Abstract
Psychological distress and biobehavioral vulnerability (e.g., arising from being older or sedentary) have independently predicted immune responses to influenza vaccination in older adults. Recent research examining basal inflammatory markers suggests that, rather than having additive effects, distress and vulnerability interact with each other. The present study tested the interactions between distress and age, sex, education, BMI, sleep quality, and physical activity over up to 8 years in older adults (N = 134; M age = 74 years) who received annual influenza vaccinations. Measured vaccination responses were changes from baseline in antibody to the three vaccine components, interleukin (IL)-6, and b2-microglobulin. As predicted, the most robust effects were interactions between distress and vulnerability. BMI interacted with stable individual differences in distress to predict antibody response (t(132) = 3.09, p < 0.003), such that only the combination of low BMI and low distress was associated with a more robust antibody response. Likewise, changes in physical activity over time interacted with changes in distress (t(156) = 2.96, p < 0.004), such that only the combination of increased physical activity and decreased distress was associated with a more robust antibody response. Finally, there was a smaller tendency for age to interact with stable individual differences in distress (t(130) = 2.46, p < 0.015), such that distress was more strongly associated with post-vaccination IL-6 at older ages. The synergistic effects of distress and other forms of vulnerability are an important direction for future research and a target for interventions to improve immunological health in older adults.
Keywords: influenza vaccination, inflammation, aging, adiposity, exercise, stress, depression, distress
Vaccination against influenza in older adults has the potential to provide protection against negative health consequences, including hospitalizations and deaths. However, many older adults do not mount robust antibody responses to the vaccine. When antibody production following vaccination is low, the individual may still be vulnerable to infection and the negative health consequences thereof (Gardner et al., 2001; Hannoun et al., 2004; Webster, 2000). In addition, antibody responses are not the only immune response to vaccination. Vaccination can also induce inflammatory responses. Most side effects of influenza vaccination are related to inflammatory responses, from minor, local responses such as pain to more serious systemic responses such as fever, neuritis, and myelitis. Inflammatory and acute phase responses to vaccination are often transient and last only a matter of days, but there are large individual differences in degree of response, with some people showing increases in inflammatory markers and others, decreases. Furthermore, individual differences – including psychological factors – also affect duration of response, such that increases in inflammatory markers may be evident weeks after vaccination in some people (Bernstein et al., 1998; Carty et al., 2006; Glaser et al., 2003; Segerstrom et al., 2008; Trzonkowski et al., 2004; Tsai et al., 2005). Changes in antibody and inflammatory markers after vaccination are independent of each other (Bernstein et al., 1998; Krakauer & Russo, 2001).
Stress and distress have been associated with both poor antibody responses and higher inflammatory markers in older adults. For example, studies of dementia caregivers have found that caregiving is associated with lower antibody response and, in some cases, higher IL-6 after vaccination (Kiecolt-Glaser et al., 1996; Segerstrom et al., 2008; Vedhara et al., 1999). However, some people may be more immunologically sensitive to the negative effects of stress and distress than others. In studies of healthy middle-aged and older adults, various measures of psychosocial well-being or distress were more strongly related to basal markers of systemic inflammation in women, those who slept poorly, those who did not engage in moderate-intensity physical activity, and those with less education (Friedman et al., 2005; Morozink et al., 2010; Morris et al., 2011; Rethorst et al., 2011; Steptoe et al., 2008). Synergistic effects of distress and demographic and behavioral variables may explain why some studies have not found main effects of stress or distress on antibody responses to vaccine (e.g., Moynihan et al., 2004).
Other qualities that may increase vulnerability include older age and higher BMI. Even within older age, aging is associated with poorer antibody responses to vaccination and higher basal markers of systemic inflammation (Goodwin et al., 2006; Harris et al., 1999). Higher BMI is associated with higher markers of systemic inflammation, particularly IL-6, due to the presence of active macrophages in adipose tissue, particularly in visceral fat (O’Connor et al., 2009). Therefore, older age and higher BMI may also predispose to higher inflammatory and activation markers after vaccination.
The present study used data from a longitudinal study of psychosocial factors and immunological responses to vaccination in older adults to test the combinations of demographic and behavioral vulnerabilities with psychological distress as predictors of antibody to vaccine components, as well as markers of immune activation and inflammation (β2 microglobulin (β2μ) and interleukin (IL) -6). β2μ is an element of the major histocompatibility complex shed primarily by activated lymphocytes. It is elevated in serum during infection and inflammation, correlates with C reactive protein during influenza infection, and is found in higher levels in serum in healthy older compared with younger adults (Cooper et al., 1984; Zissis et al., 2001). IL-6 is a pleiotropic cytokine involved in the inflammatory cascade and produced by many somatic cells; it is also elevated during inflammation and correlated with C reactive protein (Papanicolaou et al., 1998). We hypothesized that the effects of stress and distress would interact with vulnerability factors to predict undesirable immunological outcomes after vaccination (lower antibody response and higher inflammatory and activation markers). With regard to vulnerabilities, we tested a set of stable demographic vulnerabilities that included age, gender, education, and BMI, and a set of time-varying behavioral vulnerabilities that included physical activity and sleep quality. The specific hypotheses were as follows:
Antibody and inflammatory and activation marker changes after vaccination are independent of each other.
Vulnerability to undesirable immunological outcomes after vaccination will be associated with older age, female gender, less education, high BMI, poor sleep quality, and low physical activity. Each of these characteristics has predicted undesirable immunological outcomes either alone or in combination with psychosocial factors.
The combination of distress and vulnerability will be the best predictor of such outcomes, such that distress is more strongly related to undesirable outcomes among vulnerable individuals.
Method
Participants
Participants were 134 older adults who were recruited through the clinics and the volunteer subject pool of the Sanders-Brown Center on Aging and who had data at baseline (pre-vaccination) and follow-up from at least one annual influenza vaccination during this longitudinal study. The sample was on average 74 years old at enrollment (range = 60 – 91), slightly more female than male (58% vs. 42%), and well educated, although there was a large range in years of education (M = 16 years, range = 7 – 22). The sample was predominantly white (96%), with the remainder African-American (4%). All participants were married at enrollment, though no couples were included in order to avoid dependency in the data. There was a small minority (4%) of smokers in the sample.
Procedure
All procedures were approved by the University of Kentucky Institutional Review Board. Participants were recruited continuously from 2001 to 2007. Those older adults who had expressed interest in participating in research to the Center on Aging were contacted by phone and screened. Inclusion criteria were: age 60 or older, married, and willing to be vaccinated against influenza. Exclusion criteria were: diseases that affect the immune system (e.g., autoimmune disease, cancer); chemotherapy or radiation in the 5 years prior to enrollment; immunomodulatory medications including opiates and steroids; or more than two of the following classes of medications: psychotropics, antihypertensives, hormone replacement, or thyroid supplements. These criteria exclude major influences on immune responses and allow reasonably healthy older adults into the study. More restrictions on medication would result in a sample of older adults that is unrepresentative of the population.
Participants were interviewed semi-annually in their homes. In advance of each interview, they were sent a health behaviors questionnaire that they completed daily on 3 consecutive days and returned to the interviewer. Participants received a $10 gift card following each interview during a first phase of the study (2001-2006); this amount was increased to $20 during a second phase (2006-2008). Vaccinations were performed annually from 2001 to 2008. Nurses administered the commercially available, seasonal, trivalent influenza vaccine in the participant’s home or in the clinic, whichever the participant preferred. Blood draws were taken immediately prior to vaccination and at 2 and 4 weeks following vaccination.
Measures
Demographics
Subjects reported at their first visit on their date of birth, years of education, and gender.
Medications
A medication list was provided to the interviewer at each visit. Each medication was coded by an RN into medication classes. The medication classes of interest for the present report were beta-blockers (e.g., atenolol, metoprolol, propanolol) and statins (e.g., simvastatin, atorvastatin) and were coded as taken or not taken at each visit. Beta-blockers (taken at 18% of all person-years) were of particular concern with regard to the degree to which they could contribute to extraneous variance in antibody responses via autonomic pathways (see Sanders et al., 2001, for a review), and statins (taken at 38% of all person-years) were of particular concern with regard to the degree to which they could contribute to extraneous variance in inflammatory and activation markers, particularly those related to the acute phase response (see O’Connor et al., 2009, for a review). Therefore, all antibody analyses included beta-blockers as time-varying covariates, and all inflammatory and activation marker analyses included statins as time-varying covariates.
Health variables
Participants provided their height and weight, and BMI was calculated from these reports. Because these data were collected beginning in 2002, a small number of people (N = 13) who completed only one visit before dropping out were missing BMI data. These missing data were replaced with the sex-specific means. (Note that excluding these participants from analysis did not affect the results reported below.) Participants also reported on their smoking history at the first visit.
At each visit, participants reported on their physical activity and sleep quality daily on each of 3 consecutive days. The mean across the 3 days was used as the visit-level measure. Sleep quality was measured with a single item reading “How well did you sleep last night?” with anchors 0 = “much better than usual” and 100 = “much worse than usual”. Physical activity was operationalized the product of the metabolic equivalent of task (MET) and the time spent in the task across the three days, calculated from the number of minutes the person engaged in physical activity, weighted by the intensity of the activity (MET-minutes). Based on the formula used in the Leisure Time Exercise Questionnaire, we assigned 3 METs for low-intensity, 5 METs for moderate-intensity, and 9 METs for high-intensity physical activity (Godin & Shephard, 1985).
Psychological distress
At each visit, participants completed the Geriatric Depression Scale (GDS; Yesavage et al., 1982-1983) and the Perceived Stress Scale (PSS; Cohen et al., 1983). Both measures capture aspects of distress from the affective domain (e.g., “how often have you felt nervous and ‘stressed’?” from the PSS and “do you frequently feel like crying?” from the GDS) and the cognitive domain (e.g., “how often have you felt that you were unable to control the important things in your life?” from the PSS and “do you feel that your situation is hopeless?” from the GDS). We computed a composite measure of psychological distress from these 40 items by rescaling items from the GDS (range = 0-1) to the scaling of the PSS (range = 0-4). We used PROC VARCOMP in SAS to estimate the variances associated with items, years, people, and their interactions and then used these variance components to estimate reliability (equations #4 and #5 from Cranford et al., 2006). The composite measure was reliable both over people (α = .94) and over time (i.e., within people, β = .61). The lower reliability over time is typical of measures of adjustment (Cranford et al., 2006).
Antibody response
Antibodies against the three vaccine components (H1N1, H3N2, and B) in each study year were analyzed separately using a hemagglutination inhibition assay. Serial dilutions of sera from 1:8 to 1:2048 were performed and titers defined as the inverse of the highest dilution causing complete inhibition of agglutination. Because all subjects had previously been vaccinated against influenza and antibody response following vaccination in a partially seropositive sample depends highly on prior antibody levels, a log2 transformation and correction for baseline antibody level developed by Beyer et al. (2004) was applied to the post-vaccination response for each component. This approach yielded normally distributed response variables that were independent of baseline antibody levels.
Antibody responses were also dependent on the specific strain of each component administered in that year (H1N1: F(2,142) = 26.55, p <.0001; H3N2: F(5,177) = 18.18, p<.0001; B: F(5,174) = 10.31, p<.0001), so the corrected scores were then standardized within strain. The corrected, standardized scores were reasonably correlated with each other and there were no specific hypotheses regarding the specific strains, so the three scores were averaged to create a composite antibody response variable (α = .65) for analysis. Higher scores represent larger antibody responses.
Inflammatory and activation markers
IL-6 was assayed using high-sensitivity ELISA (R & D Systems). Median inter-assay CV across all years was 3.8 (range = 3.1-7.5) and median intra-assay CV was 2.4 (range = 1.1-4.7). β2μ was assayed using ELISA (ALPCO Diagnostics). Median inter-assay CV across all years was 4.2 (range = 2.7-8.8) and median intra-assay CV was 2.9 (range = 2.5-3.6). A small number of samples were re-run due to high CV or high concentrations (run at dilution). IL-6 was subjected to a log10 transformation to improve normality; the distribution of β2μ did not require transformation. Assays were performed using the same materials within study years; therefore, change over time within study year (i.e., pre-versus post-vaccination value) was not confounded with materials. Because materials can substantially affect mean values (Kiecolt-Glaser & Glaser, 1995), variables were standardized within study years.
Data analysis
Visits were matched to vaccination years based on the shortest time between the two. Most visits (70%) were conducted within 60 days of vaccination. Because participants had varying numbers of vaccinations (17 had 1 year; 69, 2 years; 23, 3 years; 4, 4 years; 5, 5 years; 1, 6 years; 1, 7 years; and 2, 8 years), the data were analyzed using multi-level models with vaccination years at the lower level and people at the upper level. Such models easily accommodate unbalanced designs (Snijders & Bosker, 1999). The gamma weight with its standard error and associated t test are reported. Gamma weights are analogous to unstandardized beta weights in regression. To aid in interpretation, η2 calculated from the F test associated with each effect is also reported; η2 is analogous to R2.
Measures of sleep quality, physical activity, and distress were separated into their between-person “trait” and within-person “state” variance (Enders & Tofighi, 2007) by taking the mean across vaccination years to yield a person-level predictor that reflects that person’s trait level of the variable and, for each person, the deviation from their own mean at each vaccination year to yield a year-level predictor that reflects that person’s state change in that variable. For those people with only one year of data, that deviation score equals 0, and therefore “state” estimates derive from deviation scores from people with multiple years of data.
The data analysis proceeded in three steps. The first analyses entered the demographic or behavioral vulnerability factors alone as predictors of immune responses. The second analyses entered psychological distress variables alone as predictors. The third analyses entered the interactions between vulnerability factors and psychological distress as predictors, controlling for main effects. Because there were four vulnerability factors within each set of analyses (age, gender, education and BMI in the demographic predictors and state and trait physical activity and sleep quality in the behavioral predictors), and because we did not have specific predictions regarding which specific factor within a set would be associated with vulnerability, a family-wise Bonferroni error correction was applied to all analyses, setting alpha at .0125.
To interpret significant interactions, a trimmed model that included the involved variables (main effects and interactions at both levels) was run, and slopes generated from these models are represented in the figures. All interactions remained significant in these trimmed models. Estimates from trimmed models are more likely to generalize to other samples because they do not rely on potentially idiosyncratic effects of other terms in the model. Additional trimmed models recentering the appropriate variables were used to generate terms testing the significance of simple main effects (Aiken & West, 1991).
With regard to covariates, beta-blockers affected antibody responses to the vaccine, with those taking these medications having a lower antibody response than those not taking the medications (γ = −0.28, SE = 0.12, t(188) = −2.36, p < .02), but beta-blockers were not associated with IL-6 or β2μ (both p > .50). Statins tended to be associated with IL-6 following vaccination, with those not taking the medications having slightly higher IL-6, but this was not a significant effect (p > .05), and taking statins did not affect antibody response (p > .50) or β2μ (p > .40). Consistent with a priori model construction, however, beta-blockers were retained in all antibody response models and statins were retained in all inflammatory and activation marker models. The corrected antibody variable was already corrected for baseline values, but all inflammatory and activation marker analyses also controlled for baseline values.
Results
Immune responses to vaccination
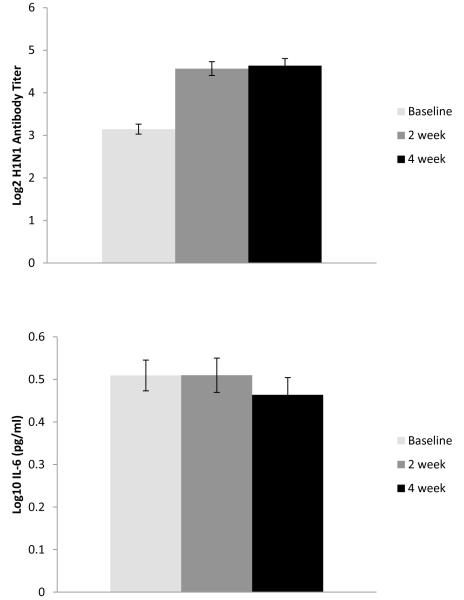
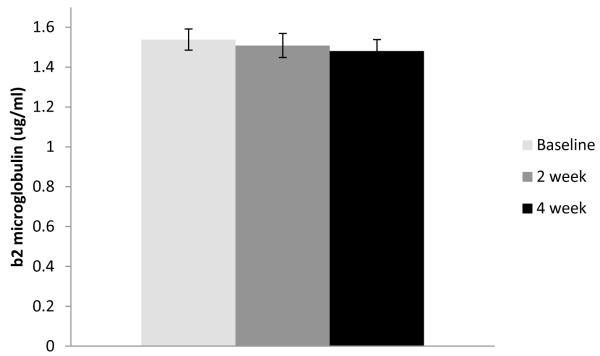
For illustrative purposes, the unstandardized means of the immune responses to vaccination for antibody (only the H1N1 component is shown), IL-6, and β2μ are given in Figure 1. These data are taken from the first year of vaccination data for all participants to ensure correct standard errors and t tests but are representative of all person-years. All antibody titers increased significantly between baseline (i.e., pre-vaccination) and 2-week and 4-week follow-ups (in paired t tests, all p < .0001). Mean levels of inflammatory and activation markers (IL-6 and β2μ) did not change significantly from baseline to either follow-up. However, there were substantial individual differences in change in these parameters from baseline to either time point (IL6: M change = 0.06, SD = 0.45, range = −2.30 to +1.80 log10 pg/ml; β2μ M change = 0.04, SD = 0.40, range = −0.96 to +1.78 μg/ml). Therefore, all subsequent analyses were performed on peak responses, that is, the highest value at either follow-up period.
Figure 1.
Mean changes in antibody titer, IL-6, and b2m from pre-vaccination baseline to 2- and 4-week follow-ups.
Independence of immune responses to vaccination
Table 1 shows the relationships among the 3 immune responses to vaccination. These relationships were derived from models predicting (1) corrected peak antibody from baseline and peak IL-6 and β2μ, respectively, (2) peak IL-6 from baseline IL-6 and baseline and peak β2μ, and (3) peak β2μ from baseline β2μ and baseline and peak IL-6. As predicted, the antibody and inflammatory and activation responses were independent of each other. Neither baseline nor peak values of IL-6 and β2μ were significantly associated with antibody responses, accounting for no more than 1% of the variance. In contrast, the two inflammatory and activation parameters were related to each other in similar ways: higher levels of peak activation in one parameter were associated with higher levels of peak activation in the other parameter, after controlling for both parameters’ baseline values. Peak values of one parameter accounted for approximately 6% of the variance in the other parameter.
Table 1.
Relationships among immune responses to influenza vaccination. Estimates are gamma weights from multi-level models with their associated standard errors.
| Outcome |
Predictors |
|||
|---|---|---|---|---|
| Baseline IL-6 | Peak IL-6 | Baseline b2m | Peak b2m | |
| Antibody | −0.037 (0.05)a | 0.049 (0.05)a | −0.098 (0.07)a | −0.027 (0.06)a |
| η 2 | .002 | .005 | .012 | .001 |
| Peak IL-6 | 0.56 (0.05)b | - | −0.16 (0.07)b | 0.21 (0.06)b |
| η 2 | .43 | .02 | .06 | |
| Peak b2m | −0.09 (0.05)c | 0.17 (0.05)b | 0.72 (0.05)b | - |
| η 2 | .02 | .06 | .50 | |
df = 186, p > .05
df = 183, p < .05
df = 183, p < .10
Effects of individual vulnerabilities
Models were tested with the between-person predictors of age, education, gender, and BMI. As shown in Table 2, there were some statistically significant relationships among these variables, but none was of the magnitude to create concern about collinearity in the model. Although more educated people tended to have higher antibody responses, this effect did not meet the corrected p value (γ = 0.046, SE = 0.020, t(137) = 2.31, p < .02, η2 = .04). Although older people tended to have higher peak β2μ after vaccination, this effect did not meet the corrected p value (γ = 0.018, SE = 0.009, t(136) = 2.03, p < .04, η2 = .03), and there were not significant effects of other vulnerabilities. None of the individual vulnerabilities predicted peak IL-6 after vaccination.
Table 2.
Correlations among person-level measures of vulnerability and resilience (n = 134)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Age | - | −.18* | .05 | −.24* | −.08 | .12 | .01 |
| 2. Gender | - | −.12 | −.07 | −.18* | −.05 | .17* | |
| 3. Education | - | −.13 | .17* | −.16 | .03 | ||
| 4. BMI | - | −.18* | −.16 | .03 | |||
| 5. Average (“trait”) physical activity | - | .05 | −.29* | ||||
| 6. Average (“trait”) sleep quality | - | .01 | |||||
| 7. Average (“trait”) distress | - | ||||||
Note.
p < .05. Gender was coded 0 = male and 1 = female.
Effects of behavioral vulnerabilities
Models were tested with both between-person “trait” and within-person “state” physical activity and sleep quality predictors. Worse state sleep quality tended to be associated with better antibody responses to vaccination, but this effect did not meet the corrected p value (γ = 0.0040, SE = 0.0057, t(163) = 2.36, p < .02, η2 = .03). Likewise, more state physical activity tended to be associated with lower peak IL-6, but this effect did not meet the corrected p value (γ = −0.00022, SE = 0.00009, t(160) = 2.37, p < .02, η2 = .03). None of the behavioral vulnerabilities predicted peak β2μ after vaccination.
Interactions between vulnerabilities and psychological distress
We predicted that effects of psychological distress on lower antibody and higher inflammatory and activation marker responses would be evident in people with individual or behavioral vulnerabilities. Consistent with the prediction that not all people may be vulnerable to immunological effects of distress, there were no significant main effects of either “trait” or “state” distress on antibody or inflammatory and activation marker responses.
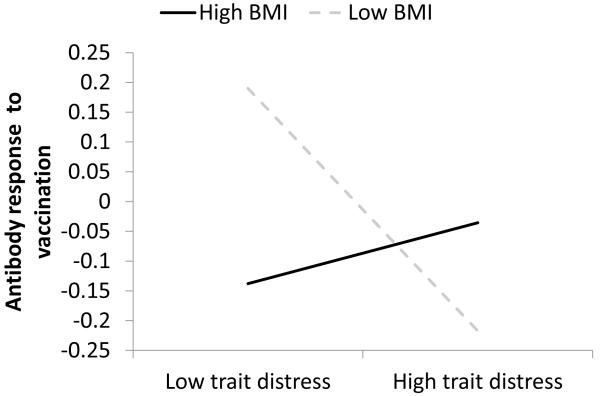
Also consistent with this prediction, there were statistically significant interactions between vulnerability and distress. For antibody responses, among the individual vulnerabilities, there was a significant interaction between BMI and trait distress (γ = 0.27, SE = 0.09, t(132) = 3.09, p < 0.003, η2 = .07). Figure 2 graphs the distress-antibody slope from 1 SD above to 1 SD below the mean on BMI (low BMI ≈ 22; high BMI ≈ 32). In this interaction, there was a significant simple main effect of distress when BMI was low (γ = −1.74, SE = 0.58, t(138) = −2.99, p < .004), such that people with low BMI and low distress had higher antibody responses. The simple main effect of distress at high BMI was not significant (p < .45). Examined another way, there was a simple main effect of BMI at low distress (γ = −0.03, SE = 0.02, t(138) = −2.03, p < .05), but not at high distress (p < .13). In short, low BMI was associated with higher antibody responses than high BMI, but only when people were also low in distress. High distress abrogated the desirable effect of low BMI.
Figure 2.
Slopes of trait distress on standardized (M=0, SD=1), peak, baseline-corrected antibody response at high (+1 SD) and low (−1 SD) levels of BMI.
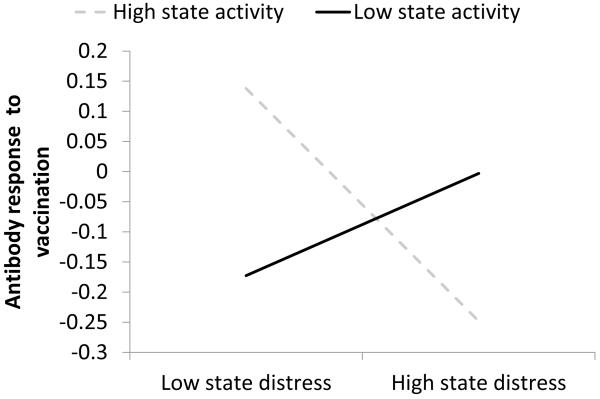
In addition, among the behavioral vulnerabilities, there was a significant interaction between state physical activity and state distress predicting antibody response (γ = −0.0077, SE = 0.0026, t(156) = −2.96, p < 0.004, η2 = .05). Figure 3 graphs the distress-antibody slope at 1 SD above and below the mean on state physical activity (low physical activity ≈ 500 MET-minutes lower than average; high physical activity ≈ 500 MET-minutes higher than average). In this interaction, there was a significant simple main effect of distress when physical activity was higher than average (γ = −3.56, SE = 1.48, t(172) = −2.41, p < .02), such that when people had decreased distress, they also had a higher antibody response with increased physical activity. The simple main effect of distress when people had decreased physical activity was not significant (p < .23). Examined another way, there was a simple main effect of physical activity at low distress (γ = .00031, SE = 0.00014, t(172) = 2.19, p < .03) but not at high distress (p < .10). In short, above-average physical activity was associated with higher antibody responses than below-average physical activity, but only when people were also low in distress. High distress abrogated the desirable effect of above-average physical activity.
Figure 3.
Slopes of trait distress on standardized (M=0, SD=1), peak, baseline-corrected antibody response at high (+1 SD) and low (−1 SD) levels of state physical activity
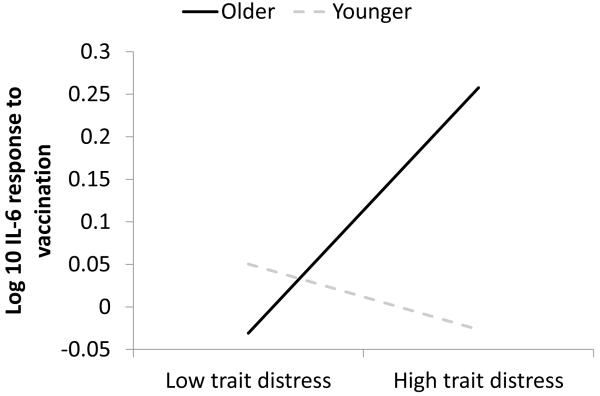
For IL-6 responses, there was an interaction between age and trait distress that was just above the corrected p value (γ = 0.19, SE = 0.08, t(130) = 2.46, p < 0.015, η2 = .04). Figure 4 illustrates this interaction by graphing the distress-IL-6 slope for people at 1 SD above and below the mean age (older ≈ 81.4 years; younger ≈ 68.6 years). In this interaction, there were not significant simple main effects of distress at older age (p < .10) or younger age (p < .50). However, looked at another way, there was a significant simple main effect of age at high distress (γ = 0.02, SE = 0.01, t(136) = 2.19, p < .03), such that age was positively related to IL-6 response at high distress; the simple main effect of age at low distress was not significant (p < .62). In short, older age was associated with higher IL-6 responses, but only when people were also high in distress. Low distress abrogated the undesirable effect of older age.
Figure 4.
Slopes of trait distress on standardized (M=0, SD=1), peak IL-6 after vaccination at older (+1 SD) and younger (−1 SD) ages
Discussion
Consistent with our predictions, antibody and inflammatory and activation marker responses after influenza vaccination were independent of each other and accordingly had different predictors. However, in both cases, the best predictors of antibody and inflammatory and activation marker responses were the combination of distress and vulnerability. In the case of antibody response, the two metabolic variables (BMI and physical activity) interacted with distress. At the “trait” level (i.e., across all study years), people with lower BMI had higher antibody responses, but only if they were also low in distress. At the “state” level (i.e., with regard to changes across study years), people who were exercising more (where a 1 SD difference was approximately the equivalent of 1 hour of walking daily) had higher antibody responses, but only if they were also experiencing low distress relative to their usual levels. In the case of inflammatory and activation markers, there was some indication that the relevant vulnerability factor was age. Even within this sample of older adults, older age tended to be associated with a larger β2μ response to vaccination and a larger IL-6 response, but this latter effect was only evident when distress was high.
Based on the patterns of interactions between vulnerability and distress in studies examining markers of basal inflammatory markers (Friedman et al., 2005; Morozink et al., 2010; Morris et al., 2011; Rethorst et al., 2011; Steptoe et al., 2008), we predicted that the effects of distress would be stronger among vulnerable people. However, the interactions that predicted antibody response took a different form. Instead, the effects of distress were stronger among less vulnerable people, because low vulnerability (i.e., low BMI or high physical activity) was only associated with better antibody response when distress was also low. When distress was high, more and less vulnerable individuals had similar, low antibody responses to vaccination. As noted above, distress abrogated the protective effects of low BMI and high physical activity.
Increases in physical activity may improve antibody response to vaccination in older adults (Kohut et al., 2004; Woods et al., 2009), but these results suggest that these increases may be most effective when distress is also low. Kohut and colleagues provided a slightly different take on this phenomenon by testing distress as a mediator of exercise effects, finding that exercise improved psychological health and that these improvements partially mediated exercise effects. The results of that mediational test and the present moderated effects tend to suggest the same thing: more physical activity is better for antibody response mainly in the presence of low distress.
Consistent with studies of markers of basal inflammatory makers, the interaction between age and distress took the expected form predicting increases in IL-6 after vaccination. When people were low in distress, there was little difference between younger and older people, but younger people were more resistant to the negative effects of distress on IL-6 response to vaccine than older people.
These findings have important implications for the development of multimodal interventions to improve health in older adults. Influenza vaccination, on average, prevents hospitalizations and deaths in older adults (Jefferson et al., 2010), but those older adults who mount a smaller antibody response to vaccination may be at higher risk. HI titers of 40 have been suggested to provide moderate protection again infection (Hannoun et al., 2004); in the present study, only 21.4% of person-years exceeded this threshold for the H1N1 component; 57.8% for the H3N2 component; and 54.3% for the B component. Conversely, levels of serum IL-6 above 3.16 pg/ml have been suggested to increase mortality risk in older adults (Harris et al., 1999); in the present study, 37.5% of person-years reached this threshold after vaccination. Therefore, there is significant room for immunological improvement among these older adults. With the exception of age, all of the factors that affected antibody and inflammatory and activation marker responses (BMI, physical activity, and distress) are modifiable. Furthermore, the analyses that demonstrated correlation between changes in physical activity and distress and changes in antibody responses in effect use each person as his or her own control. They therefore provide evidence that modification of these psychosocial and biobehavioral factors may result in immunological change. The most important message from these data, however, is that more than one factor could be targeted to optimize effects. Increased physical activity, weight loss to normal BMI, and improved coping with stress and distress would appear to be an optimal combination in this population. Another approach would be to target distress in older adults who already have other protective factors, as these findings suggest that distress may suppress the protective effects of other factors such as physical activity. Finally, the availability of a high-dose influenza vaccine offers a pharmacological alternative for older adults with persistent distress, vulnerability, or both. The high-dose vaccine can improve antibody responses in older adults, although local and potentially systemic inflammatory reactions may also be increased (Falsey et al., 2009).
One factor that could not be assessed in the present study was the dynamic effect of aging, as opposed to the static effect of age at study entry. Because specific antigenic content of each vaccine component changed across years, and samples were analyzed in annual batches, effects of study year were confounded with aging. Although effects of vaccine composition cannot be controlled, with regard to activation and inflammation, future studies could archive samples until the end of the study or, where residual samples are archived, re-run all samples together using the same materials in order to be able to assess the effects of aging independent of study years. In addition, measures of physical activity, sleep quality, and BMI were very general assessments of these biobehavioral factors. For example, BMI was used as a proxy for adiposity, but adiposity may be better assessed with combined with other measures such as waist circumference (O’Connor et al., 2009). On the other hand, the daily assessment of sleep quality and physical activity may have resulted in more accurate reporting than retrospective reports.
Most studies of vaccine response in older adults have focused on psychosocial (e.g., stress; Kiecolt-Glaser et al., 1996; Moynihan et al., 2004) or behavioral (e.g., physical activity; Keylock et al., 2007; Kohut et al., 2002) predictors of responses to vaccination, but to our knowledge, this is the first time they have been examined in combination. Studies of basal inflammation in middle-aged and older adults reported that the effects of distress were stronger in more vulnerable individuals (Friedman et al., 2005; Morozink et al., 2010; Morris et al., 2011; Rethorst et al., 2011; Steptoe et al., 2008), and a post-vaccination marker of inflammation roughly followed this same pattern. However, with regard to the antibody response, the effects of distress were strong in less vulnerable individuals, suggesting different pathways and interactions (i.e., between neural and neuroendocrine pathways) for the humoral and inflammatory responses. The synergistic effects of distress and other forms of vulnerability are an important direction for future research on immunological health in older adults.
Acknowledgements
This study was supported by the Dana Foundation, UK HealthCare, and NIH (AG026307-R01, AG028383-P30, RR02602-M01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Suzanne C. Segerstrom, Department of Psychology, University of Kentucky.
Jaime K. Hardy, Department of Psychology, University of Kentucky
Daniel R. Evans, Department of Psychology, University of Kentucky
Richard N. Greenberg, Department of Internal Medicine, University of Kentucky
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage; Thousand Oaks, CA: 1991. [Google Scholar]
- Bernstein ED, Gardner EM, Aburtyn E, Gross P, Murasko DM. Cytokine production after influenza vaccination in a healthy elderly population. Vaccine. 1998;16:1722–1731. doi: 10.1016/s0264-410x(98)00140-6. [DOI] [PubMed] [Google Scholar]
- Beyer WE, Palache AM, Lüchters G, Nauta J, Osterhaus AD. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus Res. 2004;103:125–132. doi: 10.1016/j.virusres.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Carty CL, Heagerty P, Nakatama K, McClung EC, Lewis J, Lum D, Boespflug E, McCloud-Gehring C, Soleimani BR, Ranchalis J, Bacus TJ, Furlong CE, Jarvik GP. Inflammatory response after influenza vaccination in men with and without carotid artery disease. Arterioscler Thromb Vasc Biol. 2006;26:2738–2744. doi: 10.1161/01.ATV.0000248534.30057.b5. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Beh. 1983;24:385–396. [PubMed] [Google Scholar]
- Cooper EH, Forbes MA, Hambling MH. Serum β2-microglobulin and C reactive protein concentrations in viral infections. J Clin Path. 1984;37:1140–1143. doi: 10.1136/jcp.37.10.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, Bolger N. A procedure for evaluating sensitivity to within-person change: Can mood measures in diary studies detect change reliably? Pers Soc Psychol Bull. 2006;32:917–929. doi: 10.1177/0146167206287721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychol Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, Singer BH, Ryff CD. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci. 2005;102:18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EM, Bernstein ED, Dran S, Munk G, Gross P, Abrutyn E, Murasko DM. Characterization of antibody responses to annual influenza vaccination over four years in a healthy elderly population. Vaccine. 2001;19:4610–4617. doi: 10.1016/s0264-410x(01)00246-8. [DOI] [PubMed] [Google Scholar]
- Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- Godin G, Shepard RJ. A simple method to assess exercise behavior in the community. Can J App Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- Goodwin K, Viboud C, Simonsen L. Antibody respons to influenza vaccination in the elderly: A quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103:133–138. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Di Pietranouj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly (Review) Cochrane Library. 2010;(2) doi: 10.1002/14651858.CD004876.pub3. [DOI] [PubMed] [Google Scholar]
- Keylock KT, Lowder T, Leifheit KA, Cook M, Mariani RA, Ross K, Kim K, Chapman-Novakofski K, McAuley E, Woods JA. Higher antibody, but not cell-mediated, responses to vaccination in high physically fit elderly. J Appl Physiol. 2007;102:1090–1098. doi: 10.1152/japplphysiol.00790.2006. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Measurement of immune response. In: Cohen S, Kessler RC, Gordon LU, editors. Measuring stress: A guide for health and social scientists. Oxford University Press; New York: 1995. pp. 213–229. [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut ML, Arntson BA, Lee W, Rozeboom K, Yoon KJ, Cunnick JE, McElhaney J. Moderate exercise improves antibody response to influenza vaccination in older adults. Vaccine. 2004;22:2298–2306. doi: 10.1016/j.vaccine.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol: Med Sci. 2002;57A:M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- Krakauer T, Russo C. Serum cytokine levels and antibody response to influenza vaccine in the elderly. Immunopharm Immunotox. 2001;23:35–41. doi: 10.1081/iph-100102565. [DOI] [PubMed] [Google Scholar]
- Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychol. 2010;29:626–635. doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AA, Zhao L, Ahmed Y, Stoyanova N, De Staercke C, Hooper WC, Gibbons G, Din-Dzietham R, Quyyumi A, Vaccariona V. Association between depression and inflammation – differences by race and sex: The META-Health Study. Psychosom Med. 2011;73:462–468. doi: 10.1097/PSY.0b013e318222379c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan JA, Larson MR, Treanor J, Duberstein PR, Power A, Shore B, Ader R. Psychosocial factors and the response to influenza vaccination in older adults. Psychosom Med. 2004;66:950–953. doi: 10.1097/01.psy.0000140001.49208.2d. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimistrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Beh Imm. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicoloau DA, Wilder RL, Manolagas C, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Rethorst CD, Moynihan J, Lyness JM, Heffner KL, Chapman BP. Moderating effects of moderate-intensity physical activity in the relationship between depressive symptoms and interleukin-6 in primary care patients. Psychosom Med. 2011;73:265–269. doi: 10.1097/PSY.0b013e3182108412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VM, Kasprowicz DJ, Kohn AP, Swanson MA. Neurotransmitter receptors on lymphocytes and other lymphoid cells. In: Ader R, Felton DL, Cohen N, editors. Psychoneuroimmunology. 3rd ed. Academic Press; San Diego, CA: 2001. pp. 161–196. [Google Scholar]
- Segerstrom SC, Schipper LJ, Greenberg RN. Caregiving, repetitive thought, and immune response to vaccination in older adults. Brain Beh Imm. 2008;22:744–752. doi: 10.1016/j.bbi.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel Analysis. Sage; London: 1999. [Google Scholar]
- Steptoe A, O’Donnell K, Badrick E, Kumari M, Marmot M. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: The Whitehall II Study. Am J Epidemiol. 2008;167:96–102. doi: 10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Myśliwska J, Godlewska B, Szmit E, □ukaszuk K, Więckiewicz J, Brydak L, Machała M, Landowsky J, Myśliwski A. Immune consequences of the spontaneous pro-inflammatory status in depressed elderly patients. Brain Beh Imm. 2004;18:135–148. doi: 10.1016/S0889-1591(03)00111-9. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Hanson NQ, Straka RJ, Hoke TR, Ordovas JM, Peacock JM, Arends VL, Arnett DK. Effect of influenza vaccine on markers of inflammation and lipid profile. J Lab Clin Med. 2005;145:323–327. doi: 10.1016/j.lab.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Cox NKM, Wilcock GK, Perks P, Hunt M, Anderson S, Lightman SL, Shanks NM. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet. 1999;353:627–631. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- Webster RG. Immunity to influenza in the elderly. Vaccine. 2000;18:1686–1689. doi: 10.1016/s0264-410x(99)00507-1. [DOI] [PubMed] [Google Scholar]
- Woods JA, Keylock KT, Lowder T, Vieira VJ, Zelkovich W, Dumich S, Colantuano K, Lyons K, Leifheit K, Cook M, Chapman-Novakofski K, McAuley E. Cardiovascular exercise training extends influenza vaccine seroprotection in sedentary older adults: The Immune Function Intervention Trial. J Am Geriatrics Soc. 2009;57:2183–2191. doi: 10.1111/j.1532-5415.2009.02563.x. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatric Res. 1982–1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zissis M, Afroudakis A, Glanopoulos G, Palermos I, Boura X, Michopoulos S, Archimandritis A. B2 microglobulin: Is it a reliable marker of activity in inflammatory bowel disease? Am J Gastroentorol. 2001;96:2177–2183. doi: 10.1111/j.1572-0241.2001.03881.x. [DOI] [PubMed] [Google Scholar]