Abstract
Aldosterone stimulates the endothelin-1 gene (Edn1) in renal collecting duct (CD) cells by a mechanism involving the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR). The goal of the present study was to determine if the synthetic glucocorticoid dexamethasone affected Edn1 gene expression and to characterize GR binding patterns to an element in the Edn1 promoter. Dexamethasone (1 μM) induced a 4-fold increase in Edn1 mRNA in mIMCD-3 inner medullary CD cells. Similar results were obtained from cortical collecting duct-derived mpkCCDc14 cells. RU486 inhibition of GR completely blocked dexamethasone action on Edn1. Similarly, 24h transfection of siRNA against GR reduced Edn1 expression by approximately 50%. However, blockade of MR with either spironolactone or siRNA had little effect on dexamethasone induction of Edn1. Cotransfection of MR and GR siRNAs together had no additive effect compared to GR-siRNA alone. The results indicate that dexamethasone acts on Edn1 exclusively through GR and not MR. DNA affinity purification studies revealed that either dexamethasone or aldosterone resulted in GR binding to the same hormone response element in the Edn1 Edn1 promoter. The Edn1 hormone response element contains three important sequence segments. Mutational analysis revealed that one of these segments is particularly important for modulating MR and GR binding to the Edn1 hormone response element.
Keywords: Dexamethasone, glucocorticoid response element, endothelin-1, collecting duct, glucocorticoid receptor
Introduction
Endothelin-1 (ET-1) is a 21 amino acid signaling peptide involved in a wide variety of biological processes including development, homeostasis, cell-cycle control and inflammation [1]. The spatial and temporal production of ET-1 is highly regulated in order to maintain proper control over these diverse functions. The primary mechanism controlling ET-1 concentrations occurs at the level of gene transcription [1]. Previously, we and others have reported that the ET-1 gene (Edn1) was under direct transcriptional control by the mineralocorticoid aldosterone in the kidney [2, 3]. Interestingly, the action of aldosterone on Edn1 was mediated not only by the classical mineralocorticoid receptor (MR), but also by the glucocorticoid receptor (GR).
MR and GR are both ligand-dependent transcription factors that share extensive structural homology and identical consensus sequences [4]. However, the expression and function of MR is far more restricted than GR. Most notably, MR is expressed in polarized epithelial cells involved in sodium transport including the aldosterone-sensitive cells of the distal nephron and collecting duct in the kidney [5]. In these cells, MR plays a vital role in the maintenance of sodium homeostasis and blood pressure control through the transcriptional regulation of genes involved in transepithelial sodium transport [6–8]. In contrast, GR is ubiquitously expressed in the body and is estimated to modulate 10% of the genes within the human genome [9–11]. Glucocorticoids are involved in a wide variety of physiological processes including the stress response, immune function, reproduction, behavior, and metabolism. The importance of GR is underscored by the fact that exogenous and synthetic glucocorticoids represent one of the most widely used classes of therapeutic compounds due to their efficacy in the treatment of inflammatory, autoimmune and proliferative disorders.
Renal collecting duct cells express both MR and GR in vivo [12]. These cells also express 11β-hydroxysteroid dehydrogenase type II (11βHSD-2). Aldosterone is not a substrate for this enzyme, so 11βHSD-2 acts only on endogenous glucocorticoids, such as cortisol, producing 11-keto metabolites that do not activate MR or GR [13, 14]. Therefore, the functional role of GR in renal collecting duct cells is not well defined [8]. However, the absence of 11βHSD-2 in renal collecting duct cells can have important detrimental effects. For example, glucocorticoid hormones can bind to MR with similar affinity to aldosterone [4, 15] resulting in inappropriate salt retention and hypertension in human patients [16, 17].
Aldosterone can also bind to GR [18, 19]. Therefore, it is possible that aldosterone mediates its action through both MR and GR in 11-βHSD2 expressing cells of the collecting duct. Support for this hypothesis is found in transgenic mice that overexpress GR. These animals exhibited an increase in Scnn1a (αENaC) levels in the collecting duct and a decrease in urinary aldosterone levels, demonstrating a transient GR-dependent change in sodium balance in vivo [20]. In our own studies, aldosterone stimulated both MR and GR binding to a single high affinity hormone response element (termed HRE2) in the Edn1 promoter [2]. Similar receptor binding patterns have been observed for other aldosterone target genes involved in sodium balance, such as Atp1a, Scnn1a, Sgk1 and Per1 [21–25]. Therefore, it is not surprising that both MR and GR stimulate sodium transport in collecting duct cells [19, 26].
While there is mounting evidence suggesting that GR participates in aldosterone action in the kidney, it is not known whether GR acts in concert with MR or if GR functions independently. GR could conceivably function by binding to an alternative response element or by a non-genomic action. The goal of the present study was to determine if GR stimulates Edn1 expression in the mIMCD-3 collecting duct cell line. Since mIMCD-3 collecting duct cells express 11βHSD-2, selective GR action on Edn1 was evaluated using dexamethasone. Dexamethasone is a synthetic glucocorticoid that is not subject to inactivation by 11βHSD-2. Dexamethasone has an additional advantage for study of selective GR activation because it exhibits a very high affinity for GR [27]. In this report we show that dexamethasone activates Edn1 expression via GR binding to HRE2, and that sequence changes in HRE2 alter GR binding to the element.
Experimental
Cell culture and hormone treatment
The mpkCCDc14 cells are a mouse cortical collecting duct cell line and were a kind gift of Dr. Alain Vandewalle [28]. The mIMCD-3 cells are a mouse inner medullary collecting duct cell line and were purchased from American Type Culture Collection. All cells were maintained in DMEM/F12 plus 10% FBS and 50 μg/ml gentamicin. For all hormone experiments, cells were plated on 6-well Costar Transwell plates (Corning Inc.). Cells were grown 24 h past confluency and changed to DMEM/F12 plus 10% charcoal-dextran stripped FBS (Invitrogen) for another 24 h prior to hormone treatments. Aldosterone, dexamethasone, spironolactone and RU486 were purchased from Sigma-Aldrich, prepared in 100% ethanol and stored at −20 °C until use. Cells were treated with vehicle (ethanol), 1 μM dexamethasone or 1 μM aldosterone for 1 h. For inhibitor studies, cells were treated with agonist plus RU486 (10 μM) or spironolactone (10 μM). The final concentration of ethanol in all experiments was 0.1%.
Steady-state mRNA determination
Hormone studies were conducted as described above on growth-arrested confluent cell monolayers grown in 6-well Costar Transwell plates. Total RNA (2 μg) was isolated from cells using TRIzol® Reagent (Invitrogen), treated with DNase I (Ambion) to eliminate genomic DNA, and reverse transcribed using oligo dT, random hexamers and Superscript™ III (Invitrogen). No reverse transcriptase served as a negative control in the cDNA reaction. Resulting cDNAs (32 ng) were used as templates in duplicate quantitative real-time PCR (QPCR) reactions run on an Applied Biosystems QPCR machine. No template cDNA was used a negative control in QPCR experiments. Cycle threshold (CT) values were normalized against β-actin (actb) and relative quantification was performed using the ΔΔCT method [29]. TaqMan® (Applied Biosystems) primer/probe sets were used and are listed in Supplemental Table 1. QPCR studies were run with standardized conditions using the ΔΔCT method.
Hormone receptor siRNA knockdown
MR-siRNA (J-061269-09 NR3C2), GR-siRNA (J-045970-10 NR3C1) and control non-targeting siRNA against luciferase (#2 D-001210-02-05) were purchased from Dharmacon (Lafayette, CO, USA). Cells were seeded at a density of 75,000 cells per cm2 on 6-well Transwell plates (Corning Incorporated) and transfected for 24 h with 2 μM siRNA in 6 μl of DharmaFect 4. At the time of transfection cells were switched to phenol-red free DMEM/F12 plus 10% charcoal dextran stripped FBS. After 24 h the cells were treated with 1 μM dexamethasone or vehicle for 1 h. RNA was extracted and processed as described above for QPCR.
DNA affinity purification assay and Western analysis
Hormone experiments were conducted in mIMCD-3 cells as described above except that cells were grown in 200 mm dishes (Corning). Cells were treated with vehicle (0.15% ethanol), aldosterone, or dexamethasone. Cytoplasmic and nuclear extracts were obtained using the NE-PER® kit (Pierce Biotechnology) and DNA affinity purification assays (DAPAs) were performed as described previously [2]. In brief, double stranded DNA probes homologous to the wild-type or mutated Edn1 HRE2 were biotinylated on 5′ ends (sequences detailed in Figure 5B). Probes were immobilized on 50 μl of streptavidin coated agarose beads and incubated with 175 μg of nuclear extract in the presence of freshly prepared protease inhibitors (Roche) for 1 h at room temperature with end-over-end rotation. Beads were pelleted and supernatants were removed and assayed for input controls by Western blotting for actin. Pelleted beads were washed four times with ice-cold PBS plus protease inhibitors. After the final wash, all liquid was aspirated from the beads with flat-headed gel loading tips (USA Scientific) and 50 μl of 2x LDS (BioRad) plus βME. Samples were boiled for 5 min, chilled on ice, and loaded onto a 7.5% Tris-HCl SDS-PAGE Ready Gel (Biorad) for electrophoresis. Proteins were transferred to PVDF overnight and blocked with 2% Rodeo blocker plus 0.05% saddle soap (USB) in TBS. The monoclonal MR antibody was a kind gift of Drs. Elise and Celso Gomez-Sanchez and was used at a 1:100 dilution [30]. The GR and actin antibodies were purchased from Santa Cruz and used at 1:5000 and 1:200 dilutions, respectively. Blots were washed with blocking solution and developed with Rodeo Western Detection Reagents (USB). Equal loading was controlled for by Bradford assay and input control Westerns against actin.
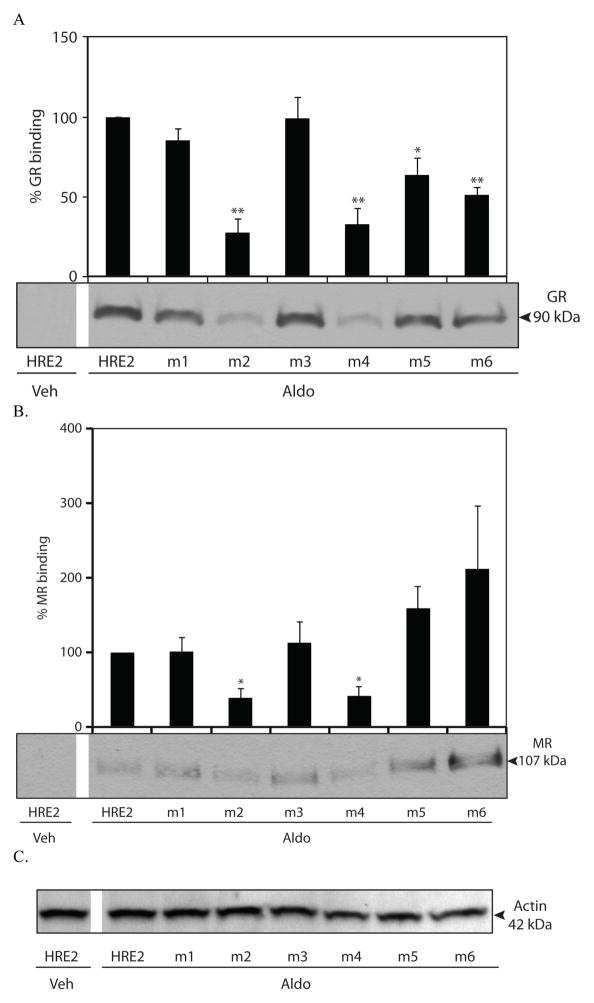
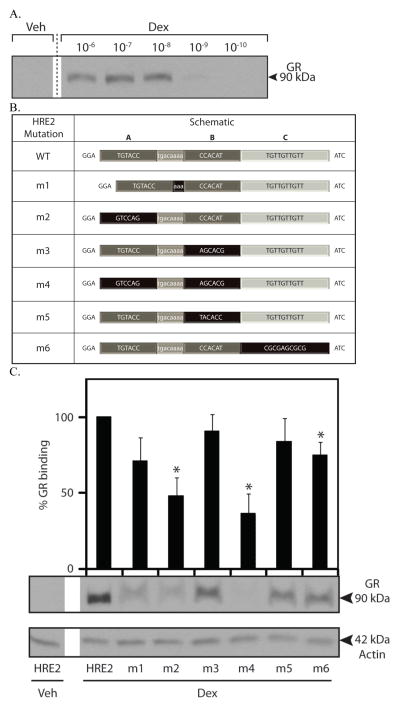
Figure 5.
Aldosterone-dependent GR and MR binding to HRE2 mutants. DAPAs were performed on nuclear protein extracts from mIMCD-3 cells treated with vehicle (Veh) or 1 μM aldosterone (Aldo) for 1 h. Changes in GR (A) or MR (B) binding to the Edn1 HRE2 wild-type or mutated probes are shown. Panel C. Actin loading control for the nuclear extract. Densitometric values are indicated above each band and are shown as percent (%) GR or MR binding. HRE2 wild-type probe in the presence of aldosterone is set to 100%. (*p < 0.05, ** < 0.001, n ≥ 3)
Statistics
Unless otherwise stated, all experiments were performed in duplicate in at least three independent studies. Statistical significance was determined using a two-tailed Student’s t test and p < 0.05 was considered significant.
Results
Dexamethasone-dependent Edn1 expression in collecting duct cells
Mineralocorticoid stimulation resulted in increases in both MR and GR bound to the HRE2 site in the proximal promoter region of the Edn1 gene and blockade of either receptor resulted in a reduction in aldosterone-dependent edn1 gene expression [2]. QPCR was performed to evaluate the steady-state mRNAs for both Nr3c1 (GR) and Nr3c2 (MR). In mIMCD-3 cells, GR was more than an order of magnitude higher than the expression of MR (data not shown).
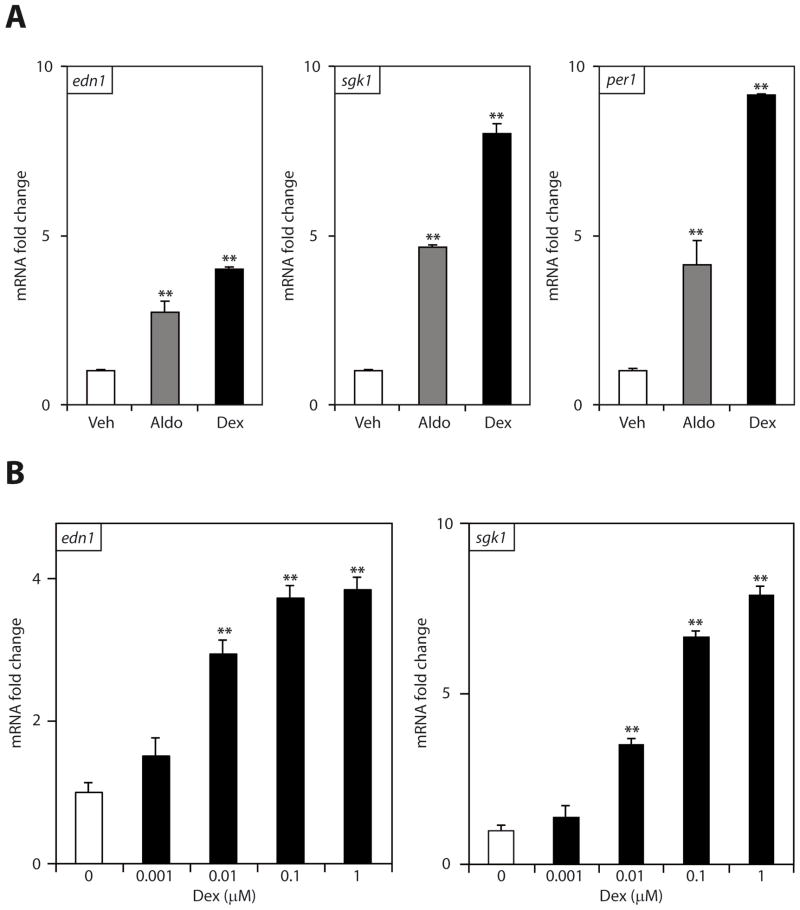
In order to gain an understanding of the role of GR in modulating Edn1 gene expression, mIMCD-3 cells were treated with dexamethasone, aldosterone or vehicle. Dexamethasone is a synthetic glucocorticoid that acts predominately through GR and is not a substrate for 11βHSD-2. Consistent with previous work [2, 31], 1 μM aldosterone stimulated a 2.7 ± 0.3 fold increase in Edn1 mRNA (Figure 1A). An equimolar concentration of dexamethasone stimulated a 3.9 ± 0.2 fold increase in Edn1. Two other established aldosterone response genes Sgk1 and Per1 were also analyzed, and both genes demonstrated substantially greater increases in mRNA levels in response to dexamethasone than with aldosterone (Figure 1A). The robust stimulation of aldosterone-response genes by dexamethasone suggested that Edn1 might be responsive to the hormone at lower concentrations. Therefore, cells were treated with increasing concentrations of dexamethasone (1 nM – 1 μM) for 1 h. Dexamethasone resulted in a dose-dependent increase in Edn1 mRNA expression that was statistically significant at concentrations as low as 10 nM and maximum Edn1 induction was achieved with 100 nM of dexamethasone (Figure 1B). In contrast, a concentration of 100 nM was required for aldosterone to achieve convincing increases in either Edn1 or Sgk1 mRNA [2].
Figure 1.
Dexamethasone-stimulated gene expression in mIMCD-3 cells. Steady state Edn1, Sgk1 and Per1 mRNA was measured by QPCR, normalized to Actb (β-actin) and expressed as mRNA fold change relative to vehicle ± SE. (*p < 0.05, **p < 0.001 relative to vehicle; n ≥ 3) Panel A. Confluent monolayers of mIMCD-3 cells were treated with vehicle (veh, open bars) or 1 μM dexamethasone (dex, closed bars) for 1 h. As a positive control, cells were also treated with 1 μM aldosterone (aldo, gray bars) for 1 h and data are shown as a reference. Panel B. Cells were treated with dexamethasone concentrations ranging from 0 to 1 μM.
A similar study was conducted in mpkCCDc14 cells to determine if dexamethasone stimulation of Edn1 was a general property of collecting duct cells. This cell line also expresses functional 11βHSD-2 and both MR and GR in relatively high abundance [28]. Dexamethasone (1 μM) stimulated increases in Edn1, Sgk1 and Per1 mRNAs at 1 h, and the trend for higher gene expression in the presence of dexamethasone compared to aldosterone was readily apparent (data not shown).
Inhibition of hormone receptors
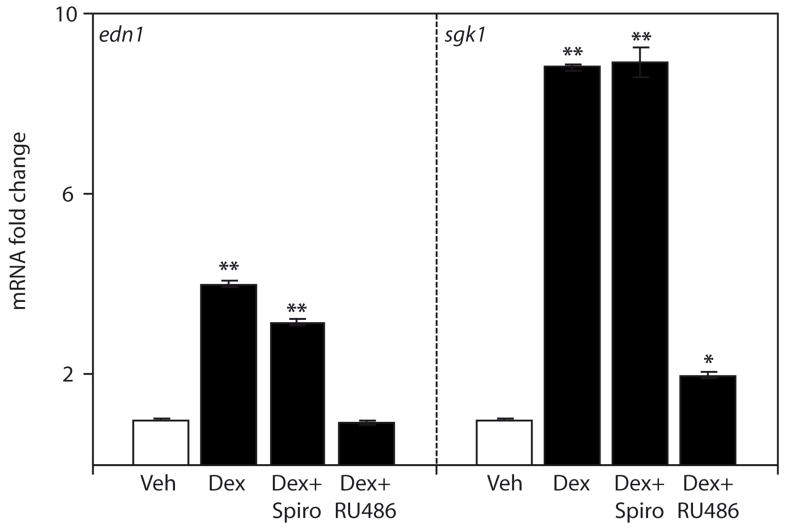
The effect of MR or GR antagonism on dexamethasone-stimulated Edn1 and Sgk1 expression was evaluated by treatment with either spironolactone or RU486, respectively. RU486 is a GR antagonist with little affinity for MR. Treatment of mIMCD-3 cells with RU486 effectively blocked dexamethasone induction of Edn1 mRNA, and greatly reduced dexamethasone induction of Sgk1 mRNA (Figure 2). In contrast, antagonism of MR with spironolactone had no apparent effect on dexamethasone-mediated Sgk1 and resulted in only a modest reduction in Edn1 expression (Figure 2). However, spironolactone is known to have moderate antagonistic effects on GR [32], and our previous report showed spironolactone induced GR nuclear translocation and binding to the Edn1 promoter [2]. Taken together, these data suggest that the vast majority of dexamethasone-dependent Edn1 gene expression was mediated through GR and not MR.
Figure 2.
Effect of pharmacological blockade of MR and GR on dexamethasone-induced Edn1 gene expression in mIMCD-3 cells. Pharmacological inhibition of MR or GR on dexamethasone-stimulated Edn1 mRNA was evaluated in mIMCD-3 cells treated with vehicle (veh, open bars), 1 μM dexamethasone (dex, closed bars), or 1 μM dexamethasone in the presence of 10 μM spironolactone (spiro) or 10 μM RU486 for 1 h. Edn1, and Sgk1 mRNA levels were determined by QPCR, normalized to actb (β-actin) and expressed as mRNA fold change relative to vehicle ± SE. (*p < 0.05, **p < 0.001 relative to vehicle; n ≥ 3)
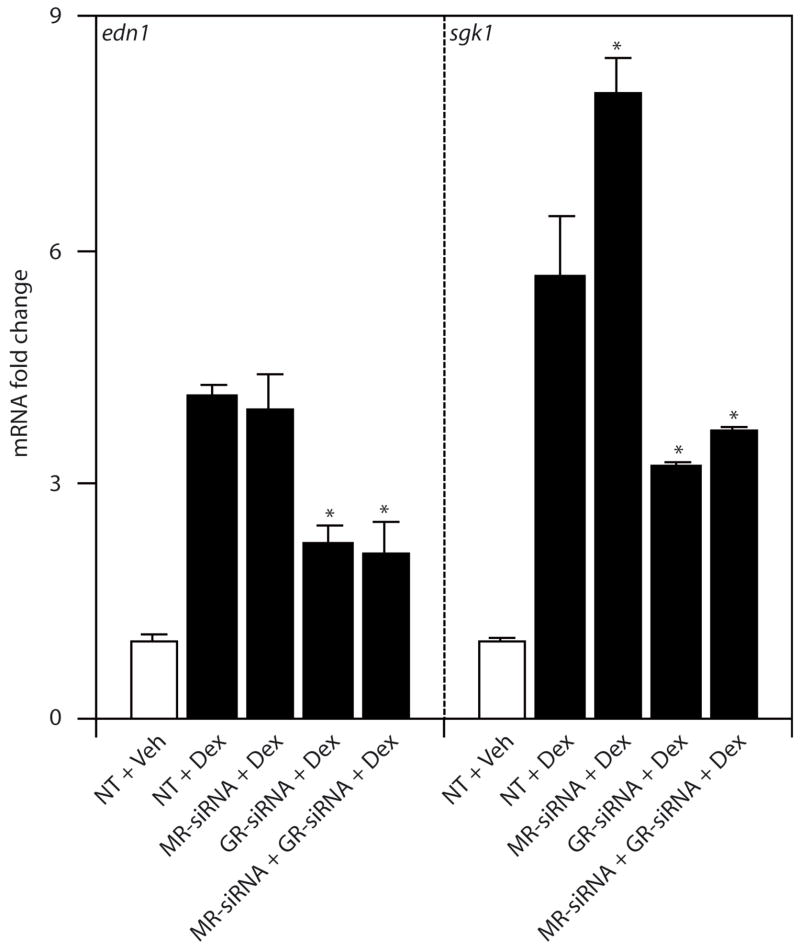
Since the pharmacological inhibitors have overlapping specificities, the effect of MR- or GR-siRNA knockdown was evaluated in mIMCD-3 cells to provide an independent and highly specific approach to studying receptor action. Transfection of either MR- or GR-siRNA resulted in approximately 90% knockdown of their respective mRNAs [25]. In the presence of 1 μM dexamethasone, siRNA blockade of GR reduced dexamethasone-dependent induction of Edn1 and Sgk1 mRNA levels by approximately 50% (Figure 3). Western analysis showed a marked reduction in GR protein levels resulting from application of siRNA, but the signal was not abrogated suggesting residual receptors remained (data not shown). Conversely, siRNA of blockade of MR failed to inhibit dexamethasone-induced Edn1 mRNA levels (Figure 3). Dexamethasone-dependent Sgk1 mRNA was reproducibly higher in the presence of MR-siRNA, but the significance of this isolated observation is unclear. Importantly, cotransfection of MR- and GR-siRNAs together had no additive effect on Edn1 or Sgk1 mRNA compared to GR-siRNA alone. Therefore, as expected, these data indicate that dexamethasone action was mediated through GR establishing that mIMCD-3 cells can serve as an appropriate model for glucocorticoid action on Edn1.
Figure 3.
Effect of MR-siRNA or GR-siRNA on dexamethasone-induced gene expression in mIMCD-3 cells. Cells were transiently transfected with non-target (NT), MR- or GR-siRNA for 24 h prior to a 1 h vehicle (Veh) or 1 μM dexamethasone (Dex) treatment. Changes in mRNA were measured by QPCR as above. Values are expressed as mean fold change relative to vehicle treated cells transfected with NT-siRNA ± SE. (*p < 0.05 vs. NT-siRNA + Dex, n=3)
Dexamethasone recruits GR to the Edn1 promoter
Both MR and GR are recruited to the Edn1 HRE2 in response to aldosterone treatment [2]. Therefore, we tested whether dexamethasone would also result in recruitment of GR to the same HRE2 in the Edn1 promoter. In order to investigate GR binding to HRE2, DNA affinity purification assays were performed on nuclear extracts from mIMCD-3 cells treated increasing concentrations of dexamethasone (1 nM – 1 μM). Concentrations of dexamethasone as low as 10 nM resulted in detectable levels of GR binding to the Edn1 HRE2 (Figure 4A).
Figure 4.
Dexamethasone-dependent binding of GR to the Edn1 HRE2. A. GR binding to the Edn1 HRE2 region was evaluated with DNA affinity purification assays (DAPA) performed on mIMCD-3 cells treated with varying concentrations of dexamethasone (0.1 nM to 1 μM) for 1 h. B. Mutations to the Edn1 HRE2 DAPA probes. Three putative receptor binding half-sites are indicated as A, B and C. The spacer region is shown in light grey with white text. Mutated regions are highlighted in black. C. DAPAs were performed using HRE2 mutant probes on mIMCD-3 cells treated with 1 μM dexamethasone for 1 h. Densitometric values are indicated above each band and are shown as percent (%) GR binding. GR binding to the HRE2 wild-type probe in the presence of dexamethasone is set to 100%. (*p < 0.05, n ≥ 3)
HRE2 is a complex response element that has three plausible receptor binding half-sites designated HRE2A, HRE2B and HRE2C (Figure 4B). In order to determine which of the three half sites mediate GR binding, a series of mutations probes containing HRE2 DAPA mutations were synthesized. Nuclear extracts were prepared from mIMCD-3 cells exposed to dexamethasone (1 μM) or vehicle for 1 h. Dexamethasone stimulated the recruitment of GR to the wild-type HRE2 DAPA probe (Figure 4C). In the first experiment, five nucleotides were deleted from the spacer region between HRE2A and HRE2B. This resulted in only a moderate decrease in dexamethasone-dependent GR binding (Figure 4C, lane m1). HRE2C has the strongest homology to a GR half-site, so it was mutated to an unrelated sequence. Surprisingly, only a modest decrease in GR binding to the probes was observed (Figure 4C, lane m6). The specific sequence of HRE2B presented a problem because differing bases could arguably contribute to GR dimer binding with either HRE2A or in the reverse orientation with HRE2C. Therefore, DAPA probes were designed to mutate HRE2B to an unrelated sequence and to simply reverse the orientation of HRE2B in a manner intended to destroy both potential half-sites. Again neither mutation resulted in a significant decrease in GR binding (Figure 4C, lanes m3 and m5). However, changing the upstream HRE2A half-site to an unrelated sequence, or mutating both HRE2A and HRE2B half-sites together abolished more than half of the dexamethasone-dependent GR binding (Figure 4C, lanes m2 and m4). Although HRE2A bears the least sequence homology to the consensus HRE sequence, the evidence clearly indicated that the HRE2A element is required for stable dexamethasone-dependent GR binding. Additional DAPA experiments were also conducted to determine if MR could bind to the HRE2 wild type or mutant probes in the presence of dexamethasone. Dexamethasone failed to activate MR in mIMCD-3 cells and no receptor binding was observed in those experiments (data not shown).
Next, the ability of aldosterone to activate MR and GR binding to each set of HRE2 mutant probes was investigated using nuclear extracts from mIMCD-3 cells with 1 μM aldosterone (Figure 5). In this experiment, the two receptors might be viewed as in competition with one another. Consistent with our previous report, aldosterone resulted in greater recruitment of both MR and GR to the wild-type HRE2 (Figure 5). Mutation to HRE2A alone or together with HRE2B resulted in a significant decrease in both MR and GR binding. In summary, the evidence indicates that the HRE2A half-site is the key sequence for high affinity binding of hormone receptors at HRE2.
Discussion
The studies presented here demonstrated that dexamethasone stimulated Edn1 gene expression in mIMCD-3 and mpkCCDc14 collecting duct cells. Pharmacological inhibition and siRNA knockdown studies confirmed that the action of dexamethasone on Edn1 mRNA was mediated specifically though GR. Dexamethasone-stimulated gene expression of Edn1 in mIMCD-3 and mpkCCDc14 cells was consistently more robust than aldosterone-stimulated gene expression using equal molar hormone concentrations. Furthermore, dexamethasone-dependent GR activation resulted in greater stimulation of the other aldosterone target genes tested Sgk1 and Per1. We previously mapped HRE2 within the Edn1 promoter region. HRE2 contained three putative receptor binding half-sites [2]. DAPA experiments identified HRE2A as the half-site required for high affinity dexamethasone-dependent GR binding. HRE2A is equally important for MR and GR binding in the presence of aldosterone.
The magnitude of dexamethasone-dependent gene transcription in both mIMCD-3 and mpkCCDc14 cells was higher than aldosterone-dependent gene transcription under the experimental conditions tested. Similarly, other investigators have shown that dexamethasone activation of GR resulted in a robust increase in sodium transport in primary cultures of rat inner medullary collecting ducts that exceeded the response achieved by aldosterone [26]. Elimination of the HRE2A half-site resulted in reduced dexamethasone-dependent GR binding and reduced MR and GR binding in response to aldosterone. Therefore, it is likely that both MR and GR compete for binding to the Edn1 gene HRE. The apparent difference in the magnitude of dexamethasone-induced Edn1 mRNA compared to aldosterone induction may be explained by differences in transcriptional machinery recruited to GR versus MR. Precedent for this type of mechanism has been observed for the elongation factor ELL (11, 19-lysine rich leukemia) that activates MR, but strongly represses GR [33]. Alternatively, MR and GR are known to form functional heterodimers in the presence of aldosterone [24, 34, 35] and these receptor complexes exhibit distinct transcriptional properties compared to either receptor acting alone [24, 36]. Finally, ligand-mediated conformational changes in GR may contribute to the observed differences in transcription activation. Mineralocorticoids and glucocorticoids are chemically very similar, but each ligand induces different conformational changes in the receptor [36–38]. For example, aldosterone induces a stronger interaction between the N-terminal domain and ligand binding domain compared to cortisol [39]. Aldosterone, but not cortisol causes an exclusive interaction of MR with RNA helicase A [39]. Moreover, cortisol and dexamethasone stimulate different changes in sodium transport in cortical collecting duct cells [38] suggesting that dexamethasone has unique transactivation properties for GR.
Previously, we mapped a GR and MR binding to HRE2 in the promoter 5′ proximal regulatory region of the Edn1 gene [2]. Chromatin immunoprecipitation experiments showed aldosterone-dependent binding of both hormone receptors to the region of the Edn1 gene promoter containing HRE2 in mIMCD-3 cells. Moreover, HRE2 was a far higher affinity site than a nearby element called HRE1. HRE2 is unique in that it contains three plausible hormone receptor binding half-sites that differ in both sequence and spacing. The present study shows that the upstream HRE2A half-site is the most important for dexamethasone-dependent GR binding. HRE2A is also central in aldosterone-dependent activation of both GR and MR. Given that hormone receptors prefer to bind in a dimeric conformation, it is possible that MR and GR dimers are recruited to HRE2A and HRE2B. Alternatively, the region containing HRE2B and HRE2C half-sites appears to constitute a conventional GR or MR dimer response element. Therefore, it is also possible that binding of an unknown protein at HRE2A is required for the recruitment or stabilization of a hormone receptor dimer binding to the HRE2B/HRE2C site. Additional studies will be required to confirm the location of the hormone receptor dimer and to identify the hypothetical protein bound at HRE2A.
The present study shows that dexamethasone stimulates Edn1 mRNA transcription in two independent renal collecting duct cells lines. While collecting duct cells express 11βHSD-2 and are not typically activated by endogenous glucocorticoids, ET-1 and dexamethasone have documented interactions in other cells types. For example, glucocorticoids decrease ET-1 binding to vascular smooth muscle cells from wild-type and spontaneously hypertensive rats [40, 41]. Dexamethasone-induced Edn1 mRNA may also be clinically relevant in sodium transporting epithelia. Dexamethasone is commonly used in the clinic as an anti-inflammatory and immunosuppressant agent. However, many side effects are known to occur including alterations in sodium transport epithelia in the eye resulting in ocular hypertension and open-angle glaucoma. Dexamethasone is known to induce ET-1 in the trabecular meshwork and increased levels of ET-1 have been directly linked to the etiology of open angle glaucoma [42]. Dexamethasone can also stimulate ENaC mediated sodium transport in collecting duct cells [19] and may contribute to unwanted pharmacological actions such as hypertension and electrolyte disorders. Alternatively, dexamethasone activated Edn1 mRNA in collecting duct cells may mitigate ENaC-dependent sodium reabsorption since ET-1 is known to directly block ENaC activity [43, 44].
Supplementary Material
HIGHLIGHTS.
Dexamethasone positively regulates the endothelin-1 gene via GR in mIMCD-3 cells.
GR binding is dependent on a single high affinity half-site located within HRE2.
Dexamethasone recruits more GR to the Edn1 promoter compared to aldosterone.
Both MR and GR compete for the high affinity HRE2A half-site.
Acknowledgments
We greatly appreciate the technical support from Dr. Marc Bailly in preparing this manuscript. We also thank Drs. Elise and Celso Gomez-Sanchez for the gift of anti-MR antibody. This work was supported by American Heart Association Predoctoral and Postdoctoral Fellowships to LRS and MLG, respectively. Major support for this work was provided by Public Health Service RO1-DK82680 to CSW and BDC.
ABBREVIATIONS
- β 11 HSD-2
11β-hydroxysteroid dehydrogenase type II
- DAPA
DNA affinity purification assay
- ET-1
Endothelin-1 peptide
- GR
Glucocorticoid receptor
- HRE
Hormone response element
- MR
Mineralocorticoid receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stow LR, Jacobs ME, Wingo CS, Cain BD. Endothelin-1 gene regulation. FASEB J. 2011;25:16–28. doi: 10.1096/fj.10-161612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stow LR, Gumz ML, Lynch IJ, Greenlee MM, Rudin A, Cain BD, et al. Aldosterone modulates steroid receptor binding to the endothelin-1 gene (edn1) J Biol Chem. 2009;284:30087–96. doi: 10.1074/jbc.M109.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong S, Brennan FE, Young MJ, Fuller PJ, Cole TJ. A direct effect of aldosterone on endothelin-1 gene expression in vivo. Endocrinology. 2007;148:1511–7. doi: 10.1210/en.2006-0965. [DOI] [PubMed] [Google Scholar]
- 4.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–75. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 5.Funder JW. Mineralocorticoid receptors: distribution and activation. Heart failure reviews. 2005;10:15–22. doi: 10.1007/s10741-005-2344-2. [DOI] [PubMed] [Google Scholar]
- 6.Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombès M. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal. 2007;5:e012. doi: 10.1621/nrs.05012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller PJ. Aldosterone and DNA: the 50th anniversary. Trends Endocrinol Metab. 2004;15:143–6. doi: 10.1016/j.tem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Odermatt A, Atanasov AG. Mineralocorticoid receptors: Emerging complexity and functional diversity. Steroids. 2009;74:163–71. doi: 10.1016/j.steroids.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 9.van der Laan S, Meijer OC. Pharmacology of glucocorticoids: beyond receptors. Eur J Pharmacol. 2008;585:483–91. doi: 10.1016/j.ejphar.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 10.Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- 11.van der Laan S, Sarabdjitsingh RA, Van Batenburg MF, Lachize SB, Li H, Dijkmans TF, et al. Chromatin immunoprecipitation scanning identifies glucocorticoid receptor binding regions in the proximal promoter of a ubiquitously expressed glucocorticoid target gene in brain. J Neurochem. 2008;106:2515–23. doi: 10.1111/j.1471-4159.2008.05575.x. [DOI] [PubMed] [Google Scholar]
- 12.Todd-Turla KM, Schnermann J, Fejes-Tóth G, Naray-Fejes-Tóth A, Smart A, Killen PD, et al. Distribution of mineralocorticoid and glucocorticoid receptor mRNA along the nephron. Am J Physiol. 1993;264:F781–91. doi: 10.1152/ajprenal.1993.264.5.F781. [DOI] [PubMed] [Google Scholar]
- 13.Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–5. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 14.Grossmann C, Scholz T, Rochel M, Bumke-Vogt C, Oelkers W, Pfeiffer AF, et al. Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: a comparison of their glucocorticoid and mineralocorticoid properties. Eur J Endocrinol. 2004;151:397–406. doi: 10.1530/eje.0.1510397. [DOI] [PubMed] [Google Scholar]
- 15.Lombes M, Kenouch S, Souque A, Farman N, Rafestin-Oblin ME. The mineralocorticoid receptor discriminates aldosterone from glucocorticoids independently of the 11 beta-hydroxysteroid dehydrogenase. Endocrinology. 1994;135:834–40. doi: 10.1210/endo.135.3.8070376. [DOI] [PubMed] [Google Scholar]
- 16.Frey FJ, Odermatt A, Frey BM. Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens. 2004;13:451–8. doi: 10.1097/01.mnh.0000133976.32559.b0. [DOI] [PubMed] [Google Scholar]
- 17.Hammer F, Stewart PM. Cortisol metabolism in hypertension. Best Pract Res Clin Endocrinol Metab. 2006;20:337–53. doi: 10.1016/j.beem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Hellal-Levy C, Couette B, Fagart J, Souque A, Gomez-Sanchez CE, Rafestin-Oblin M. Specific hydroxylations determine selective corticosteroid recognition by human glucocorticoid and mineralocorticoid receptors. FEBS Lett. 1999;464:9–13. doi: 10.1016/s0014-5793(99)01667-1. [DOI] [PubMed] [Google Scholar]
- 19.Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, et al. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol. 2005;16:878–91. doi: 10.1681/ASN.2004121110. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen Dinh Cat A, Ouvrard-Pascaud A, Tronche F, Clemessy M, Gonzalez-Nunez D, Farman N, et al. Conditional transgenic mice for studying the role of the glucocorticoid receptor in the renal collecting duct. Endocrinology. 2009;150:2202–10. doi: 10.1210/en.2008-1531. [DOI] [PubMed] [Google Scholar]
- 21.Kolla V, Robertson NM, Litwack G. Identification of a mineralocorticoid/glucocorticoid response element in the human Na/K ATPase alpha1 gene promoter. Biochem Biophys Res Commun. 1999;266:5–14. doi: 10.1006/bbrc.1999.1765. [DOI] [PubMed] [Google Scholar]
- 22.Mick VE, Itani OA, Loftus RW, Husted RF, Schmidt TJ, Thomas CP. The alpha-subunit of the epithelial sodium channel is an aldosterone-induced transcript in mammalian collecting ducts, and this transcriptional response is mediated via distinct cis-elements in the 5′-flanking region of the gene. Mol Endocrinol. 2001;15:575–88. doi: 10.1210/mend.15.4.0620. [DOI] [PubMed] [Google Scholar]
- 23.Itani OA, Liu KZ, Cornish KL, Campbell JR, Thomas CP. Glucocorticoids stimulate human sgk1 gene expression by activation of a GRE in its 5′-flanking region. Am J Physiol Endocrinol Metab. 2002;283:E971–9. doi: 10.1152/ajpendo.00021.2002. [DOI] [PubMed] [Google Scholar]
- 24.Ou XM, Storring JM, Kushwaha N, Albert PR. Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J Biol Chem. 2001;276:14299–307. doi: 10.1074/jbc.M005363200. [DOI] [PubMed] [Google Scholar]
- 25.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, et al. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119:2423–34. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Husted RF, Laplace JR, Stokes JB. Enhancement of electrogenic Na+ transport across rat inner medullary collecting duct by glucocorticoid and by mineralocorticoid hormones. J Clin Invest. 1990;86:498–506. doi: 10.1172/JCI114736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebuffat AG, Tam S, Nawrocki AR, Baker ME, Frey BM, Frey FJ, et al. The 11-ketosteroid 11-ketodexamethasone is a glucocorticoid receptor agonist. Mol Cell Endocrinol. 2004;214:27–37. doi: 10.1016/j.mce.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, et al. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol. 1999;10:923–34. doi: 10.1681/ASN.V105923. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, et al. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology. 2006;147:1343–8. doi: 10.1210/en.2005-0860. [DOI] [PubMed] [Google Scholar]
- 31.Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol. 2003;285:F664–73. doi: 10.1152/ajprenal.00353.2002. [DOI] [PubMed] [Google Scholar]
- 32.Couette B, Marsaud V, Baulieu EE, Richard-Foy H, Rafestin-Oblin ME. Spironolactone, an aldosterone antagonist, acts as an antiglucocorticosteroid on the mouse mammary tumor virus promoter. Endocrinology. 1992;130:430–6. doi: 10.1210/endo.130.1.1309341. [DOI] [PubMed] [Google Scholar]
- 33.Pascual-Le Tallec L, Simone F, Viengchareun S, Meduri G, Thirman MJ, Lombès M. The elongation factor ELL (eleven-nineteen lysine-rich leukemia) is a selective coregulator for steroid receptor functions. Mol Endocrinol. 2005;19:1158–69. doi: 10.1210/me.2004-0331. [DOI] [PubMed] [Google Scholar]
- 34.Nishi M, Tanaka M, Matsuda K, Sunaguchi M, Kawata M. Visualization of glucocorticoid receptor and mineralocorticoid receptor interactions in living cells with GFP-based fluorescence resonance energy transfer. J Neurosci. 2004;24:4918–27. doi: 10.1523/JNEUROSCI.5495-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishi M, Kawata M. Dynamics of glucocorticoid receptor and mineralocorticoid receptor: implications from live cell imaging studies. Neuroendocrinology. 2007;85:186–92. doi: 10.1159/000101917. [DOI] [PubMed] [Google Scholar]
- 36.Trapp T, Holsboer F. Heterodimerization between mineralocorticoid and glucocorticoid receptors increases the functional diversity of corticosteroid action. Trends Pharmacol Sci. 1996;17:145–9. doi: 10.1016/0165-6147(96)81590-2. [DOI] [PubMed] [Google Scholar]
- 37.Hultman ML, Krasnoperova NV, Li S, Du S, Xia C, Dietz JD, et al. The ligand-dependent interaction of mineralocorticoid receptor with coactivator and corepressor peptides suggests multiple activation mechanisms. Mol Endocrinol. 2005;19:1460–73. doi: 10.1210/me.2004-0537. [DOI] [PubMed] [Google Scholar]
- 38.Kitagawa H, Yanagisawa J, Fuse H, Ogawa S, Yogiashi Y, Okuno A, et al. Ligand-selective potentiation of rat mineralocorticoid receptor activation function 1 by a CBP-containing histone acetyltransferase complex. Mol Cell Biol. 2002;22:3698–706. doi: 10.1128/MCB.22.11.3698-3706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Rogerson FM, Fuller PJ. Interdomain interactions in the mineralocorticoid receptor. Mol Cell Endocrinol. 2003;200:45–55. doi: 10.1016/s0303-7207(02)00413-6. [DOI] [PubMed] [Google Scholar]
- 40.Nambi P, Pullen M, Wu HL, Nuthulaganti P, Elshourbagy N, Kumar C. Dexamethasone down-regulates the expression of endothelin receptors in vascular smooth muscle cells. J Biol Chem. 1992;267:19555–9. [PubMed] [Google Scholar]
- 41.Provencher PH, Saltis J, Funder JW. Glucocorticoids but not mineralocorticoids modulate endothelin-1 and angiotensin II binding in SHR vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1995;52:219–25. doi: 10.1016/0960-0760(94)00168-l. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Clark AF, Yorio T. Interactions of endothelin-1 with dexamethasone in primary cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003;44:5301–8. doi: 10.1167/iovs.03-0463. [DOI] [PubMed] [Google Scholar]
- 43.Gilmore ES, Stutts MJ, Milgram SL. Src Family Kinases Mediate Epithelial Na+ Channel Inhibition by Endothelin. The Journal of biological chemistry. 2001;276:42610–7. doi: 10.1074/jbc.M106919200. [DOI] [PubMed] [Google Scholar]
- 44.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol. 2008;295:F1063–70. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.