Abstract
Objective
The aim of this multi-institutional non randomized phase II trial was to determine the efficacy and safety of single agent aflibercept (VEGF Trap), a recombinant fusion protein that blocks multiple vascular endothelial growth factor isoforms, in women with gynecologic soft tissue sarcoma.
Methods
Patients were enrolled in two cohorts each with Simon two stage designs: uterine leiomyosarcoma and carcinosarcoma of endometrial, ovarian or fallopian tube origin. Eligibility criteria included ≤ 2 prior lines of chemotherapy for metastatic disease and ECOG performance status ≤ 2. Aflibercept 4 mg/kg was administered intravenously on day 1 of a 14 day cycle. Primary endpoints were objective response and disease stabilization (Progression Free Survival (PFS) at 6 months).
Results
41 patients with uterine leiomyosarcoma and 22 patients with carcinosarcoma (19 uterine, 3 ovarian) were enrolled on study. In the leiomyosarcoma cohort, eleven (27%) patients had stable disease (SD), 4 with SD lasting at least 24 weeks. The 6 month PFS was 17%, with median time to progression (TTP) of 1.8 (95% CI:1.6–2.1) months. In the carcinosarcoma cohort, two (9%) patients had SD, one lasting > 24 weeks, median TTP was 1.6 months (95%CI: 1.1–1.7) No partial responses were observed in patients from either cohort. Grade 3 or more aflibercept related toxicity was uncommon and included hypertension, fatigue, headache and abdominal pain.
Conclusions
Single agent aflibercept has modest activity in patients with uterine leiomyosarcoma and minimal activity in women with carcinosarcoma.
Introduction
Endometrial sarcomas constitute less than 10% of all uterine malignancies [1, 2]. Carcinosarcoma (CS) and leiomyosarcoma (LMS) comprise almost 90% of all endometrial sarcomas. Median survival of women with unresectable, recurrent or advanced disease is approximately 12 months in both cases and new therapeutic options are urgently required [1–3].
Carcinosarcomas are believed to be metaplastic carcinomas rather than true sarcomas [4] and hence warrant separate clinical trials to evaluate potential efficacy of new agents. Trials of agents which have previously shown benefit in other gynecologic carcinomas to treat women with CS would seem reasonable. Conventional chemotherapy has modest activity in CS but response duration is short. In a randomized phase III trial, the combination of cisplatin and ifosfamide was associated with a higher response rate (54% v 36%) and an improved median progression-free survival (PFS) compared to ifosfamide when used alone as first line therapy. This did not, however, translate into an improvement in overall survival (OS) and toxicity was significant [5]. A second study reported an improvement in OS (from 8.4 to 13.5 months) for Ifosfamide-paclitaxel-filgrastim over ifosfamide alone [6]. More recently, a phase II trial of carboplatin plus paclitaxel reported a response rate of 54% and demonstrated a similar PFS, 7.6 months, and OS, 14.7 months to the earlier, more toxic, ifosfamide combination studies [7].
Uterine LMS has a similarly poor prognosis and palliative chemotherapy with single agents such as ifosfamide, etoposide and doxorubicin produce response rates ranging from 11–25% [8,9,10]. More recently trabectedin demonstrated response rates of 16–18% with PFS at 6 months of 30–33% in retrospective [11] and pooled analyses in heavily pre treated patients [12]. The combination of docetaxel with gemcitabine produced a response rate of 35.8% as first line treatment (median PFS 4.4 months and OS 16.1 months) [13] and 27% when administered to women who had received prior chemotherapy [14]. Given the relatively poor outcomes for these two diseases, new therapeutic approaches informed by tumor biology are clearly needed.
One potential biologic target for therapy of these cancers is the vascular endothelial growth factor (VEGF) family of proteins. Increased expression of VEGF family members is associated with disease progression and poor prognosis in many gynecologic malignancies [15,16] and VEGF pathway targeting agents have demonstrated efficacy in many tumor types. Similarly, Increased levels of VEGF in uterine LMS and other soft tissue sarcomas are associated with increased risk of progressive disease [18, 19, 20]. CS are known to over express the angiogenic protein platelet-derived growth factor (PDGF) [17] suggesting the importance of angiogenesis in this malignancy.
Aflibercept, VEGF Trap, is a recombinant fusion protein combining the Fc portion of human IgG1 with the principal extracellular ligand-binging domains of human VEGF receptors 1 and 2. It is a potent inhibitor of angiogenesis acting as a high affinity soluble decoy VEGF receptor. Compared to other VEGF inhibitors it has a high VEGF-A binding affinity (approximately 1000-fold greater than bevacizumab), the ability to bind VEGF-B, as well as placental growth factors 1 and 2 (which have independent pro angiogenic effects), and a longer circulating half-life compared with other soluble receptor constructs. Aflibercept inhibits the growth of multiple tumor types, including a rhabdomyosarcoma, in mouse xenograft models [21]. In the phase I study of single agent aflibercept a minor response was seen in a heavily pre-treated patient with metastatic uterine LMS [22].
We conducted a multi-center phase II study in two separate cohorts of women: one with advanced recurrent carcinosarcoma and a second with uterine leiomyosarcoma, to determine the efficacy (objective response rate and rate of progression-free survival at 6 months) and toxicity of aflibercept in these two patient groups
Patients and Methods
This was a single agent, multi-centre, 2 stage, phase II trial to investigate the efficacy of aflibercept in 2 cohorts of women with metastatic or recurrent gynecologic soft tissue sarcomas: women with uterine LMS and women with CS of endometrial, ovarian or fallopian tube origin, conducted by the Princess Margaret Hospital, Chicago and California Cancer Phase II Consortia. The study was conducted according to Good Clinical Practice guidelines, with full research ethics board approval at each of the participating institutions. All patients provided written informed consent before study entry. The two cohorts were enrolled simultaneously and independently.
Eligibility
Patients with histologically or cytologically confirmed metastatic/unresectable or locally advanced uterine LMS or CS, who had received up to 2 prior lines of chemotherapy for recurrent or metastatic disease were eligible for this trial. Eligibility criteria included: age ≥ 18 years, life expectancy ≥3 months weeks; Eastern Cooperative Group (ECOG) performance status≤ 2; adequate hematologic, hepatic and renal function with at least one measurable lesion; previous radiotherapy was allowed provided it was completed>4 weeks before study entry; full dose anticoagulation was permitted provided there was no active bleeding and the patient was on a stable dose of low molecular weight heparin or the INR was less than 3 (with a target INR of less than 3) for patients on oral anti-coagulation. Exclusion criteria included: prior treatment with anti-angiogenic agents; history of bowel obstruction, gastrointestinal perforation or fistula within the previous 28 days; bleeding diathesis; clinically significant cardiovascular disease, myocardial infarction or New York Heart Association Class III or greater, congestive heart failure within the preceding 6 months; pulmonary embolism, deep venous thrombosis or cerebrovascular accident within 6 months prior to study drug administration; uncontrolled hypertension; diagnosis of any second malignancy within the last 5 years (except for treated non-melanoma skin cancer); brain metastases; invasive procedures or major surgery within 28 days of study drug administration, core biopsy within 7 days or anticipation of major surgery during the course of the study; History of hypersensitivity to Chinese Hamster ovary cell products or other recombinant human antibodies and significant proteinuria (≥500mg/24 hours) were also exclusion criteria.
Study design and treatment
Patients received Aflibercept 4 mg/kg administered intravenously over 1 hour on day 1 of each 14 day cycle. No pre-medication was required. Patients were monitored for toxicity and the dose of aflibercept could be reduced one dose level to 3mg/kg with one further dose reduction to 2 mg/kg being permitted. Treatment was discontinued for disease progression, patient choice or toxicity.
Evaluation of toxicity and tumor response
Baseline evaluations included medical history, physical examination, laboratory tests (hematology, urinalysis, coagulation, blood chemistry), ECG (if indicated), and pregnancy test and were performed within 7 days of administration of protocol therapy. Physical exam, lab tests (hematology, biochemistry and urinalysis), evaluation of toxicity according to NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 3 were performed on day 1 of each cycle. Blood pressure and pulse were measured every cycle with measurements made prior to commencing aflibercept, at 30 minutes into the infusion and 30 minutes post infusion during cycle 1 and 2. Radiologic assessment of measureable disease was performed by computed tomography (CT) or magnetic resonance imaging (MRI) within 28 days prior to registration and every 4 cycles (8 weeks). Response was defined using Response Evaluation Criteria in Solid Tumors (RECIST) [23].
Statistical Methods and Endpoints
The primary endpoints of this study were the objective response rate as determined by RECIST criteria [23] and the incidence of progression-free survival (PFS) at 6 months [24]. Secondary endpoints included evaluation of toxicity, time-to-progression (TTP) and overall survival (OS). Separate analyses were performed for patients in both cohorts uterine LMS and CS. The same statistical two stage design was applied to both cohorts. In the first stage, 21 patients would be entered. If no more than 1 objective response (≤ 5%) and/or ≤ 2 (≤ 10%) patients were alive and progression-free at 6 months the drug would be rejected at the end of the first stage of accrual. If at least one response or > 2 patients were alive and progression free an additional 20 patients would be accrued. If ≥ 5 responses (> 12%) was seen or ≥ 8 patients were alive and progression-free at 6 months amongst 41 evaluable patients the treatment would be considered sufficiently active to warrant further testing. This design yields ≥ 90% power to detect a true objective response rate of ≥ 20%, and yields at least 94% power to detect a true 6-month PFS rate ≥ 30% (median PFS of 3.5 months). It yields ≥ .91probability of a negative result if the true objective response rate is ≤ 5% and the true 6-month PFS rate is ≤ 10% (median PFS of 1.8 months), with approximately 0.47 probability, at least, of early negative stopping in this case. These last two probabilities are calculated assuming that tumor response rate and PFS rate are uncorrelated. If they are positively correlated, as is likely, the probabilities will be slightly higher. Survival statistics were estimated using the Kaplan-Meier method.
Results
Between January 2006 and January 2010, 63 patients, 41 with uterine leiomyosarcoma and 22 with carcinosarcoma were accrued to this phase II study at 15 participating institutions within the Princess Margaret Hospital, University of Chicago and California Cancer Phase II consortia. At the time of this analysis all patients were off study. Patients discontinued the study in the LMS cohort for documented progression (30/41), adverse events (6/41), patient choice (2/41) 4 death, two potentially related to study drug (4/41). In the CS cohort 15 patients discontinued the study for PD, 4 patients for adverse events, 2 for patient choice and there were 2 deaths (both progressive disease). Sixty-two patients were evaluable for toxicity (one patient withdrew consent prior to receiving study drug) and 55 were evaluable for response. Clinical characteristics for both cohorts are shown in Table 1.
Table 1.
Baseline Patient Characteristics
| Characteristic | Uterine Leiomyosarcoma N=41 |
Carcinosarcoma N=22 |
|---|---|---|
| Age (years) | ||
| Median | 58 | 63 |
| Range | 38–78 | 38–78 |
| Primary site | ||
| Uterus | 41 (100) | 19 (86) |
| Ovary | 3 (14) | |
| No of disease sites | ||
| 1 | 8 (20) | 5 (23) |
| 2 | 5 (12) | 2 (9) |
| 3+ | 28 (68) | 15 (68) |
| Disease site | ||
| Nodes | 9 (22) | 8 (36) |
| Soft tissue | 9 (22) | 9 (41) |
| Lung | 25 (61) | 7 (32) |
| Pelvis | 17 (41) | 10 (45) |
| Liver | 9 (22) | 8 (36) |
| ECOG PS | ||
| 0 | 24 (59) | 6 (27) |
| 1 | 16 (39) | 13 (59) |
| 2 | 1 (2) | 3 (14) |
| No of prior chemotherapy regimens | ||
| 0 | 16 (39) | 2 (9) |
| 1 | 18 (44) | 14 (64) |
| 2 | 6 (15) | 5 (23) |
| 3 | 1 (2) | 1 (5) |
| Prior Therapy | ||
| Adj Chemotherapy | 7 (17) | 10 (45) |
| Systemic Chemotherapy for | 20 (49) | 9 (41) |
| Radiotherapy | 14 (34) | 8 (36) |
| Endocrine | 2 (5) | 0 |
Treatment administration
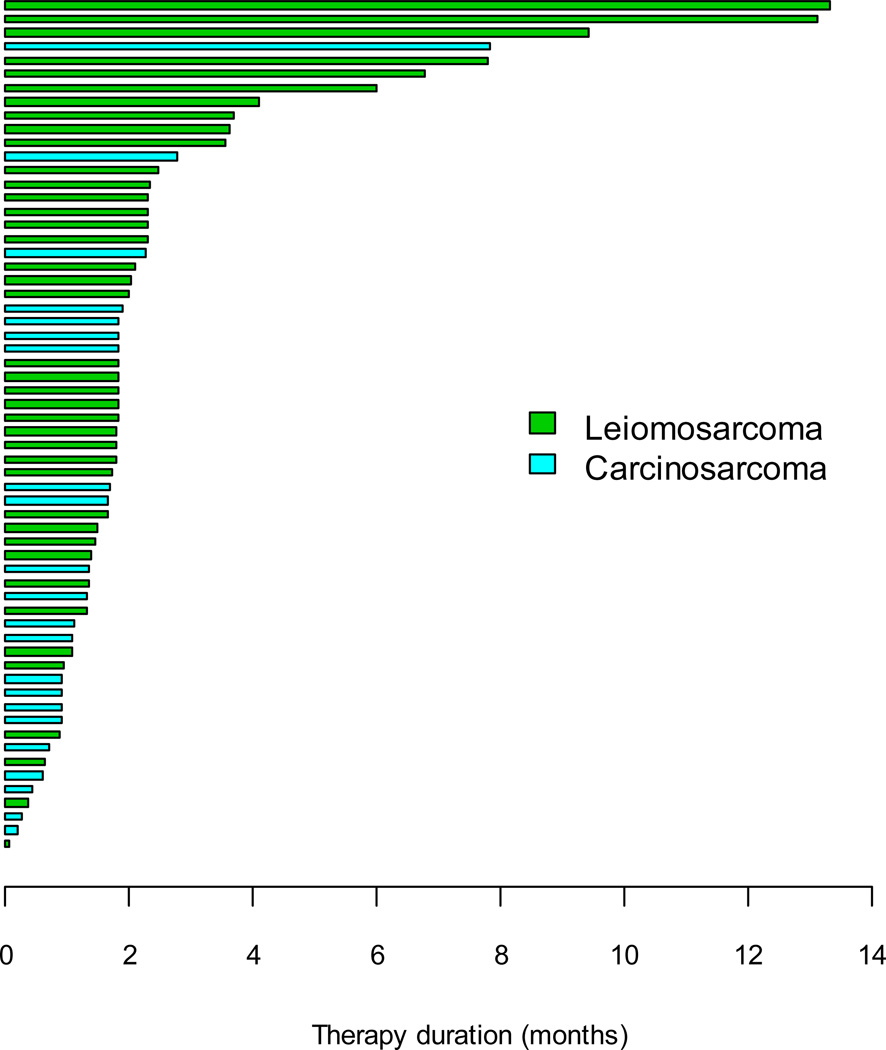
Sixty -two patients received a total of 311 treatment cycles. The median number of cycles was 4 for the LMS cohort (range 1 to 28 cycles) and 2 (range 1 to 15) cycles for the CS cohort. Five patients required one dose reduction and an additional 2 required two dose reductions secondary to toxicity. 85% of patients achieved over 90% of planned dose intensity. Figure 1 shows a waterfall plot of the duration of therapy for both groups of patients.
Figure 1.
Waterfall plot of duration of therapy (CS and LMS)
Toxicity
Potentially aflibercept related adverse events encountered are presented in Table 2. Fatigue (50%), hypertension (42%), headache (35%) and voice alteration (31%) were the most common non-hematological adverse events. Grade 3 or higher adverse events, potentially related to aflibercept, include hypertension (24%), fatigue (6%) and headache (5%). Hematological drug related events were mostly grade 1 or 2 although grade 3 anemia and lymphopenia were reported. 37% of patients experienced proteinuria, grade 3 in four (6%) patients.
Table 2.
Most frequent adverse events, NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 3, related to Aflibercept (62 patients, 311 cycles of treatment)
| Adverse Event | Grade 1/2 | ≥Grade 3 | Patients Total No. (%) |
|---|---|---|---|
| Non-hematologic | |||
| Fatigue | 27 (44) | 4 (6) | 31 (50) |
| Hypertension | 11 (18) | 15 (24) | 26 (42) |
| Voice alteration | 18 (29) | 1 (2) | 19 (31) |
| Headache | 19 (31) | 3 (5) | 22 (35) |
| Nausea | 12 (19) | 2 (3) | 14 (23) |
| Joint pain | 13 (21) | - | 13 (21) |
| Constipation | 8 (13) | 1 (2) | 9 (15) |
| Abdominalpain | 7 (11) | 2 (3) | 9 (15) |
| Vomiting | 8 (13) | - | 8 (13) |
| Mucositis | 7 (11) | 1 (2) | 8 (13) |
| Anorexia | 6 (10) | 1 (2) | 7 (11) |
| Hematology | |||
| Hemoglobin | 5 (8) | 1 (2) | 6 (10) |
| Leukocyte | 16 (26) | 16 (26) | |
| Lymphocytes | 6 (10) | 1 (2) | 7 (11) |
| Biochemistry | |||
| Proteinuria | 19 (31) | 4 (6) | 23 (37) |
| Elevation ALP | 6 (10) | 6 (10) | |
| Creatinine | 5 (8) | 5 (8) | |
One patient on study died of infection and a second died of cerebral hemorrhage. Both adverse events were felt to be potentially drug related.
Response and survival
Uterine leiomyosarcoma (LMS)
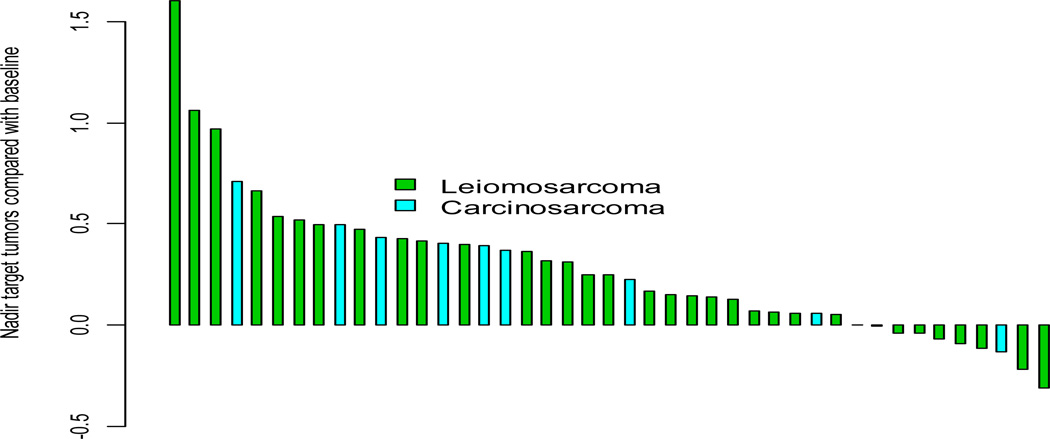
Thirty-seven patients with uterine LMS were evaluable for response. Four patients (10.8%) had stable disease (SD) for > 24 weeks, a further 11 (29.7%) had SD and 25 (60%) patients had progressive disease (PD) as their best response. No objective response was seen, Figure 2. The study proceeded to second stage accrual after 3 patients experienced SD > 24 weeks in the first stage of accrual.
Figure 2.
Waterfall plot for target lesions (CS and LMS)
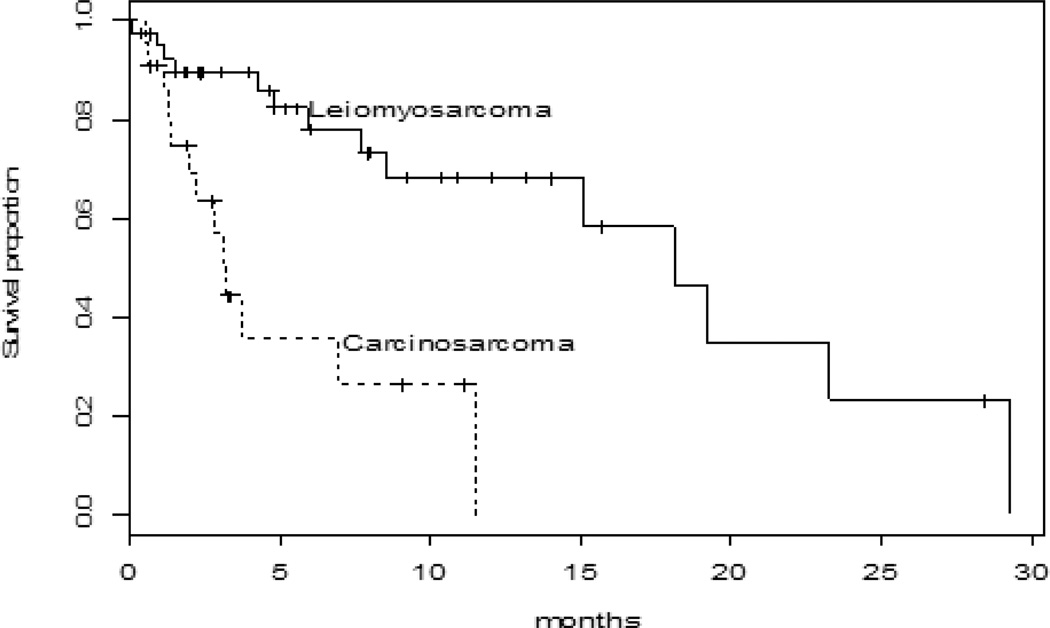
The 6 month progression-free rate was 17% (range 6–32%). Median TTP was 1.8 months (95% CI:1.6–2.1 months). At a median follow up of 5.3 months14 patients have died, median OS was 18.1 months (95% CI: 8.5-not reached) with 6 and 12 month OS rates of 78% (95% CI: 58–89%) and 68% (95% CI: 46–83%) respectively, Figure 3.
Figure 3.
Kaplan Meier progression free survival curve
Carcinosarcoma (CS)
Eighteen patients with CS were evaluable for response. One patient (5.6%), endometrial CS, had SD > 24 weeks, 1 (5.6%) further patient had SD and 16 (88.9%) had PD as their best response. No objective response was seen and thus this arm of the trial was closed without an expansion cohort (Figure 2). Median TTP was 1.6 months (95% CI: 1.1–1.7 months). At a median follow up of 5.3 months, 13 patients have died. Median OS was 3.2 months (95% CI: 1.4-not reached) with 6 month OS rate of 36% (95% CI; 13–59%) Figure 3.
Discussion
The prognosis for women with advanced or metastatic CS and uterine LMS is poor and targeting angiogenesis an attractive therapeutic option. Given that the biology of these two diseases differs this study was designed to evaluate aflibercept in two distinct cohorts. No objective response was seen in either cohort. Since PFS at 3 and 6 months has been proposed as an end-point in soft tissue sarcoma phase II studies, together with the perception that biological agents may be more likely to induce SD rather than a RECIST criteria objective response, this trial had a dual endpoint of PFS rate at 6 months [24]. In the cohort of women with uterine LMS recruitment proceeded to the second stage after three SD >24 weeks were observed. One further prolonged SD was observed in the second stage, the study was closed following a futility analysis after 37 response evaluable patients were accrued. The PFS rate at 6 months was 17%, indicating a modest level of activity in a predominantly pre-treated population of patients. There were a number of patients with uterine LMS who received aflibercept for 8 months or more (figure 1) it is not clear whether survival and duration of therapy was due to alfibercept or whether this reflects individual tumor biology. There are a subset of uterine LMS which exhibit a more indolent growth and are more likely to be hormone sensitive [25]. The hormonal status of uterine LMS should be considered when designing future studies. Among women with CS two patients had SD and sixteen PD as their best response. Aflibercept demonstrated minimal activity and following a futility analysis, which determined it was unlikely to proceed to second stage accrual, the study was closed after 18 response evaluable patients had been enrolled. In a Gynecologic Oncology Group (GOG) phase II study of the receptor tyrosine kinase (RTK) inhibitor sunitinib in women with recurrent or persistent uterine LMS 2 patients had a PR (8.7%) with 4 (17.4%) remaining progression free at 6 months [26]. In this, sunitinib, study all of the patients had received at least one prior line of chemotherapy for advanced disease compared to 61% of patients in the current trial. In a further study, thalidomide was associated with a 7% PFS at 6 months [20]. This compares to 30.7% and 33% PFS at 6 months with trabectedin in a pooled analysis and retrospective series respectively [11,12]. Response rates of 20% have been seen with single agent gemcitabine [27]. An objective response rate of 27% and 52% PFS at 6 months was seen for the combination of gemcitabine and docetaxel in the treatment of patients who had received one prior line of chemotherapy [14]. Single agent pazopanib, which targets VEGF, PDGF receptors and c-kit, among others, demonstrated promising activity as a single agent in a cohort of pre treated patients with soft tissue LMS. In this study the PFS at 3 months was 44% for the LMS cohort, meeting predetermined efficacy criteria for further investigation [28]. To date, although reasonably well tolerated, the efficacy displayed by single agent anti-angiogenic therapies in uterine LMS does not warrant their adoption in the clinic. It remains to be seen whether combination of these agents with chemotherapy (or used as maintenance therapy) would lead to an improved outcome over chemotherapy alone.
In a phase II study of the RTK inhibitor sorafenib in women with uterine CS the 6 month PFS was 13% [29], 62.5% of patients had received prior chemotherapy. The lack of efficacy to anti-angiogenic agents in CS contrasts with that observed in patients with epithelial ovarian cancer where bevacizumab demonstrated a single agent response rate of 16% and a 53% SD rate [30]. In a phase II study of imatinib mesylate (Gleevec) in women with CS only one patient (4%) had SD, the remaining 17 evaluable patients progressing [31]. This compares to an 8.3% response rate and a median PFS of 1.8 months for second line treatment with gemcitabine/ docetaxel and response rates of 5–35% to single agent cytotoxic agents in pre-treated CS [32, 33]. Better understanding of the biology and new approaches to treating this aggressive disease continue to be urgently required. Aflibercept was reasonably well tolerated in these patient populations. However fatigue was seen in 50% of patients (mostly grade ≤ 2), hypertension was observed in 42% of patients (24% experiencing grade 3 or higher). Two patients died on study from potentially drug related adverse events, infection and cerebral haemorhage. No bowel perforations were observed. The side effect profile was similar to that observed in the phase II study in patients with pre –treated adenocarcinoma of the lung [34]. In the phase II trial of afliberceptin patients with metastatic urothelial cancer drug related adverse events followed a similar pattern but were less common than in the other studies in other patient populations [35].
Aflibercept demonstrated minimal activity in women with carcinosarcoma and only modest activity in those with uterine leiomyosarcoma, toxicity was not insignificant. Further investigation of single agent aflibercept, in these two groups of women are not warranted. Future combination studies including anti angiogenic agents may be of interest, however better understanding of the biology of these cancers is essential to inform future trial design.
Highlights.
In this study we investigate the efficacy of the anti-angiogenic aflibercept in 2 groups of women with uterine sarcoma.
Single agent aflibercept has minimal activity in women with recurrent or metastatic carcinosarcoma
Single agent aflibercept has modest activity (stable disease rate 27%) in women with metastatic or recurrent uterine leiomyosarcoma
Acknowledgments
Support: The Cancer Therapeutics Evaluation Program, US National Cancer Institute N01-CM-62203, NO1-CCM-07003-74 and NO1-CM-62209
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
None of the authors of this manuscript have any conflict of interest to declare.
References
- 1.Kanjeekal S, Chambers A, Fung Kee Fung M, et al. Systemic therapy for advanced uterine sarcoma: A systematic review of the literature. Gynecologic Oncology. 2005;97:624–637. doi: 10.1016/j.ygyno.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 2.Clark MA, Fisher C, Judson I, et al. Soft-Tissue Sarcomas in Adults. N Eng J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 3.Gadducci A, Sartori E, Landoni F, et al. The prognostic relevance of histological type in uterine sarcomas: a Cooperation Task Force (CTF) multivariate analysis of 249 cases. Eur J Gynaecol Oncol. 2002;23:295–299. [PubMed] [Google Scholar]
- 4.McCluggage WG. Uterine carcinosarcomas (malignant mixed Mullarian tumors) are metaplastic carcinomas. Int J Gynecol. Cancer. 2002;12:687–690. doi: 10.1136/ijgc-00009577-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Sutton G, Brunetto VL, Kilgore L, et al. A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2000;79:147–153. doi: 10.1006/gyno.2000.6001. [DOI] [PubMed] [Google Scholar]
- 6.Homesley H, Filiaci V, Markman M, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: A Gynecologic Oncology Group study. J Clin. Oncol. 2007;25:526–531. doi: 10.1200/JCO.2006.06.4907. [DOI] [PubMed] [Google Scholar]
- 7.Powell MA, Filiaci VL, Rose PG. A phase II evaluation of paclitaxel and carboplatin in the treatment of carcinosarcoma of the uterus: a Gynecologic Oncology Group study. J Clin. Oncol. 2010;28:2727–2731. doi: 10.1200/JCO.2009.26.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton GP, Blessing JA, Barrett RJ, et al. Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol. 1992;166:556–559. doi: 10.1016/0002-9378(92)91671-v. [DOI] [PubMed] [Google Scholar]
- 9.Slayton RE, Blessing JA, Angel C, et al. Phase II trial of etoposide in the management of advanced and recurrent leiomyosarcoma of the uterus: a Gynecologic Oncology Group Study. Cancer Treat Rep. 1987;71:1303–1304. [PubMed] [Google Scholar]
- 10.Sutton G, Blessing J, Hanjani P, et al. Phase II evaluation of liposomal doxorubicin (Doxil) in recurrent or advanced leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecologic Oncology. 2005;96:749–752. doi: 10.1016/j.ygyno.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Sanfilippo R, Grosso F, Jones RL, et al. Trabectedin in advanced uterine leiomyosarcomas: A retrospective case series analysis from two reference centers. Gynecol Oncol. 2011 doi: 10.1016/j.ygyno.2011.08.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Judson I, Blay J, Chawla S, et al. Trabectedin in the treatment of advanced uterine leiomyosarcomas: results of a pooled analysis of five single-agent phase II studies using the recommended dose. J Clin Oncol. 2010;28 p. 15s suppl; abstr 10028. [Google Scholar]
- 13.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecologic Oncology. 2008;109:329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensley ML, Blessing JA, DeGeest K, Abulafia O. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecologic Oncology. 2008;109:323–328. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsuhashi A, Suzuka K, Yamazawa K, et al. Serum vascular endothelial growth factor (VEGF) and VEGF-C levels as tumor markers in patients with cervical carcinoma. Cancer. 2005;103:724–730. doi: 10.1002/cncr.20819. [DOI] [PubMed] [Google Scholar]
- 16.Kamat AA, Merritt WM, Coffey D, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res. 2007;13:7487–7495. doi: 10.1158/1078-0432.CCR-07-1017. [DOI] [PubMed] [Google Scholar]
- 17.Ramondetta LM, Burke TW, Jhingran A, et al. A phase II trial of cisplatin, ifosfamide, and mesna in patients with advanced or recurrent uterine malignant mixed mullerian tumors with evaluation of potential molecular targets. Gynecologic Oncology. 2003;90:529–536. doi: 10.1016/s0090-8258(03)00332-9. [DOI] [PubMed] [Google Scholar]
- 18.Arita S, Kikkawa F, Kajiyama H, et al. Prognostic importance of vascular endothelial growth factor and its receptors in the uterine sarcoma. Int. J. Gynecol Cancer. 2005;15:329–336. doi: 10.1111/j.1525-1438.2005.15225.x. [DOI] [PubMed] [Google Scholar]
- 19.Pakos EE, Goussia AC, Tsekeris PG, et al. Expression of vascular endothelial growth factor and its receptor, KDR/Flk-1, in soft tissue sarcomas. Anticancer Res. 2005;25:3591–3596. [PubMed] [Google Scholar]
- 20.McMeekin DS, Sill MW, Kathleen M, Darcy KM, Stearns-Kurosawa DJ. A phase II trial of thalidomide in patients with refractory leiomyosarcoma of the uterus and correlation with biomarkers of angiogenesis: A gynecologic oncology group study. Gynecologic Oncology. 2007;106:596–603. doi: 10.1016/j.ygyno.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad. Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupont J, Rothenberg ML, Spriggs DR, et al. Safety and pharmacokinetics of intravenous VEGF Trap in a phase I clinical trial of patients with advanced solid tumors. Proc Am Soc Clin Oncol. 2005;23:199s. [Google Scholar]
- 23.Therasse P, Arbuck S, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors; European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Van Glabbeke M, Verweij J, Judson I, et al. Progression-free rate as the principal end-point for phase II trials in soft tissue sarcoma. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 25.Koivisto-Korander R, Butzow R, Koivisto AM, Leminen A. Immunohistochemical studies on uterine carcinosarcoma, leiomyosarcoma, and endometrial stromal sarcoma: expression and prognostic importance of ten different markers. Tumour Biol. 2011;32(3):451–459. doi: 10.1007/s13277-010-0138-1. [DOI] [PubMed] [Google Scholar]
- 26.Hensley ML, Sill MW, Dennis R, Scribner DR, Jr, et al. Sunitinib malate in the treatment of recurrent or persistent uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecologic Oncology. 2009;115:460–465. doi: 10.1016/j.ygyno.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Look KY, Sandler A, Blessing JA, et al. Phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma: a Gynecologic Oncology Group (GOG) study. Gynecol Oncol. 2004;92:644–647. doi: 10.1016/j.ygyno.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor in patients with relapsed of refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 29.Nimeiri HS, Oza AM, Morgan RJ. A phase II study of sorafenib in advanced uterine carcinoma/carcinosarcoma: A trial of the Chicago, PMH, and California Phase II Consortia. Gynecologic Oncology. 2010;117:37–40. doi: 10.1016/j.ygyno.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burger RA, Sill MW, Monk BJ, et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 31.Huh WK, Sill MW, Darcy KM, et al. Efficacy and safety of imatinib mesylate (Gleevec®) and immunohistochemical expression of c-Kit and PDGFR-β in a Gynecologic Oncology Group Phase Il Trial in women with recurrent or persistent carcinosarcomas of the uterus. Gynecologic Oncology. 2010;117:248–254. doi: 10.1016/j.ygyno.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Miller BE, Blessing JA, Stehman FB, Shahin MS, et al. A phase II evaluation of weekly gemcitabine and docetaxel for second-line treatment of recurrent carcinosarcoma of the uterus: A gynecologic oncology group study. Gynecologic Oncology. 2010;118(2010):139–144. doi: 10.1016/j.ygyno.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Sutton GP, Blessing JA, Homesley HD, Malfetano JH. A phase II trial of ifosfamide and mesna in patients with advanced or recurrent mixed mesodermal tumors of the ovary previously treated with platinum-based chemotherapy: a Gynecologic Oncology Group study. Gynecol. Oncol. 1994;53:24–26. doi: 10.1006/gyno.1994.1081. [DOI] [PubMed] [Google Scholar]
- 34.Leighl NB, Luis E, Raez LE, Besse B, et al. A multicenter, phase 2 study of vascular endothelial growth factor Trap (aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. Journal of Thoracic Oncology. 2010;10:1054–1059. doi: 10.1097/jto.0b013e3181e2f7fb. [DOI] [PubMed] [Google Scholar]
- 33.Twardowski P, Stadler WM, Frankel P, et al. Phase II study of aflibercept (VEGF-Trap) in patients with recurrent or metastatic urothelial cancer, a California Cancer Consortium Trial. Urology. 2010;76:923–926. doi: 10.1016/j.urology.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]