and filtered using a Butterworth low-pass filter at 6Hz. Muscle activations collected during walking were normalized to the maximum activation values collected during the maximum contraction trials for each muscle.
Vasti EMG activations were analyzed with toe-off, the initiation of swing phase, defined as the beginning of a gait cycle (Fig. 1B). EMG data for the VM and VL muscles were synchronized with vertical ground reaction force and knee flexion angle to determine muscle activation onset times relative to heel strike. Muscle activations were detected using a threshold function: a muscle was determined to be “on” if its EMG signal exceeded the greater of 3 std devs of its resting EMG value or 2% of the larger peak activation between the VM and VL muscles.25 The 3 std devs from rest threshold alone produced multiple spurious EMG onset times prior to heelstrike in some subjects, while in some subjects with weak VM activation, neither the 3 std devs from rest nor the 2% of VM activation identified the burst of activity prior to heelstrike. We found the combination provided a reliable method to identify the clear burst of activity of each muscle prior to heel strike.
VL:VM activation ratio was determined from peak normalized activations of the muscles over the entire gait cycle.27 A VL:VM activation ratio >1.0 represents a larger normalized activation magnitude of the VL muscle. Vastus medialis activation delay was calculated as the difference between the VL and VM activation onset times. A positive VM delay indicated activation of the VL prior to VM activation. The VL:VM activation ratio and VM activation delay measurements from all gait trials of a subject were averaged.
We evaluated the relationships between VL:VM activation ratio and patellar tracking measures, and VM activation delay, in pain-free controls and all PF pain subjects grouped together, and in PF pain subjects classified as normal trackers and maltrackers. Linear regression models were used to test for the significance of a relationship (p < 0.05). Average VL:VM activation ratios were compared between the pain-free controls and all PF pain subjects grouped together, and between the pain-free controls and PF pain subjects classified into maltracking and normal tracking groups. Significant differences between the groups were assessed with two-tailed, unpaired t-tests (p < 0.05 for the pain-free controls and all PF pain subjects grouped together; p < 0.017 post Bonferroni correction for the pain-free controls and PF pain subjects classified into maltracking and normal tracking groups).
RESULTS
Sex-specific maltracking thresholds corresponding to the 75th percentiles were 12.4° (males) and 16.0° (females) for patellar tilt (Fig. 2A), and 69.3% (males) and 74.2% (females) for bisect offset (Fig. 2B). Ten of 39 PF pain subjects were classified as maltrackers using these thresholds (4 males, 6 females). Among the 10, 6 had both abnormal tilt and abnormal bisect offset, 3 had only abnormal tilt, and 1 had only abnormal bisect offset.
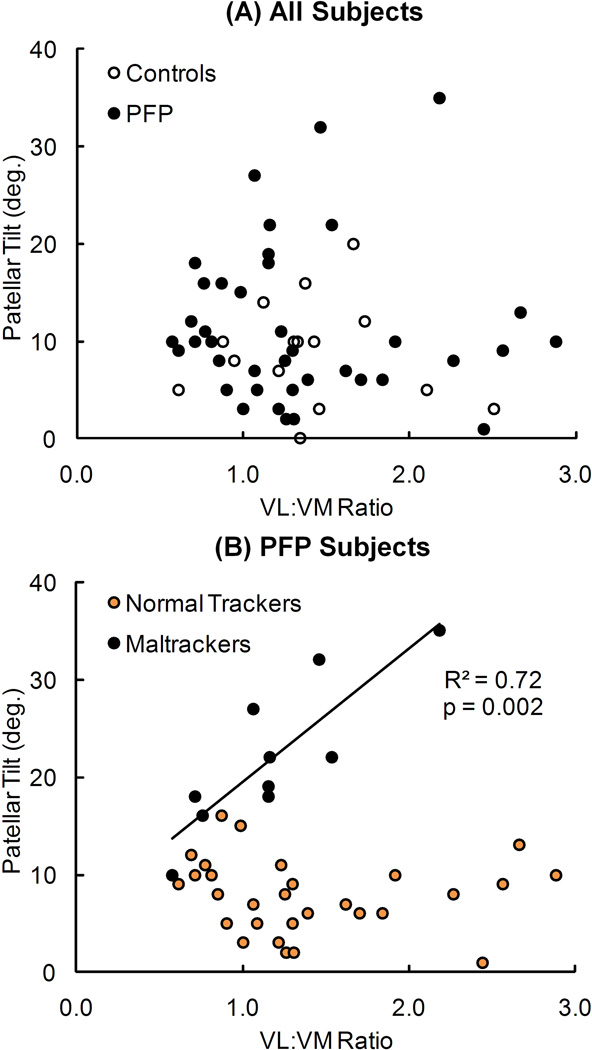
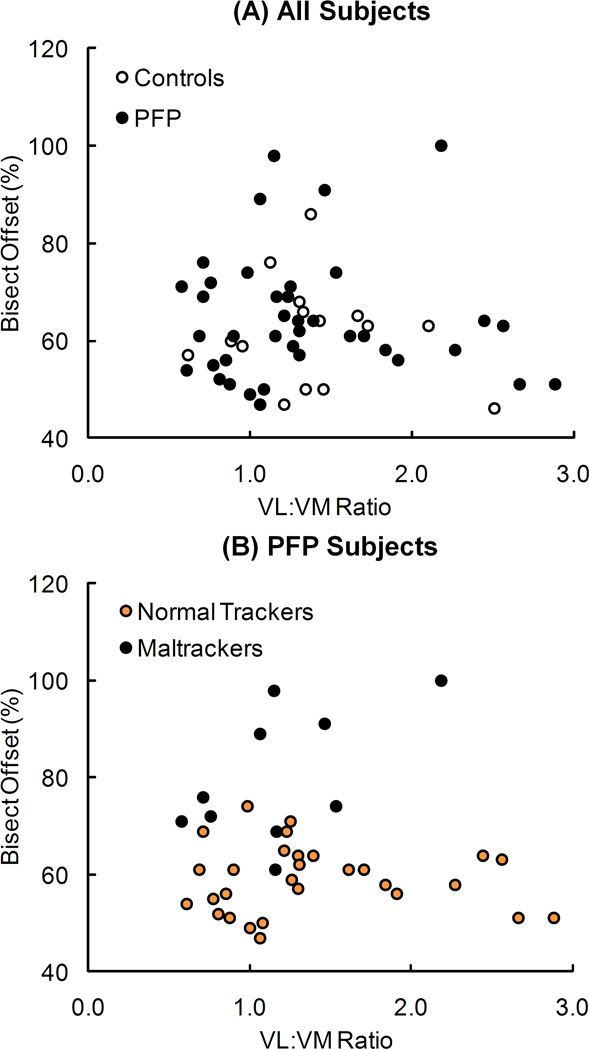
Patellar tilt was significantly correlated with VL:VM activation ratio only in maltracker PF pain subjects (R2 = 0.72, p = 0.002) (Fig. 3). No significant correlations occurred between patellar tilt and VL:VM activation ratio in the pain-free controls, all PF pain subjects grouped together, and normal tracker PF pain subjects. There were no significant correlations between bisect offset and VL:VM activation ratio for any of the groups evaluated (Fig. 4).
Figure 3.
Relationship between patellar tilt and VL:VM activation ratio for (A) pain-free controls and all PF pain (PFP) subjects, and (B) PFP subjects classified into normal tracking and maltracking groups. The regression line represents a significant relationship (R2 = 0.72, p = 0.002) in maltracking PFP subjects. No significant relationships existed between tilt and VL:VM activation ratio in pain-free controls (R2 = 0.01, p = 0.70), all PFP subjects (R2 < 0.01, p = 0.949), or normal tracking PFP subjects (R2 = 0.01, p = 0.652).
Figure 4.
Relationship between bisect offset and VL:VM activation ratio for (A) pain-free controls and all PF pain (PFP) subjects, and (B) PFP subjects classified into normal tracking and maltracking groups. No significant relationships existed between offset and VL:VM activation ratio in pain-free controls (R2 = 0.03, p = 0.551), all PFP subjects (R2 < 0.01, p = 0.821), normal tracking PFP subjects (R2 < 0.01, p = 0.761), or maltracking PFP subjects (R2 = 0.30, p = 0.102).
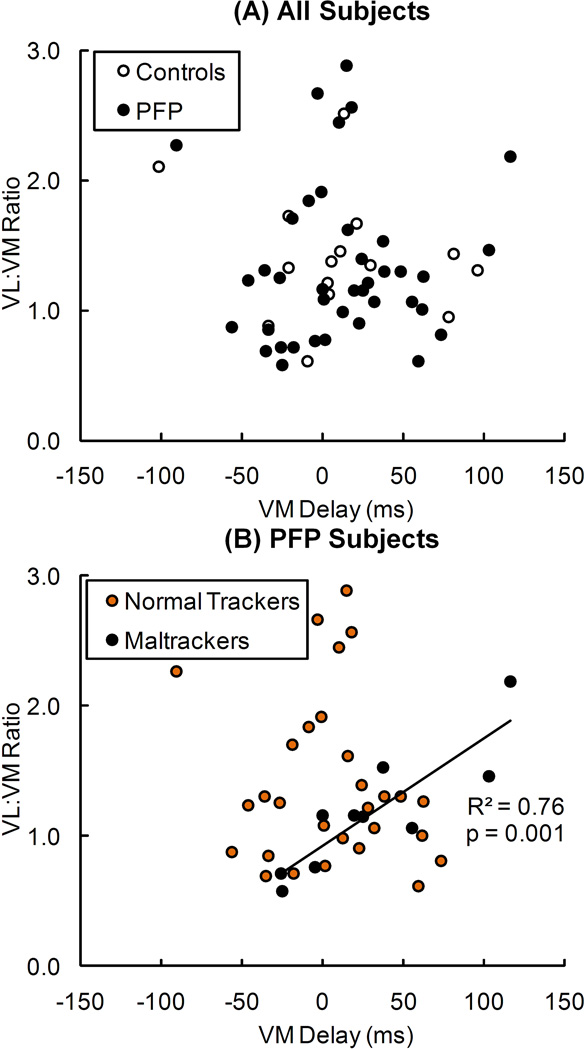
VL:VM activation ratio was significantly correlated with VM activation delay only in maltracker PF pain subjects (R2 = 0.76, p = 0.001) (Fig. 5). No significant correlations occurred between VL:VM activation ratio and VM activation delay in the pain-free controls, all PF pain subjects grouped together, and PF pain subjects classified as normal trackers.
Figure 5.
Relationship between VL:VM activation ratio and VM activation delay in (A) pain-free controls and all PF pain (PFP) subjects, and (B) PFP subjects classified into normal tracking and maltracking groups. The regression line represents a significant relationship (R2 = 0.76, p = 0.001) in maltracking PFP subjects. No significant relationships existed between VL:VM activation ratio and VM activation delay in pain-free controls (R2 = 0.06, p = 0.399), all PFP subjects (R2 = 0.01, p = 0.548), or normal tracking PFP subjects (R2 = 0.01, p = 0.659).
No differences were found in the means of VL:VM activation ratios between the pain-free controls and all PF pain subjects (p = 0.724). Average ± SD VL:VM activation ratios for pain-free controls and all PF pain subjects were 1.40 ± 0.47 and 1.34 ± 0.60, respectively. No significant differences were found in the means of VL:VM activation ratios between the pain-free controls and maltracker PF pain subjects (p = 0.254), between the pain-free controls and normal tracking PF pain subjects (p = 0.979), and between the maltracking and normal tracking PF pain subjects (p = 0.325).
DISCUSSION
Our results demonstrate a relationship between patellar tilt and VL:VM activation ratio only in PF pain subjects classified as maltrackers (Fig. 3), providing new evidence relating maltracking to vasti activation imbalance using a combination of weightbearing MRI and gait analysis. Although studies have reported large VL:VM activation ratio31 in PF pain subjects compared to pain-free controls, evidence relating VL:VM activation ratio to patellar maltracking is limited. One previous study27 concluded that maltracking was not related to VM activation imbalance, and reported an inverse relationship between vasti activation ratio and patellar tracking.27 Our conclusions differ from this study, probably because the previous study evaluated patellar tracking during supine MRI under minimal (~15% body weight) weightbearing conditions and because in the previous study, all PF pain subjects were grouped together. When we lump all the pain-free controls and PF pain subjects together, we find an inverse relationship between vasti activation ratio and patellar tracking, consistent with the previous study.27
Our study clarifies the prevalence of vasti activation imbalance in PF pain subjects. No consensus exists regarding altered VL:VM activation ratio in PF pain subjects compared to pain-free controls. Previous studies tested the differences between the means of VL:VM activation ratios for pain-free controls and all PF pain subjects grouped together. Using this test, Santos et al.29 and Souza and Gross31 concluded that PF pain subjects exhibit abnormal VL:VM activation ratios compared to pain-free controls, while Powers et al.28 reported no difference between the two groups. We found no difference between VL:VM activation ratios for pain-free controls and all PF pain subjects grouped together. Powers et al.28 attributed the conflicting findings to methodological differences among studies, such as EMG normalization. We theorize that the conflicting findings may be due to the varying percentage of maltracking PF pain subjects recruited in these studies. In our study, only ~26% (10 of 39) PF pain subjects were maltrackers. Santos et al.29 and Souza and Gross31 studies might have included a greater percentage of maltracking PF pain subjects than our or the Powers et al.28 study, resulting in significant differences in means of VL:VM activation ratios between the two groups.
Although normal tracking PF pain subjects displayed a large range of VL:VM activation ratios (Fig. 3), no correlations exitsed between tracking measures and activation ratios in normal trackers. A possible explanation is differences in PF joint geometry,33 patella alta alignment,8 or abnormal tensioning in the lateral retinacula5 and/or the medial PF ligament1 between subjects classified as normal trackers and maltrackers. As a result, tracking measures may be insensitive to VM activation imbalance in normal tracking PF pain subjects. Our study complements previous work done in understanding the role of the vasti muscles in PF pain subjects, including contributions to knee extension torque,24 relative activations during open and closed kinetic chain exercises,32 and relationships between muscle cross section area, insertion location, and fiber orientation to patellar tracking.19,22,23
We also investigated potential relationships between VL:VM activation ratio and VM activation delay. We found a relationship between the two only in maltracking PF pain subjects (Fig. 5). Measurement of VM activation delay during functional tasks requires synchronization of EMG data with joint kinematics and ground reaction forces.25 However, VL:VM activation ratio can be obtained by attaching surface EMG electrodes on a patient without the need to measure joint motion or ground reaction force and is easier to perform in clinical settings.
A limitation of our study is that patellar tracking measures and vasti activations were measured during separate activities. It is difficult to acquire simultaneous VM activation delay and VL:VM activation ratio measurements during a backrest-assisted weightbearing squat; the vasti muscle are active as soon as a subject positions himself/herself against the backrest. Reproducing a walking activity during MRI is not feasible. Another potential limitation is that we measured the activity of the entire VM muscle and not the isolated vastus medialis oblique (VMO) fibers.27–29 It is difficult to distinguish between the VMO and vastus medialis longus activations using surface electrodes, and a recent article concluded that there is insufficient evidence to isolate the VM into separate bundles.30 A third potential limitation is that our results were based on data acquired from an open-configuration MRI scanner, an expensive device available in few clinics around the world. A clinically feasible method for accurate classification of subjects based on patellar tracking measured under weightbearing conditions remains a challenge. Another potential limitation is our definition of the 75th percentile of the Weibull model as maltracking thresholds; although this choice is subjective, small changes to the maltracking thresholds (e.g., ± 5 percentage points) do not change the significant relationships reported in our study. In addition, we cannot rule out the influence of increased medial femoral rotation due to altered muscle recruitment at the hip6 or excessive foot pronation13 on patellar maltracking in our subjects.
We showed a direct relationship between patellar tracking and VL:VM activation ratio in a subset of the PF pain population, those classified as maltrackers. Our results imply that clinical interventions targeting vasti muscle imbalance may improve patellar tracking only in maltracking subjects, highlighting the importance of accurate classification of PF pain subjects prior to the selection of a clinical intervention.
ACKNOWLEDGEMENTS
Financial support provided by NIH (EB005790-05) and the Office of Research and Development (Rehabilitation R&D Service grant #A2592R), Department of Veterans Affairs.
REFERENCES
- 1.Amis AA, Firer P, Mountney J, et al. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10:215–220. doi: 10.1016/s0968-0160(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 2.Baldini A, Anderson JA, Cerulli-Mariani P, et al. Patellofemoral evaluation after total knee arthroplasty. Validation of a new weight-bearing axial radiographic view. J Bone Joint Surg Am. 2007;89:1810–1817. doi: 10.2106/JBJS.E.00432. [DOI] [PubMed] [Google Scholar]
- 3.Bigley RF, Gibeling JC, Stover SM, et al. Volume effects on fatigue life of equine cortical bone. J Biomech. 2007;40:3548–3554. doi: 10.1016/j.jbiomech.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Brechter JH, Powers CM. Patellofemoral joint stress during stair ascent and descent in persons with and without patellofemoral pain. Gait Posture. 2002;16:115–123. doi: 10.1016/s0966-6362(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 5.Clifton R, Ng CY, Nutton RW. What is the role of lateral retinacular release? J Bone Joint Surg Br. 2010;92:1–6. doi: 10.1302/0301-620X.92B1.22909. [DOI] [PubMed] [Google Scholar]
- 6.Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43:584–588. doi: 10.1136/bjsm.2008.053553. [DOI] [PubMed] [Google Scholar]
- 7.Crowninshield RD, Brand RA. A physiologically based criterion of muscle force prediction in locomotion. J Biomech. 1981;14:793–801. doi: 10.1016/0021-9290(81)90035-x. [DOI] [PubMed] [Google Scholar]
- 8.Davies AP, Costa ML, Donnell ST, et al. The sulcus angle and malalignment of the extensor mechanism of the knee. Journal of Bone and Joint Surgery-British. 2000;Volume 82B:1162–1166. doi: 10.1302/0301-620x.82b8.10833. [DOI] [PubMed] [Google Scholar]
- 9.DeHaven KE, Lintner DM. Athletic injuries: comparison by age, sport, and gender. Am J Sports Med. 1986;14:218–224. doi: 10.1177/036354658601400307. [DOI] [PubMed] [Google Scholar]
- 10.Devereaux MD, Lachmann SM. Patello-femoral arthralgia in athletes attending a Sports Injury Clinic. Br J Sports Med. 1984;18:18–21. doi: 10.1136/bjsm.18.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Draper CE, Besier TF, Fredericson M, et al. Differences in patellofemoral kinematics between weight-bearing and non-weight-bearing conditions in patients with patellofemoral pain. J Orthop Res. 2011;29:312–317. doi: 10.1002/jor.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Draper CE, Besier TF, Santos JM, et al. Using real-time MRI to quantify altered joint kinematics in subjects with patellofemoral pain and to evaluate the effects of a patellar brace or sleeve on joint motion. J Orthop Res. 2009;27:571–577. doi: 10.1002/jor.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffey MJ, Martin DF, Cannon DW, et al. Etiologic factors associated with anterior knee pain in distance runners. Med Sci Sports Exerc. 2000;32:1825–1832. doi: 10.1097/00005768-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Elias JJ, Cech JA, Weinstein DM, Cosgrea AJ. Reducing the lateral force acting on the patella does not consistently decrease patellofemoral pressures. Am J Sports Med. 2004;32:1202–1208. doi: 10.1177/0363546503262167. [DOI] [PubMed] [Google Scholar]
- 15.Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30:447–456. doi: 10.1177/03635465020300032501. [DOI] [PubMed] [Google Scholar]
- 16.Guo Z, De Vita R. Probabilistic constitutive law for damage in ligaments. Med Eng Phys. 2009;31:1104–1109. doi: 10.1016/j.medengphy.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Haldar A, Mahadevan S. Probability, Reliability and Statistical Methods in Engineering Design. New York, NY: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 18.Hurschler C, Provenzano PP, Vanderby R., Jr Application of a probabilistic microstructural model to determine reference length and toe-to-linear region transition in fibrous connective tissue. J Biomech Eng. 2003;125:415–422. doi: 10.1115/1.1579046. [DOI] [PubMed] [Google Scholar]
- 19.Jan MH, Lin DH, Lin JJ, et al. Differences in sonographic characteristics of the vastus medialis obliquus between patients with patellofemoral pain syndrome and healthy adults. Am J Sports Med. 2009;37:1743–1749. doi: 10.1177/0363546509333483. [DOI] [PubMed] [Google Scholar]
- 20.Kadaba MP, Ramakrishnan HK, Wooten ME. Measurement of Lower Extremity Kinematics During Level Walking. Journal of Orthopaedic Research. 1990;8:383–392. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- 21.Kujala UM, Jaakkola LH, Koskinen SK, et al. Scoring of patellofemoral disorders. Arthroscopy. 1993;9:159–163. doi: 10.1016/s0749-8063(05)80366-4. [DOI] [PubMed] [Google Scholar]
- 22.Lin YF, Lin JJ, Cheng CK, et al. Association between sonographic morphology of vastus medialis obliquus and patellar alignment in patients with patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2008;38:196–202. doi: 10.2519/jospt.2008.2568. [DOI] [PubMed] [Google Scholar]
- 23.Lin YF, Lin JJ, Jan MH, et al. Role of the vastus medialis obliquus in repositioning the patella: a dynamic computed tomography study. Am J Sports Med. 2008;36:741–746. doi: 10.1177/0363546507312171. [DOI] [PubMed] [Google Scholar]
- 24.Makhsous M, Lin F, Koh JL, et al. In vivo and noninvasive load sharing among the vasti in patellar malalignment. Med Sci Sports Exerc. 2004;36:1768–1775. doi: 10.1249/01.mss.0000142302.54730.7f. [DOI] [PubMed] [Google Scholar]
- 25.Pal S, Draper CE, Fredericson M, et al. Patellar Maltracking Correlates With Vastus Medialis Activation Delay in Patellofemoral Pain Patients. Am J Sports Med. 2011;39:590–598. doi: 10.1177/0363546510384233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perotto A, Delagi EF, Iazzetti J, Morrison D. Anatomical Guide for the Electromyographer. Springfield, IL: Charles C. Thomas; 2005. [Google Scholar]
- 27.Powers CM. Patellar kinematics, part I: the influence of vastus muscle activity in subjects with and without patellofemoral pain. Phys Ther. 2000;80:956–964. [PubMed] [Google Scholar]
- 28.Powers CM, Landel R, Perry J. Timing and intensity of vastus muscle activity during functional activities in subjects with and without patellofemoral pain. Phys Ther. 1996;76:946–955. doi: 10.1093/ptj/76.9.946. discussion 956-967. [DOI] [PubMed] [Google Scholar]
- 29.Santos EP, Bessa SNF, Lins CAA, et al. Electromyographic activity of vastus medialis obliquus and vastus lateralis muscles during functional activities in subjects with patellofemoral pain syndrome. Revista Brasileira De Fisioterapia. 2008;12:304–310. [Google Scholar]
- 30.Smith TO, Nichols R, Harle D, Donell ST. Do the Vastus Medialis Obliquus and Vastus Medialis Longus Really Exist? A Systematic Review. Clinical Anatomy. 2009;22:183–199. doi: 10.1002/ca.20737. [DOI] [PubMed] [Google Scholar]
- 31.Souza DR, Gross MT. Comparison of vastus medialis obliquus: vastus lateralis muscle integrated electromyographic ratios between healthy subjects and patients with patellofemoral pain. Phys Ther. 1991;71:310–316. doi: 10.1093/ptj/71.4.310. discussion 317-320. [DOI] [PubMed] [Google Scholar]
- 32.Tang SF, Chen CK, Hsu R, et al. Vastus medialis obliquus and vastus lateralis activity in open and closed kinetic chain exercises in patients with patellofemoral pain syndrome: an electromyographic study. Arch Phys Med Rehabil. 2001;82:1441–1445. doi: 10.1053/apmr.2001.26252. [DOI] [PubMed] [Google Scholar]
- 33.Wu SH, Chu NK, Liu YC, et al. Relationship between the EMG ratio of muscle activation and bony structure in osteoarthritic knee patients with and without patellar malalignment. J Rehabil Med. 2008;40:381–386. doi: 10.2340/16501977-0178. [DOI] [PubMed] [Google Scholar]