Abstract
Human ketosteroid reductases of the aldo-keto reductase (AKR) superfamily, i.e. AKR1C1-4, are implicated in the biotransformation of synthetic steroid hormones. Norethynodrel (NOR, 17α-ethynyl-17β-hydroxy-estra-5(10)-en-3-one), the progestin component of the first marketed oral contraceptive, is known to undergo rapid and extensive metabolism to 3α- and 3β-hydroxy metabolites. The ability of the four human AKR1C enzymes to catalyze the metabolism of NOR has now been characterized. AKR1C1 and AKR1C2 almost exclusively converted NOR to 3β-hydroxy NOR, while AKR1C3 gave 3β-hydroxy NOR as the main product and AKR1C4 predominantly formed 3α-hydroxy NOR. Individual AKR1C enzymes also displayed distinct kinetic properties in the reaction of NOR. In contrast, norethindrone (NET), the Δ4-isomer of NOR and the most commonly used synthetic progestin, was not a substrate for the AKR1C enzymes. NOR is also structurally identical to the hormone replacement therapeutic tibolone (TIB), except TIB has a methyl group at the 7α-position. Product profiles and kinetic parameters for the reduction of NOR catalyzed by each individual AKR1C isoform were identical to those for the reduction of TIB catalyzed by the respective isoform. These data suggest that the presence of the 7α-methyl group has a minimal effect on the stereochemical outcome of the reaction and kinetic behavior of each enzyme. Results indicate a role of AKR1C in the hepatic and peripheral metabolism of NOR to 3α- and 3β-hydroxy NOR and provide insights into the differential pharmacological properties of NOR, NET and TIB.
Keywords: Ketosteroid reduction, progestin, oral contraceptive
1. Introduction
Synthetic progestogens are commonly used by women for contraception and hormone replacement therapy. Different progestogens are known to exert differential pharmacological effects, the mechanism of which is not fully understood [1]. Exogenous progestogens have different metabolic profiles and may produce metabolites with biological activities different from their parent progestogens.
The synthetic progestogen norethynodrel (NOR, 17α-ethynyl-17β-hydroxy-estra-5(10)-en-3-one) was the progestin component of the first marketed oral contraceptive [2]. Early studies show that NOR undergoes rapid metabolism upon oral administration and the principal pathway is the direct reduction of the 3-oxo group of the molecule [3, 4]. The major metabolites in plasma are the 3α-hydroxy NOR (3α-OH-NOR) and 3β-OH-NOR and their conjugates. The enzyme(s) responsible for the conversion have not been identified. The use of NOR in contraceptives is based on its progestational properties. However, NOR is also known to exert estrogenic effects [5, 6]. It is possible that the metabolites contribute to, or even, are responsible for the pharmacological effects of the parent compound.
NOR is an isomer of norethindrone (NET, 17α-ethynyl-17β-hydroxy-4-estren-3-one) [7], with the difference being the position of the double bond (Fig. 1). NET is the most commonly used progestin in contraceptives and hormone replacement therapy. NET is a minor metabolite of NOR formed by either non-enzymatic or enzymatic ketosteroid isomerization [3, 4].
Fig. 1. Chemical structures of NOR, NET and TIB and the reduction of the 3-oxo group catalyzed by AKR1C enzymes.
The four rings in the steroids are designated A, B, C, and D. α-Substituents are below the plane of the four rings, while β-substituents are above the plane of the steroid. The angular methyl groups of the steroids are β-oriented, whereas the 7-methyl and 17-ethinyl groups of the steroids are β-oriented.
NOR is also structurally related to the hormone replacement therapeutic tibolone (TIB, 7α-methyl-17α-ethynyl-17β-hydroxy-estra-5(10)-en-3-one), with the difference being the presence of the additional 7α-methyl group in TIB(Fig. 1) . TIB is used by postmenopausal women for the treatment of menopausal symptoms and in the prevention of osteoporosis [8]. TIB works as a pro-drug and exerts its tissue-specific actions due to tissue-specific metabolism into three active derivatives: a 3α-and a 3 β-hydroxymetabolite (which are moderately estrogenic) and a Δ4-isomer (which has androgenic and progestogenic properties) [9, 10]. Previously, we have shown that the tissue specific actions of TIB relyon its conversion to estrogenic 3 α-and 3β-hydroxymetabolites catalyzed by the enzymes of the aldo-keto reductase superfamily 1C subfamily (AKR1C) [11, 12].
AKR1C enzymes are cytosolic reductases that catalyze the reduction of ketosteroids to hydroxysteroids [13–15]. These enzymes have broad substrate specificity and are implicated in the transformation of natural and synthetic steroid hormones. Four human enzymes have been identified, which share high sequence homology, but have distinct reaction preferences. As such, AKR1C1 and AKR1C2 selectively reduce TIB to 3β-hydroxytibolone but not 3α-hydroxytibolone, whereas AKR1C3 and AKR1C4 reduce TIB to both the 3α- and 3β-hydroxytibolone where AKR1C3 preferentially forms 3β-hydroxytibolone and AKR1C4 preferentially forms 3α-hydroxytibolone [11]. In addition, we have found that an individual AKR1C isoform can have different stereochemical preference depending on the ketosteroid substrate. For example, AKR1C2 produces a 3α-product with 5α-dihydrotestosterone, but a 3β-product with TIB[11, 15] .
In this study, we investigated the ability of AKR1C enzymes to metabolize NOR. By comparing the reaction of NOR with that of TIB, we assess the effect of the 7α-methyl group on the stereochemistry and kinetic parameters of the reaction catalyzed by each individual AKR1C isoform. Our results indicate a role of AKR1C isoforms in the hepatic and peripheral metabolism/bioactivation of NOR to 3α- and 3β-OH-NOR. They also provide insights into the differential pharmacological properties of the three structurally related synthetic steroids NOR, NET, and TIB.
2. Materials and Methods
2.1 Materials
All steroids which include [16-3H]NOR (45.7 Ci/mmol), unlabeled NOR, NET, 3α- and 3β-OH-NOR were provided by N.V. Organon (Oss, Netherlands). Pyridine nucleotides were purchased from Roche Applied Science. All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) and were of American Chemical Society grade or better. Recombinant AKR1C1-4 enzymes were over-expressed and purified to homogeneity as previously described [16, 17]. The specific activities of AKR1C1-3 were standardized using 1-acenaphthenol as the substrate for AKR1C1-3 or androsterone as the substrate for AKR1C4. Under standard assay conditions, the specific activities were determined to be 2.1 μmol/min/mg for AKR1C1, 2.5 μmol/min/mg for AKR1C2, 2.8 μmol/min/mg for AKR1C3, and 0.21 μmol/min/mg for AKR1C4.
2.2 Product Identification and Quantitation by TLC
Products formed during the reduction of NOR catalyzed by AKR1C enzymes were prepared for TLC analyses as follows. Reaction mixtures contained 100 mM potassium phosphate buffer (pH 7.0), NOR (9.78 μM unlabeled and 0.22 μM labeled steriod), 0.9 mM NADPH, and 6% acetonitrile in 100 μl of total volume. The reaction was initiated by the addition of purified enzyme (buffer for no-enzyme control, 7.3 μg of AKR1C1, 5.0 μg of AKR1C2, 8.2 μg of AKR1C3, or 4.8 μg of AKR1C4) and incubated at 37 °C for 60 min. Reactions were terminated by the addition of 1 ml of ice-cold ethyl acetate and steroids extracted by continuous vortexing for 5 min. The organic extracts were vacuum dried and the residues were re-dissolved in 40 μl of ethyl acetate and applied to LK6D Silica TLC plates (Whatman Inc., Clifton, NJ). The chromatograms were developed in methylene chloride/acetone (90:10, v/v). Co-chromatographed reference steroids were stained by spraying with an acetic acid/sulfuric acid/anisaldehyde (100:2:1, v/v/v) solution and heating. The procedural loss was determined to be less than 4 % when the total radioactivity added was compared to the amount that was extracted with organic solvent.
Radiochromatograms were scanned with an automatic TLC-linear analyzer (Bioscan Imaging Scanner System 200-IBM with AutoChanger 3000; Bioscan, Washington, DC) as described previously [11]. The relative amount of each radioactive steroid was calculated as a percentage of the total radioactivity recovered from a single TLC lane. Blank values were subtracted. Enzyme activity was expressed as nmoles/min/mg using the specific radioactivity of [16-3H]-NOR as conversion factor. The identity of radioactive steroids on the TLC plates was verified by the staining of the co-chromatographed reference standards.
2.3. Determination of Steady State Kinetic Parameters
Initial velocities were measured fluorimetrically by monitoring the change in fluorescence emission of NADPH using a Hitachi F-2500 fluorescence spectrophotometer (Hitachi America, Ltd.; New York, NY). Excitation and emission wavelengths were set at 340 nm and 450 nm, respectively. Changes in fluorescence units were converted to nanomoles of cofactor by using standard curves of fluorescence emission versus known NADPH concentrations. Typical reaction samples contained enzyme (1.4 μg AKR1C1, 1.2 μg AKR1C2, 1.6 μg AKR1C3, or 0.8 μg AKR1C4), NADPH at a saturating concentration of 12 μM and, steroid at varied concentrations (0.1 μM – 50 μM), 100 mM potassium phosphate buffer (pH 7.0), and 4% methanol in a total volume of 1 mL. Reactions were initiated by the addition of NADPH and run at 25 °C.
Data were fitted by nonlinear regression to the equation, , where υ is the initial velocity, [E] and [S] are the total molar concentrations of the enzyme and steroid substrate, respectively, kcat (s−1) is the turnover number, and Km (μM) is the apparent Michaelis-Menten constant for the steroid substrate. When decreases in rates were noticed at higher concentrations of substrate, a modified equation that took into consideration of substrate inhibition was used, , where Ki (μM) is the apparent dissociation constant for the presumed inhibitory enzyme-substrate complex.
2.4 Molecular Modeling of NOR Binding in AKR1C1, AKR1C2 and AKR1C3
The structures of Protein Data Bank (PDB) entries 1MRQ for AKR1C1 [18], 1IHI for AKR1C2 [19], and 1S2C for AKR1C3 [20] less ligand and solvent molecules were employed as docking targets, respectively. The structure of NADPH with a puckered nicotinamide ring was built into the targets as described [21]. The coordinates for NOR were generated based on the coordinates of TIB. Previously, we have performed molecular docking of TIB to AKR1C1 and AKR1C2 using AUTODOCK [21]. Using the productive docking position of TIB in these enzymes as template, NOR was manually docked into AKR1C1, AKR1C2, and AKR1C3, and energy minimization was carried out using the AutoDock 4.2 software [22].
Briefly, the program “AutoDock Tools” was used to prepare the system, which included addition of charge and nonpolar hydrogen and assignment of rotatable bonds of the ligand, and analyze the docking simulations. The simulation space was defined so that the active site and all amino acid residues that line the steroid binding cavity are included. Atomic interaction energy on a 0.15 Å grids was calculated with the auxiliary program AutoGrid using probes corresponding to each atom type found in the ligand. Energy minimization was performed using AutoDock with a local search algorithm and the manually positioned NOR as the start point. A total of 256 independent simulations with a population size of 50 members were run for each enzyme using default parameters. After docking, the 256 conformers generated for each compound were assigned to clusters based on a tolerance of 2 Å all-atom root-mean-square deviation (rmsd) in position from the lowest-energy solution.
3. Results and Discussion
3.1. Stereochemistry
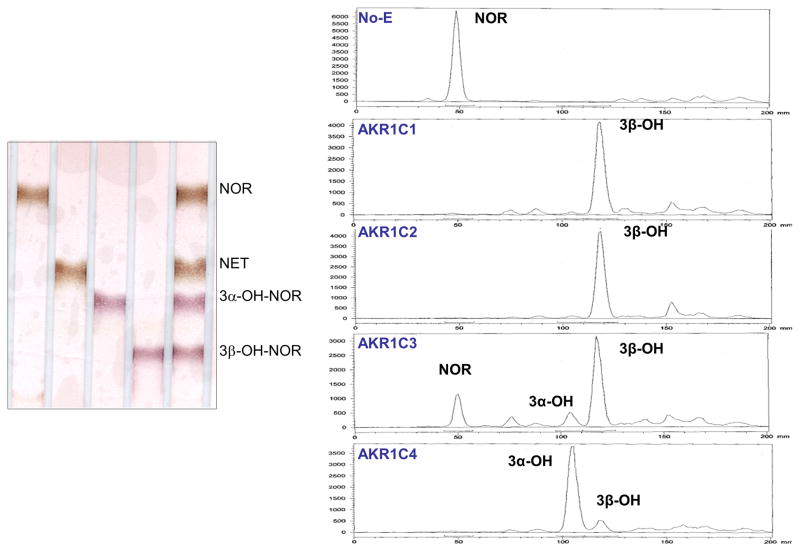
Radiolabeled NOR was incubated with each of the four recombinant AKR1C isoforms in the presence of NADPH. The organic soluble products were extracted and analyzed by TLC. Products were identified by reference to the authentic standards. NOR was found to be stable under the reaction conditions and no non-enzymatic isomerization or ketone reduction occurred in the no-enzyme control sample. Despite the high sequence identity among AKR1C1-4 enzymes, the stereochemical outcomes of the NOR reduction catalyzed by these enzymes are quite different (Fig. 2 and Table 1).AKR1C1 and AKR1C2 formed the 3 β-hydroxy product of NOR almost exclusively, AKR1C3 gave mainly the 3β-OH-NOR and a small amount of 3α-OH-NOR, and AKR1C4 generated mainly 3α-OH-NOR and a small amount of 3β-OH-NOR.
Fig. 2. TLC analyses of products of NOR reduction catalyzed by AKR1C enzymes.
Left: TLC separation of non-radiolabeled authentic steroid standards. Right: TLC radiochromatograms. Radiolabeled NOR was incubated with enzyme and NADPH as described. Reaction mixtures were extracted and analyzed by TLC.
Table 1.
Product distribution for the reduction of NOR and TIB catalyzed by AKR1C isoforms
| 3α – OH : 3β –OH | ||||
|---|---|---|---|---|
| AKR1C1 | AKR1C2 | AKR1C3 | AKR1C4 | |
| NOR | 1 : 31 | 1 : 26 | 1 : 5.7 | 6.3 : 1 |
| TIB | 3β only | 3β only | 1 : 5.4 | 4.7 : 1 |
Values for TIB were from reference [11], which were calculated based on radiometric assay.
Previously we have characterized the reduction of the structurally related TIB, which differs from NOR only by an extra 7α-methyl group. Comparison of the product profiles formed from the two steroids showed that the stereochemical preferences were quite similar when the same AKR1C isoform was used (Table 1). This suggests that the 7α-methyl had little effect on the binding position of the substrate in the active site of AKR1C enzyme.
3.2. Kinetic Properties
Steady-state kinetic parameters for the reduction of NOR by AKR1C enzyme were determined in continuous spectrofluorometric assays and are listed in Table 2 (see Supplemental material Fig. S1 for representative data). The depletion of NADPH was monitored during the assay, thus for reactions, where a mixture of products were formed, the activity measured represented the total activity.
Table 2.
Kinetic parameters of NOR and TIB as substrate of AKR1C enzymes.
| AKR1C1 | AKR1C2 | AKR1C4 | ||
|---|---|---|---|---|
| NOR | kcat (min−1) | 1.0 ± 0.1 | 8.2 ± 2.1 | 2.7 ± 0.2 |
| Km (μM) | 2.3 ± 0.2 | 1.5 ± 0.3 | 0.43 ± 0.04 | |
| kcat /Km (min−1μM−1) | 0.45 | 5.6 | 6.4 | |
| Ki (μM) | 2.3 ± 0.5 | |||
| TIB | kcat (min−1) | 0.88 ± 0.06 | 12.7 ± 3.7 | 1.83 ± 0.06 |
| Km (μM) | 0.76 ± 0.13 | 0.87 ± 0.34 | 1.02 ± 0.07 | |
| kcat /Km (min−1μM−1) | 1.2 | 14.6 | 1.8 | |
| Ki (μM) | 0.7 ± 0.29 |
Values for AKR1C3-catalyzed reactions could not be determined due to low turnover. Values for TIB were from reference [15].
Individual AKR1C1-4 enzymes displayed distinct kinetic behaviors in the reduction of NOR. AKR1C1 and AKR1C2 catalyzed rapid reduction of NOR, with AKR1C2 exhibiting potent substrate inhibition; AKR1C3 turned over NOR poorly and gave an estimated kcat lower than 0.2 min−1; while the AKR1C4 enzyme displayed the highest catalytic efficiency. Due to the slow turnover, it was not possible to accurately determine kcat and Km values for the AKR1C3 catalyzed NOR reduction.
We compared the steady state kinetic parameters for the reduction of NOR and TIB by the AKR1C enzymes (Table 2). For each individual AKR1C enzyme, the kinetic properties for the two compounds were quite similar. For example the reduction of NOR and TIB by AKR1C1 gave a similar kcat but a lower Km with TIB. AKR1C2 displayed the unique kinetic feature of potent substrate inhibition with both of the steroids, although the kcat value with NOR was 1.5 fold lower than that with TIB. AKR1C3 was turned over NOR and TIB poorly. Similar kinetic parameters were observed for the reduction of NOR and TIB catalyzed by AKR1C4, with the kcat value of NOR being 1.5 fold higher than that with TIB.
No significant enzyme-dependent oxidation of NADPH was observed from incubations of the AKR1C enzymes with NET, suggesting NET was not a substrate for these enzymes. This was expected as the AKR1C enzymes are unable to reduce the carbonyl bond in a Δ4-3-oxo structure, as indicated by testosterone acting as an inhibitor of the AKR1C enzymes [23].
3.3. Structural Insights
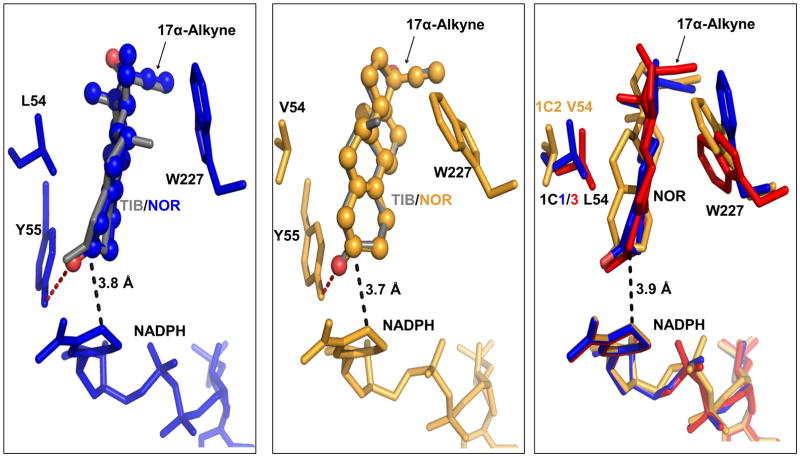
Molecular docking studies of NOR were conducted in AKR1C1, AKR1C2, and AKR1C3. Docking of NOR in AKR1C4 was not pursued, since the crystal structure of a ternary AKR1C4 complex is not currently available to generate a mature steroid binding cavity for the docking study. Productive docking positions of NOR in AKR1C1, AKR1C2 and AKR1C3 were found (Fig. 3 Left and middle panel). Our results showed that NOR could be accommodated in the active sites of AKR1C1 and AKR1C2 in almost exact positions of TIB in those enzymes [21], suggesting that the 7α-methyl group does not contribute significantly to TIB binding. This is consistent with observed similarities between NOR and TIB as substrates for AKR1C enzyme. The binding position of NOR in AKR1C3 was similar to that of NOR in AKR1C1 (Fig. 3 Right panel). Interestingly, the binding energy of NOR in AKR1C3 (−5.39 kcal/mol) was significantly higher than those of NOR in AKR1C1 (−7.14 kcal/mol) and AKR1C2 (−7.52 kcal/mol), which may be related to the low turnover of NOR catalyzed by AKR1C3. In all three enzymes, the 3-oxo group of NOR was anchored by the hydrogen bonding network formed with the catalytic tetrad (Tyr55 and His117), which brought the 3-oxo group to the close proximity to the nicotinamide ring of NADPH. In addition, the α-face of the steroid was presented to the 4-pro-R hydride of the cofactor, which could account for the stereochemical preference for the formation of 3β-hydroxy product by these enzymes.The binding interactions also included hydrophobic interactions between the steroid ring structure and the surrounding residues(e.g. L/V54 and W227).
Fig. 3. Molecular docking of NOR in AKR1C1, AKR1C2 and AKR1C3.
Left panel: productive binding positions of NOR (shown in blue and ball-and-stick representation) and TIB ( shown in grey and stick representation) in AKR1C1; Middle panel: productive binding positions of NOR (shown in orange and ball-and-stick representation) and TIB (shown in grey and stick representation) in AKR1C2; Right panel: superposition of the productive binding positions of NOR in AKR1C1 (blue), AKR1C2 (orange) and AKR1C3 (red). The stereochemical preference for the formation of 3β-hydroxy product by these enzymes can be explained. The 3-ketone of the steroid was proximal to the cofactor with the α-face of the steroid presented to the 4-pro-R hydrogen of NADPH. Structures of AKR1C1, AKR1C2 and AKR1C3 were superimposed on their backbone atoms. NADPH and active site residues (Leu/Val54, Tyr55, and Trp227) are shown in stick representation. For the purpose of clarity, not all residues involved in binding interactions are shown. Hydrogen interactions are shown by red dashed lines. The distance between the C3 of NOR and the C4 of the nicotinamide ring of NADPH are indicated by black dashed lines.
In our previous study, a low-energy nonproductive binding position of TIB was found in AKR1C2 but not in AKR1C1, which may explain the unique strong substrate inhibition observed in the reaction catalyzed by AKR1C2 [21]. The steroid is held half-way in the steroid binding pocket via hydrogen bonding and hydrophobic interactions. Again, the 7α-methyl group of TIB does not appear to significantly contribute to the binding in AKR1C2,as NOR could also be accommodated. A s imilar binding position of NORcould not be obtained with AKR1C1 and AKR1C3, possibly due to the presence of a leucine residue instead of a valine residue (of AKR1C2) at position 54.
3.4. Implications for Role of AKR1C in NOR Metabolism
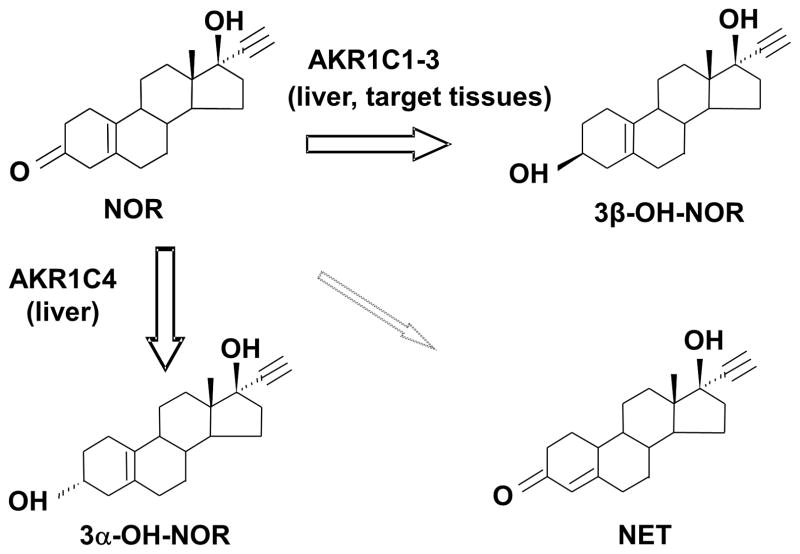
Cook et al. have shown that NOR administered orally undergoes extensive hepatic first pass metabolism primarily by reduction of the 3-oxo group, followed by conjugation [4]. The principal metabolites in plasma and urine are 3α-OH-NOR and 3β-OH-NOR, where the 3α-OH NOR predominated over the 3β-isomer, and their conjugates. A small amount of NET was also observed as a minor metabolite [24, 25]. This metabolic profile of NOR can be explained by the observed activity of AKR1C enzymes on NOR (Fig. 4). All four human AKR1C enzymes are abundantly expressed in liver and can efficiently transform NOR. AKR1C4 is the most efficient enzyme and can convert NOR to 3α-OH-NOR, whereas AKR1C1-3 can convert NOR to 3β-OH-NOR. In addition, under neutral pH non-enzymatic isomerization of NOR did not occur, and no significant enzymatic turnover of NET catalyzed by AKR1C enzymes was observed.
Fig. 4. Metabolic fate of NOR.
NOR is directly metabolized to 3α-OH-NOR by AKR1C4 and to 3β-OH-NOR by AKR1C1-3 in the liver, whereas it is mainly converted to 3β-OH-NOR by AKR1C1-3 in target tissues.
While AKR1C4 is expressed in liver only, AKR1C1-AKR1C3 are expressed in many target tissues (e.g. breast and uterus) and will be involved in the metabolism of NOR following parenteral administration [13]. These enzymes will convert NOR to mainly 3β-OH-NOR.
3.4. Insight into Differential Pharmacological Properties of NOR, NET, and TIB
Isomeric compounds NOR and NET show distinct pharmacokinetic and pharmacodynamics properties. Upon oral administration, no unchanged NOR was observed in plasma after 30 min [4]. In contrast, the biological half-life of NET in plasma was determined to be about 3 to 6.5 h [26, 27]. Further, the metabolites of NOR declined with half-lives of 14 h [4] and 45 h [28], following oral and intravenous administration of NOR, respectively, whereas NET metabolites disappeared with an average half-life of 67 h [29]. Rapid hepatic and local metabolism of NOR can be accounted for by the efficient transformation of NOR catalyzed by AKR1C enzymes. Due to the Δ4-3-oxo moiety in the structure, NET cannot be directly metabolized by AKR1C enzymes. Instead, its metabolism requires first the reduction of the 4-double bond by either the 5α-reductase or 5β-reductase, followed by the subsequent reduction of the 3-oxo group by the AKR1C enzymes to give the A-ring-reduced tetrahydrosteroids as the principal metabolites [25]. Past studies suggested that the first step in the metabolic sequence catalyzed by either 5α-reductase or 5β-reductase is rate-determining [30, 31]. Thus the metabolic transformation of NET is slower than that of NOR.
Similar to NOR, TIB undergoes rapid first pass metabolism [9]. TIB is thus considered a prodrug and exerts its tissue-specific actions via its estrogenic 3α-and 3β-hydroxymetabolite s and itsandrogenic and progestogenic Δ4-isomer[10] . The metabolic similarity between NOR and TIB suggests that the 7α-methylin TIB has minimal effect on metabolism, which is consistent with the similar stereochemistry and kinetics of the phase 1 reaction of NOR and TIB catalyzed by AKR1Cenzymes observed in this study. However , the 7α-methyl group in TIB clearly affectsthe phase 2 reaction s. For NOR, plasma and urine conjugates are composed of β-glucuronides, sulfates, and mixed conjugates, with the β-glucuronides being the predominate excreted metabolites at early time periods[4] . In contrast, TIB conjugates were only found in the sulfated form[9], suggesting the 7α-methyl group in TIB prevents the formation of β-glucuronides.
Upon administration, significant levels of NET can enter the systemic circulation and exert progestational effects at the target site [32]. In contrast, NOR is rapidly metabolized, suggesting that NOR metabolites are responsible for its biological effects [1, 4]. While there has been no direct study to show that 3α-or 3β-OH-NOR are estrogenic, the structurally related3 α- and 3β-OH-TIB were shown to haveestrogenic properties when evaluated in vitro and in vivo [33, 34]. The 7α-methyl group appeared to affect the activity of the steroid as NOR displayed reduced progestagenic and increased androgenic properties when compared to TIB [34]. When administered orally, the estrogenic activity of NOR in ovariectomized rats, which was most likely mediated via the 3α-or 3β-OH metabolites of NOR, was less than half of the estrogenic activity of TIB, suggesting that the presence of the 7α-methyl group (in TIB) increased the estrogenic properties of the 3α-or 3β-OH metabolites. The low formation of NET from NOR was believed by some to contribute to the contraceptive effectof NOR [25].
4. Conclusions
AKR1C enzymes catalyze the efficient reduction of the 3-keto group of NOR to 3α-or 3β-hydroxymetabolites. No significant non -enzymatic or enzymatic conversion of NOR to NET was observed. Individual AKR1C enzymesdisplay distinct stereochemical preference and kinetic properties with NOR, which are similar to those observed by the respective enzymef or TIB. Results suggest AKR1C enzymes play an important role inNOR metabolism. In liver, NOR is directly metabolized to 3α-OH-NOR by AKR1C4 and to 3β-OH-NOR by AKR1C1-3. In target tissues, NOR is converted only to 3β-OH-NOR by AKR1C1-3 since AKR1C4 is absent.
Supplementary Material
Highlights.
3-Keto reduction of norethynodrel to 3α- or 3β-hydroxy steroids by AKR1C1-4
Distinct stereochemistry and kinetics with individual AKR1C isoforms
Similarity between norethynodrel and tibolone as substrates of AKR1C isoforms
Differential roles of AKR1Cs in hepatic and peripheral metabolism of norethynodrel
Acknowledgments
This work was supported by a FOCUS-Junior Faculty Investigator Award from the Edna G. Kynett Memorial Foundation to Y.J. and by grants R01-DK47015 and P30-ES13408 to T.M.P from the National Institutes of Health.
Abbreviations
- AKR
aldo-keto reductase (also visit www.med.upenn.edu/akr)
- NOR
norethynodrel, 17α-ethynyl-17β-hydroxy-estra-5(10)-en-3-one
- 3α-OH-NOR
3α-hydroxy norethynodrel
- 3 β-OH-NOR
3β-hydroxynorethynodrel
- NET
norethindrone, norethisterone, 17α-ethynyl-17β-hydroxy-4-estren-3-one
- TIB
tibolone, Livial®, 7α-methyl-17α-ethynyl-17β-hydroxy-estra-5(10)-en-3-one
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68(10–13):879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Norethynodrel. IARC Monogr Eval Carcinog Risk Chem Hum. 1979;21:461–477. [PubMed] [Google Scholar]
- 3.Palmer KH, Ross FT, Rhodes LS, Baggett B, Wall ME. Metabolism of antifertility steroids. I. Norethynodrel. J Pharmacol Exp Ther. 1969;167(2):207–216. [PubMed] [Google Scholar]
- 4.Cook CE, Twine ME, Tallent CR, Wall ME, Bressler RC. Norethynodrel metabolites in human plasma and urine. J Pharmacol Exp Ther. 1972;183(1):197–205. [PubMed] [Google Scholar]
- 5.Roland M. Effects of norethynodrel on the human endometrium. Ann N Y Acad Sci. 1958;71(5):638–648. doi: 10.1111/j.1749-6632.1958.tb46794.x. [DOI] [PubMed] [Google Scholar]
- 6.Jordan VC, Jeng MH, Catherino WH, Parker CJ. The estrogenic activity of synthetic progestins used in oral contraceptives. Cancer. 1993;71(4 Suppl):1501–1505. doi: 10.1002/cncr.2820710415. [DOI] [PubMed] [Google Scholar]
- 7.Norethisterone and norethisterone acetate. IARC Monogr Eval Carcinog Risk Chem Hum. 1979;21:441–460. [PubMed] [Google Scholar]
- 8.Albertazzi P, Di Micco R, Zanardi E. Tibolone: a review. Maturitas. 1998;30(3):295–305. doi: 10.1016/s0378-5122(98)00059-0. [DOI] [PubMed] [Google Scholar]
- 9.Vos RM, Krebbers SF, Verhoeven CH, Delbressine LP. The in vivo human metabolism of tibolone. Drug Metab Dispos. 2002;30(2):106–112. doi: 10.1124/dmd.30.2.106. [DOI] [PubMed] [Google Scholar]
- 10.Kloosterboer HJ. Tibolone: a steroid with a tissue-specific mode of action. J Steroid Biochem Mol Biol. 2001;76(1–5):231–238. doi: 10.1016/s0960-0760(01)00044-9. [DOI] [PubMed] [Google Scholar]
- 11.Steckelbroeck S, Jin Y, Oyesanmi B, Kloosterboer HJ, Penning TM. Tibolone is metabolized by the 3α/3β-hydroxysteroid dehydrogenase activities of the four human isozymes of the aldo-keto reductase 1C subfamily: inversion of stereospecificity with a Δ 5(10)-3-ketosteroid. Mol Pharmacol. 2004;66(6):1702–1711. doi: 10.1124/mol.104.004515. [DOI] [PubMed] [Google Scholar]
- 12.Steckelbroeck S, Oyesanmi B, Jin Y, Lee SH, Kloosterboer HJ, Penning TM. Tibolone metabolism in human liver is catalyzed by 3α/3β-hydroxysteroid dehydrogenase activities of the four isoforms of the aldo-keto reductase (AKR)1C subfamily. J Pharmacol Exp Ther. 2006;316(3):1300–1309. doi: 10.1124/jpet.105.091587. [DOI] [PubMed] [Google Scholar]
- 13.Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351(Pt 1):67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penning TM, Jin Y, Steckelbroeck S, Lanisnik Rizner T, Lewis M. Structure-function of human 3α-hydroxysteroid dehydrogenases: genes and proteins. Mol Cell Endocrinol. 2004;215(1–2):63–72. doi: 10.1016/j.mce.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3α-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3β-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279(11):10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 16.Jin Y, Duan L, Lee SH, Kloosterboer HJ, Blair IA, Penning TM. Human cytosolic hydroxysteroid dehydrogenases of the aldo-ketoreductase superfamily catalyze reduction of conjugated steroids: implications for phase I and phase II steroid hormone metabolism. J Biol Chem. 2009;284(15):10013–10022. doi: 10.1074/jbc.M809465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burczynski ME, Harvey RG, Penning TM. Expression and characterization of four recombinant human dihydrodiol dehydrogenase isoforms: oxidation of trans-7, 8-dihydroxy-7,8-dihydrobenzo. Biochemistry. 1999;38(32):10626. doi: 10.1021/bi995085z. [DOI] [PubMed] [Google Scholar]
- 18.Couture JF, Legrand P, Cantin L, Luu-The V, Labrie F, Breton R. Human 20α-hydroxysteroid dehydrogenase: crystallographic and site-directed mutagenesis studies lead to the identification of an alternative binding site for C21-steroids. J Mol Biol. 2003;331(3):593–604. doi: 10.1016/s0022-2836(03)00762-9. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, Stayrook SE, Albert RH, Palackal NT, Penning TM, Lewis M. Crystal structure of human type III 3α-hydroxysteroid dehydrogenase/bile acid binding protein complexed with NADP(+) and ursodeoxycholate. Biochemistry. 2001;40(34):10161–10168. doi: 10.1021/bi010919a. [DOI] [PubMed] [Google Scholar]
- 20.Lovering AL, Ride JP, Bunce CM, Desmond JC, Cummings SM, White SA. Crystal structures of prostaglandin D(2) 11-ketoreductase (AKR1C3) in complex with the nonsteroidal anti-inflammatory drugs flufenamic acid and indomethacin. Cancer Res. 2004;64(5):1802–1810. doi: 10.1158/0008-5472.can-03-2847. [DOI] [PubMed] [Google Scholar]
- 21.Jin Y, Penning TM. Molecular docking simulations of steroid substrates into human cytosolic hydroxysteroid dehydrogenases (AKR1C1 and AKR1C2): insights into positional and stereochemical preferences. Steroids. 2006;71(5):380–391. doi: 10.1016/j.steroids.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Goodsell DS, Morris GM, Olson AJ. Automated docking of flexible ligands: applications of AutoDock. J Mol Recognit. 1996;9(1):1–5. doi: 10.1002/(sici)1099-1352(199601)9:1<1::aid-jmr241>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Bennett MJ, Albert RH, Jez JM, Ma H, Penning TM, Lewis M. Steroid recognition and regulation of hormone action: crystal structure of testosterone and NADP+ bound to 3α-hydroxysteroid/dihydrodiol dehydrogenase. Structure. 1997;5(6):799–812. doi: 10.1016/s0969-2126(97)00234-7. [DOI] [PubMed] [Google Scholar]
- 24.Edgren RA. Early oral contraceptive history: norethynodrel is not a prohormone. Steroids. 1994;59(1):58–59. doi: 10.1016/0039-128x(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 25.Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42(1):67–96. doi: 10.1016/0010-7824(90)90093-b. [DOI] [PubMed] [Google Scholar]
- 26.Warren RJ, Fotherby K. Radioimmunoassay of synthetic progestogens, norethisterone and norgestrel. J Endocrinol. 1974;62(3):605–618. doi: 10.1677/joe.0.0620605. [DOI] [PubMed] [Google Scholar]
- 27.Pasqualini JR, Castellet R, Portois MC, Hill JL, Kincl FA. Plasma concentrations of ethynyl oestradiol and norethindrone after oral administration to women. J Reprod Fertil. 1977;49(2):189–193. doi: 10.1530/jrf.0.0490189. [DOI] [PubMed] [Google Scholar]
- 28.Laumas KR, Murugesan K, Hingorani V. Disappearance in plasma and tissue uptake of radioactivity after an intravenous injection of (6,7-3H) norethynodrel in women. Acta Endocrinol (Copenh) 1971;66(3):385–400. doi: 10.1530/acta.0.0660385. [DOI] [PubMed] [Google Scholar]
- 29.Mills TM, Lin TJ, Braselton WE, Ellegood JO, Mahesh VB. Metabolism of oral contraceptive drugs. The formation and disappearance of metabolites of norethindrone and mestranol after intravenous and oral administration. Am J Obstet Gynecol. 1976;126(8):987–992. [PubMed] [Google Scholar]
- 30.Jin Y, Penning TM. Multiple steps determine the overall rate of the reduction of 5α-dihydrotestosterone catalyzed by human type 3 3α-hydroxysteroid dehydrogenase: implications for the elimination of androgens. Biochemistry. 2006;45(43):13054–13063. doi: 10.1021/bi060591r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin Y, Mesaros AC, Blair IA, Penning TM. Stereospecific reduction of 5β-reduced steroids by human ketosteroid reductases of the AKR (aldo-keto reductase) superfamily: role of AKR1C1-AKR1C4 in the metabolism of testosterone and progesterone via the 5β-reductase pathway. Biochem J. 437(1):53–61. doi: 10.1042/BJ20101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braselton WE, Jr, Lin TJ, Ellegood JO, Mills TM, Mahesh VB. Accumulation of norethindrone and individual metabolites in human plasma during short- and long-term administration of a contraceptive dosage. Am J Obstet Gynecol. 1979;133(2):154–160. doi: 10.1016/0002-9378(79)90467-8. [DOI] [PubMed] [Google Scholar]
- 33.Markiewicz L, Gurpide E. In vitro evaluation of estrogenic, estrogen antagonistic and progestagenic effects of a steroidal drug (Org OD-14) and its metabolites on human endometrium. J Steroid Biochem. 1990;35(5):535–541. doi: 10.1016/0022-4731(90)90196-y. [DOI] [PubMed] [Google Scholar]
- 34.de Gooyer ME, Deckers GH, Schoonen WG, Verheul HA, Kloosterboer HJ. Receptor profiling and endocrine interactions of tibolone. Steroids. 2003;68(1):21–30. doi: 10.1016/s0039-128x(02)00112-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.