Abstract
The exposure of guanine in the oligonucleotide 5’-d(TCGCT) to one-electron oxidants leads initially to the formation of the guanine radical cation G•+, its deptotonation product G(−H)• and, ultimately, to various two- and four-electron oxidation products via pathways that depend on the oxidants and reaction conditions. We utilized single or successive multiple laser pulses (308 nm, 1 Hz rate) to generate the oxidants CO3•− and SO4•− (via the photolysis of S2O82− in aqueous solutions in the presence and absence of bicarbonate, respectively) at concentrations/pulse that were ~20-fold lower than the concentration of 5’-d(TCGCT). Time-resolved absorption spectroscopy measurements following single-pulse excitation show that the G•+ radical (pKa = 3.9) can be observed only at low pH and is hydrated within ≥ 3 ms at pH 2.5, thus forming the two-electron oxidation product 8-oxo-7,8-dihydroguanosine (8-oxoG). At neutral pH, and single pulse excitation, the principal reactive intermediate is G(−H)• that at best, reacts only slowly with H2O, and lives for ≥ 70 ms in the absence of oxidants/other radicals to form base sequence-dependent intrastrand cross-links via the nucleophilic addition of N3-thymidine to C8-guanine (5'-G*CT* and 5'-T*CG*). Alternatively, G(−H)• can be oxidized further by reaction with CO3•− generating the two electron products 8-oxoG (C8 addition), and 5-carboxamido-5-formamido-2-iminohydantoin (2Ih, by C5 addition). The four-electron oxidation products, guanidinohydantoin (Gh) and spiroiminodihydantoin (Sp), appear only after a second (or more) laser pulses. The levels of all products, except 8-oxoG, which remains at a low constant value, increase with the number of laser pulses.
Introduction
Guanine is the most easily oxidizable nucleic acid base1 in DNA and is thus the primary target of oxidizing and reactive intermediates such as free radicals, UV light, and ionizing radiation.2 One of the principal one-electron oxidants found in human cells is the carbonate radical anion (CO3•−), which is overproduced at sites of inflammation via spontaneous homolysis of nitrosoperoxycarbonate.3 The primary product of one-electron abstraction from guanine is the guanine radical cation (G•+).4 The further chemical transformations of this electrophilic intermediate yields a wide spectrum of stable base modifications, which can be considered as products of multi-electron oxidation (e.g., 2, 4, and 6 electron oxidation) of guanine5, some of which are depicted in Figure 1. However, the impact of the concentrations of oxidants relative to the guanine substrate concentrations on the distributions of two- or more electron stable oxidation products is poorly understood. In cellular DNA, oxidatively generated guanine lesions occur at levels of the order of one per ~million guanines.6 These low levels of oxidized lesions pose difficulties for the design of in vitro model experiments for gaining deeper understanding of the different oxidation pathways leading to the products depicted in Figure 1 since the concentrations of oxidants are often in excess of the DNA for assuring the accurate detection and quantification of the guanine lesions formed. These conditions tend to favor the formation of multi-electron oxidation products.2,5 We developed a new approach to study pathways of oxidation based on the controlled injection of known and relatively low quantities of free radical oxidants by a single or by a train of laser pulses. Both HPLC and mass spectrometry methods were used to investigate the relationships between two- and four-electron guanine oxidation products in the 5’-d(TCGCT) oligonucleotide model system. The concentrations of oxidizing radicals were measured directly by transient absorption spectroscopy and maintained at levels ~ 20-fold lower than the concentrations of d(TCGCT) in order to minimize the formation of multi-electron oxidation products.
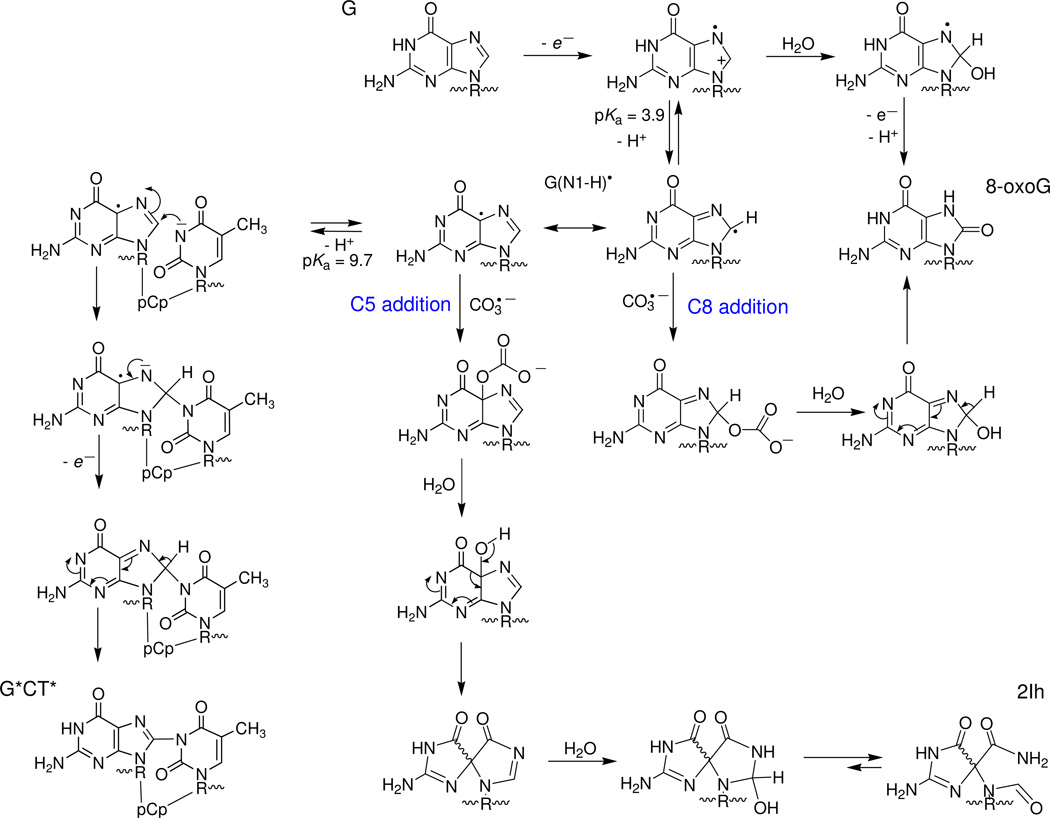
Figure 1.
Oxidatively generated guanine lesions.2,5,13 2'-deoxyribonucleosides of 8-oxo-7,8-dihydroguanosine (8-oxoG), diastereomeric 5-carboxamido-5-formamido-2-iminohydantoin (2Ih) and intrastrand cross-link in which C8-atom of guanine is covalently linked to the N3-atom of thymidine (5'-G*CT*) are products of two-electron oxidation, whereas guanidinohydantoin (Gh), diastereomeric spiroiminodihydantoin (Sp) and 2,5-diamino-4H-imidazolone (Iz) are products of four-electron oxidation; the masses of these lesions, relative to the mass of G (M), are shown in parentheses.
Photochemical methods were utilized to generate the carbonate radical anions or sulfate radicals anions as the oxidants.7–11 The former is a mild oxidant with the reduction potential12 of Eo = 1.59 V vs NHE that selectively oxidizes guanine in DNA by one-electron mechanisms, thus leading to the formation of a wide spectrum of oxidation products.8–11 The sulfate radical anion, is a significantly stronger oxidant (Eo = 2.43 V vs NHE)12. This reactive intermediate was used to oxidize guanine in 5’-d(TCGCT) at acidic pH values since the carbonate radical anion cannot be generated at low pH.
We show here that the guanine radical cation generated by the one-electron oxidation of guanine, manifests itself on microsecond time scales only under acidic pH; its lifetime of ~ 3 ms in aqueous solution at pH 2.5 is governed by a hydration mechanism that produces 8-oxo-7,8-dihydroguanosine (8-oxoG). In contrast, at pH values ≥ 7, only the deprotonation product of G•+, the neutral guanine radical, G(−H)•, with a lifetime of ~ 70 ms at pH 8, is evident. This intermediate is transformed by various reaction pathways to the different end-products shown in Figure 1. We propose mechanisms of formation and report the proportions of the products formed as a function of the number of successive laser pulses (308 nm, ~ 12 ns duration, 1 Hz repetition rate).
Experimental Methods
Materials
All chemicals (analytical grade) were used as received. The 5’-d(TCGCT) was purchased from Integrated DNA Technologies (Coralville, IA) and 5’-d(TC[8-oxoG]CT) from Bio-Synthesis (Lewisville, TX). Both oligonucleotides were purified and desalted using reversed-phase HPLC. The integrity of the oligonucleotides was confirmed by MALDI-TOF/MS analysis.
Laser Pulse Photolysis
The kinetics of oxidative reactions initiated by CO3•− or SO4•− radicals were monitored directly using a fully-computerized kinetic spectrometer system (~7 ns response time) described elsewhere.14 Briefly, an individual single laser pulse selected by an electromagnetic shutter from a train of 308 nm XeCl excimer laser pulses (~12 ns, 60 mJ/pulse/cm2, 1 Hz) was directed through a rectangular aperture (0.3×1.0 cm) into an 80 µL sample solution (87 µM oligonucleotide, 10 mM Na2S2O8 and 300 mM NaHCO3 adjusted to pH 8.0 by NaH2PO4) in a 0.2×1.0 cm quartz cell. The transient absorbance was probed along a 1 cm optical path by a light beam (75 W xenon arc lamp) oriented perpendicular to the laser beam. The signal was detected with a Hamamtsu 928 photomultiplier tube and recorded by a Tektronix TDS 5052 oscilloscope operating in its high resolution mode that provides a satisfactory signal/noise ratio after a single laser pulse. The rate constants were determined by least squares fits of the appropriate kinetic equations to the experimentally measured transient absorption profiles as described in detail elsewhere. The values reported are averages of five independent measurements. Kinetic modeling was carried out using the INTKIN software developed at the Brookhaven National Laboratory by H. A. Schwarz. The Numerical Integration method used in this program is the DVODE package written by P. N. Brown and A. C. Hindmarsh, Lawrence Livermore National Laboratory, and G. D. Byrne, Exxon Research and Engineering Co.
HPLC Isolation of Oxidatively Generated Oligonucleotide Adducts
After laser pulse irradiation, the samples were immediately subjected to reversed-phase HPLC analysis. The oxidatively modified oligonucleotides were isolated on an analytical (150 × 4 mm i.d.) ACE C18 (3 µm, 100 Å) column (MAC-MOD Analytical, Chadd Ford, PA), using a 3 – 8% linear gradient of acetonitrile in solvent containing 50 mM triethylammonium acetate (TEAA) and 5% acetonitrile for 60 min at a flow rate of 1 mL/min. The products formed in minor quantities were collected after multiple HPLC injections and combined. The HPLC fractions containing the isolated oligonucleotide adducts were thoroughly dried under vacuum to remove most of the TEAA, dissolved in water, and subjected to MALDI-TOF/MS analysis.
Mass Spectrometry
The MALDI-TOF mass spectra were recorded using a Bruker Daltonics ultrafleXtreme Instrument. In the negative mode, the matrix was a 2:1 mixture of 2’,4’,6’-trihydroxyacetophenone methanol solution (30 mg/mL) and ammonium citrate aqueous solution (100 mg/ml, whereas in the positive mode, the matrix was a 2,5-dihydroxybenzoic acid (DHB) with concentration of 50 mg/mL in 0.1% trifluoroacetic acid (TFA) solution containing 30% acetonitrile and 10 mg/mL of ammonium phosphate.
Generation of Carbonate and Sulfate Radical Anions
Sulfate radicals, SO4•−, were generated by the photodissociation of peroxodisulfate anions, S2O82− (10 mM) induced by single nanosecond 308 nm XeCl excimer laser pulses:
| (1) |
The photodissociation of the peroxodisulfate anions is rapid and thus all sulfate radicals are generated within the ~12 ns laser pulse duration. If bicarbonate is also present and in excess (300 mM HCO3−), the sulfate radicals will decay predominantly and rapidly by oxidizing bicarbonate thus resulting in the formation of formation of CO3•− radicals, a reaction which is complete within < 3 µs after actinic laser pulse.7,8 The observed transient absorption in the millisecond time range with a maximum at 600 nm is assigned to CO3•− radicals produced by the one-electron oxidation of HCO3− by SO4•− radicals:
| (2) |
that occurs with the rate constant k2 = 4.6×106 M−1s−1.7,8 This reaction was used for generation of CO3•− radicals at pH ≥ 7; the decomposition of bicarbonate with the formation of carbon dioxide,15 pK(CO2,H2O/HCO3−,H+) = 6.35 at 25°C, suppresses the generation of CO3•− radicals at pH < 7, because CO2 is not oxidized by SO4•− radical.16
Results
Overview
The carbonate radical anion was utilized as the oxidizing species at pH ≥ 7, and the sulfate radical at acidic pH. Therefore, we investigated the reactivities of G•+ (pKa = 3.9)4 and the neutral G(−H)• radical in the oligonucleotide 5'-d(TCGCT) at pH 2.5 and pH ≥ 7, respectively. At the low concentrations of CO3•− or SO4•− radicals generated by individual laser pulses ([CO3•−] or [SO4•−] << [5'-d(TCGCT)]), single-laser pulse excitation was limited to a single initial electron transfer reaction that yield either G(−H)• or G•+. Furthermore, the one-second spacing of laser pulses in our multiple laser pulse studies ensured that neither the CO3•− nor SO4•− radicals survived from pulse-to-pulse.
Single laser pulses produced only the two-electron oxidation products: the G*CT* intrastrand cross-link in which C8-atom of guanine is covalently linked to the N3-atom of thymidine, the well-known 8-oxo-7,8-dihydroguanine, and the diastereomeric 5-carboxamido-5-formamido-2-iminohydantoin (2Ih) lesions recently described by Gold,17,18 Burrows5,19 and co-workers (Figure 1). In multiple laser pulse experiments, the diastereomeric spiroiminodihydantoin (Sp), guanidinohydantoin (Gh) and 2,5-diamino-4H-imidazolone (Iz), which are products of four-electron oxidation of guanine (Figure 1), are also formed.
Effect of the Number of Laser Pulses on the Formation of Guanine Lesions
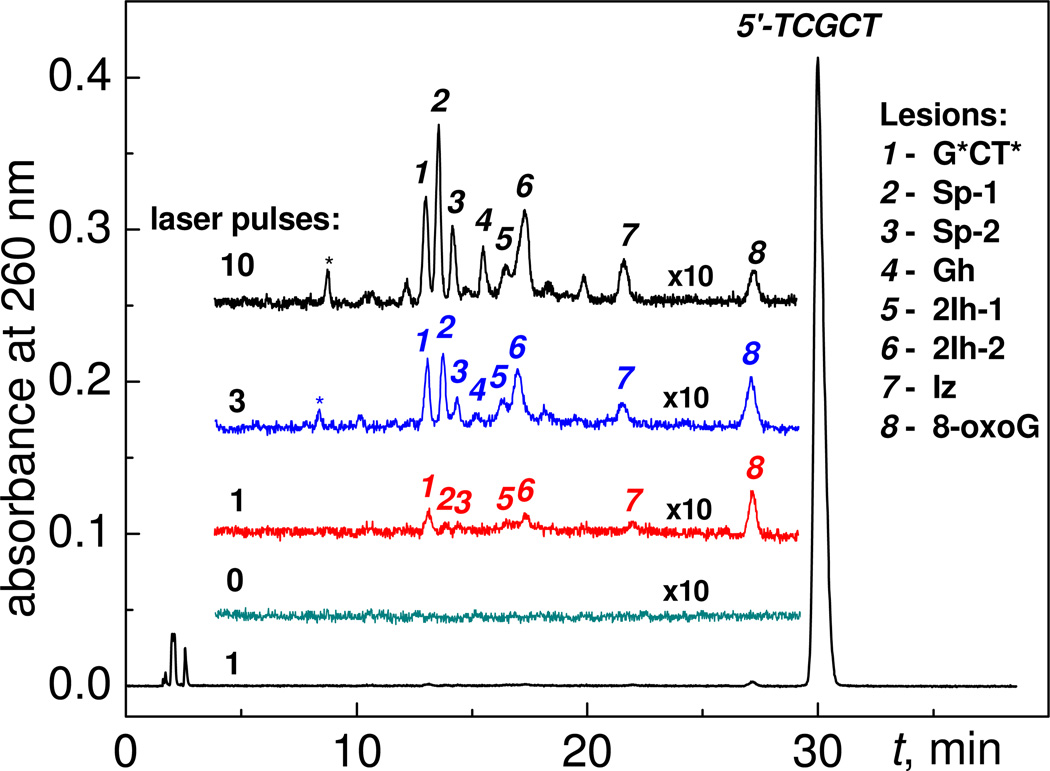
The sample solutions containing 87 µM 5’-d(TCGCT), 10 mM Na2S2O8 and 300 mM NaHCO3 were irradiated by defined numbers of successive 308 nm XeCl excimer laser pulses, and the oxidatively generated products were analyzed by reversed-phase HPLC. Typical reversed-phase chromatograms of the irradiated sample solutions after exposure to 1, 3 or 10 laser pulses are shown in Figure 2. It is evident that the amounts and the relative distributions of guanine oxidation products in the oligonucleotide 5’-d(TCGCT) depend on the number of pulses, and that there are no products in the unirradiated sample (Figure 2).
Figure 2.
Oxidatively generated end-products detected after excitation with different numbers of 308 nm nanosecond laser pulses. The irradiated 80 µL solution aliquots were air-equilibrated buffer solutions (pH 8.0) containing 87 µM 5’-d(TCGCT), 10 mM Na2S2O8, and 300 mM NaHCO3. Reversed-phase HPLC elution conditions (detection of products at 260 nm): 3 – 8% gradient of acetonitrile in aqueous solvent containing 50 mM triethylammonium acetate and 5% acetonitrile for 60 min at a flow rate of 1 mL/min. The adduct marked by the asterisk (*) has the same mass as the G*CT* adduct (M-2, labeled 1 in the figure) and was assigned to the analogous cross-linked 5'-d(T*CG*CT) product.10
In each individual experiment, the end products were separated by reversed-phase HPLC methods and identified by MALDI-TOF/MS analysis and by co-elution with authentic standards synthesized by laser flash and continuous illumination photochemical methods developed in our group and described in detail elsewhere.8–11 We found that the major products are modified oligonucleotides containing oxidatively modified guanine bases. The adducts derived from two-electron oxidations of guanine include 5’-d(TCG*CT*), 5’-d(TC[8-oxoG]CT) and the diastereomeric 5’-d(TC[2Ih]CT) eluted at 13.5 (1), 28.0 (8), and 17.0–17.8 (5,6) min, respectively (Figure 2). In turn, the adducts produced by four-electron oxidation of guanine include the diastereomeric 5'-d(TC[Sp]CT), 5’-d(TC[Gh]CT), and 5’-d(TC[Iz]CT) eluting at 14.0–14.7 (2,3), 16.0 (6), and 22.2 (7) min, respectively (Figure 2). We also observed the 5’-d(T*CG*CT) cross-linked adduct that elutes at 8.0 min (denoted by an asterisk (*) in Figure 2), which is typically formed in smaller quantities than 5'-d(TCG*CT*) product 1.10
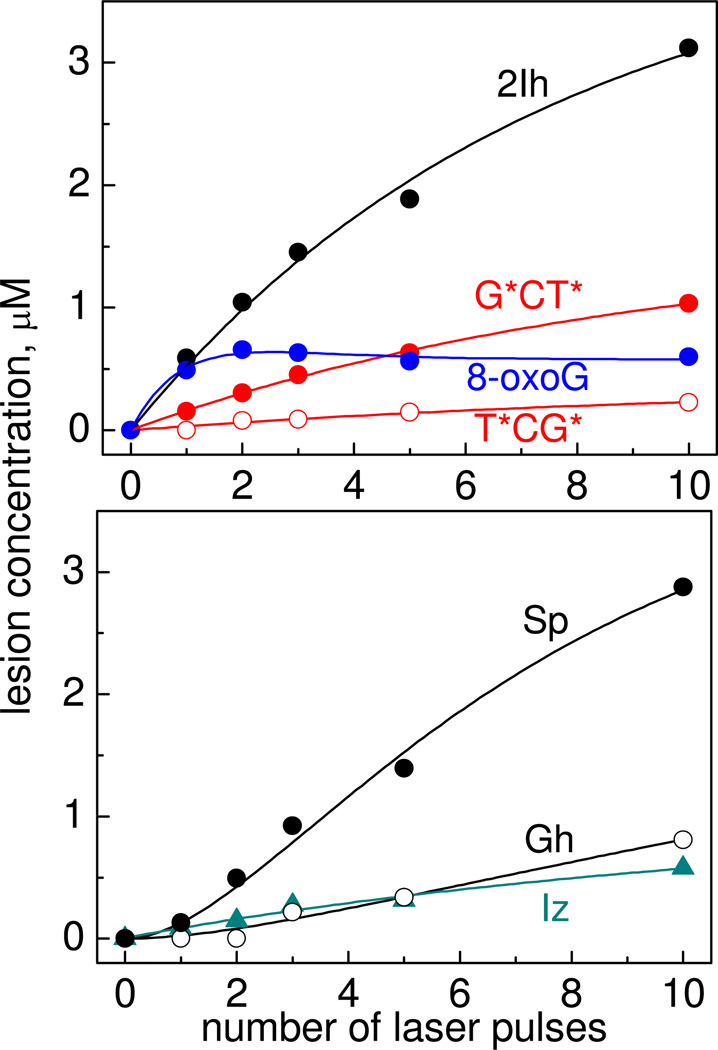
The yields of oligonucleotide adducts as a function of the number of laser pulses are shown in Figure 3.
Figure 3.
Dependence of the yields of the G*CT*, T*CG*, 8-oxoG, 2Ih, Sp, Gh and Iz oligonucleotide adducts on the number of successive 308 nm laser pulses in air-equilibrated buffer solutions (pH 8.0) containing 87 µM 5’-d(TCGCT), 10 mM Na2S2O8, and 300 mM NaHCO3. The modified oligonucleotide yields were estimated by integrating the areas under each elution band in the HPLC profiles, and utilizing the molar absorptivities of the oligonucleotide adducts at 260 nm.
Increasing the number of laser pulses induces a monotonic rise in the yields of the 5'-d(TCG*CT*) and 5’-d(TC[2Ih]CT adducts. However, the yield of the 5'-d(TC[8-oxoG]CT) product attains a maximum value after two laser pulses and slowly decreases with increasing number of pulses. The yields of the Sp- and Gh-containing oligonucleotide products on the number of laser pulses are quite different; the yields are negligible after the first laser pulse, and then gradually rise after the second pulse. Overall, these results suggest that a single laser pulse generates 5'-d(TCG*CT*), 5’-d(TC[2Ih]CT) and 5'-d(TC[8-oxoG]CT) adducts. The 8-oxoG in the latter is more reactive than guanine in the parent 5'-d(TCGCT) and thus 5'-d(TC[8-oxoG]CT) is further oxidized to yield 5'-d(TC[Sp]CT) and 5’-d(TC[Gh]CT). These reactions prevent the growth of the 5'-d(TC[8-oxoG]CT) product levels with increasing numbers of laser pulses. In agreement with this mechanism, excitation of sample solutions containing only an authentic 5'-d(TC[8-oxoG]CT) sample in a 10 mM Na2S2O8, and 300 mM NaHCO3 solution, generates 5'-d(TC[Sp]CT) and 5’-d(TC[Gh]CT adducts after irradiation with a single laser pulse. Note, that the formation of G*CT* cross-links is base sequence-dependent because the yields of 5'-G*CT* are higher than the yields of the 5'-T*CG* cross-linked products (Figure 3).
Formation of Imidazolone Lesions
Reversed-phase HPLC analysis of the oxidation products showed that oligonucleotides containing imidazolone lesions (7) are also formed (Figure 2). Increasing the number of laser pulses induces a monotonic growth of the yields of the 5'-d(TC[Iz]CT) adducts (Figure 3B). The major pathway of formation of Iz lesions is the combination reaction of G(−H)• radicals with superoxide, O2•−, and peroxyl radicals, RO2•.20,21 Here, we propose that side reactions of SO4•− radicals generate C-centered alkyl radicals of pyrimidine bases (T and C) and 2-deoxyribose,22,23 which in the presence of oxygen can serve as a source of RO2•/ O2•− radicals.24,25
Comparisons of 8-oxoG and G*CT* Product Yields at Different pH Values and Generated by Carbonate or Sulfate Radical Anions
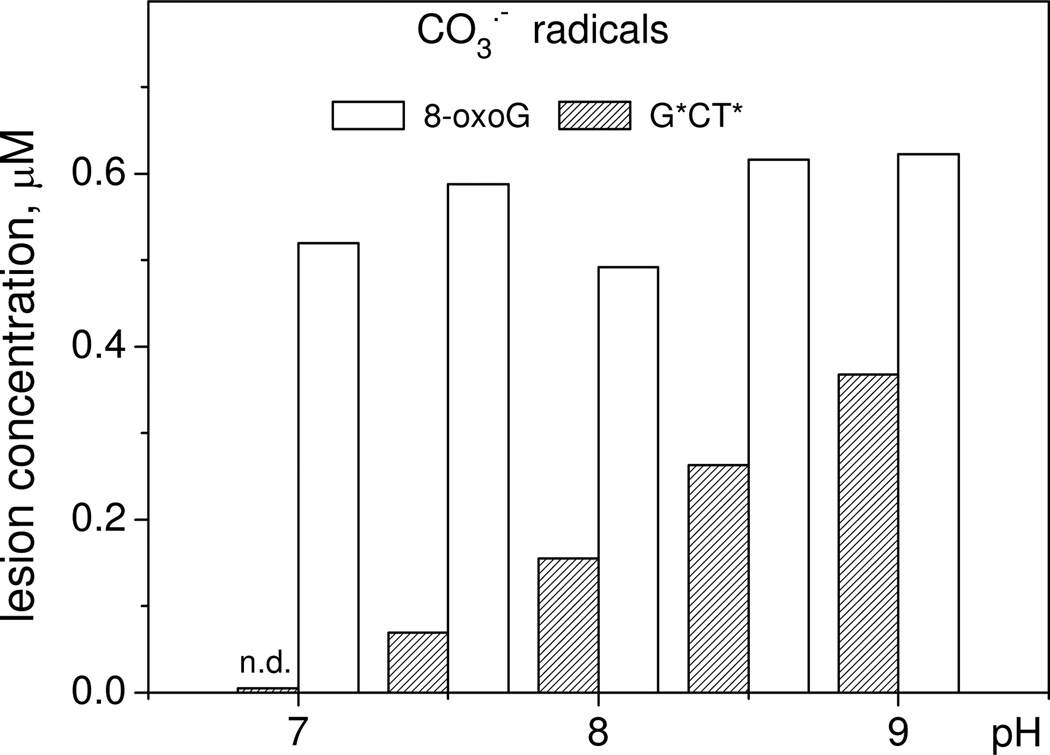
The primary molecular products of guanine oxidation are products of two-electron oxidation, which can form by two alternative pathways: (1) addition of nucleophiles to guanine radicals followed by one-electron oxidation of radical adducts, and (2) combination of guanine radicals with other free radicals.2,13 The first pathway suggests a correlation of the product yields with the solution pH, which can affect nucleophilicity/electrophilicity of the reaction partners. In turn, the reactivities of radicals (CO3•− or SO4•−) can affect product formation via the radical combination. The yields of 5'-d(TCG*CT*) adducts generated by CO3•− radicals injected by a single laser pulse excitation gradually increase with increasing pH from 7 to 9 (Figure 4) that correlates with enhancement of the thymine nucleophilicity.11 However, the yields of 5'-d(TC[8-oxoG]CT) does not change significantly and remains practically identical in the pH range in agreement with the radical combination mechanism.9
Figure 4.
Effect of pH on the generation of 5'-d(TCG*CT*) and 5'-d(TC[8-oxoG]CT) induced by CO3•− radicals produced by a single laser pulse irradiation of an 87 µM 5’-d(TCGCT), 10 mM Na2S2O8, and 300 mM NaHCO3 solution. The G*CT* adduct was not detected (n.d.) at pH 7.0.
In contrast, when the solution composition was altered to produce only sulfate radical anions by a single pulse irradiation experiments of a solution without bicarbonate anions, no 5'-d(TC[8-oxoG]CT) products were detected in the range of pH 7–8 (Figure 5).
Figure 5.
Effect of pH on the generation of 5'-d(TCG*CT*) and 5'-d(TC[8-oxoG]CT) produced by SO4•− radicals. The latter were generated by irradiation of an 87 µM 5’-d(TCGCT), 10 mM Na2S2O8 solution by a single laser pulse. The G*CT* and 8-oxoG adducts were not detected (n.d.) at pH 2.5, whereas 8-oxoG adducts were not detected at pH 7 – 8.
The oxidation of 5'-d(TCGCT) by SO4•− radicals can generate 8-oxoG lesions only in slightly acidic solutions (pH 2.5), where the guanine radicals G•+ (pKa = 3.9) exist in the cationic form and do not deprotonate to form neutral radicals, G(−H)•.4 However, the 5'-d(TCG*CT*) adducts are not formed in acidic solutions, but are generated in neutral solutions (pH 7– 8) with yields similar to those observed in the presence of HCO3− anions and grow with increasing pH as demonstrated in Figure 5. These differences in oxidative pathways of guanine oxidation by CO3•− and SO4•− radicals account for the difference in the distribution of end-products generated by these radicals with the relative yields summarized in Table 1.
Table 1.
Relative yields (%) of the 5'-d(TCG*CT*) and 5'-d(TC[8-oxoG]CT) adducts produced by CO3•− and SO4•− radicals. The latter were generated by irradiation of an 87 µM 5’-d(TCGCT), 10 mM Na2S2O8 solution (pH 2.5 or 8.0) by a single laser pulse.
| oxidant | 8-oxoG | G*CT* |
|---|---|---|
| CO3•− | 0.57 (pH 8.0) | 0.31 (pH 8.0) |
| SO4•− | 1.60 (pH 2.5) | 0.18 (pH 8.0) |
| SO4•− | ≤ 0.01 (pH 8.0) | ≤ 0.01 (pH 2.5) |
Kinetics of Guanine Radical Formation and Decay
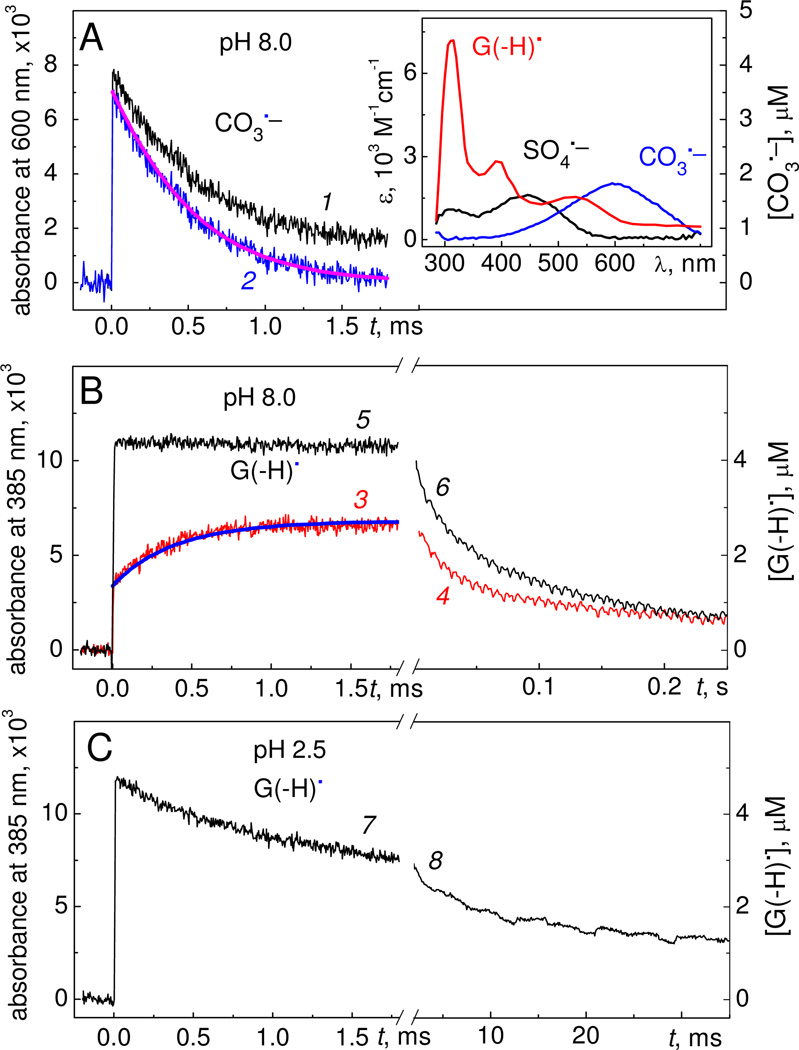
Further information about oxidative reaction pathways can be obtained by examining the kinetics of some of the reactive intermediates observed by irradiating solutions containing 87 µM 5’-d(TCGCT), 10 mM Na2S2O8 and 300 mM NaHCO3 in air-equilibrated buffer solutions. The CO3•− radicals exhibit a characteristic transient absorption band at 600 nm with the molar extinction coefficient26 ε = 1.97×103 M−1cm−1 which is greater than the ε values of the other free radicals at this wavelength (inset in Figure 6A).7,8
Figure 6.
Kinetics of free radical reactions induced by a 308 nm nanosecond single laser pulse excitation of air-equilibrated buffer solutions containing 87 µM µM 5’-d(TCGCT), 10 mM Na2S2O8 and 300 mM NaHCO3 at different values of pH (A) Transient absorbance recorded at 600 nm at pH 8.0 (black trace 1). The blue trace 2 was determined by subtraction of the G(−H)• absorbance at each time point from the black trace 1. Thus, trace 2 represents the decay of CO3•− radicals. The inset depicts the transient absorption spectra of CO3•−, SO4•− and G(−H)• radicals showing that there is some overlap in the absorption spectra of CO3•− and G(−H)• radicals at 600 nm. (B) Transient absorption profiles recorded at 385 nm and attributed to the formation and decay of G(−H)• radicals in buffer solution containing 300 mM NaHCO3, pH 8.0 (red traces 3 and 4, oxidation produced by CO3•− radicals), and without NaHCO3 (black traces 5 and 6, oxidation generated by SO4• radicals only). (C) Formation and decay of the G(−H)•+ radicals generated by SO4•− radicals (black traces 7 and 8) at pH 2.5 (in the absence of NaHCO3). The time course of formation and decay of CO3•− radicals (magenta, solid curve superimposed on the experimental data shown in blue, curve 2, Panel A) and G(−H)• radicals (blue, solid curve superimposed on the experimental data shown in red, curve 3, Panel B) involved in reactions 4 – 6 (see text) were simulated with the following best-fit rate constants: k4 = 1.5×107, k5a = 1.4×108, k5b = 1.6×108, and k6 = 3.0×108 M−1s−1, and the initial concentrations, [CO3•−]o = 3.60 µM and [G(−H)•]o = 1.25 µM.
The transient absorption spectrum of G(−H)• radicals shows a narrow absorption band at 315 nm and two less intense bands near 390 and 510 nm (inset in Figure 6A).4,27 To exclude photolysis of the sample solution by the probe light, we utilized a 360 nm cut-off filter and monitored the formation and decay of G(−H)• radicals at 385 nm. At this wavelength, the extinction coefficient of G(−H)• radicals4 (ε = 2.7×103 M−1cm−1) is greater than the molecular absorptivities of SO4•− radicals28 (ε = 1.6×103 M−1cm−1), and of CO3•− radicals (negligible at 385 nm).
At pH 8.0, the lifetime of CO3•− radicals is reduced from ~7.7 ms in the absence of 5'-d(TCGCT), to ~0.5 ms in the presence of 87 µM 5'-d(TCGCT) (blue trace 2, Figure 6A). The growth of the transient absorbance at 380 nm associated with the G(−H)• radicals is characterized by two kinetic components (red trace 3, Figure 6B). The first and fast component, which was not time-resolved in the experiment shown in Figure 6B, is assigned to the direct oxidation of 5'-d(TCGCT) by SO4•− radicals:
| (3) |
Reaction 3 contributes to the formation of G(−H)• radicals even at high concentrations of HCO3− (300 mM), because the values of k3 are typically very high, (2–3)×109 M−1s−1 and are close to the diffusion-controlled limit.7,8,29 The second millisecond component (~0.5 ms) is correlated with the decay of CO3•− radicals (blue trace 2, Figure 6A), and hence is assigned to oxidation of 5'-d(TCGCT) by CO3•− radicals:
| (4) |
However, the yield of G(−H)• radicals formed in this process (1.28 µM, calculated as a difference of the yields at 1.8 ms and 0.01 ms) is significantly less than the yield of CO3•− radicals (~3.60 µM) in reaction 2 (blue trace 2, Figure 6A). To explain this difference between the expected and observed yields of G(−H)• radicals in TC[G(−H)•]CT, we propose that the G(−H)• radicals derived from reactions 3 and 4 are rapidly oxidized by CO3•− radicals to form the two-electron oxidation products (8-oxoG and 2Ih):
| (5a) |
| (5b) |
Indeed, the simple kinetic scheme, which includes reactions 4, 5a, 5b discussed above, as well as reaction 6 describing the further oxidation of 5'-d(TC[8-oxoG]CT) by CO3•− radicals:
| (6) |
can account for the kinetics and yields of G(−H)• radicals in the presence of HCO3− anions. The simulated kinetic curves describing the decay of CO3•− radicals (magenta curve in Figure 6A) and the formation G(−H)• radicals (blue curve in Figure 6B) agree with the experimental kinetic profiles (blue trace 2 in Figure 6A and red trace 3 in Figure 6B, respectively). This scheme also predicts that the yield of 8-oxoG lesions should attain a relatively constant value after several laser flashes (Figure S1 in Supporting Information) as observed in the experiment (Figure 3).
Upon excitation with a single laser pulse and in the absence of HCO3− anions, G(−H)• radicals are formed via oxidation by SO4•− radicals (reaction 3) within < 10 µs following the 12 ns actinic laser pulse. This is evident from the rapid rise in the G(−H)• absorbance measured at 385 nm (black trace 7, Figure 6B). A simple kinetic scheme, based on reaction 3 (generation of G(−H)• radicals), reaction 7 (oxidation of G(−H)• radicals to form 8-oxoG lesions), and reaction 8 (oxidation of 8-oxoG) was developed:
| (7) |
| (8) |
This kinetic scheme predicts that the value of the rate constant k7 should be at the level of ~6×1010 M−1s−1 (Figure S2 in Supporting Information) in order to achieve yields of 8-oxoG that are comparable to those generated by CO3•− radicals (Figure 3). However, this value of k7 is unrealistic because it is by one order of magnitude greater than the diffusion-controlled limit. Therefore, it is not surprising that we did not observe any 8-oxoG lesions upon oxidation of 5'-d(TCGCT) with SO4•− radicals after a single laser pulse excitation in the absence of HCO3− anions (Figure 5).
In air-equilibrated buffer solutions (pH 8.0), the G(−H)• radicals decay relatively slowly with characteristic lifetimes of ~ 0.07 s which are not affected by the presence of oxidants (CO3•−, red trace 4, or SO4•−, black trace 6) as shown in Figure 6B. The value of the rate constant k9 ~ 15 s−1, defined by the reactions (pH 8.0):
| (9a) |
| (9b) |
can be estimated from the lifetimes of G(−H)• radicals; the value of this constant is an upper limit that defines the rate of formation of the cross-linked products after a single laser pulse excitation (Figure 5).
In acid solutions, only SO4•− radicals can be used as oxidants since HCO3− anions (precursors of CO3•− radicals) decompose to CO2 and H2O below pH 7. At pH 2.5, the formation of guanine radicals is complete within < 10 µs, and the yield of G(−H)• radicals (black trace 7, Figure 6C) is close to the yields at pH 8.0 (black trace 5, Figure 6B). At pH 2.5, the guanine radicals exist in the form of the radical cations,4 G•+ (pKa = 3.9) and decay with the characteristic lifetime of ~3 ms, or rate constant k10 ~ 3.3×102 s−1. The latter can be considered as the upper limit for the formation of 8-oxoG lesions according to the reaction:
| (10) |
Consistent with this mechanism, the 8-oxoG lesions were detected by reversed-phase HPLC after a single laser pulse excitation of the sample solutions (Figure 5). The G*CT* cross-links were not detected; the lifetime of G•+ is too short at pH 2.5 due to hydration, which limits the lifetime of G•+ to ~3 ms, and reaction 9 occurring with the characteristic time of ~70 ms can not compete with reaction 10.
Discussion
In this work, the one-electron oxidation of guanine bases in DNA sequences was initiated by CO3•− or SO4•− radicals produced by a single laser pulse excitation. The different reaction pathways established are summarized in Figure 7.
Figure 7.
One-electron oxidation of guanine bases in oligonucleotides in a GCT sequence context by CO3•− or by SO4•− radicals.
In agreement with these constraints, guanine radicals generated by SO4•− radicals at pH 2.5 (< pKa = 3.9) exist mostly in the cation form, G•+. Hydration of G•+ radicals results in the formation of 8-hydroxy-7,8-dehydroguanyl radicals (8-HO-G•), which are identical to the radical adducts derived from addition of hydroxyl radicals to the C8 position of guanine.2 The latter are reducing and are rapidly oxidized by weak oxidants such as oxygen, methylviologen, and 1,4-benzoquinone to form 8-oxoG lesions.30 The upper limit of the hydration rate constant, k10 ~ 3.3×102 s−1 was estimated from the decay of G•+ radicals recorded at 385 nm and at pH 2.5 (traces 4 and 6, Figure 6C). This is the first estimate of this rate constant by direct spectroscopic measurements; it is two orders of magnitude smaller than the rate constant of 6×104 s−1 obtained by computer modeling of the distributions of alkali-labile guanine lesions in DNA.31
The kinetics of the buildup of the G(−H)• absorption band at 625 nm band was utilized to measure the deprotonation rate constant of G•+ radicals, which varies from 1.8×107 s−1 for the free nucleoside to ≥ 3×106 s−1 in double-stranded DNA.32,33 The deprotonation of G•+ has been described in terms of a release of the N1 proton and the formation of the G(N1-H)• tautomer.34 Based on EPR studies in aqueous solutions (pH ≤ 3) at room temperature, coupled with a theoretical study, it was concluded that the G•+ radical is protonated at N1 and is indeed deprotonated by the loss of the proton at N1 to form the G(N1-H)• neutral radical at pH > 4.35 Extensive DFT calculations of various tautomeric forms of G(−H)• confirm that in aqueous solution G(N1-H)• is the most stable form of neutral guanine radical.36 Here, we propose two different pathways of G(−H)• radical decay that lead to the 8-oxoG or G*CT* lesions that are detected experimentally after irradiation of 5’-d(TCGCT) with a single laser pulse (Figure 7).
The formation of the G*CT* cross-links occur via the nucleophilic addition of thymine to the C8 position of the G(−H)• radical (Figure 7). Increasing the pH facilitate deprotonation of T-N3(H) since its pKa = 9.67,37 and thus greatly enhances the nucleophilicity of thymine due to the formation of (T-N3)−. The G(C8)-(N3)T radical adduct formed is easily oxidized by oxygen, thus resulting in the formation of the G*CT* cross-link. This adduct can also be oxidized by other mild oxidants like 1,4-benzoquinone in deoxygenated aqueous solutions.38 This mechanism does not depend on the oxidant (CO3•− or SO4•− radical) used for the generation of G(−H)• radicals and can account for a gradual growth of the yields of the G*CT* lesions with increasing pH (Figure 5). A similar nucleophilic mechanism was proposed by Perrier et al. for the formation of cross-linked products between guanine in d(TpGpT) and lysine mediated by photoexcited riboflavin in aerated solutions containing the KKK tripeptide.39
The neutral radical, G(−H)• radical, is a weaker electrophile than the radical cation G•+ and does not react directly with water which could account for the absence of 8-oxoG observed after single laser pulse excitation (Figures 2 and 5) and continuous UV illumination as reported earlier.10 In this work we demonstrate that CO3•− radicals can oxidize G(−H)• radicals. The rate constant of this reaction, k5 = (3.0±0.5)×107 M−1s−1 was obtained from analysis of the transient absorption profiles monitored by laser flash photolysis (Figure 6). This value of k5 is similar to the rate constants of the addition of oxyl radicals (e.g., O2•−, •NO2) to G(−H)• radicals.20,40 As in the case of other oxyl radicals (O2•−, •NO2), the combination of G(−H)• and CO3•− radicals occurs via the addition of CO3•− to the C8 and C5 positions of G(−H)• radicals (Figure 7). The adducts formed, which can be considered as the mono-esters of carbonic acid, H2CO3, are unstable and rapidly decompose to form the final products, 8-oxoG and the diastereomeric 2Ih lesions. The partitioning of CO3•− radical addition to either C5 or C8 of G(−H)• can be determined from the ratio of 2Ih/8-oxoG lesions which are ~ 1.2, detected after a single laser pulse (Figure 3). The DNA secondary structure can affect the ratio of the C5/C8 products. In the case of •NO2 radicals the ratio of 5-guanidino-4-imidazolone (C5 addition)/8-nitroguanine (C8 addition) gradually decreases from 3.4 in the model compound, 2’,3’,5’-tri-O-acetylguanosine, to 2.1 – 2.6 in single-stranded oligodeoxynucleotides, to 0.8 – 1.1 in double-stranded DNA.41 Here, this effect is accounted for in terms of the relative accessibilities of C5 and C8 positions of G(−H)• radicals by carbonate radicals and the further transformation of the adducts formed to end-products such as 2Ih (C5 addition) or 8-oxoG (C8 addition).
Conclusions
The one-electron oxidation of guanine bases in single-stranded oligonucleotides in the 5'-..TCGCT.. sequence context by CO3•− and SO4•− radicals generates guanine radical cations. In neutral solutions, the G•+ radicals rapidly deprotonate to form guanine neutral radicals, G(−H)•. The transformation of G(−H)• radicals to chemical end products occurs by two principal pathways: (i) combination of G(−H)• and CO3•− radicals followed by the formation of 8-oxoG and 2Ih lesions, and (ii) nucleophilic addition of thymine bases to G(−H)• followed by the formation of 5'-G*CT* and 5'-T*CG* cross-links. The cross-linking reaction is very slow and occurs with the characteristic time of ≥ 70 ms. In acid solutions (pH 2.5), the principal pathway of G•+ decay is hydration that is followed by the formation of 8-oxoG lesions.2 This hydration reaction, estimated from the decay time of the radical cation at pH 2,5, occurs within a characteristic time of ≥ 3 ms. Under physiological conditions (pH 7 – 8) the major decay pathway of the G•+ radical cation is deprotonation that occurs in double-stranded DNA within ≤ 300 ns.36,37 The ratio of the hydration/deprotonation rates of G•+ estimated from the characteristic times of these reactions suggests that the yields of 8-oxoG formed are ≤ 0.01% per Guanine radical cation generated by the one-electron oxidation mechanism. Such a low efficiency of 8-oxoG formation originating via the G•+ pathway is consistent with the lack of observation of 8-oxoG formation in our sulfate radical oxidation experiments at pH 8.0 (Table 1). Furthermore, the long lifetime of the G(−H)• radical in neutral aqueous solutions (~ 70 ms in the absence of other reactive radical intermediates, Figure 6B) is not limited by hydration that would have yielded 8-oxoG at pH 8.0. In the case of cellular DNA the levels of 8-oxoG lesions are quite small (0.3 – 4.2 8-oxoG per 106 guanines6). However, these low yields may be due to other factors (e.g., low yields of G•+ radicals and differences in reaction pathways of these radicals in cellular environments, as well as base excision repair of 8-oxoG lesions).
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Environmental Health and Sciences Grant 2 R01 ES 011589-10. Components of this work were conducted in the Shared Instrumentation Facility at NYU that was constructed with support from a Research Facilities Improvement Grant (C06 RR-16572) from the National Center for Research Resources, National Institutes of Health. The acquisition of the MALDI-TOF mass spectrometer was supported by the National Science Foundation (CHE-0958457).
Footnotes
Associated Content
S. Supporting Information
The simulated kinetics of reactions of CO3•− and G(−H)• radicals, and the yields of the 8-oxoG, 2Ih, Sp and Gh molecular products as a function of the number of laser pulses (Figure S1). The simulated kinetics of the SO4•− and G(−H)• radicals and the yields of 8-oxoG produced by a single laser pulse excitation (Figure S2). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Steenken S, Jovanovic SV. J. Am. Chem. Soc. 1997;119:617–618. [Google Scholar]
- 2.Cadet J, Douki T, Ravanat JL. Acc. Chem. Res. 2008;41:1075–1083. doi: 10.1021/ar700245e. [DOI] [PubMed] [Google Scholar]
- 3.Pacher P, Beckman JS, Liaudet L. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candeias LP, Steenken S. J. Am. Chem. Soc. 1989;111:1094–1099. [Google Scholar]
- 5.Fleming AM, Muller JG, Burrows CJ. Org. Biomol. Chem. 2011;9:3338–3348. doi: 10.1039/c1ob05112a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins AR, Cadet J, Moller L, Poulsen HE, Vina J. Arch. Biochem. Biophys. 2004;423:57–65. doi: 10.1016/j.abb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Shafirovich V, Dourandin A, Huang W, Geacintov NE. J. Biol. Chem. 2001;276:24621–24626. doi: 10.1074/jbc.M101131200. [DOI] [PubMed] [Google Scholar]
- 8.Joffe A, Geacintov NE, Shafirovich V. Chem. Res. Toxicol. 2003;16:1528–1538. doi: 10.1021/tx034142t. [DOI] [PubMed] [Google Scholar]
- 9.Crean C, Geacintov NE, Shafirovich V. Angew. Chem. Int. Ed. Engl. 2005;44:5057–5060. doi: 10.1002/anie.200500991. [DOI] [PubMed] [Google Scholar]
- 10.Crean C, Uvaydov Y, Geacintov NE, Shafirovich V. Nucleic Acids Res. 2008;36:742–755. doi: 10.1093/nar/gkm1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crean C, Lee YA, Yun BH, Geacintov NE, Shafirovich V. ChemBioChem. 2008;9:1985–1991. doi: 10.1002/cbic.200800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huie RE, Clifton CL, Neta P. Radiat. Phys. Chem. 1991;38:477–481. [Google Scholar]
- 13.Shafirovich V, Geacintov NE. In: Radical and radical ion reactivity in nucleic acid chemistry. Greenberg M, editor. Hoboken, New Jersey: John Willey&Sons, Inc.; 2009. pp. 325–355. [Google Scholar]
- 14.Shafirovich V, Dourandin A, Huang W, Luneva NP, Geacintov NE. J. Phys. Chem. B. 1999;103:10924–10933. [Google Scholar]
- 15.Harned HC, Bonner FC. J. Am. Chem. Soc. 1945;67:1026–1031. [Google Scholar]
- 16.Neta P, Huie RE, Ross AB. J. Phys. Chem. Ref. Data. 1988;17:1027–1284. [Google Scholar]
- 17.Ye W, Sangaiah R, Degen DE, Gold A, Jayaraj K, Koshlap KM, Boysen G, Williams J, Tomer KB, Ball LM. Chem. Res. Toxicol. 2006;19:506–510. doi: 10.1021/tx0600144. [DOI] [PubMed] [Google Scholar]
- 18.Ye W, Sangaiah R, Degen DE, Gold A, Jayaraj K, Koshlap KM, Boysen G, Williams J, Tomer KB, Mocanu V, Dicheva N, Parker CE, Schaaper RM, Ball LM. J. Am. Chem. Soc. 2009;131:6114–6123. doi: 10.1021/ja8090752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghude P, Schallenberger MA, Fleming AM, Muller JG, Burrows CJ. Inorganica Chim Acta. 2011;369:240–246. doi: 10.1016/j.ica.2010.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misiaszek R, Crean C, Joffe A, Geacintov NE, Shafirovich V. J. Biol. Chem. 2004;279:32106–32115. doi: 10.1074/jbc.M313904200. [DOI] [PubMed] [Google Scholar]
- 21.Crean C, Geacintov NE, Shafirovich V. Chem. Res. Toxicol. 2008;21:358–373. doi: 10.1021/tx700281e. [DOI] [PubMed] [Google Scholar]
- 22.Lomoth R, Naumov S, Brede O. J. Phys. Chem. A. 1999;103:6571–6579. [Google Scholar]
- 23.Aravindakumar CT, Schluchmann MN, Rao BSM, von Sonntag J, von Sonntag C. Org. Biomol. Chem. 2003;1:401–408. doi: 10.1039/b209626a. [DOI] [PubMed] [Google Scholar]
- 24.von Sonntag C, Schuchmann H-P. Angew. Chem. Int. Ed. Engl. 1991;30:1229–1253. [Google Scholar]
- 25.Tallman KA, Tronche C, Yoo DJ, Greenberg MM. J. Am. Chem. Soc. 1998;120:4903–4909. [Google Scholar]
- 26.Czapski G, Lymar SV, Schwarz HA. J. Phys. Chem. A. 1999;103:3447–3450. [Google Scholar]
- 27.Crean C, Geacintov NE, Shafirovich V. J Phys Chem B. 2009;113:12773–12781. doi: 10.1021/jp903554n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McElroy WJ. J. Phys. Chem. 1990;94:2435–2441. [Google Scholar]
- 29.Candeias LP, Steenken S. J. Am. Chem. Soc. 1993;115:2437–2440. [Google Scholar]
- 30.Candeias LP, Steenken S. Chem. Eur. J. 2000;6:475–484. doi: 10.1002/(sici)1521-3765(20000204)6:3<475::aid-chem475>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.Giese B, Spichty M. ChemPhysChem. 2000;1:195–198. doi: 10.1002/1439-7641(20001215)1:4<195::AID-CPHC195>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi K, Tagawa S. J. Am. Chem. Soc. 2003;125:10213–10218. doi: 10.1021/ja036211w. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi K, Yamagami R, Tagawa S. J. Phys. Chem. B. 2008;112:10752–10757. doi: 10.1021/jp804005t. [DOI] [PubMed] [Google Scholar]
- 34.Steenken S. Chem. Rev. 1989;89:503–520. [Google Scholar]
- 35.Bachler V, Hildenbrand K. Radiat. Phys. Chem. 1992;40:59–68. [Google Scholar]
- 36.Adhikary A, Kumar A, Becker D, Sevilla MD. J. Phys. Chem. B. 2006;110:24171–24180. doi: 10.1021/jp064361y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knobloch B, Linert W, Sigel H. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7459–7464. doi: 10.1073/pnas.0501446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crean C, Geacintov NE, Shafirovich V. Free Radic. Biol. Med. 2008;45:1125–1134. doi: 10.1016/j.freeradbiomed.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perrier S, Hau J, Gasparutto D, Cadet J, Favier A, Ravanat JL. J. Am. Chem. Soc. 2006;128:5703–5710. doi: 10.1021/ja057656i. [DOI] [PubMed] [Google Scholar]
- 40.Misiaszek R, Crean C, Geacintov NE, Shafirovich V. J. Am. Chem. Soc. 2005;127:2191–2200. doi: 10.1021/ja044390r. [DOI] [PubMed] [Google Scholar]
- 41.Joffe A, Mock S, Yun BH, Kolbanovskiy A, Geacintov NE, Shafirovich V. Chem. Res. Toxicol. 2003;16:966–973. doi: 10.1021/tx025578w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.