Abstract
BACKGROUND
Lesions in the insula and basal ganglia can be risky to resect due to their depth and proximity to critical structures, particularly in the dominant hemisphere. Transsylvian approaches shorten the surgical distance to these lesions, preserve perisylvian temporal and frontal cortex, and minimize brain transgression.
OBJECTIVE
We report our experience with transsylvian-transinsular approaches to vascular lesions.
METHODS
The anterior approach opened the sphenoidal and insular portions of the Sylvian fissure and exposed the limen insulae and short gyri, whereas the posterior approach opened the insular and opercular portions of the Sylvian fissure and exposed the circular sulcus and long gyri.
RESULTS
41 patients with vascular lesions (24 arteriovenous malformations (AVM) and 17 cavernous amlformations (CM)) were treated surgically with a transsylvian-transinsular approach. Complete resection was obtained in 87.5% of AVMs and 95% of CMs. Permanent neurologic morbidity related to surgery was observed in 2 AVM patients (5%), with the remaining 39 patients (95%) improved or unchanged postoperatively (modified Rankin Scale scores 0–2 in 83%). There were no new language deficits in patients with dominant hemisphere lesions.
CONCLUSION
Transsylvian-transinsular approaches safely expose vascular pathology in or deep to the insula while preserving overlying eloquent cortex in the frontal and temporal lobes. The anterior transsylvian-transinsular approach can be differentiated from the posterior approach based on technical differences in splitting the Sylvian fissure and anatomical differences in final exposure. Discriminating patient selection and careful microsurgical technique are essential.
INTRODUCTION
Splitting the proximal Sylvian fissure is one of the most important maneuvers for neurosurgeons because it opens the gateway to the basal subarachnoid space and circle of Willis, which is essential to treating a significant number of brain aneurysms. Medially-directed subarachnoid dissection progresses from the distal Sylvian cistern down to the proximal Sylvian cistern and into the carotid cistern where aneurysm dissection can continue. Subarachnoid dissection that progresses in the other direction – laterally, posteriorly, and superiorly – splits the distal Sylvian fissure, opens the operculum, and exposes the insula. Transsylvian approaches are important for exposing lesions in the insula and basal ganglia, including the extreme capsule, claustrum, external capsule, putamen, and globus pallidus. Surgical resection of lesions in this territory is considered risky because of their depth, eloquence, and proximity to other critical anatomy like the internal capsule, thalamus, language cortex, distal middle cerebral artery (MCA), and middle cerebral veins.1, 2 Transsylvian-transinsular approaches are rarely used, and most published surgical experiences are with tumors3–11 with little written about the application of these approaches to vascular lesions.12–16 In this report, we examine our experience with transsylvian-transinsular approaches to cavernous malformations (CMs) and arteriovenous malformations (AVMs), and describe microsurgical techniques and associated results.
CLINICAL MATERIAL AND METHODS
Data Collection
This study was approved by the Institutional Review Board of the University of California, San Francisco, and conducted in compliance with Health Insurance Portability and Accountability Act regulations. The database of the Vascular Neurosurgery service was retrospectively reviewed to identify patients with insular or basal ganglia CMs or AVMs treated with a transsylvian-transinsular approach. Medical records, radiographic studies, intraoperative photographs, and clinical follow-up evaluations were retrospectively reviewed. Outcomes were assessed using the modified Rankin Scale (mRS) score preoperatively, postoperatively (6 weeks), and at late follow-up.
Surgical Technique
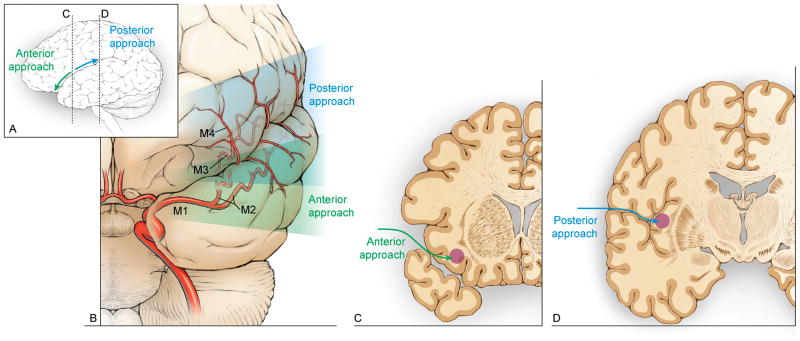
Two distinct variations of the transsylvian-transinsular approach were used: anterior and posterior. Both approaches require wide splitting of the Sylvian fissure to expose the insular cortex but they differ in the direction of the Sylvian fissure split (Figure 1). With the anterior transsylvian-transinsular approach, the dissection parallels the sphenoidal portion of the Sylvian cistern and is directed at the sphenoidal (M1) and insular (M2) segments of the MCA. Final exposure accesses the limen insulae and short gyri. With the posterior transsylvian-transinsular approach, the dissection parallels the opercular cleft and is directed along the opercular (M3) segments of the MCA. Final exposure separates the opercular surfaces of the frontal, parietal, and temporal lobes and accesses the circular sulcus and long gyri of the insula. Both approaches preserve frontal, parietal, and temporal cortex around the Sylvian fissure but access the lesion by transgressing insular cortex, working through the candelabra of MCA vessels.
Figure 1.
(A) The anterior transsylvian-transinsular approach follows the sphenoidal portion of the Sylvian cistern and (B) is directed at the sphenoidal (M1) and insular (M2) segments of the MCA. The posterior transsylvian-transinsular approach follows the opercular cleft and is directed along the opercular (M3) segments of the MCA. (C) Final exposure of the anterior transsylvian-transinsular accesses the limen insulae and short gyri. (D) Final exposure of the posterior transsylvian-transinsular separates the opercular surfaces of the frontal, parietal, and temporal lobes and accesses the circular sulcus and long gyri of the insula.
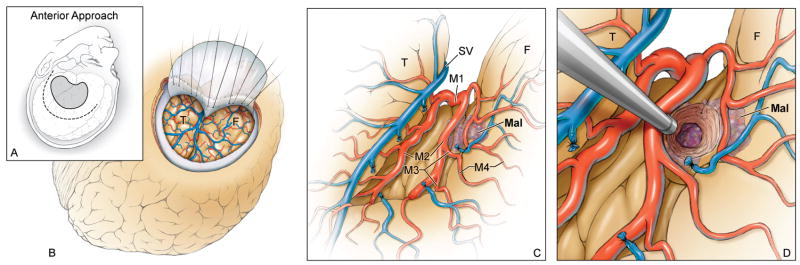
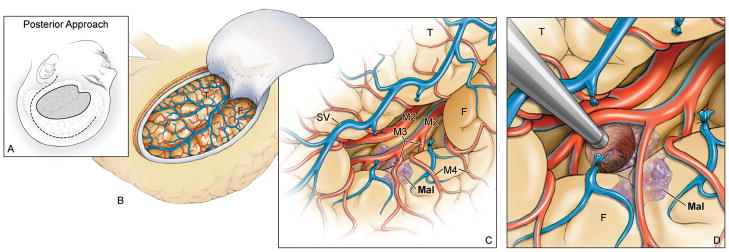
Both transsylvian-transinsular approaches use a pterional craniotomy for access to the Sylvian fissure. With the anterior approach, the head position is the same as for an MCA aneurysm. The head is rotated 15–20 degrees away from the side of the lesion and extended 20 degrees (Figure 2). This head position aligns the plane of the proximal Sylvian fissure vertically, allowing frontal and temporal lobes to fall naturally to either side as the fissure is split like pages in a book that rests on its binding. This eliminates the need for retractors during the Sylvian fissure dissection. With the posterior approach, the head is positioned nearly laterally to align the plane of the opercular cleft vertically, again allowing the frontal and temporal lobes to separate naturally to either side (Figure 3).
Figure 2.
(A) With the anterior transsylvian-transinsular approach, the head position, skin incision (dashed line), and craniotomy (solid line) are the same as a for an MCA aneurysm. (B) A standard pterional craniotomy with drilling of the sphenoid wing exposes the sphenoidal portion of the Sylvian fissure. (C) A wide Sylvian fissure split exposes the MCA bifurcation, the insular segments (M2), the limen insulae, and short gyri. (D) A small cortical incision is needed to reach lesions not on the insular surface. Abbreviations: F = frontal lobe; T = temporal lobe; Mal = malformation; SV = Sylvian vein; M1 = sphenoidal segment of MCA; M3 = opercular segment of MCA; M4 = cortical segment of MCA.
Figure 3.
(A) With the posterior transsylvian-transinsular approach, the head is positioned nearly laterally to align the plane of the opercular cleft vertically. A question mark skin incision (dashed line) is used and the pterional craniotomy is extended posteriorly to incorporate more parietal and posterior temporal bone (solid line). (B) The craniotomy exposes the entire Sylvian fissure, from the pterion proximally to the operculum distally. (C) Dissection begins at the same place as with the anterior approach, but proceeds in the opposite direction. Cortical (M4) and opercular arteries (M3) are followed down to the depths of the opercular cleft to reach the long gyri of the insula. (D) Lesions not on the insular surface are accessed through a small cortical incision. Abbreviations: F = frontal lobe; T = temporal lobe; Mal = malformation; SV = Sylvian vein; M2 = insular segment of MCA.
A standard pterional skin incision is used with the anterior approach, whereas a “question mark” incision is used with the posterior approach to allow for a more posterior exposure of the angular gyrus. Similarly, a standard pterional craniotomy is used with the anterior approach, whereas additional parietal and posterior temporal bone is incorporated into the craniotomy with the posterior approach.
With the anterior transsylvian-transinsular approach, the Sylvian fissure is split with the same technique as an unruptured MCA aneurysm. Dissection typically begins below the pars triangularis of the inferior frontal gyrus, where the distal Sylvian fissure is widest. Cortical arachnoid is incised and superficial Sylvian veins are mobilized to the temporal side of the fissure. Dissection proceeds from distal to proximal, transitioning at the temporal pole from cortical to sphenoidal arachnoid. A cortical artery is followed into the operculum to develop the plane of separation between frontal and temporal lobes. Opercular arteries are followed down to insular arteries and the trunks of the MCA at its bi- or trifurcation. Spreading dissection from inside-out separates the frontal and temporal lobes. Finally, arterial branches are mobilized frontally or temporally. This dissection exposes the MCA bifurcation and the insular segments, through which the limen insulae and short gyri are then exposed.
With the posterior transsylvian-transinsular approach, dissection typically begins at the same place as with the anterior approach, but proceeds in the opposite direction. Cortical arachnoid is incised posteriorly, following the fissure below the pars triangularis, pars opercularis, precentral gyrus, and the gyral bridge between the lower ends of pre-and postcentral gyri. Dissection proceeds from proximal to distal, mobilizing veins and following cortical arteries down into the fissure. Separation of frontal and temporal lobes is more difficult here than in the proximal Sylvian fissure because the Sylvian cistern ends and the opercular surfaces are adherent. Splitting the proximal Sylvian fissure first and extending posteriorly into the distal fissure can sometimes facilitate the opening, but the complex of overlying veins often prevents continuous dissection without venous sacrifice. Opercular arteries are followed down to the depths of the opercular cleft to reach the long gyri of the insula. The exposure in the distal Sylvian fissure is narrow and constrained, and unlike with the proximal Sylvian fissure, often requires one retractor to widen the view.
RESULTS
Patients
During a 13-year period from August 1997 to August 2010, 41 patients with vascular lesions were treated surgically with a transsylvian-transinsular approach. The mean patient age was 36 years (range 11–68 years), with 20 men and 21 women. This cohort included 24 patients with AVMs (Table 1) and 17 patients with CMs (Table 2). All patients were operated on by the senior author (MTL).
Table 1.
Summary of patients with arteriovenous malformations.
| Patient | Age | Sex | Presentation | Lesion | Side | Location | Previous Tx | Approach | Resection | MRS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop | Early | Late | ||||||||||
| 1 | 45 | M | Headaches | AVM | L | Insula | None | Anterior | Complete | 1 | 2 | 3 |
| 2 | 33 | M | Hemorrhage | AVM | L | Insula | None | Anterior | Complete | 4 | 4 | 2 |
| 3 | 15 | F | Hemorrhage (2) | AVM | L | Basal Ganglia | None | Anterior | Incomplete | 2 | 3 | 2 |
| 4 | 53 | M | Hemorrhage | AVM | L | Insula | None | Anterior | Complete | 4 | 1 | 1 |
| 5 | 26 | F | Hemorrhage | AVM | L | Insula & Basal ganglia | Surgery, GK | Anterior | Complete | 5 | 4 | 2 |
| 6 | 40 | M | Headaches | AVM | L | Insula | None | Anterior | Complete | 1 | 0 | 0 |
| 7 | 68 | F | Hemorrhage | AVM | L | Basal Ganglia | None | Anterior | Complete | 4 | 3 | 3 |
| 8 | 61 | M | Hemorrhage | AVM | L | Insula | None | Anterior | Complete | 2 | 2 | 1 |
| 9 | 29 | F | Hemorrhage | AVM | L | Insula | None | Anterior | Complete | 4 | 3 | 3 |
| 10 | 47 | M | Hemorrhage | AVM | L | Insula & Basal ganglia | None | Anterior | Complete | 5 | 4 | 4 |
| 11 | 28 | M | Incidental | AVM | R | Insula | None | Posterior | Complete | 0 | 0 | 0 |
| 12 | 33 | F | Hemorrhage | AVM | L | Insula | GK | Posterior | Complete | 5 | 4 | 3 |
| 13 | 12 | M | Hemorrhage | AVM | R | Insula | None | Posterior | Incomplete | 1 | 1 | 0 |
| 14 | 47 | F | Hemorrhage | AVM | R | Insula | Surgery | Posterior | Complete | 3 | 3 | 2 |
| 15 | 11 | M | Hemorrhage | AVM | L | Insula | None | Posterior | Complete | 1 | 1 | 1 |
| 16 | 47 | M | Hemorrhage | AVM | R | Insula | None | Posterior | Complete | 2 | 2 | 1 |
| 17 | 28 | M | Hemorrhage | AVM | L | Insula | None | Posterior | Complete | 2 | 2 | 2 |
| 18 | 33 | F | Hemorrhage | AVM | L | Insula | None | Posterior | Complete | 3 | 3 | 2 |
| 19 | 48 | F | ↑Numbness | AVM | R | Insula | None | Posterior | Complete | 1 | 1 | 1 |
| 20 | 16 | F | Hemorrhage | AVM | L | Insula | Surgery, GK | Posterior | Complete | 4 | 3 | 2 |
| 21 | 42 | M | Seizures | AVM | R | Insula | None | Posterior | Complete | 1 | 1 | 1 |
| 22 | 19 | F | Seizures | AVM | R | Insula | None | Posterior | Complete | 0 | 0 | 0 |
| 23 | 26 | M | Hemorrhage | AVM | L | Insula & Basal ganglia | None | Posterior | Complete | 1 | 3 | 2 |
| 24 | 23 | M | Hemorrhage | AVM | L | Insula & Basal ganglia | None | Posterior | Incomplete | 5 | 4 | 3 |
Abbreviations: M = male; F = female; L = left; R = right; Tx = treatment; MRS = modified Rankin Scale score; Preop = preoperative; ↑ = increasing.
Table 2.
Summary of patients with cavernous malformations.
| Patient | Age | Sex | Presentation | Lesion | Side | Location | Previous Tx | Approach | Resection | MRS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop | Early | Late | ||||||||||
| 25 | 45 | F | Hemorrhage | CM | L | Basal ganglia | Surgery | Anterior | Complete | 2 | 3 | 2 |
| 26 | 49 | F | Seizures | CM | L | Basal ganglia | None | Anterior | Complete | 1 | 0 | 0 |
| 27 | 23 | M | Seizures | CM | R | Insula | None | Anterior | Complete | 1 | 0 | 0 |
| 28 | 54 | F | Hemorrhage | CM | L | Basal ganglia | None | Anterior | Complete | 2 | 3 | 2 |
| 29 | 32 | M | Hemorrhage | CM | L | Basal ganglia | None | Anterior | Complete | 1 | 1 | 1 |
| 30 | 52 | F | Hemorrhage | CM | R | Basal ganglia | None | Anterior | Complete | 3 | 3 | 2 |
| 31 | 59 | F | Hemorrhage | CM | R | Basal ganglia | None | Anterior | Complete | 1 | 0 | 0 |
| 32 | 48 | F | Hemorrhage | CM | R | Insula | None | Anterior | Complete | 1 | 0 | 0 |
| 33 | 36 | F | Hemorrhage | CM | R | Insula | None | Anterior | Complete | 1 | 0 | 0 |
| 34 | 30 | M | Seizures | CM | L | Insula | None | Posterior | Complete | 1 | 0 | 0 |
| 35 | 19 | M | Hemorrhage | CM | L | Insula & Basal ganglia | None | Posterior | Complete | 2 | 0 | 0 |
| 36 | 19 | M | Hemorrhage | CM | R | Basal ganglia | None | Posterior | Incomplete | 2 | 4 | 1 |
| 37 | 17 | F | Hemorrhage | CM | R | Insula | None | Posterior | Complete | 3 | 1 | 1 |
| 38 | 52 | M | Hemorrhage | CM | L | Insula | None | Posterior | Complete | 2 | 1 | 1 |
| 39 | 52 | F | Hemorrhage | CM | R | Insula & Basal ganglia | None | Posterior | Complete | 3 | 3 | 3 |
| 40 | 54 | F | Seizures | CM | L | Insula | None | Posterior | Complete | 1 | 1 | 1 |
| 41 | 46 | F | Seizures | CM | L | Basal ganglia | None | Posterior | Complete | 1 | 1 | 1 |
Abbreviations: M = male; F = female; L = left; R = right; Tx = treatment; MRS = modified Rankin Scale score; Preop = preoperative; ↑ = increasing.
Sensorimotor deficits related to hemorrhage were the most common presenting symptoms. Overall, 18 patients with AVMs (75%) and 12 patients with CMs (71%) presented with clinical findings and radiographic evidence of hemorrhage. Seizures were the next most common presentation in 7 patients. Patients with AVMs presented with more significant deficits than patients with CMs, with mean preoperative mRS scores of 2.5 and 1.6, respectively. Three AVM patients presented in coma after AVM rupture.
AVMs were predominantly located in the insula (18 patients, 75%), whereas CMs were located deeper – 8 in the basal ganglia (47%), 7 in the insula (41%), and 2 in both (12%). The mean diameter was 2.4 cm for AVMs and 2.3 cm for CMs. AVM grading is shown in Table 3. AVMs were fed predominantly by MCA branches, with minor contributions from insular and lenticulostriate perforators.
Table 3.
Grading of insular and basal ganglia arteriovenous malformations.
| Grade | Spetzler-Martin | Spetzler-Martin, Modified | Supplementary | |||
|---|---|---|---|---|---|---|
| I | 0 | 0% | 0 | 0% | 3 | 13% |
| II | 8 | 33% | 8 | 33% | 8 | 33% |
| III | 10 | 42% | 10 | 42% | ||
| III− | 5 | 21% | ||||
| III | 1 | 4% | ||||
| III+ | 4 | 17% | ||||
| IV | 6 | 25% | 6 | 25% | 3 | 13% |
| V | 0 | 0% | 0 | 0% | 0 | 0% |
|
| ||||||
| Total | 24 | 24 | 24 | |||
Surgical Management of AVMs
Four patients had previous interventions related to their AVMs. Three of these patients had prior surgery, including one hematoma evacuation, one decompressive hemicraniectomy, and one partial resection. Two of these operated patients were treated subsequently with stereotactic radiosurgery. One additional patient was treated with radiosurgery. Of the three patients treated radiosurgically, two patients hemorrhaged during the latency period and one had incomplete AVM obliteration.
Fifteen patients (62%) underwent preoperative embolization. The anterior transsylvian-transinsular approach was performed in 10 AVM patients (42%) (Figures 4 and 5) and the posterior approach was performed in 14 AVM patients (58%). Selection of the anterior versus posterior approach was based on AVM location. There were no significant differences in subgroups with regard to patient age, preoperative neurological condition, or AVM anatomy with the exception of side – all of the anterior approaches but only half of the posterior approaches were performed on the left side. Language mapping was used in one patient and frameless navigation was used in 4 patients (BrainLab North America, Westchester, IL).
Figure 4.
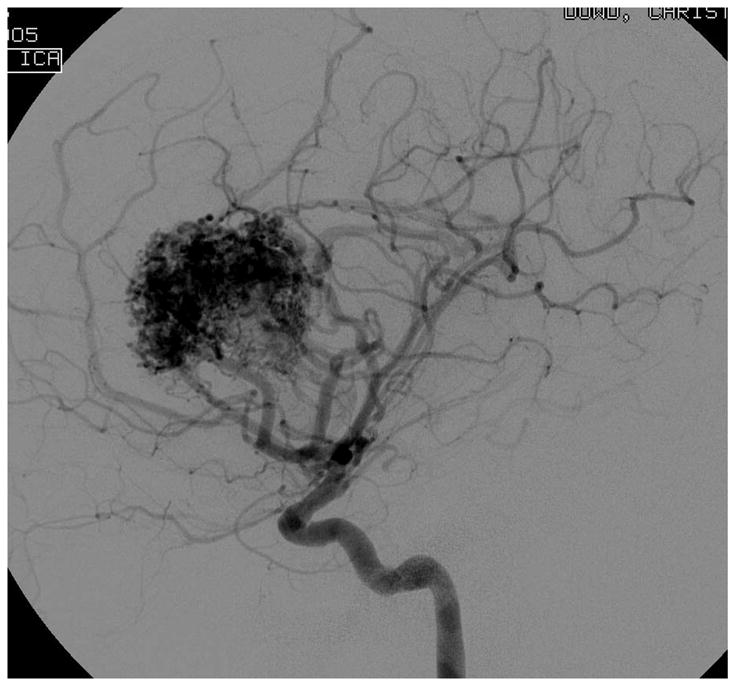
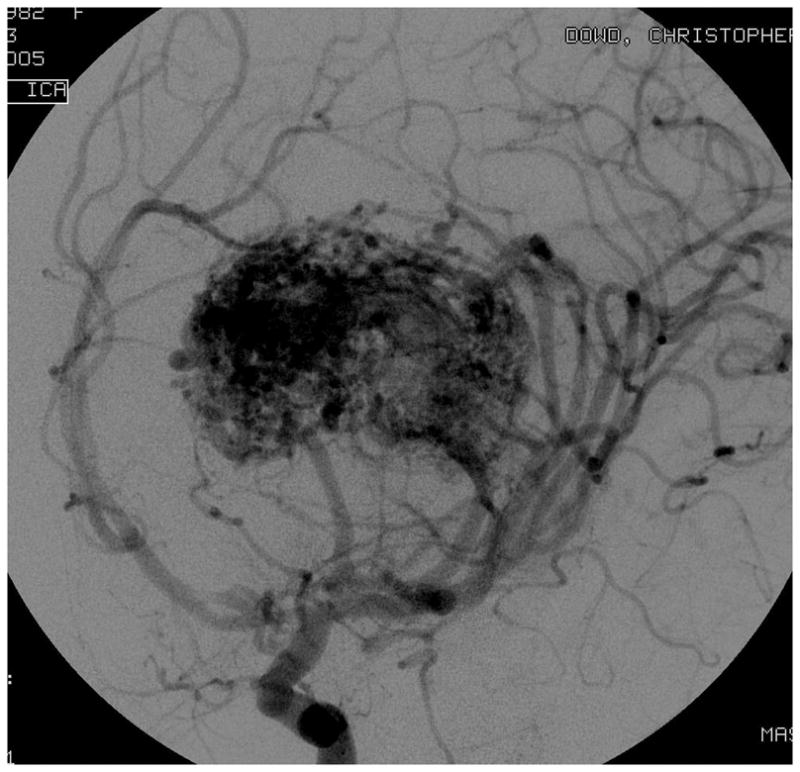
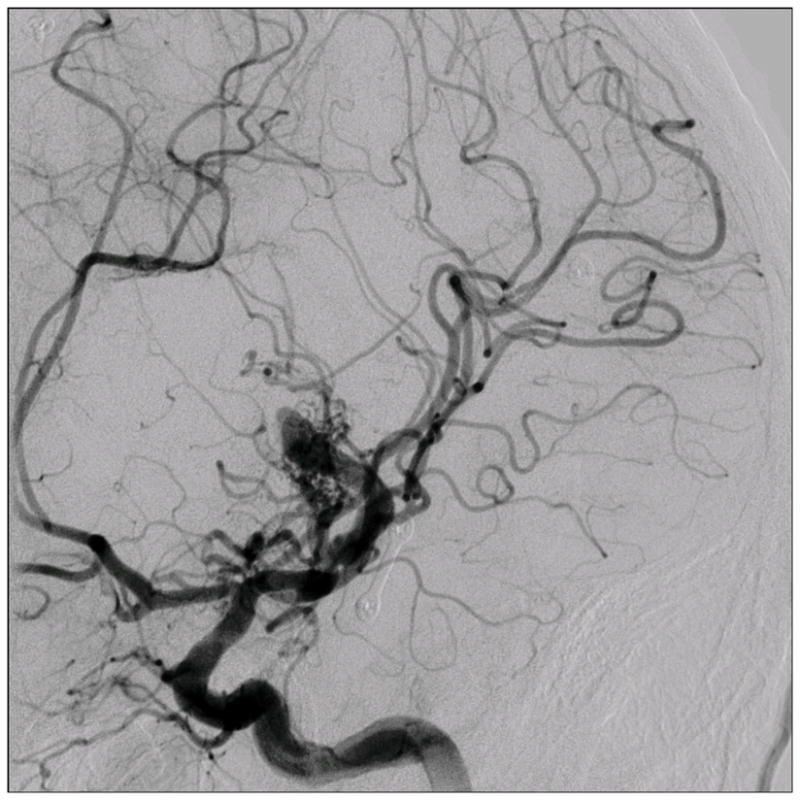
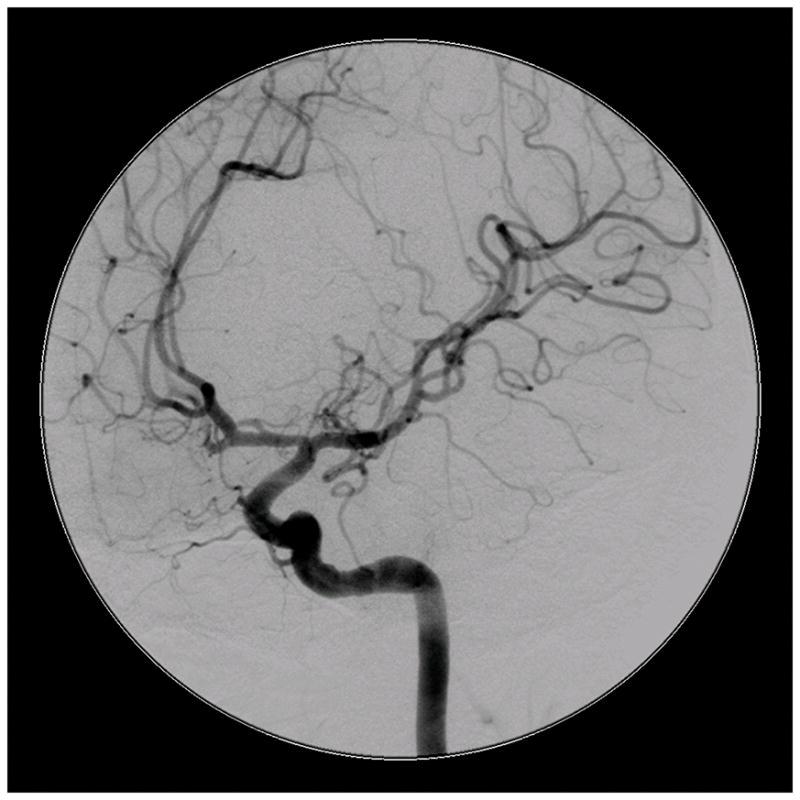
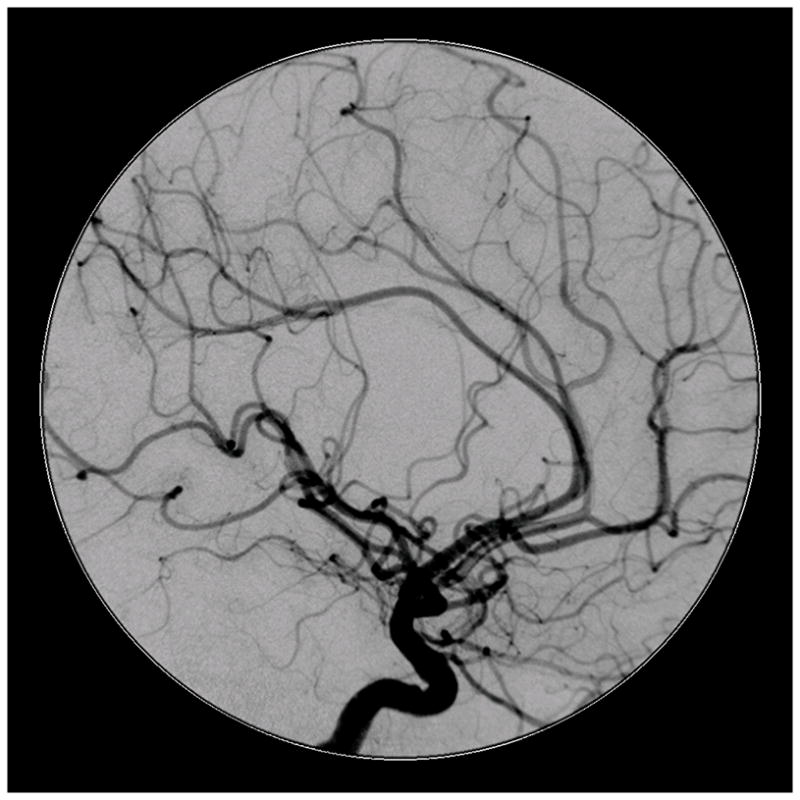
Patient 5 presented comatose with a ruptured left anterior insular and basal ganglia AVM. (A) Axial computed tomography angiography demonstrated a large hematoma in the basal ganglia with midline shift. She underwent emergency decompressive hemicraniectomy, after which digital subtraction angiography showed a modified Spetzler-Martin grade III+ AVM (left internal carotid artery injection, (B) lateral and (C) anterior oblique views). (D) Her AVM was significantly smaller 3 years after Gamma Knife radiosurgery. Her AVM was resected completely through an anterior transsylvian-transinsular approach, as confirmed by postoperative angiography (left ICA injection, (E) anterior oblique and (F) lateral views).
Figure 5.
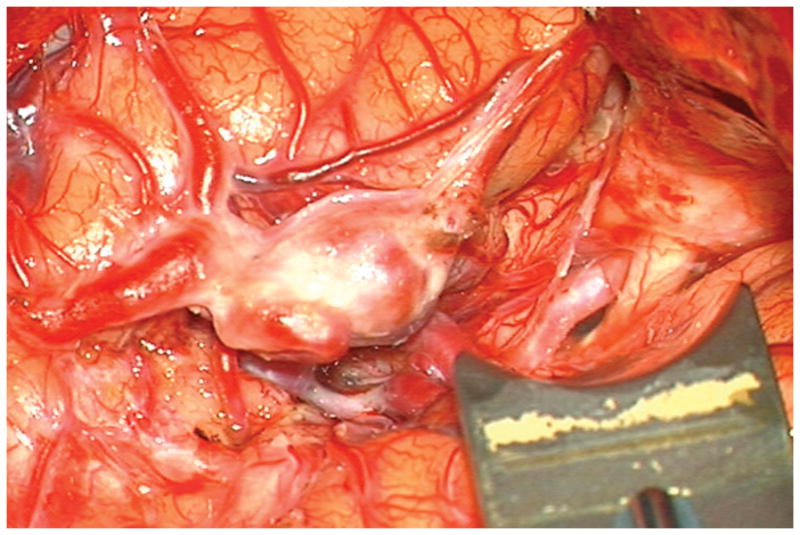
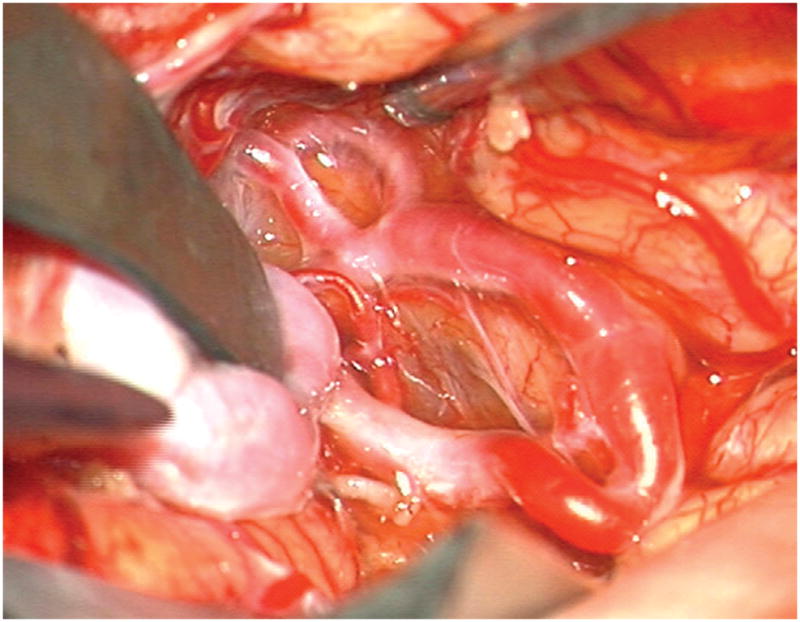
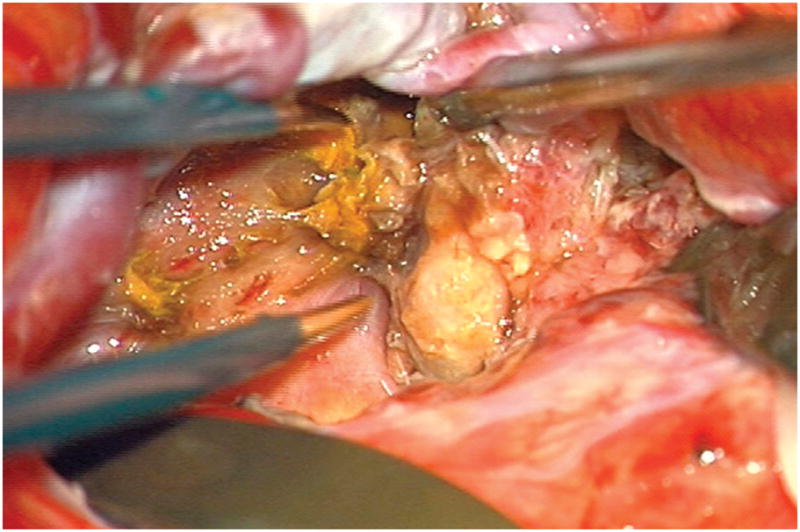
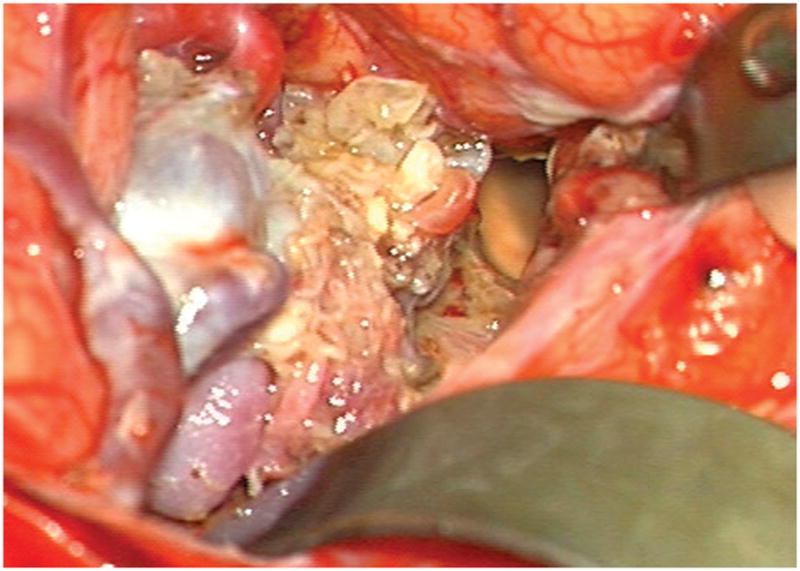
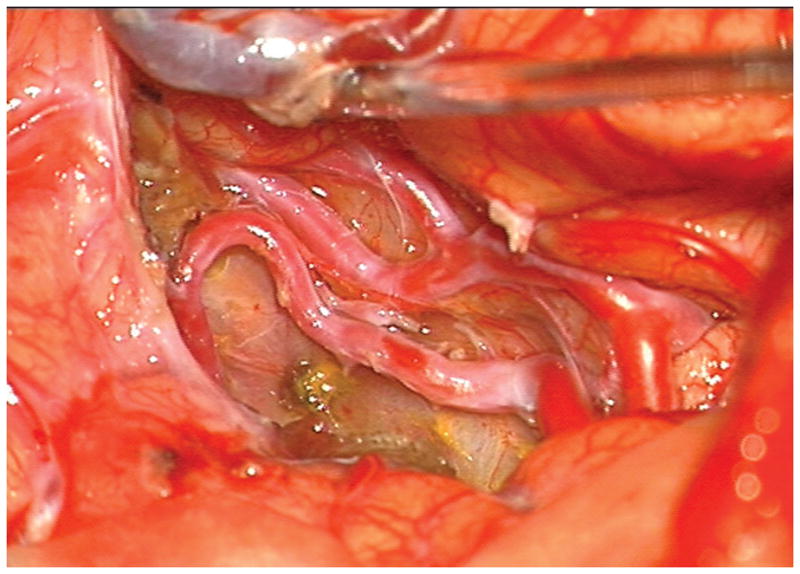
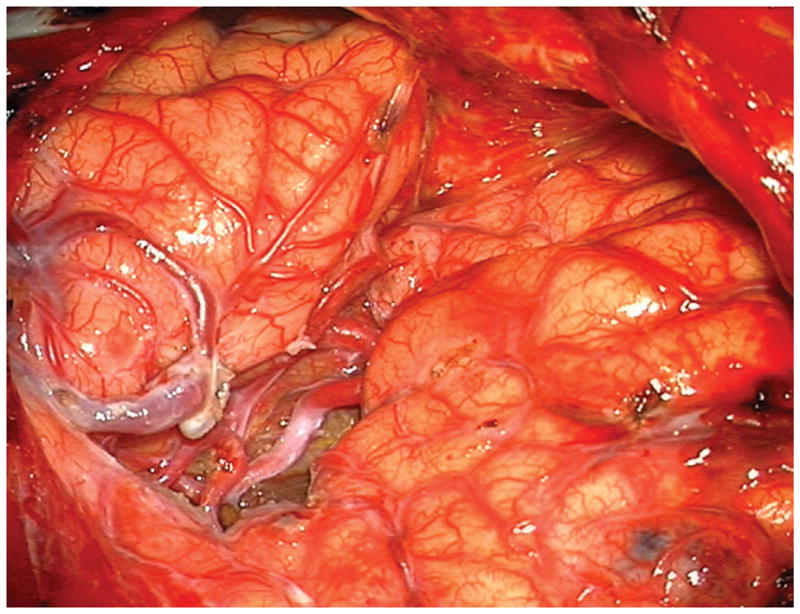
(A) Widely splitting the left proximal Sylvian fissure exposed the draining venous varix, MCA branches, supraclinoid ICA, and optic nerve (Patient 5). (B) Retraction of the varix exposed the MCA bifurcation and its branches, and the inferior margin of the AVM was visualized deep to the M2 MCA segments in the limen insulae. (C) A gliotic plane of dissection around the anterior border to the nidus resulted from previous hemorrhage and radiosurgery. (D) The medial border of the nidus connected to the lateral ventricle. (E) En passage and uninvolved arteries in the operculum were carefully preserved, as seen after AVM resection. (F) The anterior transsylvian-transinsular approach preserved language cortex and the patient awoke with no new language deficits.
Complete AVM resection was accomplished in 21 patients and confirmed with postoperative angiography. Three patients required two planned surgical stages, and two patients had unexpected residual AVM that hemorrhaged postoperatively, necessitating a second operation. Three patients had small, ventricular AVM remnants that were incompletely resected and subsequently treated with Gamma Knife radiosurgery. Of these, two were obliterated completely and one is within the latency period.
Surgical Management of CMs
Only one patient had undergone previous treatment of her CM and presented with a recurrence. The anterior transsylvian-transinsular approach was used in 9 patients (53%) and the posterior approach was used in 8 patients (47%) (Figures 6 and 7). There were no significant differences in subgroups with regard to patient age, preoperative neurological condition, or CM anatomy. Language mapping was used in one patient and motor mapping was used in one patient. In contrast to AVMs, frameless navigation was used in all patients with CMs. Complete resection was achieved in all but one patient (94%), and was confirmed with late magnetic resonance imaging 12 months postoperatively. The incomplete resection was a large, atypical CM that bled significantly during the surgery, had neoplastic features pathologically, and required subsequent radiosurgery.
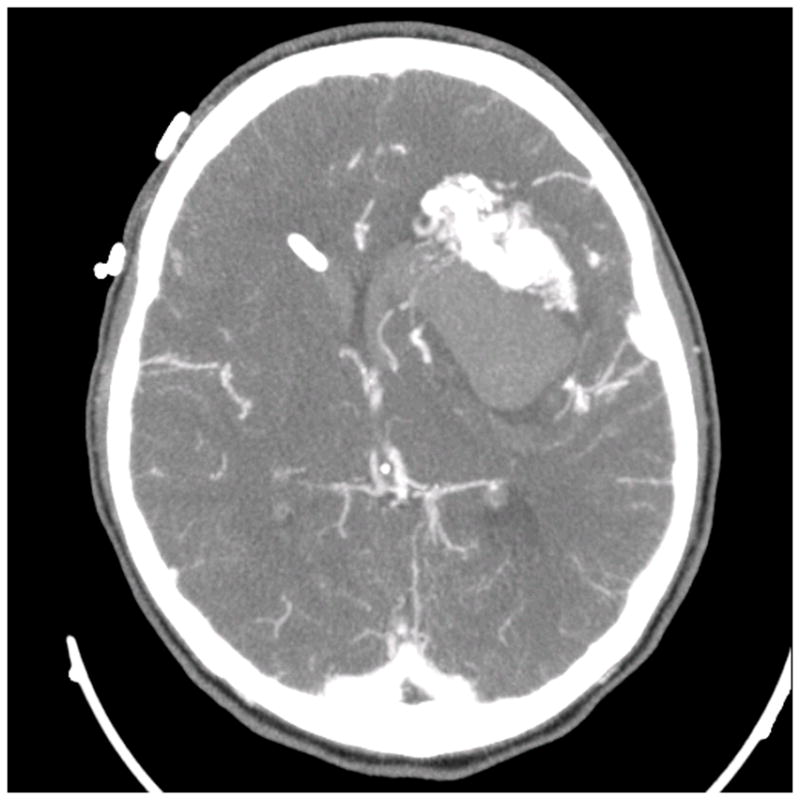
Figure 6.
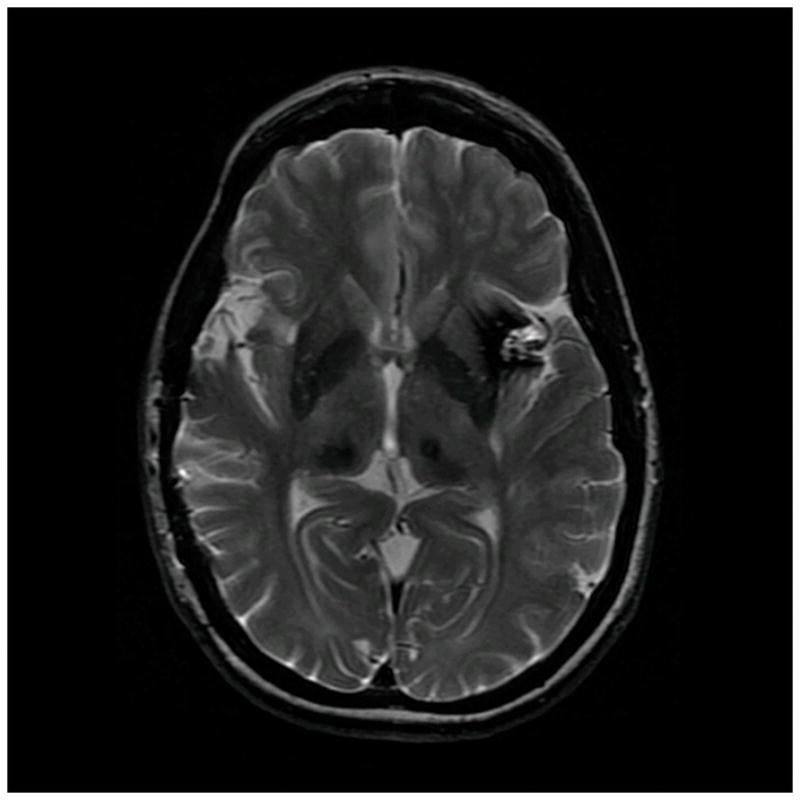
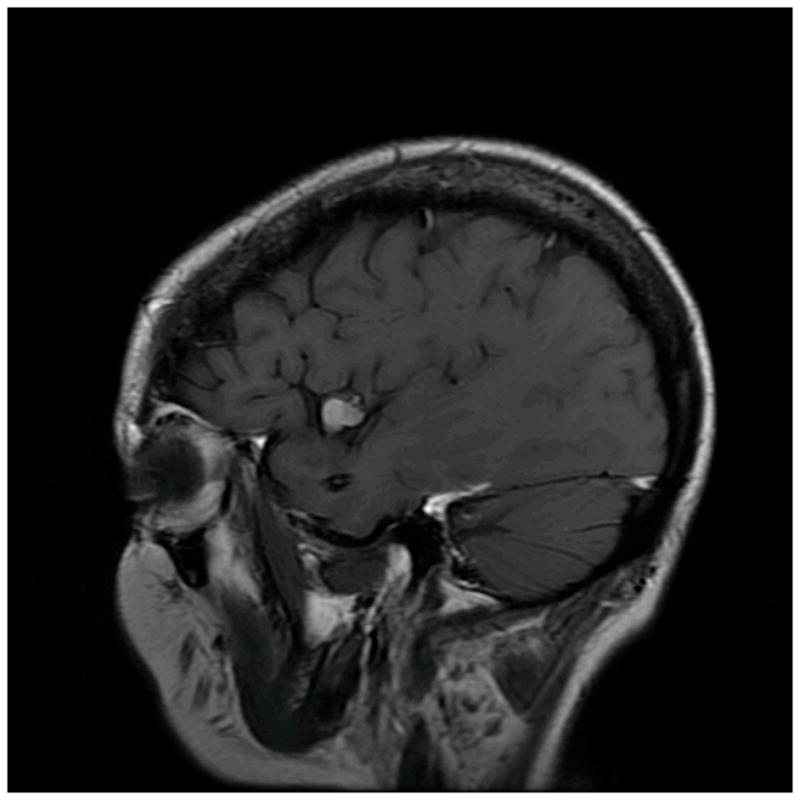
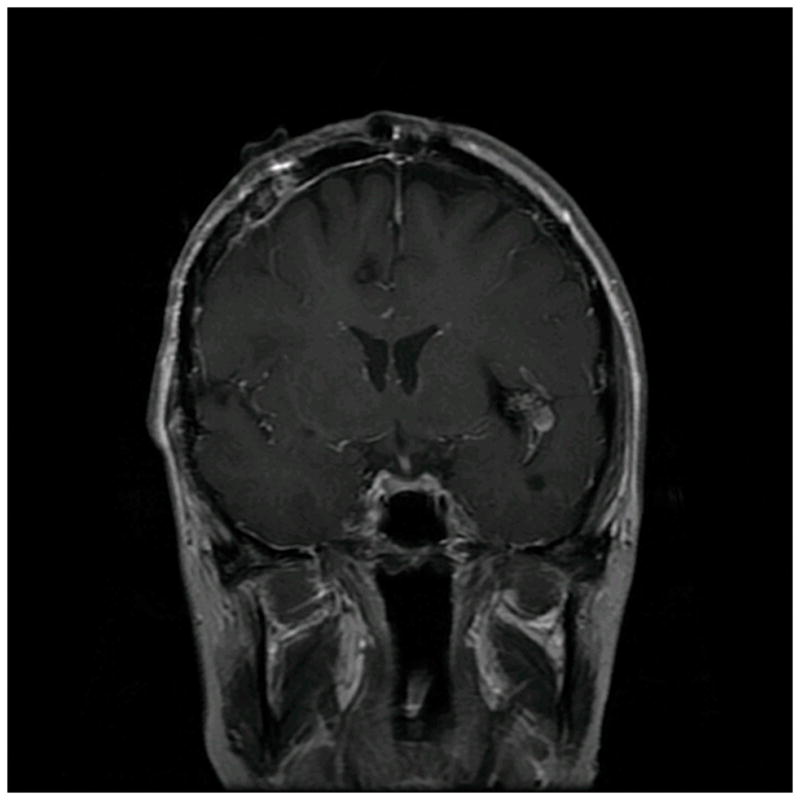
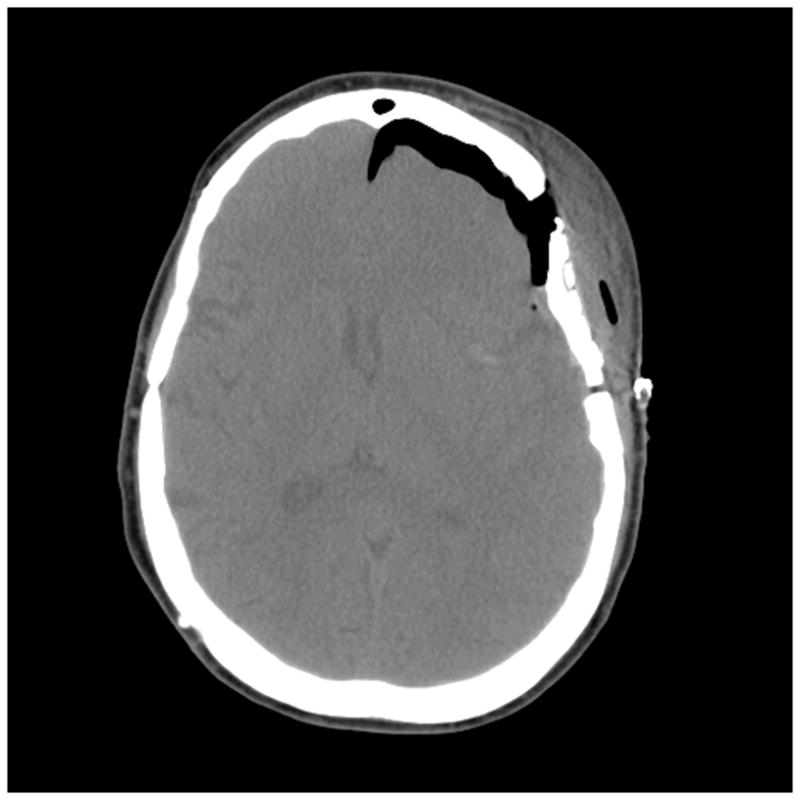
Patient 40 presented with seizures from this left insular cavernous malformation, seen on (A) axial T2-weighted, (B) sagittal T1-weighted, and (C) coronal T1-weighted MR images. The CM was resected completely through a posterior transsylvian-transinsular approach, (D) as seen on postoperative axial CT scan.
Figure 7.
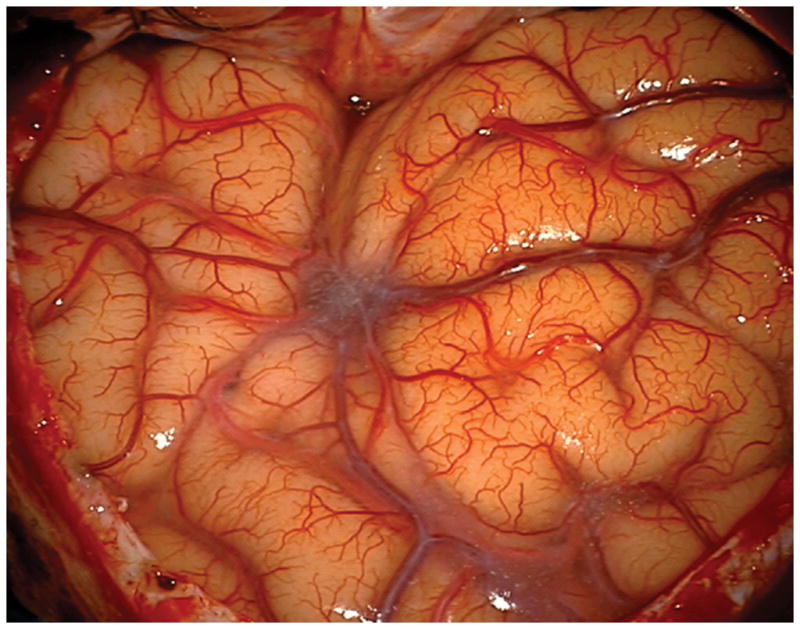
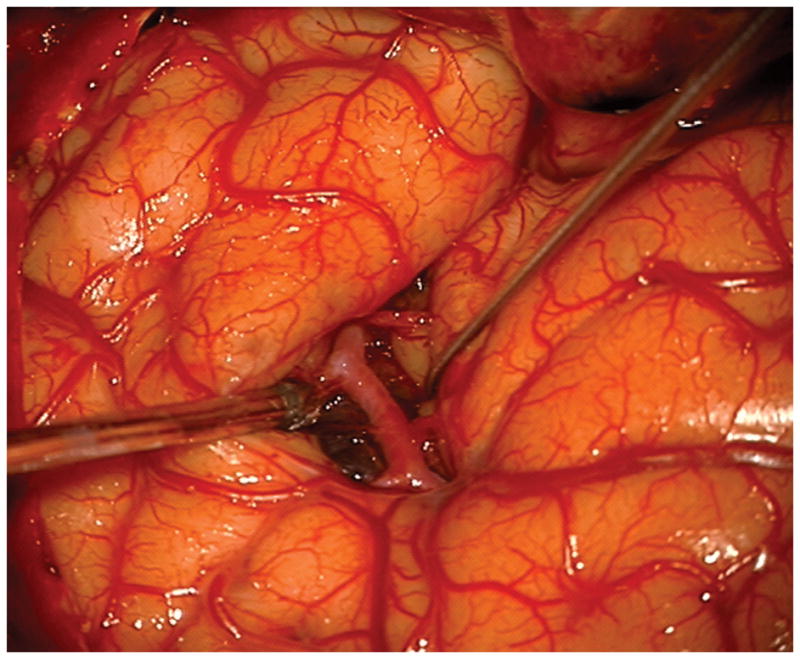
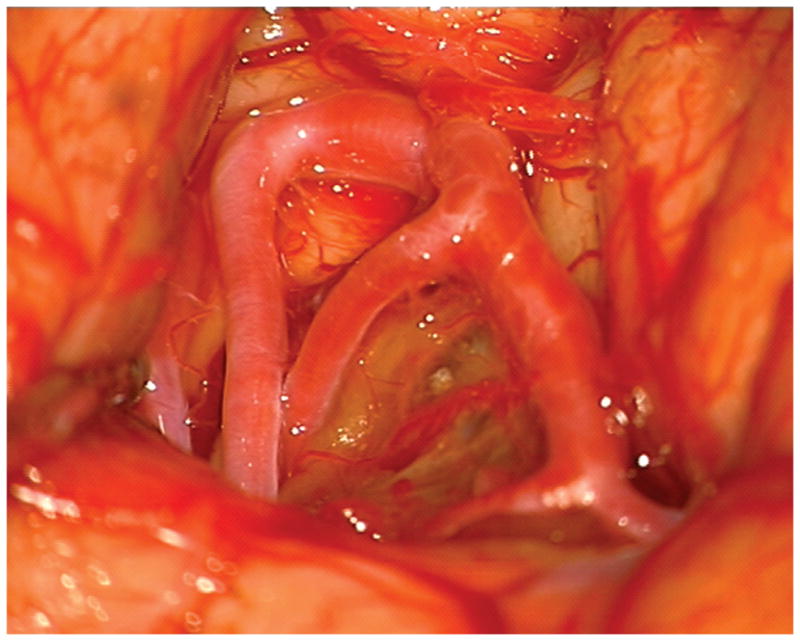
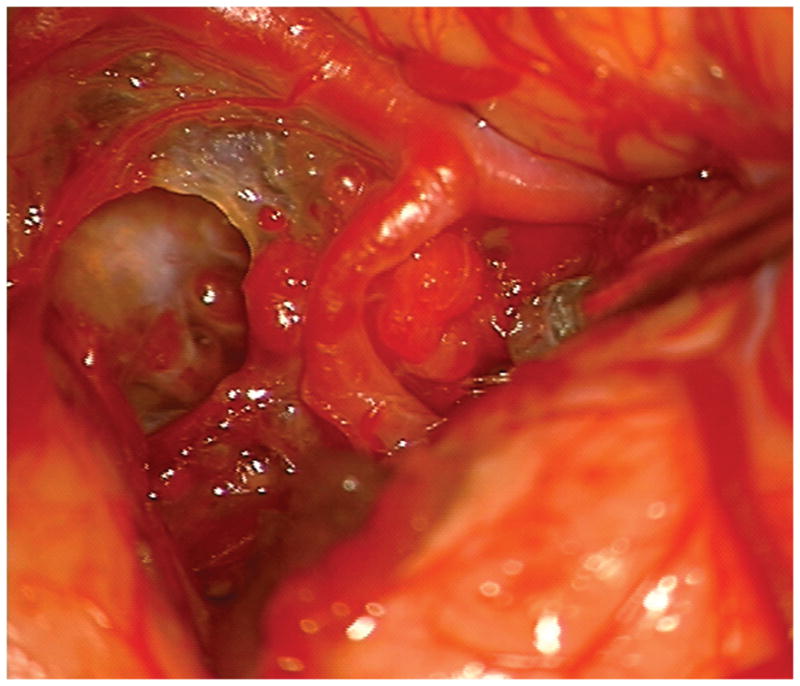
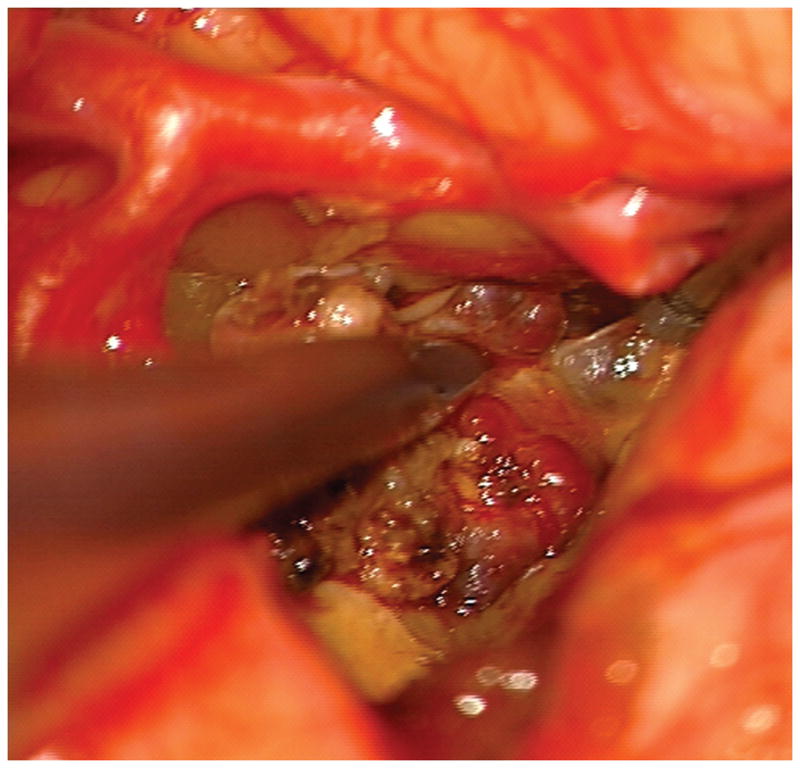
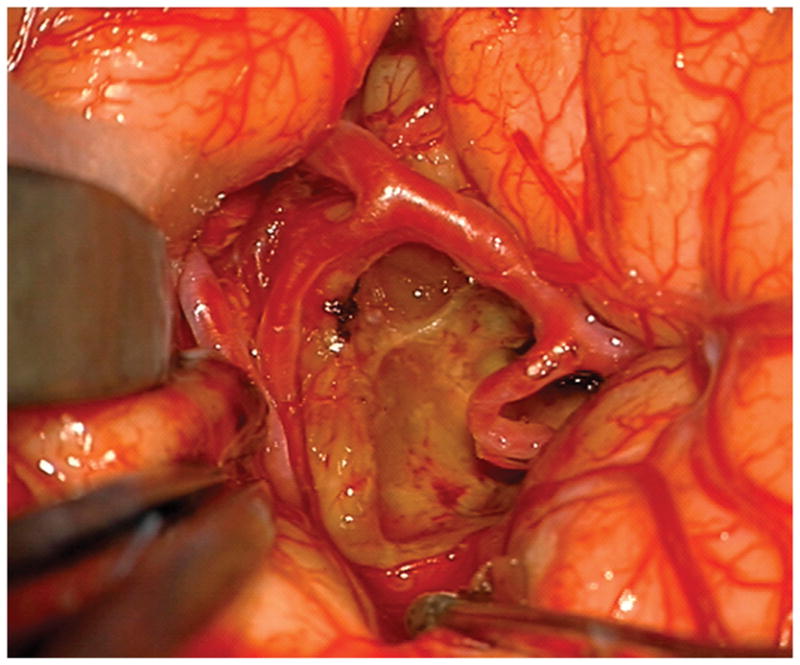
(A) The absence of overlying Sylvian veins facilitated splitting the distal Sylvian fissure, (B) which exposed the opercular segments of MCA (Patient 40). (C) The cavernous malformation came to the surface of the long gyrus and was apparent under the arterial branches. (D) Evacuation of intracapsular hematoma decompressed the lesion and facilitated the dissection in the gliotic plane. (E) The posterior transsylvian-transinsular approach preserved perisylvian cortex and overlying MCA branches.
Outcomes
There was no surgical mortality in this patient series. Six patients were neurologically worse after surgery, of whom 4 patients recovered completely (transient neurological morbidity, 9.8%) and 2 patients did not (permanent neurological morbidity, 4.9%). Both of these patients had AVMs; one deteriorated after a postoperative hemorrhage from unexpected residual AVM and the other had new hemiparesis after resecting an insular/basal ganglia AVM adjacent to the internal capsule. There were no new language deficits in patients with dominant hemisphere lesions. Overall, good outcomes (mRS 0-2) were observed in 34 patients (83%) and poor outcomes (mRS 3-4) in 7 patients (17%). Relative to neurological baseline, 39 patients (95%) were improved or unchanged after treatment. The mean length of post-operative follow-up for all patients was 19.3 months.
Patient outcomes after CM resection with the anterior transsylvian-transinsular approach were not significantly different from those with the posterior approach (Table 4). With AVMs, neurological morbidity was similar with anterior and posterior approaches, but patients were more likely to improve after the anterior approach.
Table 4.
Summary of neurological outcomes after anterior and posterior transsylvian-transinsular approaches.
| Anterior Approach | Posterior Approach | Total | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Arteriovenous Malformations
| |||||
| Improved | 8 | 80% | 7 | 50% | 15 |
| Unchanged | 1 | 10% | 6 | 43% | 7 |
| Worse | 1 | 10% | 1 | 7% | 2 |
| Total | 10 | 14 | 24 | ||
|
| |||||
| Cavernous Malformations
| |||||
| Improved | 6 | 67% | 5 | 63% | 11 |
| Unchanged | 3 | 33% | 3 | 38% | 6 |
| Worse | 0 | 0% | 0 | 0% | 0 |
| Total | 9 | 8 | 17 | ||
|
| |||||
| Overall
| |||||
| Improved | 14 | 74% | 12 | 55% | 26 |
| Unchanged | 4 | 21% | 9 | 41% | 13 |
| Worse | 1 | 5% | 1 | 5% | 2 |
| Total | 19 | 22 | 41 | ||
DISCUSSION
Surgical Approaches to the Insula and Basal Ganglia
Lesions in or deep to the insula are difficult to access because of overlying frontal, temporal, and parietal cortex. The eloquence of this cortex, particularly in the dominant hemisphere, heightens the risks of surgical exposure. Fortunately, the Sylvian fissure provides a subarachnoid corridor that can be opened microsurgically to access the insula. Our experience with the transsylvian-transinsular approach demonstrates that it can be utilized safely and effectively with vascular pathologies. In addition, our experience demonstrates that the anterior transsylvian-transinsular approach can be differentiated from the posterior approach based on technical differences in splitting the Sylvian fissure and anatomical differences in final exposure. The anterior approach opens the sphenoidal and insular portions of the Sylvian fissure and exposes the limen insulae and short gyri, whereas the posterior approach opens the insular and opercular portions of the Sylvian fissure and exposes the circular sulcus and long gyri. Both approaches avoid transgression of temporal, frontal, or parietal cortex, and therefore limit brain transgression to insular cortex.
The transsylvian-transinsular approaches are obvious choices for lesions located in insular cortex, but the choice is less clear for lesions in the basal ganglia. Alternatives include transcortical or transsulcal approaches, the infrafrontal-supracarotid approach,17 and the contralateral transcallosal approach.18 The anterior transinsular approach exposes the lateral head of caudate nucleus and the anterior limb of internal capsule, and is ideal when the lesion thins overlying insular cortex and displaces caudate head medially. The contralateral transcallosal approach is optimal for lesions along the medial head of caudate nucleus that present to an ependymal surface in lateral ventricular wall and displace the nucleus laterally. The posterior transinsular approach exposes the wedge of basal ganglia lateral to the internal capsule harboring the claustrum, extreme capsule, putamen, and globus pallidus. This approach is ideal when the lesion or associated hematoma thins overlying insular cortex. The genu and posterior limb of internal capsule define the medial extent of this exposure and accounted for neurological morbidity in one of our patients. The supracarotid-infrafrontal approach offers a more antero-inferior approach than transsylvian approaches, transgressing the posterior aspect of medial orbital gyrus directly above internal carotid artery bifurcation rather than insular cortex. The orbitozygomatic craniotomy is required for this approach, allowing the microscope to be angled low and medially over the eye. This approach is preferred when there is a thick layer of overlying insular cortex and only a thin layer of overlying medial orbital gyrus.
Technical Considerations
Good results with transsylvian approaches depend on a meticulous splitting of the Sylvian fissure that respects pial planes, overlying middle cerebral veins, branches of the MCA, and lenticulostriate and insular perforating arteries. Pial preservation ensures a clean separation of the frontal and temporal lobes, and also protects cortical function in eloquent operculum. The distal Sylvian fissure is often covered by an obstructive tangle of veins formed by the confluence of temporal veins draining anteriorly to the sphenoparietal sinus, frontoparietal veins draining superiorly to the superior sagittal sinus, and temporal veins draining posteriorly to the transverse sinus. It might be tempting to sacrifice a middle cerebral vein or tributary to gain opercular access, but compromise of venous outflow at this confluence can cause venous infarction. The working corridor of transsylvian-transinsular approaches places the insular segments of MCA between the neurosurgeon and the lesion. These arteries do not supply CMs, but often must be dissected away from them. With AVMs, these arteries may be uninvolved or continue en passage to supply distal territories. These normal arteries must be carefully distinguished from pathological arteries and protected.
A surgical corridor lined with eloquent and vascular structures can be made less hazardous by widening the Sylvian fissure split. The proximal Sylvian fissure splits naturally because large arterial trunks and a generous Sylvian cistern separate frontal and temporal lobes. The distal Sylvian fissure is more difficult to split because the opercular surface becomes more adherent as arterial caliber diminishes and the Sylvian cistern terminates. Splitting the distal Sylvian fissure is often facilitated by also splitting the proximal fissure; however, the advantages of a wide dissection must be weighed against the risks of venous compromise or cortical injury. Widening the surgical corridor enhances visualization and maneuverability while reducing the ill effects of brain retraction.
Arterialized draining veins on the cortical surface are invaluable landmarks guiding the dissection to insular AVMs. Associated hematomas are also invaluable surgical adjuncts. The combination of a draining vein and a hematoma help localize small insular AVMs, and evacuation of the hematoma creates additional working space. 75% of AVM patients presented with hemorrhage, and the presence of a hematoma was an important selection factor. Intraoperative navigation was used in just 4 AVM patients (17%). CMs lack draining veins, were slightly smaller on average than the AVMs in this series, and did not have associated hematomas. For these reasons, intraoperative navigation is more critical with CMs and was used in every case. Hemosiderin-stained insular cortex provides guiding landmarks, and a gradient of hemosiderin can be followed transcortically to deeper lesions. Surgical trajectories are carefully planned to maximize subarachnoid dissection through Sylvian fissure and insular sulci, and to minimize brain transgression.
Overall, 63% of lesions in this experience were located in the dominant hemisphere and speech outcomes were excellent, demonstrating that surgical dissection within the insular cortex is well tolerated. Transient speech deficits during the first postoperative week are not uncommon and are due to cerebral edema. These deficits resolve completely by early follow-up, and therefore our methodology comparing preoperative and early postoperative mRS scores misses these transient deficits. Transsylvian-transinsular approaches preserve perisylvian cortex where this critical language function resides. Our use of awake craniotomy and intraoperative speech mapping was limited to just two cases, both large lesions where some transcortical transgression was considered. Confining the microsurgical dissection to the perisylvian subarachnoid spaces obviates the need for mapping in most cases.
Operating on AVMs in the dominant insula is inherently risky. Consequently, patients in this surgical series were highly selected. AVM patients were selected based on Spetzler-Martin and supplementary grades.19–21 Overall, 24 patients with insular and basal ganglia AVMs were selected for surgery from a larger cohort of 46 patients, the remainder of whom were observed or treated with stereotactic radiosurgery. 75% of surgical AVMs were low-grade (Spetzler-Martin grades II or III). High-risk AVMs, with modified Spetzler-Martin grade III+20 or grade IV, had other clinical features like young patient age, presentation with hemorrhage, or compact nidus that offset their high Spetzler-Martin grades. These other clinical features were reflected in low supplementary grades that predict more favorable surgical outcomes,21 with 88% having supplementary grades I – III. Similarly, cavernous malformation patients were selected based upon presentation with hemorrhage (71%), young patient age, and presentation of the CM on or right below the surface of the insular cortex. Patients were advised as to the risks of surgical therapy and the natural history of their lesion, and ultimately decided on their own management.
Our use of staged resections in 3 of 24 patients (12.5%), and incomplete resections in 3 additional patients (12.5%) are significantly higher rates than observed with other AVMs elsewhere, reflecting a cautious surgical attitude. In general, we recommend a cautious surgical attitude towards insular and basal ganglia AVMs, and consider radiosurgery for many patients with these AVMs when the surgical risks of causing new language deficits are high.
CONCLUSION
Transsylvian-transinsular approaches safely expose vascular pathology in or deep to the insula while preserving overlying eloquent cortex in the frontal and temporal lobes. The anterior transsylvian-transinsular approach can be differentiated from the posterior approach based on technical differences in splitting the Sylvian fissure and anatomical differences in final exposure.
Acknowledgments
This research is funded in part by the National Institutes of Health, R01 NS034949 (WLY) and P01 NS044155 (Center for Cerebrovascular Research).
Footnotes
Disclosures: The authors have no personal or financial interests in the drugs, materials, or devices described in this article.
The authors have reviewed and beautifully illustrated their extensive experience with transsylvian-transinsularapproaches. The report is valuable as it contains important data on their experience not only regarding microsurgical tactics, but also regarding patient selection. It is very valuable that experienced colleagues are explicit not only with how to meet technical challenges, but also with the importance and methods of selecting patient who are likely to benefit from technically demanding surgery. One very important feature of surgical decision making is that we make individual treatment decision. We do not accept a high number of patients “needed to treat” for one to benefit.
Tiit Mathiesen, Stockholm, Sweden
This is a very nice, well written description of Dr. Lawton’s experience with vascular lesions (AVMs and cavernous angiomas) in the insula and basal ganglia that were approached through either an anterior or posterior approach through the Sylvian fissure. During a 13-year period, Dr. Lawton has operated on 24 AVMs and 17 cavernous angiomas in this fashion and his results have been excellent. Although the surgical approaches described in this paper are fairly standard, the authors emphasize some important points. They point out that opening the distal Sylvian fissure completely is much more difficult than opening the proximal fissure because the Sylvian cistern posteriorly is much narrower and it is rather difficult to separate the frontal and temporal opercule in these areas. I always fear damaging the peri-Sylvian speech areas when operating on the dominant hemisphere and this is why I refer some of these posterior insula and basal ganglia AVMs for radiosurgery when they are small enough. Interestingly, my experience has been that those AVMs that are actually in the Sylvian fissure, particularly the large ones, result in more generous CSF spaces around the AVM which makes it easier to open the fissure in these cases. Many of these lesions are fed, as the authors point out, by vessels en passage, which makes it imperative to dissect completely the M-3 branches in this region taking only the short side branches to the AVM and preserving the main trunk. At times, when encountering significant bleeding with some of these lesions, I have opted to place one or more temporary clips proximally to allow fast but careful dissection in a dry field of the branches en passage that can run in close relationship to the AVM. It is remarkable that the senior author did not produce any significant speech deficit operating on these complex lesions even though over half of them were in the dominant hemisphere.
The approach to cavernous malformations is of course much more straight forward and the point that I emphasize to the residents with these lesions is that the surgeon should not sacrifice any significant arteries or veins under the impression that they are either feeding or draining the malformation. Any veins of significant size are usually part of the venous angioma that is so frequently associated with these lesions and any significant artery that appears to feed the cavernous angioma invariably passes around it to go to distal brain. Incidentally, I am surprised that the authors can be so sure that they resected “completely” 95% of the cavernous angiomas based on the postoperative MRIs. When resecting cavernomas from critical areas such as the basal ganglia, and particularly, the brainstem, I make an effort to not remove any of the surrounding hemosiderin-stained brain. This frequently results in a confusing postoperative MRI frequently read by the neuro-radiologist on a report that unfortunately gets to the patient all too often, as “residual cavernoma cannot be completely ruled out”.
In brief, I congratulate Dr. Lawton and his colleagues on a well illustrated, well written report of an excellent surgical series.
Roberto C. Heros, Miami, Florida
References
- 1.Bertalanffy H, Gilsbach JM, Eggert HR, Seeger W. Microsurgery of deep-seated cavernous angiomas: report of 26 cases. Acta Neurochir (Wien) 1991;108(3–4):91–99. doi: 10.1007/BF01418515. [DOI] [PubMed] [Google Scholar]
- 2.Gross BA, Duckworth EA, Getch CC, Bendok BR, Batjer HH. Challenging traditional beliefs: microsurgery for arteriovenous malformations of the basal ganglia and thalamus. Neurosurgery. 2008 Sep;63(3):393–410. doi: 10.1227/01.NEU.0000316424.47673.03. discussion 410–391. [DOI] [PubMed] [Google Scholar]
- 3.Heffez DS. Stereotactic transsylvian, transinsular approach for deep-seated lesions. Surg Neurol Aug. 1997;48(2):113–124. doi: 10.1016/s0090-3019(96)00463-6. [DOI] [PubMed] [Google Scholar]
- 4.Moshel YA, Marcus JD, Parker EC, Kelly PJ. Resection of insular gliomas: the importance of lenticulostriate artery position. J Neurosurg Nov. 2008;109(5):825–834. doi: 10.3171/JNS/2008/109/11/0825. [DOI] [PubMed] [Google Scholar]
- 5.Nagata S, Sasaki T. The transsylvian trans-limen insular approach to the crural, ambient and interpeduncular cisterns. Acta Neurochir (Wien) Aug. 2005;147(8):863–869. doi: 10.1007/s00701-005-0554-y. [DOI] [PubMed] [Google Scholar]
- 6.Schramm J, Aliashkevich AF. Surgery for temporal mediobasal tumors: experience based on a series of 235 patients. Neurosurgery. 2007 Feb;60(2):285–294. doi: 10.1227/01.NEU.0000249281.69384.D7. discussion 294–285. [DOI] [PubMed] [Google Scholar]
- 7.Lang FF, Olansen NE, DeMonte F, et al. Surgical resection of intrinsic insular tumors: complication avoidance. J Neurosurg Oct. 2001;95(4):638–650. doi: 10.3171/jns.2001.95.4.0638. [DOI] [PubMed] [Google Scholar]
- 8.Hentschel SJ, Lang FF. Surgical resection of intrinsic insular tumors. Neurosurgery. 2005 Jul;57(1 Suppl):176–183. doi: 10.1227/01.neu.0000163603.70972.ab. discussion 176–183. [DOI] [PubMed] [Google Scholar]
- 9.Malak R, Bouthillier A, Carmant L, et al. Microsurgery of epileptic foci in the insular region. J Neurosurg Jun. 2009;110(6):1153–1163. doi: 10.3171/2009.1.JNS08807. [DOI] [PubMed] [Google Scholar]
- 10.Kaya RA, Turkmenoglu O, Ziyal IM, Dalkilic T, Sahin Y, Aydin Y. The effects on prognosis of surgical treatment of hypertensive putaminal hematomas through transsylvian transinsular approach. Surg Neurol. 2003 Mar;59(3):176–183. doi: 10.1016/s0090-3019(02)01043-1. discussion 183. [DOI] [PubMed] [Google Scholar]
- 11.Jianwei G, Weiqiao Z, Xiaohua Z, Qizhong L, Jiyao J, Yongming Q. Our experience of transsylvian-transinsular microsurgical approach to hypertensive putaminal hematomas. J Craniofac Surg Jul. 2009;20(4):1097–1099. doi: 10.1097/scs.0b013e3181abbf09. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi T, Aoki N, Sakai T, Mizutani H. “Transinsular approach” for the treatment of a medial temporal arteriovenous malformation. Neurosurgery. 1993 May;32(5):863–866. doi: 10.1227/00006123-199305000-00026. discussion 866. [DOI] [PubMed] [Google Scholar]
- 13.Tirakotai W, Sure U, Benes L, Krischek B, Bien S, Bertalanffy H. Image-guided transsylvian, transinsular approach for insular cavernous angiomas. Neurosurgery. 2003 Dec;53(6):1299–1304. doi: 10.1227/01.neu.0000093496.61236.66. discussion 1304–1295. [DOI] [PubMed] [Google Scholar]
- 14.Mathiesen T, Edner G, Kihlstrom L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg Jul. 2003;99(1):31–37. doi: 10.3171/jns.2003.99.1.0031. [DOI] [PubMed] [Google Scholar]
- 15.Conrad M, Schonauer C, Morel C, Pelissou-Guyotat I, Deruty R. Computer-assisted resection of supra-tentorial cavernous malformation. Minim Invasive Neurosurg Jun. 2002;45(2):87–90. doi: 10.1055/s-2002-32485. [DOI] [PubMed] [Google Scholar]
- 16.Duffau H. Intraoperative direct subcortical stimulation for identification of the internal capsule, combined with an image-guided stereotactic system during surgery for basal ganglia lesions. Surg Neurol Mar. 2000;53(3):250–254. doi: 10.1016/s0090-3019(00)00183-x. [DOI] [PubMed] [Google Scholar]
- 17.Waldron JS, Lawton MT. The supracarotid-infrafrontal approach: surgical technique and clinical application to cavernous malformations in the anteroinferior Basal Ganglia. Neurosurgery. 2009 Mar;64(3 Suppl):86–95. doi: 10.1227/01.NEU.0000335647.71014.07. discussion 95. [DOI] [PubMed] [Google Scholar]
- 18.Chang EF, Gabriel RA, Potts MB, Berger MS, Lawton MT. Supratentorial cavernous malformations in eloquent and deep locations: surgical approaches and outcomes. J Neurosurg. 2010 Jul 2;2:2. doi: 10.3171/2010.5.JNS091159. [DOI] [PubMed] [Google Scholar]
- 19.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg Oct. 1986;65(4):476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 20.Lawton MT. Spetzler-Martin Grade III arteriovenous malformations: surgical results and a modification of the grading scale. Neurosurgery. 2003 Apr;52(4):740–748. doi: 10.1227/01.neu.0000053220.02268.9c. discussion 748–749. [DOI] [PubMed] [Google Scholar]
- 21.Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery. 2010 Apr;66(4):702–713. doi: 10.1227/01.NEU.0000367555.16733.E1. discussion 713. [DOI] [PMC free article] [PubMed] [Google Scholar]