Abstract
Objective
Sleep homeostasis is altered in major depressive disorder (MDD). Pre-to post-sleep decline in waking auditory evoked potential (AEP) amplitude has been correlated with sleep slow wave activity (SWA), suggesting that overnight changes in waking AEP amplitude are homeostatically regulated in healthy individuals. This study investigated whether the overnight change in waking AEP amplitude and its relation to SWA is altered in MDD.
Method
Using 256-channel high-density electroencephalography, all-night sleep polysomnography and single-tone waking AEPs pre-and post-sleep were collected in 15 healthy controls (HC) and 15 non-medicated individuals with MDD.
Results
N1 and P2 amplitudes of the waking AEP declined after sleep in the HC group, but not in MDD. The reduction in N1 amplitude also correlated with fronto-central SWA in the HC group, but a comparable relationship was not found in MDD, despite equivalent SWA between groups. No pre-to post-sleep differences were found for N1 or P2 latencies in either group. These findings were not confounded by varying levels of alertness or differences in sleep variables between groups.
Conclusion
MDD involves altered sleep homeostasis as measured by the overnight change in waking AEP amplitude. Future research is required to determine the clinical implications of these findings.
Keywords: major depressive disorder, auditory evoked potentials, sleep, homeostasis, slow-wave sleep
Introduction
Sleep disturbances in major depressive disorder (MDD) have both diagnostic and functional significance (1–3). Alterations in sleep continuity (4), rapid eye movement (REM) sleep (1, 5), and non-REM (NREM) sleep have been demonstrated in MDD. In NREM sleep, slow wave activity (SWA; sleep EEG power in the 1–4.5Hz range) has been established as a marker of sleep homeostasis, as it increases in proportion to prior wakefulness and declines with sleep (6–9). Regulation of these homeostatic processes, as measured by SWA, has been shown to be aberrant in MDD (10–12).
In addition to sleep SWA, waking auditory evoked potentials (AEPs), a readily collected measure of auditory processing, have also been linked to homeostatic function. A recent investigation demonstrated that the amplitudes of AEP N1 and P2 components significantly decline pre-to post-sleep, most prominently over a fronto-central region of the cortex (13). Additionally, the overnight change in N1 amplitude correlated with SWA from that night, suggesting sleep-related homeostatic regulation of these AEP components.
Given the demonstrated abnormalities in MDD related to sleep homeostasis, combined with the evidence that overnight change in waking AEP amplitude is a sleep-related homeostatic process, pre-to post-sleep changes in waking AEP amplitude could provide valuable information regarding the altered regulation of sleep homeostasis observed in MDD. Therefore, the current study employed a common auditory oddball technique and spectral analysis of sleep using high-density EEG (hdEEG) in healthy controls and individuals with MDD.
Aims of the study
This study investigated the overnight change in auditory evoked potential amplitude in healthy controls and participants with major depressive disorder (MDD). We hypothesized that the control group would demonstrate a pre-to post sleep decline in N1 and P2 amplitude that would correlate with sleep slow wave activity, while the MDD group would fail to show a comparable sleep-related decline in N1 or P2 amplitude.
Material and methods
Participants
30 right-handed individuals, including 15 MDD participants and 15 healthy controls (HC), were recruited from a larger study on sleep homeostasis in depression. MDD was diagnosed via the Structured Clinical Interview for DSM-IV Axis I disorders (SCID) (14), and global depression severity was evaluated with the clinician-administered 17-item Hamilton Rating Scale for Depression (15). MDD participants were unipolar, non-psychotic, and medication-free for ≥ 6 months prior to the study. HC participants were evaluated with the non-patient version of the SCID to rule out current or past psychiatric disorders (16).
All participants provided informed consent and were instructed to maintain regular sleep-wake schedules, avoid napping, and to limit the use of caffeinated or alcoholic beverages to no more than 1 drink per day and none after 2 p.m. for the duration of the study. Adherence was verified via sleep diaries and wrist motor actigraphy (Actiwatch, Mini-Mitter, Bend, OR). This study was approved by the Institutional Review Board of the University of Wisconsin-Madison.
Study design
All participants underwent in-laboratory polysomnography (PSG) prior to their experimental night, which served to both acclimate participants to the laboratory environment and to rule out clinically significant sleep-disordered breathing (apnea-hypopnea index > 10/hr) or sleep-related movement disorders (periodic limb movement-arousal index > 10/hr). PSG included standard monitoring with electrooculogram (EOG), sub-mental electromyogram (EMG), electrocardiogram (ECG), bilateral tibial EMG, respiratory inductance plethysmography, pulse oximetry, and a position sensor. To reduce potential confounds caused by low total sleep time and sleep fragmentation, individuals with sleep efficiency (total sleep time/time in bed) < 80% were excluded from the study.
On the experimental night, participants arrived at the laboratory between 20:00 and 21:00 and were outfitted with 256-channel hdEEG (Electrical Geodesics Inc., Eugene, OR). Participants sequentially performed a Psychomotor Vigilance Task (PVT) (17), an auditory oddball task, and a visuo-motor learning task (18), then slept undisturbed in the laboratory starting within one hour of their reported usual bedtime. The following morning, participants were woken up after at least 7 hours of sleep and performed the tasks from the previous evening in the same sequential order. The morning tasks were performed at least 30 minutes after awakening to minimize effects of sustained drowsiness after waking (19). All AEP and sleep hdEEG recordings were collected with vertex-referencing, using NetStation software (Electrical Geodesics Inc., Eugene, OR) and DC amplifiers with200Hz analog low-pass filters. To increase the signal-to-noise ratio, all hdEEG processing and analyses were restricted to the 185 channels overlaying the scalp (20).
Auditory oddball task
AEPs for each participant were gathered using a standard oddball paradigm (13, 21, 22), delivering sinusoidal tones of 50 msec duration and frequencies of 800 Hz (standard) or 560 Hz (target) using E-Prime (Psychology Software Tools Inc., Pittsburgh, PA). A total of 96 standard and 24 target stimuli were presented at each time point with an inter-stimulus interval that varied randomly between 1900 and 3190 msec. Tones were presented binaurally at an unchanging, comfortable volume through circumaural headphones or earbuds. Throughout each recording, participants were instructed to sit comfortably with eyes open, fixating at a point 90 cm in front of them, while counting the number of low-frequency (target) tones.
To ensure a high signal-to-noise ratio when averaging the evoked responses, data processing and analysis for each time point were restricted to the 96 standard tones. The AEP recordings were sampled at 500Hz, first-order high-pass filtered (Kaiser type FIR, 0.1 Hz), bandpass filtered (Kaiser type FIR, 1–15 Hz), and segmented (100 msec pre-to 500 msec post-stimulus). Data cleaning was performed automatically, whereby channels in each segment were marked as artifact if the maximum amplitude difference of the entire segment was greater than 100 μV or less than .05 μV, or if the maximum amplitude difference within a moving window of 80 msec was greater than 150 μV (for ocular artifacts). Segments were removed if they contained more than 20 artifactual channels or any ocular artifacts. Artifactual channels in the remaining segments were replaced using spherical spline interpolation. Visual inspection of the data was then performed using topographical maps and synoptic plots, but no further channels or segments were removed. The mean percentage of trials retained for AEP averaging was at least 80% of total segments for each group (HC: 82.11 ± 14.65%; MDD: 85.38 ± 16.80%), consistent with prior studies (23, 24). Finally, data from the 185 channels were re-referenced using linked mastoids and baseline corrected by subtracting the average voltage during the 100 msec preceding the tone.
The peak N1 and P2 amplitudes for each channel were identified as the most negative value between 40 and 140 msec and the most positive value between 142–262 msec post-stimulus, respectively (13). Latencies to N1 and P2 peaks were additionally identified for analysis. The average of a fronto-central cluster of channels was calculated for pre-and post-sleep recordings for overnight comparisons by time. To measure global brain activation of the AEPs (13, 25, 26), global mean field power (GMFP) was calculated as the root mean square of the instantaneous voltage distribution across all 185 channels.
Sleep recordings
All-night hdEEG recordings were sampled at 500 Hz, first-order high-pass filtered (Kaiser type, 0.6 Hz), downsampled to128 Hz, band-pass filtered (2-way least-squares FIR, 1–40 Hz), and average-referenced. Semi-automatic artifact rejection was conducted to remove channels with high-frequency noise or interrupted contact with the scalp for the majority of the recording. Spectral analysis was performed for each channel in consecutive 6-second epochs (Welch’s averaged modified periodgram with a Hamming window). Sleep staging was based on 6 bipolar mastoid-referenced channels (F3, F4, C3, C4, O1, and O2), sub-mental EMG and EOG, and was performed by a registered polysomnographic technologist in 30-second epochs according to standard critera using Alice® Sleepware (Philips Respironics, Murrysville, PA) (27).
Statistics
Overnight differences (pre-vs. post-sleep) in N1 and P2 amplitude and latency of the AEP within each group were examined using 2-tailed, paired t-tests. To correct for multiple comparisons of the topographical hdEEG data, statistical non-parametric mapping with suprathreshold cluster tests (STCT) was employed (13, 28). For a cluster of channels to be significant, it had to be larger than 95% of the clusters in the maximal cluster size distribution. To compare pre-sleep AEP latency and amplitude, as well as polysomnographic variables between groups, 2-tailed unpaired (homoscedastic) t-tests were used. For correlational analyses, AEP and hdEEG sleep data were first cleaned for outliers using a threshold of ± 2.5 standard deviations from the mean at each channel. Statistical dependence between variables at each channel and frequency bin was assessed using linear regression. Descriptive statistics are expressed as mean ± standard deviation. Statistical analyses were performed using MATLAB (The MathWorks Inc., Natick, MA) and STATISTICA (StatSoft Inc., Tulsa, OK).
Results
Demographic, clinical, and polysomnographic data
Table 1 displays descriptive statistics and P values for between group comparisons. MDD and HC groups did not differ in age. Both groups consisted of male and female participants, with a slightly higher proportion of women in the MDD group (10/15 vs. 8/15). HRSD-17 scores for MDD participants ranged from 8 to 25, indicating mild to moderate depression severity (15). Mean number of lifetime major depressive episodes was 10.1 ± 11.9 (range: 1–30), and mean duration of the current episode was 7.2 ± 3.2 weeks. 6 MDD participants met criteria for melancholic and 3 for atypical depression subtype. In addition to their principal diagnosis of MDD, 6 participants had comorbid anxiety disorders (5 generalized anxiety disorder, 1 post-traumatic stress disorder). No participants had current or a past history of drug or alcohol dependence. On the initial screening PSG, MDD and HC groups did not demonstrate significantly different indices of sleep-disordered breathing (AHI) or sleep fragmentation due to nocturnal myoclonus (PLM-AI).
Table 1.
Demographic, clinical, and polysomnographic data
| HC (N = 15) | MDD (N = 15) | P | |
|---|---|---|---|
| Sex (male/female) | 7/8 | 5/10 | |
| Age (years) | 21.40 (1.55) | 22.40 (2.67) | 0.220 |
| HRSD-17 | -- | 16.27 (5.02) | |
| AHI* (#/hr.) | 0.57 (1.12) | 0.54 (0.73) | 0.939 |
| PLM-AI* (#/hr.) | 2.23 (2.85) | 2.36 (2.85) | 0.902 |
| TST (min.) | 430.30 (28.74) | 409.20 (56.38) | 0.207 |
| WASO (min.) | 25.97 (21.33) | 25.73 (21.56) | 0.976 |
| AI (#/hr.) | 7.56 (4.13) | 11.34 (5.07) | 0.033 |
| SE (%) | 91.48 (5.59) | 90.25 (7.01) | 0.598 |
| SOL (min.) | 15.10 (15.49) | 17.73 (12.86) | 0.612 |
| N1 (%) | 5.90 (3.79) | 7.05 (4.02) | 0.426 |
| N2 (%) | 58.47 (7.06) | 54.71 (11.71) | 0.296 |
| N3 (%) | 16.53 (5.71) | 14.81 (10.32) | 0.577 |
| SWA | 45.08 (38.25) | 41.36 (26.51) | 0.759 |
| REM (%) | 19.13 (6.11) | 23.42 (7.28) | 0.092 |
| REML (min.) | 95.40 (37.64) | 89.10 (50.89) | 0.703 |
HC, healthy control; MDD, major depressive disorder; HRSD-17, Hamilton Rating Scale for Depression (17-item); AHI, apnea-hypopnea index; PLM-AI, periodic limb movement-arousal index; TST, total sleep time; WASO, wake (time) after sleep onset; AI, arousal index; SE, sleep efficiency; N1/2/3, NREM stage 1/2/3 (% of TST); SWA, slow wave activity (EEG power in 1–2.33 Hz range); REM, stage REM (% of TST); REML, REM latency Values are displayed as mean (standard deviation). 2-tailed, independent samples t-tests were used.
Values are from initial screening night. All other polysomnographic variables reflect the experimental night.
There was no difference between HC and MDD groups for lights-off (23:04 ± 41 min. vs. 23:06 ± 18 min., P = 0.841)or lights-on (06:46 min. ± 39 vs. 06:47 ± 49 min., P = 0.956). Additionally, no significant differences between groups were observed for total sleep time (TST), wake after sleep onset, sleep efficiency, sleep onset latency, amount of NREM sleep (% of TST), or REM latency. However, the MDD group exhibited a higher arousal index (T = 2.24, P = 0.033) and trended toward a greater percentage of REM sleep (T = 1.75, P = 0.092). There was no significant difference in the amount of all-night global SWA between MDD and HC groups. Additional exploratory analyses demonstrated no significant differences in global SWA between groups for any NREM episode, nor significant differences in topographic SWA for all-night or NREM episodes1–4.
Overnight change in N1 and P2 amplitude
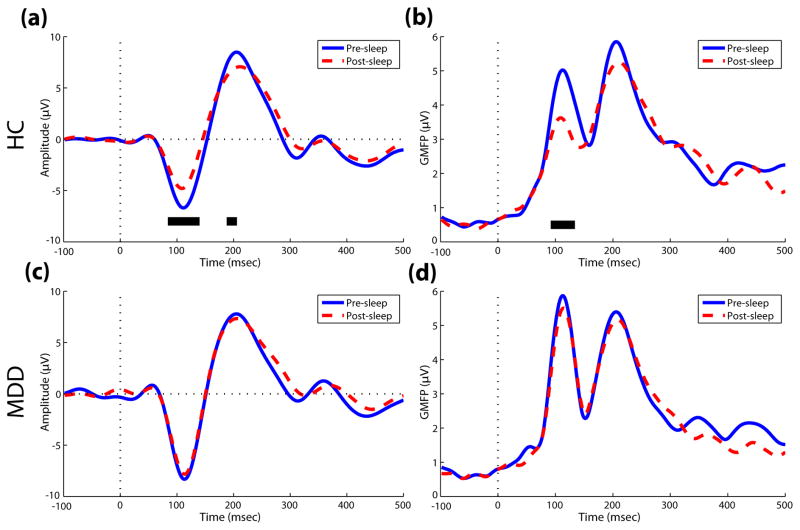
The auditory oddball task was administered at similar times for HC and MDD groups (Pre-sleep: 21:31 ± 25 min. vs. 21:29 ± 15 min., P = 0.793; Post-sleep: 07:25 ± 43 min. vs. 07:30 ± 42 min., P = 0.722). Consistent with previous research (13), the HC group demonstrated a significant decline in AEP amplitude pre-to post-sleep within the N1 (84–140 msec) and P2 (188–206 msec) ranges (Fig. 1a). GMFP, a measure of global brain activation following the auditory stimulus, also showed a significant overnight reduction in N1 (92–134 msec) amplitude for HC participants (Fig. 1b). However, the MDD group did not show a comparable overnight reduction in N1 or P2 amplitude (Figs. 1c and 1d). When comparing mean AEP amplitude between groups, no significant pre-sleep difference was observed for N1 or P2 amplitude, but the MDD group had greater post-sleep N1 amplitude compared to controls (−7.37 ± 4.96 vs. −4.26 ± 2.95, P = 0.046, averaged across 108–128 msec post-stimulus). For latencies of the AEP peaks, no overnight differences were observed for N1 or P2 components in either group.
Fig. 1.
Overnight comparison of AEP amplitude by time in healthy control (HC) and depressed (MDD) groups. (a) Pre-and post-sleep AEP raw amplitude from the average of a fronto-central cluster of channels and (b) global mean field power (GMFP) in controls. (c,d) Identical raw amplitude and GMFP plots for the MDD group. Dotted vertical lines signify stimulus onset. Samples in the N1 and P2 ranges with significant pre-vs. post-sleep differences (P < .05) pre are denoted by a solid black line.
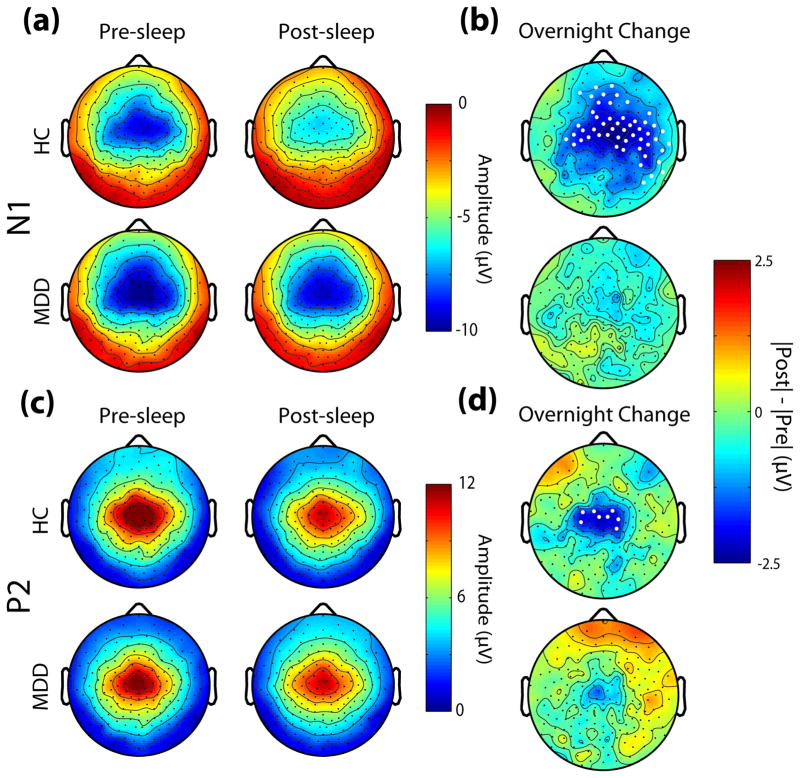
Topographic representations of AEP amplitude pre-and post-sleep, as well as the overnight change, are displayed in Fig. 2. For the N1 component, both HC and MDD groups showed the typical scalp distribution of fronto-central negativity with peripheral polarity reversal (13, 29, 30) (Fig. 2a), but a significant overnight reduction in amplitude within a fronto-central group of channels (using STCT) was observed only in the HC group (Fig. 2b). For the P2 component, the typical scalp distribution of the signal was again observed in both groups (Fig. 2c), while an overnight reduction in amplitude was observed for the HC group, but not for the MDD group (Fig. 2d).
Fig. 2.
Topographic distribution of pre-and post-sleep AEP amplitude and overnight change. (a) Pre-and post-sleep values of the peak amplitude at each channel within the N1 range for HC and MDD groups. (b) Overnight change in N1 amplitude for each channel, represented as the absolute difference. Channels with significant overnight differences using STCT are denoted by white dots. (c,d) Same as in (a,b) for the P2 range.
With the same topographic analysis stratified by sex, females and males within each group showed similar patterns of overnight change in AEP amplitude and latency. The overnight change in AEP amplitude was also similar for MDD participants with and without comorbid anxiety. In addition, there were no significant differences in pre-vs. post-sleep vigilance level as measured by the PVT for either group, and AEP latency did not correlate with PVT values pre-or post-sleep.
Topographic correlations
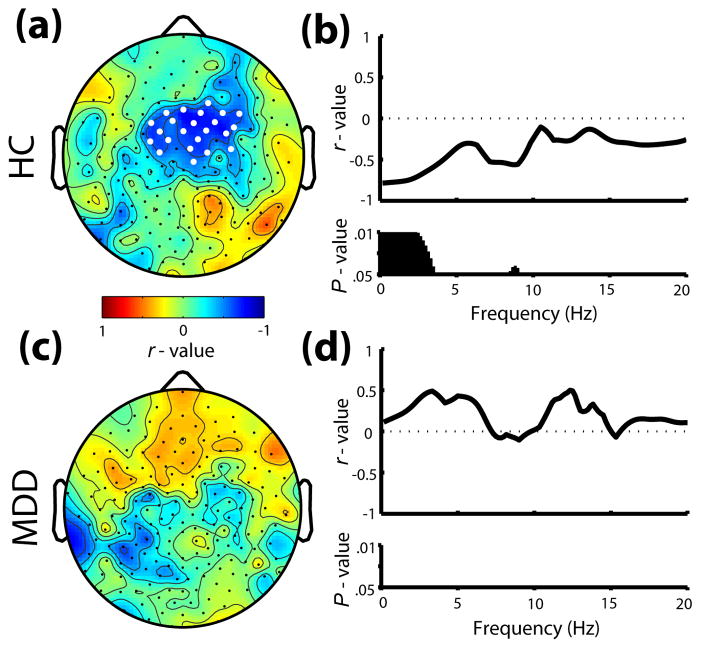
Given the overnight change in AEP amplitude specific to a fronto-central region, as well as previous research demonstrating a relationship between the overnight change in N1 amplitude and low-frequency SWA (1–2.33Hz) in healthy controls (13), topographic correlations were performed for both groups. Consistent with Hulse et al. (13), a fronto-central cluster of channels showed a significant correlation between the decline in N1 amplitude and average all-night low-frequency SWA in the HC group (Fig. 3a). When examined across a range of spectral activity during sleep using a representative fronto-central channel, the overnight decline in N1 amplitude in healthy controls correlated most significantly with frequencies <4Hz, along with a correlation in the alpha range around 9 Hz (Fig. 3b). In contrast, change in N1 amplitude in the MDD group did not correlate topographically with low-frequency SWA (Fig 3c), or any other frequency <20Hz (Fig. 3d).
Fig. 3.
Relationship between overnight change (%) in N1 amplitude and average all-night sleep EEG power. (a) Topographic correlations (r – values) of N1 change with SWA (1–2.33Hz) at each channel for the HC group. White dots signify channels with P < .05. (b) Frequency-specific correlations from a representative fronto-central channel for HC. Black bars denote frequency bins with P < .05. (c,d) Same as in (a,b) for the MDD group.
No significant correlations were found between either the overnight change in P2 amplitude or GMFP measures of global brain activation, and SWA for either group. In addition, no significant correlations were observed for either group between the overnight change in N1 or P2 amplitude and standard polysomnographic variables (listed in Table 1). Within the MDD group, neither the pre-or post-sleep AEP amplitude, nor the overnight change in amplitude, significantly correlated with global depression severity as measured by the HRSD-17.
Discussion
Using a standard waking auditory oddball task pre-and post-sleep, this study replicated previous findings by Hulse et al. (13) with a separate group of healthy individuals, showing that auditory evoked potential (AEP) N1 and P2 amplitudes decline after a night of sleep and that the overnight change in N1 amplitude is proportional to the amount of slow wave activity (SWA) from that night. When applying the same technique to a group of participants with major depressive disorder (MDD), however, neither a comparable overnight decline in N1 or P2 amplitude, nor a relationship between overnight AEP amplitude change and SWA was observed.
According to PSG variables from the experimental night, MDD and HC groups showed comparable sleep profiles. The elevated arousal index and REM sleep amount in MDD compared to HC are consistent with previous studies (1, 5, 31). Despite these differences, none of the PSG variables correlated with the overnight change in AEP amplitude, suggesting that overall sleep architecture was not differentially impacting the AEP results across groups.
Varying states of vigilance or attention, such as sleep inertia experienced in the morning after awakening (32), could have impacted AEP amplitude or latency, but all participants were given sufficient time in the morning before beginning the tasks (19), supported by equivalent PVT and AEP latency values pre-vs. post-sleep and between groups (21, 33, 34). Moreover, PVT values did not correlate with pre-or post-sleep AEP amplitude values. Studies have also demonstrated an impact of antidepressant medication on auditory processing in animals and humans (35–37). Because MDD participants were free of pharmacological treatment for at least 6 months prior to the study, it is unlikely that antidepressant medication biased the results observed in this study. In addition, although the MDD group included two more females than the HC group, no differences were observed in terms of overnight change in AEP amplitude when conducting stratified analyses. However, because prior literature has suggested SWA in MDD may be different in women versus men (11, 38), sex-related effects on homeostatic processes, including overnight change in AEP, may be an important area of future investigation.
Previous literature regarding baseline comparisons of AEP N1 and P2 components between MDD and healthy controls has yielded conflicting results. Increased N1 latency and amplitude (39, 40), and decreased P2 amplitude have been demonstrated (39), whereas other studies have shown no difference in N1 amplitude and increased P2 amplitude (41, 42). It is possible that unmeasured factors such as time of day of the recordings, which may affect both circadian and/or homeostatic processes (43, 44), influenced the observed AEP amplitude and latency in previous studies. For example, Kerkhof and colleagues (45) previously demonstrated that morning-type individuals had greater N1-P2 amplitude overall compared to evening-types, suggesting that intrinsic circadian preference may affect AEP amplitude. Although AEP recordings and sleep times were similar between MDD and HC groups in this investigation, circadian phase was not rigorously measured in our study design, and thus differential circadian modulation of AEP amplitude between groups could potentially explain the observed difference in pre-to post-sleep amplitude changes. However, bed times and waking times, as well as time of administering the oddball task did not significantly differ between groups. To clarify the degree to which circadian and homeostatic factors contribute to overnight changes in AEP amplitude, the use of constant routine and/or forced desynchrony protocols may be beneficial in future research.
This study replicates previous work (13) demonstrating the overnight decline in N1 amplitude correlates with SWA in healthy controls, which suggests that this AEP measure reflects a sleep homeostatic process. However, it is noteworthy that in this study, MDD and HC had similar SWA values, both all-night and across NREM episodes, despite lack of overnight decline in N1 amplitude in the MDD group. Because SWA has been widely used as a measure of sleep homeostasis (6–9), one might expect alterations in sleep homeostasis to be reflected by different SWA values between HC and MDD groups. However, our results of similar SWA values between HC and MDD groups are not dissimilar with other reports that have failed to demonstrate significant differences in baseline SWA in MDD relative to healthy subjects (12, 46, 47). In addition, SWA in MDD is moderated by effects of age and sex (11, 12, 48), and thus the lack of difference in SWA between HC and MDD groups in this study may have been due to the narrow age range of participants, different sex distributions, and/or inadequate power to detect such differences between groups. An alternative hypothesis for our findings would be that SWA may be present, but dysfunctional in MDD, which could explain the lack of relationship between overnight change in N1 amplitude and SWA for the MDD group. In this case, overnight change in AEP amplitude could be a more sensitive measure for or reflect a different aspect of sleep homeostasis in MDD that is not fully captured by SWA. To more critically examine the homeostatic nature of AEP amplitude and its relationship to SWA, future studies would benefit from delivering a challenge to normal sleep, such as sleep delay or restriction protocols (12), in both MDD and control samples.
Several studies have linked both AEP N1 and P2 components with central auditory plasticity (49–53). In the present study, a greater decline in N1, but not P2, amplitude was associated with greater SWA from that night in healthy controls. In accordance with previous literature (13, 34, 50, 53), it is likely that the N1 and P2 components involve separate processes, especially when considering their relationship to sleep. Given that sleep SWA has also been linked to synaptic plasticity (8, 54, 55), and MDD may involve deficiencies in plastic processes (56–58), it is possible that the present findings, particularly involving the AEP N1 component, reflect alterations in sleep-regulated synaptic plasticity in MDD.
This study was designed to be an initial investigation of the overnight change in AEP amplitude in a broad sample of unipolar, non-psychotic MDD participants. Given the heterogeneous nature of MDD (59, 60), we investigated the pattern of overnight AEP change in the subgroup of MDD individuals with comorbid anxiety. However, when comparing this subgroup with the entire MDD sample, similar patterns were observed, suggesting that the alterations in the overnight change in AEP were likely related to the primary diagnosis of MDD, rather than comorbid disorders. Furthermore, although no correlations between HRSD-17 scores and AEP values were found in this study, it is possible that global severity or specific symptomatology, such as suicidality, may play a role in sleep-related regulation of auditory processing in MDD (42, 61). Thus, examination of overnight changes in AEP amplitude in more severe MDD participants without comorbidity may further clarify the relationship between homeostatic regulation of AEP components and MDD.
Along with sleep and spontaneous waking EEG measures of homeostatic processes (3, 62, 63), waking AEP techniques have been shown to predict response to therapeutic sleep deprivation (64, 65), and, particularly when measuring the loudness-dependence of the AEP (LDAEP), response to pharmacotherapy (66–68). Given the robust findings of research using LDAEP measures, in combination with the results of the present study, it would be intriguing to examine whether overnight changes in the LDAEP could further reveal MDD subgroups of diagnostic or prognostic value.
Significant Outcomes.
In healthy controls, N1 and P2 amplitudes of the auditory evoked potential (AEP) were reduced after a night of sleep, and greater overnight reduction in N1 amplitude was related to greater sleep slow wave activity (SWA).
for the major depressive disorder (MDD) group, neither a significant overnight decline in AEP amplitude, nor a comparable relationship with sleep SWA was observed.
The results were not confounded by varying levels of alertness or differences in sleep variables between groups.
Limitations.
This study involved a broad sample of MDD participants with variable depression severity and a moderate proportion of comorbid anxiety disorders.
This study was not designed to distinguish the impact of circadian rhythms on overnight changes in AEP amplitude.
Multi-tone AEP paradigms could further elucidate sleep-related abnormalities in MDD.
Acknowledgments
This research was funded by the National Institute of Mental Health (5P20MH077967 to GT and RB, and F30MH082601 to EL). The authors would like to thank the polysomnographic technologists from Wisconsin Sleep for working with participants overnight and scoring the sleep recordings, Meredith Rumble, PhD and Elizabeth Frei, PhD for conducting the SCIDs, the undergraduate research assistants for their help collecting the data, Tim Wanger for his assistance in processing the hdEEG sleep data, Jennifer Noe and Kate Sprecher for their logistical contributions, and Brady Riedner, PhD for his helpful feedback in conducting additional analyses and manuscript revisions.
Footnotes
Declaration of Interest
Dr. Plante reports having owned stock in Pfizer, and having received honoraria from Oakstone Medical Publishing and royalties from Cambridge University Press. Dr. Tononi has consulted for Sanofi-Aventis and Takeda, and he is currently the David P. White Chair in Sleep Medicine at the University of Wisconsin – Madison, endowed by Phillips Respironics. Dr. Tononi has also received unrelated research support from Phillips Respironics. Dr. Benca has consulted for Merck and Sanofi-Aventis. The other authors have indicated no financial conflicts of interest.
References
- 1.BENCA R, OBERMEYER W, THISTED R, GILLIN J. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. discussion 69–70. [DOI] [PubMed] [Google Scholar]
- 2.AMERICAN PSYCHIATRIC ASSOCIATION. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. AMERICAN PSYCHIATRIC ASSOCIATION TASK FORCE ON DSM-IV. [Google Scholar]
- 3.STEIGER A, KIMURA M. Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res. 2010;44:242–52. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 4.KUPFER DJ, FRANK E, GROCHOCINSKI VJ, GREGOR M, MCEACHRAN AB. Electroencephalographic sleep profiles in recurrent depression. A longitudinal investigation. Arch Gen Psychiatry. 1988;45:678–81. doi: 10.1001/archpsyc.1988.01800310088011. [DOI] [PubMed] [Google Scholar]
- 5.KUPFER DJ, FOSTER FG. Interval between onset of sleep and rapid-eye-movement sleep as an indicator of depression. Lancet. 1972;2:684–6. doi: 10.1016/s0140-6736(72)92090-9. [DOI] [PubMed] [Google Scholar]
- 6.BORBÉLY AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 7.ACHERMANN P, DIJK DJ, BRUNNER DP, BORBÉLY AA. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull. 1993;31:97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 8.TONONI G, CIRELLI C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 9.VYAZOVSKIY V, OLCESE U, LAZIMY Y, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–78. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BORBÉLY A. The S-deficiency hypothesis of depression and the two-process model of sleep regulation. Pharmacopsychiatry. 1987;20:23–9. doi: 10.1055/s-2007-1017069. [DOI] [PubMed] [Google Scholar]
- 11.ARMITAGE R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl. 2007:104–15. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 12.BROWER KJ, HOFFMANN R, CONROY DA, ARNEDT JT, ARMITAGE R. Sleep homeostasis in alcohol-dependent, depressed and healthy control men. Eur Arch Psychiatry Clin Neurosci. 2011 doi: 10.1007/s00406-011-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.HULSE BK, LANDSNESS EC, SARASSO S, et al. A postsleep decline in auditory evoked potential amplitude reflects sleep homeostasis. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FIRST M, SPITZER R, GIBBON M, WILLIAMS J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 15.HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960 Feb;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FIRST M, SPITZER R, GIBBON M, WILLIAMS J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Non-patient Edition (SCID-I/NP) [Google Scholar]
- 17.DINGES D, PACK F, WILLIAMS K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 18.LANDSNESS E, CRUPI D, HULSE B, et al. Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32:1273–84. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.TASSI P, MUZET A. Sleep inertia. Sleep Med Rev. 2000;4:341–53. doi: 10.1053/smrv.2000.0098. [DOI] [PubMed] [Google Scholar]
- 20.GONCHAROVA II, MCFARLAND DJ, VAUGHAN TM, WOLPAW JR. EMG contamination of EEG: spectral and topographical characteristics. Clin Neurophysiol. 2003;114:1580–93. doi: 10.1016/s1388-2457(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 21.HERRMANN CS, KNIGHT RT. Mechanisms of human attention: event-related potentials and oscillations. Neurosci Biobehav Rev. 2001;25:465–76. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 22.SALISBURY DF, COLLINS KC, MCCARLEY RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophr Bull. 2010;36:991–1000. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HALL J. New Handbook of Auditory Evoked Responses. Boston: Pearson Education, Inc; 1992. [Google Scholar]
- 24.DE BEER NA, VAN DE VELDE M, CLUITMANS PJ. Clinical evaluation of a method for automatic detection and removal of artifacts in auditory evoked potential monitoring. J Clin Monit. 1995;11:381–91. doi: 10.1007/BF01616744. [DOI] [PubMed] [Google Scholar]
- 25.LEHMANN D, SKRANDIES W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol. 1980;48:609–21. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- 26.ESSER SK, HUBER R, MASSIMINI M, PETERSON MJ, FERRARELLI F, TONONI G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69:86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 27.IBER C, ANCOLI-ISRAEL S, CHESSONN A, QUAN S. The AAASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 28.NICHOLS TE, HOLMES AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SIMSON R, VAUGHAN HG, RITTER W. The scalp topography of potentials associated with missing visual or auditory stimuli. Electroencephalogr Clin Neurophysiol. 1976;40:33–42. doi: 10.1016/0013-4694(76)90177-2. [DOI] [PubMed] [Google Scholar]
- 30.IŞOǦLU-ALKAÇ U, KEDZIOR K, KARAMÜRSEL S, ERMUTLU N. Event-related potentials during auditory oddball, and combined auditory oddball-visual paradigms. Int J Neurosci. 2007;117:487–506. doi: 10.1080/00207450600773509. [DOI] [PubMed] [Google Scholar]
- 31.BRITTON WB, HAYNES PL, FRIDEL KW, BOOTZIN RR. Polysomnographic and subjective profiles of sleep continuity before and after mindfulness-based cognitive therapy in partially remitted depression. Psychosom Med. 2010;72:539–48. doi: 10.1097/PSY.0b013e3181dc1bad. [DOI] [PubMed] [Google Scholar]
- 32.FERRERA M, DE GENNARO L, FERLAZZO F, CURCIO G, CRISTIANI R, BERTINI M. Topographical changes in N1-P2 amplitude upon awakening from recovery sleep after slow-wave sleep deprivation. Clin Neurophysiol. 2002;113:1183–90. doi: 10.1016/s1388-2457(02)00146-3. [DOI] [PubMed] [Google Scholar]
- 33.NÄÄTÄNEN R, PICTON T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 34.CROWLEY K, COLRAIN I. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol. 2004;115:732–44. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 35.JUCKEL G, HEGERL U, MOLNÁR M, CSÉPE V, KARMOS G. Auditory evoked potentials reflect serotonergic neuronal activity--a study in behaving cats administered drugs acting on 5-HT1A autoreceptors in the dorsal raphe nucleus. Neuropsychopharmacology. 1999;21:710–6. doi: 10.1016/S0893-133X(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 36.MAXWELL CR, EHRLICHMAN RS, LIANG Y, et al. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006;316:315–24. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- 37.HEGERL U, BOTTLENDER R, GALLINAT J, KUSS HJ, ACKENHEIL M, MÖLLER HJ. The serotonin syndrome scale: first results on validity. Eur Arch Psychiatry Clin Neurosci. 1998;248:96–103. doi: 10.1007/s004060050024. [DOI] [PubMed] [Google Scholar]
- 38.ARMITAGE R, HOFFMANN R, TRIVEDI M, RUSH A. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000;95:201–13. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 39.ROTH W, PFEFFERBAUM A, KELLY A, BERGER P, KOPELL B. Auditory event-related potentials in schizophrenia and depression. Psychiatry Res. 1981;4:199–212. doi: 10.1016/0165-1781(81)90023-8. [DOI] [PubMed] [Google Scholar]
- 40.URRETAVIZCAYA M, MORENO I, BENLLOCH L, et al. Auditory event-related potentials in 50 melancholic patients: increased N100, N200 and P300 latencies and diminished P300 amplitude. J Affect Disord. 2003;74:293–7. doi: 10.1016/s0165-0327(02)00016-2. [DOI] [PubMed] [Google Scholar]
- 41.BRUDER GE, TENKE CE, TOWEY JP, et al. Brain ERPs of depressed patients to complex tones in an oddball task: relation of reduced P3 asymmetry to physical anhedonia. Psychophysiology. 1998;35:54–63. [PubMed] [Google Scholar]
- 42.KEMP AH, PE BENITO L, QUINTANA DS, et al. Impact of depression heterogeneity on attention: an auditory oddball event related potential study. J Affect Disord. 2010;123:202–7. doi: 10.1016/j.jad.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 43.VAN DONGEN HP, DINGES DF. Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. J Sleep Res. 2003;12:181–7. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 44.SCHWARTZ JR, ROTH T. Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol. 2008;6:367–78. doi: 10.2174/157015908787386050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.KERKHOF GA, KORVING HJ, WILLEMSE-VD GEEST HM, RIETVELD WJ. Diurnal differences between morning-type and evening-type subjects in self-rated alertness, body temperature and the visual and auditory evoked potential. Neurosci Lett. 1980;16:11–5. doi: 10.1016/0304-3940(80)90093-2. [DOI] [PubMed] [Google Scholar]
- 46.MENDELSON WB, SACK DA, JAMES SP, et al. Frequency analysis of the sleep EEG in depression. Psychiatry Res. 1987;21:89–94. doi: 10.1016/0165-1781(87)90067-9. [DOI] [PubMed] [Google Scholar]
- 47.ARMITAGE R, CALHOUN JS, RUSH AJ, ROFFWARG HP. Comparison of the delta EEG in the first and second non-REM periods in depressed adults and normal controls. Psychiatry Res. 1992;41:65–72. doi: 10.1016/0165-1781(92)90019-y. [DOI] [PubMed] [Google Scholar]
- 48.ARMITAGE R, HOFFMANN R, FITCH T, TRIVEDI M, RUSH AJ. Temporal characteristics of delta activity during NREM sleep in depressed outpatients and healthy adults: group and sex effects. Sleep. 2000;23:607–17. [PubMed] [Google Scholar]
- 49.TREMBLAY K, KRAUS N, MCGEE T, PONTON C, OTIS B. Central auditory plasticity: changes in the N1-P2 complex after speech-sound training. Ear Hear. 2001;22:79–90. doi: 10.1097/00003446-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 50.CLAPP WC, KIRK IJ, HAMM JP, SHEPHERD D, TEYLER TJ. Induction of LTP in the human auditory cortex by sensory stimulation. Eur J Neurosci. 2005;22:1135–40. doi: 10.1111/j.1460-9568.2005.04293.x. [DOI] [PubMed] [Google Scholar]
- 51.ROBERTSL BOSNYAK D, SHAHIN A, LJ T. Neuroplastic Adaptations of the Auditory System in Musicians and Nonmusicians. In: Syka J, Merzenich M, editors. Plasticity and Signal Representation in the Auditory System. Springer; 2005. pp. 343–50. [Google Scholar]
- 52.ZAEHLE T, CLAPP WC, HAMM JP, MEYER M, KIRK IJ. Induction of LTP-like changes in human auditory cortex by rapid auditory stimulation: an FMRI study. Restor Neurol Neurosci. 2007;25:251–9. [PubMed] [Google Scholar]
- 53.BAUMANN S, MEYER M, JÄNCKE L. Enhancement of auditory-evoked potentials in musicians reflects an influence of expertise but not selective attention. J Cogn Neurosci. 2008;20:2238–49. doi: 10.1162/jocn.2008.20157. [DOI] [PubMed] [Google Scholar]
- 54.HUBER R, ESSER S, FERRARELLI F, MASSIMINI M, PETERSON M, TONONI G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS One. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MASSIMINI M, TONONI G, HUBER R. Slow waves, synaptic plasticity and information processing: insights from transcranial magnetic stimulation and high-density EEG experiments. Eur J Neurosci. 2009;29:1761–70. doi: 10.1111/j.1460-9568.2009.06720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CASTRÉN E. Is mood chemistry? Nat Rev Neurosci. 2005;6:241–6. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- 57.NORMANN C, SCHMITZ D, FÜRMAIER A, DÖING C, BACH M. Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry. 2007;62:373–80. doi: 10.1016/j.biopsych.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 58.NISSEN C, HOLZ J, BLECHERT J, et al. Learning as a model for neural plasticity in major depression. Biol Psychiatry. 2010;68:544–52. doi: 10.1016/j.biopsych.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 59.KEMP AH, PE BENITO L, QUINTANA DS, et al. Impact of depression heterogeneity on attention: an auditory oddball event related potential study. J Affect Disord. 2010;123:202–7. doi: 10.1016/j.jad.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 60.OSTERGAARD SD, JENSEN SO, BECH P. The heterogeneity of the depressive syndrome: when numbers get serious. Acta Psychiatr Scand. 2011 doi: 10.1111/j.1600-0447.2011.01744.x. [DOI] [PubMed] [Google Scholar]
- 61.ASHTON H, GOLDING J, MARSH V, THOMPSON J, HASSANYEH F, TYRER S. Cortical evoked potentials and clinical rating scales as measures of depressive illness. Psychol Med. 1988;18:305–17. doi: 10.1017/s0033291700007856. [DOI] [PubMed] [Google Scholar]
- 62.HEMMETER U, HEMMETER-SPERNAL J, KRIEG J. Sleep deprivation in depression. Expert Rev Neurother. 2010;10:1101–15. doi: 10.1586/ern.10.83. [DOI] [PubMed] [Google Scholar]
- 63.LANDSNESS EC, GOLDSTEIN MR, PETERSON MJ, TONONI G, BENCA RM. Antidepressant effects of selective slow wave sleep deprivation in major depression: A high-density EEG investigation. J Psychiatr Res. 2011 doi: 10.1016/j.jpsychires.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.KASPER S, KATZINSKI L, LENARZ T, RICHTER P. Auditory evoked potentials and total sleep deprivation in depressed patients. Psychiatry Res. 1988;25:91–100. doi: 10.1016/0165-1781(88)90162-x. [DOI] [PubMed] [Google Scholar]
- 65.DANOS P, KASPER S, SCHOLL H, et al. Clinical response to sleep deprivation and auditory-evoked potentials--preliminary results. Pharmacopsychiatry. 1994;27:70–1. doi: 10.1055/s-2007-1014281. [DOI] [PubMed] [Google Scholar]
- 66.GALLINAT J, BOTTLENDER R, JUCKEL G, et al. The loudness dependency of the auditory evoked N1/P2-component as a predictor of the acute SSRI response in depression. Psychopharmacology (Berl) 2000;148:404–11. doi: 10.1007/s002130050070. [DOI] [PubMed] [Google Scholar]
- 67.JUCKEL G, POGARELL O, AUGUSTIN H, et al. Differential prediction of first clinical response to serotonergic and noradrenergic antidepressants using the loudness dependence of auditory evoked potentials in patients with major depressive disorder. J Clin Psychiatry. 2007;68:1206–12. doi: 10.4088/jcp.v68n0806. [DOI] [PubMed] [Google Scholar]
- 68.MULERT C, JUCKEL G, BRUNNMEIER M, et al. Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98:215–25. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]