Abstract
Objectives
Identifying the genes responsible for chemotherapy toxicity in Drosophila melanogaster may allow for the identification of human orthologs that similarly mediate toxicity in humans. In order to develop Drosophila melanogaster as a model of dissecting chemotoxicity, we first need to develop standardized high throughput toxicity assays and prove that inter-individual variation in toxicity as measured by such assays is highly heritable.
Methods
We developed a method for the oral delivery of commonly used chemotherapy drugs to Drosophila. Post-treatment female fecundity displayed a dose dependent response to varying levels of the chemotherapy drug delivered. We fixed the dose for each drug at a level that resulted in a 50% reduction in fecundity and used a paternal half-sibling heritability design to calculate the heritability attributable to chemotherapy toxicity assayed via a decrease in female fecundity. Chemotherapy agents tested were carboplatin, floxuridine, gemcitabine hydrochloride, methotrexate, mitomycin C, and topotecan hydrochloride.
Results
We found that six currently widely prescribed chemotherapeutic agents lowered fecundity in D. melanogaster in both a dose dependent and highly heritable manner. The following heritability estimates were found: carboplatin – 0.72, floxuridine – 0.52, gemcitabine hydrochloride – 0.72, methotrexate – 0.99, mitomycin C – 0.64, and topotecan hydrochloride – 0.63.
Conclusions
The high heritability estimates observed in this study, irrespective of the particular class of drug examined, suggest that human toxicity may also have a sizable genetic component.
Keywords: Genetic polymorphism, carboplatin, floxuridine, gemcitabine hydrochloride, methotrexate, mitomycin C, topotecan hydrochloride, chemotherapy toxicity, heritability
INTRODUCTION
Chemotherapy agents, most often used in cancer therapy, are some of the most toxic medications administered to humans [1]. Many patients are forced to switch medications and undergo extensive dose adjustments before they find an appropriate cancer therapy cocktail; this process can be painful for the patient, can take a long time, and meanwhile the disease progresses [2]. Chemotherapy induced toxicity is variable among patients, this variation has largely been attributed to the type of drug, age, gender, and diet of the patient, disease development, tumor biology, and other environmental factors [2, 3, 4, 5, 6, 7]. Others argue that individual treatment responses may be complex genetic traits controlled by several genes in concert with the environment[8]. Although there are many known genetic polymorphisms associated with chemotherapy induced toxicity in humans (reviewed in Supplementary Table 1), for any given drug all such known polymorphisms only account for a small fraction of the total variation in toxicity. The failure of known polymorphisms to explain the bulk of toxicity variation could be because toxicity has a low heritability, or alternatively toxicity may be like many other complex traits in humans where the bulk of known heritable variation cannot be explained by known polymorphisms. In order to distinguish between these two possibilities, heritability estimates are required.
Directly estimating the heritability (i.e., the proportion of the phenotypic variation in a trait attributable to transmittable genetic factors[10]) of chemotherapy treatment outcomes in humans is difficult, if not impossible. Healthy human patients cannot be ethically given chemotherapeutic agents at the doses required to carry out such a study, and cohorts of cancer patients given identical dosing and treatment schedules are rare. In addition, hundreds of pairs of blood-related patients (for example full-sibs) each receiving the same treatment for the same length of time, under similar environmental conditions are rarely available. A comprehensive heritability study can be easily performed in a model system, such as D. melanogaster. Drosophila melanogaster has been proposed as a model to screen for drug induced reproductive adverse effects due to its similarities to the mammalian reproductive system, including putative sex hormones and conserved proteins involved in genitourinary development[11]. In addition in instances in which we know the genetic pathway mediating a drug’s uptake, there is often one-to-one gene conservation of that pathway between humans and flies. For example the primary genes that mediate MTX efficacy (i.e., ABCB1, ABCB4, ABCB5, ABCB11, RFC1, FPGS, GGH, TYMS, DHFR, ATIC, MTHFR, and MTR) each have a single ortholog in flies (namely CG10181, CG10226, CG3879, CG8523, CG1119, CG2543, CG32155, CG3181, CG14887, CG11089, CG7560, CG10621, and CG10623). This fact is not surprising, as chemotherapy drugs tend to target fairly basal cell-level processes. Nonetheless high heritability estimate of the toxicity phenotype in flies suggests that chemotoxic drug targets can be identified in Drosophila, these genes may be worth a closer examination in humans.
The majority of chemotherapeutic agents work by blocking rapidly dividing cell processes, such as cancerous growths[12]. In adult D. melanogaster, perhaps the only naturally rapidly diving cell processes are oogenesis and spermatogenesis. Therefore, we predict the number of eggs a female D. melanogaster is able to produce, or the number of offspring a male can produce, following a controlled bout of chemotherapy will monotonically depend on the delivered dose. Of course, once a suitable model of chemotoxicity is developed in flies, heritability estimates[10], QTL [14; http://www.flyrils.org] and association mapping (http://mackay.gnets.ncsu.edu/MackaySite/DGRP.html), and genetic manipulations are more routine than in most other systems[15].
We developed a system for delivering chemotherapeutic drugs to flies and assaying their impact on male spermatogenesis and/or female oogenesis. We used a half-sib breeding design to estimate the heritability of toxicity for the six drugs that appeared to primarily impact oogenesis. We observed atypically high levels of genetic, relative to environmental, variation for the toxicity of six different commonly used chemotherapy medications in Drosophila melanogaster. The highly heritable nature of chemotoxicity response opens up the possibility of identifying the genes mediating this response using the powerful genetic tools available in flies. For the subset of those genes having clear human orthologs, those orthologs are strong candidates for similarly mediating toxicity in humans. Irrespective of the genetic details of chemotoxicity in flies, the observation that the post treatment reduction in fecundity are among the most heritable characters ever observed in Drosophila suggests that a large fraction of the inter-individual variation in efficacy and toxicity may be similarly genetic in origin in humans.
METHODS
Drosophila melanogaster Population Genetics
Drosophila melanogaster populations used for this experiment were taken from two outbred populations derived from a set of 15 inbred P-free stocks representing a worldwide sample obtained from the Bloomington and Tucson stock centers[14]. All dosing and drug delivery experiments were performed on flies from generations 40–50 post-founder of these populations (http://www.flyrils.org). All heritability experiments were performed on flies from generations 55–68 of these populations. For each drug, the dosing and heritability experiments were performed on the same population (population A was used for methotrexate, mitomycin C, and topotecan experiments; population B was used for gemcitabine hydrochloride, floxuridine, carboplatin, cisplatin, and cytribine experiments).
Drug Therapy Assay – Drug Delivery and Dose Response
We carried out dosing experiments in order to determine the dose response effect of each chemotherapy agent. The cancer chemotherapeutic agents used are pure chemical compounds of carboplatin, floxuridine, gemcitabine hydrochloride, methotrexate, mitomycin C and topotecan, obtained from LKT Laboratories, Inc. (lktlabs.com). Eight doses (varying 36-fold plus a placebo, no drug, control) of each chemotherapy agent were mixed with liquid food (Supplementary Table 3), spread onto a 1 cm × 2 cm piece of Whatman filter paper (cat.# 3030 392), and placed into an agar plug at the bottom of a plastic exposure vial. Twelve 3-day-old males and females were placed in each of six exposure vials per dose, exposed to drug/food or placebo/food filter papers for three days, and then transferred to recovery vials (Supplementary Table 4) for one day. Following recovery, four females were chosen at random from each recovery vial and placed individually in separate “lay-out” vials (Supplementary Table 4), allowed to lay eggs for three days, and discarded. Two-weeks after the mothers were discarded from the lay-out vials the offspring were recovered and counted. In order to validate that oral delivery of food, we also carried out injections of chemotherpeutic agents into the haemolymph of the Drosophila [16]. We could achieve similar levels of fecundity knockdown for both, injected and orally treated flies by varying the dose (Supplementary Figure 1). We further observed that the variation in fecundity between 200 outbred flies subjected to one assay or the other were not significantly different between assays (p>0.07 via an F-test on log-transformed data. This led to our adoption of the simpler oral delivery method.
Drug Therapy Assay – Heritability
A single male was mated to five virgin females, of which three females were chosen at random to lay eggs in vials with food (recipe is listed in Supplementary Table 4). Three offspring from each female were chosen at random and separately exposed to a chemotherapy treatment. Chemotherapy agents were mixed with liquid food to achieve the final concentration (molarity) given in Table 1 and delivered the same way as in the dose response experiment. After three days of exposure, the females were placed in separate recovery vials with two males in each vial for three days. Adult flies were discarded after three days and their offspring were allowed to mature for 14 days. Adult offspring of each female were counted.
Table 1.
Chemotherapeutic drugs examined, effects on female fecundity and heritability
| Chemotherapy Agent Delivery | Results of Chemotherapy Delivery | Heritability | ||||
|---|---|---|---|---|---|---|
| Name | Molarity (mM)1 | Fertility Reduction2 | Females with | Standard Error4 | ||
| 95% CI | Zero Fertility | Heritability3 | ||||
| Methotrexate | 0.110 | 74% | [70, 77] | 6.30% | 0.99 | 0.148 |
| Gemcitabine HCl | 0.334 | 60% | [56, 64] | 2.68% | 0.72 | 0.157 |
| Carboplatin | 0.404 | 41% | [38, 44] | 2.84% | 0.72 | 0.106 |
| Mitomycin C | 1.346 | 66% | [62, 70] | 14.08% | 0.64 | 0.157 |
| Topotecan HCl | 0.109 | 42% | [38, 46] | 2.33% | 0.63 | 0.183 |
| Floxuridine | 0.041 | 55% | [51, 58] | 0.37% | 0.52 | 0.153 |
| No Drug | 0.000 | 0%2 | [−4, 4] | 4.17% | 0.32 | 0.145 |
In the liquid food suspension delivered to the flies.
Relative to mock, drug free, fertility. The average number of offspring in the mock treatment was 78.
Calculated using the described half-sib cross.
Standard errors calculated according to Becker, pg 48[13]
Statistical Analysis
We used a half-sib breeding design to estimate the narrow sense heritability for each of eight drugs and their respective controls (placebo). Each heritability experiment consisted of 30 sires each mated to 3 dams with 3 of each dam’s female offspring exposed to a drug. We carried out a Nested Analysis of Variance on ln(φ+1), where φ is the observed fecundity of each female, in R (http://www.r-project.org/). We employed a log transformation to the raw fecundity as the variance in fecundity appeared to be a function of the average fecundity, a commonly phenomena for complex traits [10]. Observed mean squares were equated to their expectations, variance components estimated, and narrow sense heritability estimated as four times the variance between half-sib families divided by the total phenotypic variance [10]. Standard errors on the heritability were estimated based on the mean square values for the dams and sires and the total variance (Becker, pg 47–48) [13].
RESULTS
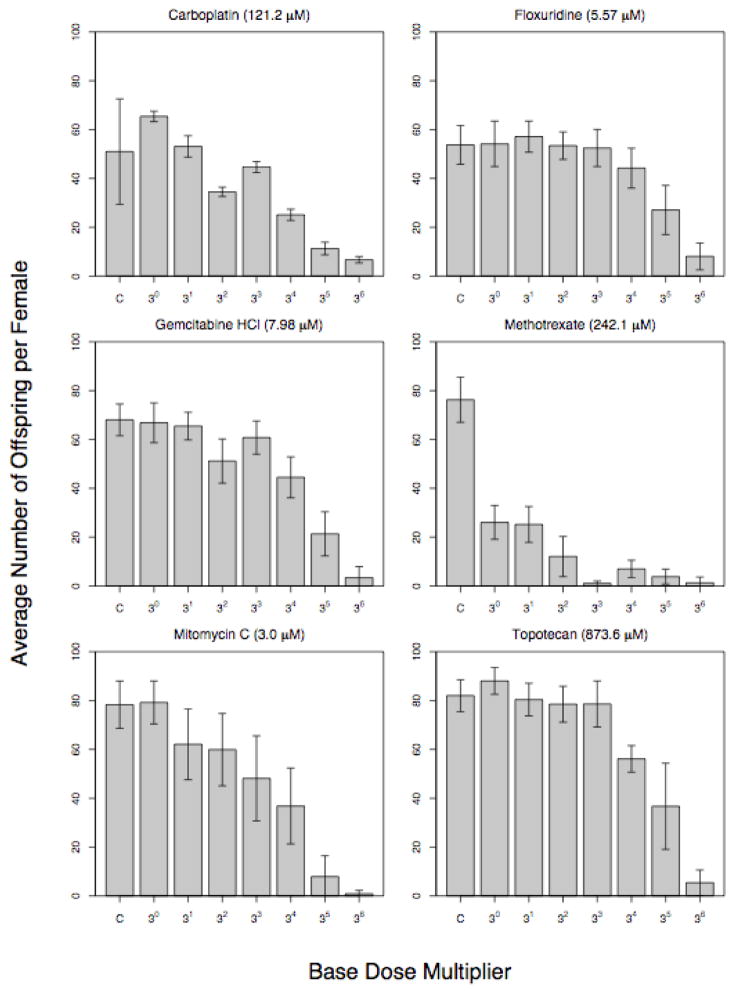
In order to establish whether the chemotherapeutic agent resulted in a dose response adverse effect on oogenesis or spermatogenesis (or both), we performed a number of assays. We attempted to deliver 20 different chemotherapy drugs to Drosophila: five drugs were not water soluble, one drug was fatal to the adult flies (despite no apparent drop in fertility), and seven appeared to have no effect on fecundity at the maximum delivered dose (Supplementary Table 2 and Supplementary Figure 2). For the subset of six drugs that we were able to assay in a high-throughput manner, we observed that increasing concentrations of the drug were associated with monotonic decreases in fecundity (Figure 1). At very high doses, many females were unable to lay any eggs, whereas at low doses fecundity was statistically indistinguishable from the control treatment.
Figure 1.
Results of dosing experiments. Each chemotherapy agent was orally administered to D. melanogaster. We define toxicity as the reduction in the average number of offspring produced by individual females following exposure to chemotherapeutic drug (relative to controls). In all cases, the first column is the control, which had no chemotherapy added to the food, but was otherwise treated identically. The base for each drug is listed in the parenthesis next to the drug name.
We decided to estimate the heritability of toxicity for the six drugs that primarily impacted female oogenesis. In order to determine the heritability of the fecundity knock-down associated with any chemotherapeutic drug, we chose a single dose that produced close to a 40–75% reduction in fecundity; higher doses were generally associated with a large fraction of females having zero offspring making it difficult to measure heritability, while low doses implied that we could simply measure wild-type variations in fecundity. We carried out a paternal half-sibling breeding design and estimated narrow-sense heritability from the paternal half-sib variance component, widely considered the best method of excluding maternal effects from the heritability estimate[13]. The heritability of fertility for the six drugs we assayed varied from ~50–100%, with a mean of 70% (Table 1). In D. melanogaster, fewer than 1% of heritable traits have heritability estimates higher than 70% [17]; suggesting that the heritability estimates of inter-individual variation in response to chemotherapy drugs we observed are atypically high. All drug associated heritability estimates were larger in magnitude than the control groups, varying from 1.6 to 3.1 fold more heritable. The standard errors associated with heritability estimates were similar to those obtained from comparable half-sib heritability designs[14].
DISCUSSION
The six drugs we chose to examine are representative of those in current use in clinical settings and represent a broad of spectrum of mechanism of action of chemotherapeutic drugs (Supplementary Table 2). Of major concern is the possibility that there are non-specific effects of the chemicals on fecundity. Although several lines of evidence argue against non-specific effects explaining the bulk of the phenomena observed in this study, the chemotherapeutics used in this assay were in pure chemical form and always compared to a mock treatment, thus observed effects are attributable specifically to these compounds. It seems likely that the effects of these compounds are specific to oogenesis. Based on casual observations of post-treatment female Drosophila they appear phenotypically normal, their mating behavior is normal, and they do not appear obviously lethargic or sick. A previously published paper showed that the eggs laid by female Drosophila post methotrexate treatment were significantly reduced in number and were visibly damaged, images in the paper show that treated female’s ovaries were withered [18]. Finally, we have exposed male Drosophila to the same doses of each of the chemotherapeutic agents, and observe no reduction in post-treatment fecundity, suggesting that these drugs have no obvious large lasting effects on spermatogenesis, nor do they impact a male’s ability to carry out a highly ritualized series of courtship behaviors sensitive to male quality.
Several of these drugs have known genetic polymorphisms in humans that mediate toxicity or efficacy (Supplementary Table 1), but the total fraction of variation in toxicity attributable to these known polymorphisms is likely small (and difficult to estimate from the primary literature). High heritability measures obtained in this study show not only that there is a large genetic component to toxicity but also that the environment of the exposure and fly rearing was well controlled; however, with such high heritability measures, the genetic component of toxicity in a more variable environment is still likely to be very large.
It may be argued that a Drosophila model does not sufficiently represent the complex tissue specific pharmacodynamics of the chemotherapeutic agents in humans. However, as most basic cellular processes are conserved among eukaryotic species [19], and the chemotherapy drugs of this study tend to act on conserved cellular level targets [12], there may be as many parallels as differences. The resolution to this debate will ultimately require the identification of the genes mediating the observed toxicity in flies, that toxicity is highly heritable allows for the possibility of a genetic dissection of this phenotype in Drosophila and the ability to directly address this dichotomy.
Overall, the present study shows that there is a large genetic component to chemotherapy toxicity in Drosophila. Based on greater than five hundred characters with heritability measures in Drosophila reviewed by Roff and Mousseau in 1986 only 1% have heritabilities greater than 70%, three of the six toxicity characters of this study had h2>70% [17]. The six drugs studied had widely varying modes of actions, hence the high heritability is associated with chemotherapy drugs in general, and not any specific drug or class of drug. It is interesting to speculate that the genetics of chemotoxicity may be similar in flies and humans and that the variability in treatment toxicity among human patients is similarly genetic in origin. Our observation of high heritability of chemotoxicity in Drosophila may have direct bearing on a debate currently going on in human genetic circles: Is inter-individual variation in chemo drug toxicity largely genetic or environmental [2, 3, 4, 5, 6, 7, 8].
Our long-term goal is to identify the actual genes (and nucleotides) underlying the genetic component of inter-individual variation in toxicity. To accomplish this goal, we will genetically dissect toxicity QTL using a collection of ~1600 recombinant inbred lines derived from an advanced generation synthetic populations of D. melanogaster [14; http://www.flyrils.org]. By identifying genes that offer protection against chemotherapy toxicity in flies, we can examine human orthologs via targeted resequencing in small cohorts to determine if they similarly modulate chemotherapy toxicity. Drosophila genetics may accelerate the identification of novel genes in humans that mediate chemotherapeutic induced toxicities. This work will also contribute to a new era of cancer therapy where chemotherapy regimens are personalized to maximize efficacy and minimize toxicity [6, 9].
Supplementary Material
Acknowledgments
This work was supported by NIH GM085251 to ADL.
Footnotes
Conflicts of interest: none declared
References
- 1.Hardman JG, Limberd LE, Molinoff PB, Ruddon RW. Goodman and Gillman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996. [Google Scholar]
- 2.Rothenberg M, Merepol N, Poplin E, Cutsem E, Wadler S. Mortality Associated With Irinotecan Plus Bolus Fluorouracil/Leucovorin: Summary Findings of an Independent Panel. Journal of Clinical Oncology. 2001;18:3801–3807. doi: 10.1200/JCO.2001.19.18.3801. [DOI] [PubMed] [Google Scholar]
- 3.Lee W, Lockhart C, Kim R, Rothenberg M. Cancer Pharmacogenomics: Powerful Tools in Cancer Chemotherapy and Drug Development. The Oncologist. 2005;10:104–111. doi: 10.1634/theoncologist.10-2-104. [DOI] [PubMed] [Google Scholar]
- 4.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Human Reproduction Update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 5.Gajewski J, Ho W, Nimer S, Hirji K, Gekelman L, Jacobs A, et al. Efficacy of intensive chemotherapy for acute myelogenous leukemia associated with a preleukemic syndrome. Journal of Clinical Oncology. 1989;7:1637–1645. doi: 10.1200/JCO.1989.7.11.1637. [DOI] [PubMed] [Google Scholar]
- 6.Watters J, McLeod H. Cancer pharmacogenomics: current and future applications. Biochimica et Biophysica Acta. 2003;1603:99–111. doi: 10.1016/s0304-419x(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 7.Wecker L, Watts S, Faingold C, Dunaway G, Crespo L. Brody’s Human Pharmacology: Molecular to Clinical. 5. Philadelphia: Mosby/Elsevier; 2010. [Google Scholar]
- 8.Cirulli E, Goldstein D. Uncovering the roles of rare variants in common disease through whole-genome sequencing. 2010;11:415–425. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]
- 9.Pui C, Relling M, Evans W. Role of Pharmacogenomics and Pharmacodynamics in the Treatment of Acute Lymphoblastic Leukemia. Best Practice & Research Clinical Haematology. 2003;15:741–756. doi: 10.1053/beha.2002.0225. [DOI] [PubMed] [Google Scholar]
- 10.Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. 4. Addison Wesley Longman; Harlow, Essex: 1996. [Google Scholar]
- 11.Avanesian A, Semnani S, Jafari M. Can Drosophila melanogaster represent a model system for the detection of reproductive adverse drug reactions? Drug Discovery Today. 2009;15:761–766. doi: 10.1016/j.drudis.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Kerbel R, Kamen A. The Anti-Angiogenic Basis of Metronomic Chemotherapy. Nature. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 13.Becker WA. Manual of Quantitative Genetics. 5. Academic Enterprises; 1992. [Google Scholar]
- 14.Macdonald S, Long A. Joint Esimates of Quantitative Trait Locus Effect and Frequency Using Synthetic Recombinant Populations of Drosophila melanogaster. Genetics. 2007;176:1261–1281. doi: 10.1534/genetics.106.069641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nature Reviews Genetics. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 16.Lazzaro B, Sceurman B, Clark A. Genetic basis of natural variation in D. melanogaster antibacterial immunity. Science. 2004;303:1873–1876. doi: 10.1126/science.1092447. [DOI] [PubMed] [Google Scholar]
- 17.Roff D, Mousseau T. Quantitative genetics and fitness: lessons from Drosophila. Heredity. 1987;58:103–118. doi: 10.1038/hdy.1987.15. [DOI] [PubMed] [Google Scholar]
- 18.Affleck J, Neumann K, Wong L, Walker V. The effects of methotrexate on Drosophila development, female fecundity, and gene expression. Toxicological Sciences. 2006;89(2):495–503. doi: 10.1093/toxsci/kfj036. [DOI] [PubMed] [Google Scholar]
- 19.Ashburner M, Ball C, Blake J, Botstein D, Butler H, Cherry M, et al. Gene Ontology: tool for the unification of biology. Nature Genetics. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekhart C, Rodenhuis S, Smits P, Beijnen J, Huitema A. Relations between polymorphisms in drug-metabolizing enzymes and toxicity of chemotherapy with cycclophosphamide, thiotepa and carboplatin. Pharmacogent Genomics. 2008;18(11):1009–15. doi: 10.1097/FPC.0b013e328313aaa4. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Sun X, Sun N, Qin S, Cheng H, Feng J, et al. Association between polymorphisms of ERCC1 and XPD and clinical response to platinum-based chemotherapy in advanced non-small cell lung cancer. Am J Clin Oncol. 2010;33(5):489–94. doi: 10.1097/COC.0b013e3181b9cedc. [DOI] [PubMed] [Google Scholar]
- 22.Booton R, Ward T, Ashcroft L, Morris J, Heighway J, Thatcher N. ERCC1 mRNA expression is not associated with response and survival after platinum-based chemotherapy regimens in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2(10):902–6. doi: 10.1097/JTO.0b013e318155a637. [DOI] [PubMed] [Google Scholar]
- 23.Steffensen K, Waldstrom M, Jeppesen U, Brandslund I, Jakobsen A. Prediction of response to chemotherapy by ERCC1 immunohistochemistry and ERCC1 polymorphism in ovarian cancer. Int J Gynecol Cancer. 2008;18(4):702–10. doi: 10.1111/j.1525-1438.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 24.Sun N, Sun X, Chen B, Cheng H, Feng J, Cheng L, Lu Z. MRP2 and GSTP1 polymorphisms and chemotherapy response in advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2010;65:437–446. doi: 10.1007/s00280-009-1046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng H, Sun N, Sun X, Chen B, Li F, Feng J, et al. Polymorphisms in hMSH2 and hMLH1 and response to platinum-based chemotherapy in advanced non-small-cell lung cancer patients. Acta Biochim Ciophys Sin. 2010;42:311–317. doi: 10.1093/abbs/gmq023. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama E, Kaniwa N, Kim SR, Hasegawa R, Saito Y, Ueno H, et al. Population Pharmacokinetics of gemicitabine and its metabolite in Japanese cancer patients. Clin Pharmacokinet. 2010;49(8):549–558. doi: 10.2165/11532970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki S, Kobunai T, Kitayama J, Nagawa H. DNA methylation and sensitivity to antimetabolites in cancer cell lines. Oncology. 2008;19:407–412. [PubMed] [Google Scholar]
- 28.Barbour K, Hoganson D, Berger S, Berger F. A naturally occurring tyrosine to histidine replacement at residue 33 of human thymidylate synthase confers resistance to 5-Fluoro-2′-deoxyuridine in mammalian and bacterial cells. Molecular Pharmacology. 1992;42:242–248. [PubMed] [Google Scholar]
- 29.Yawata A, Kim SR, Miyajima A, Kubo T, Ishida S, Saito Y, Nakajima Y, et al. Polymorphic tandem repeat sequences of the thymidylate synthase gene correlates with cellular-based sensitivity to fluoropyrimidine antitumor agents. Cancer Chemother Pharmacol. 2005;56:465–472. doi: 10.1007/s00280-005-1018-z. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Javle M, Dong X, Eng C, Abbruzzese J, Li D. Gemcitabine metabolic and transporter gene polymorphisms are associated with drug toxicity and efficacy in patients with locally advanced pancreatic cancer. Cancer. 2010:5325–5335. doi: 10.1002/cncr.25282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metharom E, Galettis P, Manners S, Jelinek M, Liauw W, Souza P, et al. The pharmacological advantage of prolonged dose rate gemciatavine is restricted to patients with variant alleles of cytidine deaminase c. 79A>C. Asia-Pacific Journal of Clinical Oncology. 2011;7:65–74. doi: 10.1111/j.1743-7563.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 32.Maring J, Wachters F, Slijfer M, Maurer M, Boezen M, Uges D, et al. Parmacokinetics of gemcitabine in non-small-cell lung cancer patients: impact of the 79 A>C cytidine deaminase polymorphism. Eur J Clin Pharmacol. 2010;66:611–617. doi: 10.1007/s00228-010-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugiyama E, Kaniwa N, Kim SR, Hasegawa R, Saito Y, Ueno H, Okusaka T, et al. Clin Pharmacokinet. 2010;49(8):549–558. doi: 10.2165/11532970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Yonemore K, Ueno H, Okusaka T, Yamamoto N, Ikeda M, Saijo N, et al. Clin Cancer Res. 2005;11(7):2620–2624. doi: 10.1158/1078-0432.CCR-04-1497. [DOI] [PubMed] [Google Scholar]
- 35.Ueno H, Kuyosawa K, Kaniwa N. Pharmacogenomics of gemcitabine: can genetic studies lead to tailor-made therapy? British Journal of Cancer. 2007;97:145–151. doi: 10.1038/sj.bjc.6603860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Si S, Liao Q, Zhao Y, Hu Y, Zhang Q, You L. Relationship between single nucleotide polymorphisms in the deoxycitidine kinase gene and chemosensitivity of gemcitabine in six pancreatic cancer cell lines. Chin Med J. 2011;124(3):419–422. [PubMed] [Google Scholar]
- 37.Gusella M, Pasini F, Bolzonella C, Meneghetti S, Barile C, Bononi A, et al. British Journal of Clinical Pharmacology. 2010;71(3):437–444. doi: 10.1111/j.1365-2125.2010.03838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka M, Okazaki T, Suzuki H, Abbruzzese J, Li D. Association of multi-drug resistance gene polymorphisms with pancreatic cancer outcome. Cancer. 2011:744–751. doi: 10.1002/cncr.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alberola V, Sarries C, Rosell R, Taron M, Penas R, Camps C, et al. Clinical Lung Cancer. 2004;5(6):360–365. doi: 10.3816/clc.2004.n.014. [DOI] [PubMed] [Google Scholar]
- 40.Kalari K, Hebbring S, Chai H, Li L, Kocher JP, Wang L, et al. BMC Genomics. 2010;11(357):1–14. doi: 10.1186/1471-2164-11-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rau T, Erney B, Gores R, Eschenhagen T, Beck J, Langer T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clin Pharmacol Ther. 2006;80(5):468–76. doi: 10.1016/j.clpt.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Wessels JA, Kooloos WM, De Jonge R, De Vries-Bouwstra JK, Allaart CF, Linssen A, et al. Arthritis Rheum. 2006;54(9):2830–9. doi: 10.1002/art.22032. [DOI] [PubMed] [Google Scholar]
- 43.Vella N, Aiello M, Russo A, Scalisi A, Spandidos D, Toffoli G, et al. Genetic profiling and ovarian cancer therapy. Molecular Medicine Reports. 2011;4:771–777. doi: 10.3892/mmr.2011.512. [DOI] [PubMed] [Google Scholar]
- 44.Kotnik B, Grabnar I, Grabar P, Dolzan V, Jazbec J. Association of gentic polymorphism in the folate metabolic pathway with methotrexate pharmacokinetics and toxicity in chilhood acute lymphoblastic leukaemia and malignant lymphoma. Eur J Clin Pharmacol. 2011:1–14. doi: 10.1007/s00228-011-1046-z. [DOI] [PubMed] [Google Scholar]
- 45.Kurzawski M, Pawlik A, Safranow K, Herczynska M, Drozdzik M. 677C>T and 1298A>C MTHFR polymorphisms affect methotrexate treatment outcome in rheumatoid arthritis. Pharmacogenomics. 2007;8(11):1551–9. doi: 10.2217/14622416.8.11.1551. [DOI] [PubMed] [Google Scholar]
- 46.Seidemann K, Book M, Zimmermann M, Meyer U, Welte K, Stanulla M, Reiter A. MTHFR 677 (C>T) polymorphism is not relevant for prognosis or therapy-associated toxicity in pediatric NHL: results from 484 patients of multicenter trial NHL-BFM 95. Ann Hematl. 2006;85(5):291–300. doi: 10.1007/s00277-005-0072-2. [DOI] [PubMed] [Google Scholar]
- 47.Hughes LB, Beasley TM, Patel H, Tiwari HK, Morgan SL, Baggott JE. Racial or ethnic differences in allele frequencies of single-nucleotide polymorphisms in the methylenetetrahydrofolate reductase gene and their influence on response to methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2006;65(9):1213–8. doi: 10.1136/ard.2005.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drozdzik M, Rudas T, Pawlik A, Gornik W, Kurzawski M, Herczynska M. Reduced folate carrier-1 80G>A polymorphism affects methotrexate treatment outcome in rheumatoid arthritis. Pharmacogenomics J. 2007;7(6):404–7. doi: 10.1038/sj.tpj.6500438. [DOI] [PubMed] [Google Scholar]
- 49.Traver R, Horikoshi T, Danenber K, Stadlbauer T, Danenberg P, Ross D, Gibson N. NAD(P)H: Quinone oxidoreductase gene expression in human colon carinoma cells: characterization of a mutation which modulates DT-diaphorase activity and mitomycin sensitivity. Cancer Research. 1992;52:797–802. [PubMed] [Google Scholar]
- 50.Jamieson D, Wilson K, Pridgeon S, Margetts J, Edmondson R, Leung H, Knox R, et al. NAD(P)H: Quinone oxidoreductase 1 and NRH: Quinone oxicdoreductase 2 activity and expression in bladder and ovarian cancer and lower NRH: Quinoone oxidoreductase 2 activity associated with an NQO2 exon 3 single-nucleotide polymorphism. Clin Cancer Res. 2007;13(5):15841590. doi: 10.1158/1078-0432.CCR-06-1416. [DOI] [PubMed] [Google Scholar]
- 51.Basu S, Brown J, Flannigan M, Gill J, Loadman P, Martin S, et al. NAD(P)H: Quinone oxidoreductase-1 C609T polymorphism analysis in human superficial bladder cancers: relationship of genotype status to NQ01 phenotype and clinical response to mitomycin C. Int J or Oncology. 2004;25(4):921–928. [PubMed] [Google Scholar]
- 52.Fleming R, Drees J, Loggie B, Russell G, Geisigner K, Morris R, et al. Clinical significase of a NAD(P)H: quinone oxidoreductase 1 polymorphism in patients with disseminated peritoneal cancer receiving intraperitoneal hyperthermic chemotherapy with mitomycin C. Pharmacogenetics. 2002;12(1):31–37. doi: 10.1097/00008571-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Mrozek E, Kolesar J, Young D, Allen J, Villalona-Calero M, Shapir CL. Phase II study of sequentially administered low-dose mitomycin C (MMC) and irinotecan (CPT-11) in women with metastatic breast cancer (MBC) Annals of Oncology. 2008;19:1417–1422. doi: 10.1093/annonc/mdn154. [DOI] [PubMed] [Google Scholar]
- 54.Deeken J, Robey R, Shukla S, Steadman K, Chakraborty A, Poonkuzhali B, et al. Identification of compounds that correlate with ABCG2 transporter function in the national cancer institue anticancer drug screen. Mol Pharmacol. 2009;76:946–956. doi: 10.1124/mol.109.056192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim IS, Kim HG, Kim B, Eom HS, Kong SY, Shin HJ, et al. ABCG2 Q141K polymorphism is associated with chemotherapy-induced diarrhea in patients with diffuse large B-cell lymphoma who received frontline rituximab plus cyclophosphamide/doxorubicin/vincristine/prednisone chemotherapy. Cancer Sci. 2008;99(12):2496–2501. doi: 10.1111/j.1349-7006.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crouthamel M, Wu D, Yang Z, Ho R. A novel MDR1 G1199T variant alters drug resistance and efflux transport activity of P-glycoprotein in recombinant hek cells. Journal of Pharmaceutical Sciences. 2006;96(12):2767–2777. doi: 10.1002/jps.20743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.