Abstract
In 1991, the Biophysics Research Institute at the Medical College of Wisconsin was among the first groups to develop functional Magnetic Resonance Imaging (fMRI). Our story is unique on a few levels: We didn’t have knowledge of the ability to image human brain activation with MRI using blood oxygenation dependent (BOLD) contrast until early August of 1991 when we attended the Society for Magnetic Resonance in Medicine (SMRM) meeting in San Francisco, yet we produced our first BOLD-based maps of motor cortex activation about a month later. The effort started with two graduate students, Eric Wong and myself. Only a few days prior to that extremely important SMRM meeting, we had developed human echo planar imaging (EPI) capability in-house. Wong designed, built, and interfaced a head gradient coil made out of sewer pipe, wire, and epoxy to a standard GE 1.5 T MRI scanner. Also, a few months prior to building this human head gradient coil he developed the EPI pulse sequences and image reconstruction. All of these efforts were towards a different goal – for demonstration of Wong’s novel approach to perfusion imaging in the human brain. Following SMRM, where a plenary lecture by Tom Brady from MGH opened our eyes to human brain activation imaging using BOLD contrast, and where we learned that EPI was extremely helpful if not critical to its success, we worked quickly to achieve our first results on September 14, 1991. The story is also unique in that Jim Hyde had set up the Biophysics Research Institute to be optimal for just this type of rapidly advancing basic technology research. It was well equipped for hardware development, had open and dynamic collaborative relationships with other departments, hospitals on campus, and GE, and had a relatively flat hierarchy and relaxed, flexible, collegial atmosphere internally. Since these first brain activation results, MCW Biophysics has continued to be at the forefront of functional MRI innovation, having helped to pioneer real time fMRI, high-resolution fMRI, and functional connectivity mapping.
Introduction
Serendipity played a large role in the timing and rapidity of the development of Functional Magnetic Resonance Imaging (fMRI) at the Medical College of Wisconsin (MCW). The group at MCW was likely the third, behind the Massachusetts General Hospital (MGH) and the University of Minnesota (UM), to perform a successful fMRI experiment, and was the first to have their fMRI results make it into publication (Bandettini et al., 1992). MGH and UM published their papers on June 15, 1992 and July 1, 1992 respectively in PNAS (Kwong et al., 1992a; Ogawa et al., 1992). The next groups with fMRI publications were Yale (Blamire et al., 1992), Gottingen, Germany (Frahm et al., 1992), and the National Institutes of Health (Turner et al., 1993).
“Luck is when preparation meets opportunity.” This quote originated with the Roman philosopher Seneca and was one of my dad’s favorites. The MCW group was extremely lucky, as we were perfectly prepared for the opportunity that was going to rapidly reveal itself. How did fMRI start so early and so quickly at MCW - a relatively small research group in a moderately known medical college? Here I try to look back to shed light on that question, and hopefully convey the charged scientific atmosphere and extreme excitement at the time. Revolutions in methodology like this come along perhaps once in a career. At MCW, Eric Wong and myself, at the start of our careers, were swept up in this revolution. Eric Wong was finishing his Ph.D. thesis work on MRI gradient coil design and about to enter the final medical school phase of the Medical Scientist Training Program (MSTP). I was a second year Ph.D. student searching for a thesis project. We were extremely lucky as we had prepared and were running with this opportunity.
Other key individuals were Jim Hyde, Andrzej Jesmanowicz, Scott Hinks, Ron Tikofsky, and Lloyd Estowski. Jim Hyde was mine and Eric’s zthesis advisor and chair of the Biophysics Research Institute (BRI). The BRI became the Department of Biophysics several years later. Research at the BRI was and is divided between Electron Paramagnetic Resonance (EPR) research and Magnetic Resonance Imaging (MRI) and spectroscopy (MRS) research. Hyde, who established his international reputation with EPR method innovation and coil development, was still spending most of his time with this specialty and a small amount of time on high resolution MRI with home-built coils. The MRI research part of the BRI was relatively small. Andrzej Jesmanowicz was working on all aspects of MRI development - from RF coils to processing methods. Scott Hinks, my co-advisor, was a new scientist at the Applied Science Lab at GE Medical Systems, 15 miles west of the Medical College of Wisconsin. Ron Tikofsky was in charge of the single photon emission tomography (SPECT) resource at Froedtert Memorial Lutheran Hospital, an associated hospital on campus. Lloyd Estowski was the MR technologist employed by the Biophysics Research Institute who was an important element in the success of many of the very late-night fMRI experiments that took place during these early years.
Many others at MCW became involved as fMRI grew. One defining characteristic of the early days of fMRI at MCW, and perhaps at other new fMRI centers elsewhere, was a tangible openness and enthusiasm as well as very few obstacles that stood in the way of accomplishing things. Things got done – rapidly. At MCW this positive atmosphere was in no small part to Hyde’s vision and setup of the BRI, and the BRI’s respected status at the medical school and hospital campus.
In this article, the scientific context, the key developments, and the primary people associated with the inception and early growth of fMRI at MCW are described. A timeline is sketched, and then the most important factors of the early fMRI success at MCW are discussed.
Timeline (early 80’s –1995)
1985 – 1989
Jim Hyde started a research program in MRI development at the BRI in about 1985. By the late 80’s the BRI had developed an international reputation in MRI coil development and had NIH funding. Hyde had earlier developed an international reputation in EPR resonator development. The transition between EPR and MRI was made possible by the loop gap resonator. He recognized that the ratio of the dimensions of the resonator to the size of the object being studied was about the same and therefore realized that loop gap resonators should work for both EPR and MRI.
One of the first GE Signa 1.5T scanners produced was installed at Froedtert Memorial Hospital and was reserved for BRI students and professors on weekends in the afternoon and weekday evenings after about 9:00 PM. In August of 1986, Eric Wong entered the Medical Scientist Training Program (MSTP) at MCW. In August of 1989, I entered graduate school in the Biophysics Research Institute at MCW.
January – June, 1991
Exploring possible avenues for a Ph.D. thesis project, I began experiments to detect activation-induced changes in MRI signal using diffusion imaging with b = 50 s/mm2 and standard multi-shot acquisition. According to the Intravoxel Incoherent Motion (IVIM) model put forth by Le Bihan et al (Le Bihan et al., 1986; Le Bihan and Turner, 1992; Turner et al., 1990), perfusion attenuates signal in a similar fashion to diffusion when using a “b value” in the range of 30 to 50 s/mm2. The hypothesis was that flow of blood through capillaries mimicked, statistically, very rapid diffusion that would have been observable (as would any activation-related changes in this apparent diffusion coefficient) using low b values. I was not successful in these experiments for a number of reasons. Among these reasons is that multi-shot imaging with diffusion weighting without navigators causes significant temporal instability, masking any small change. Even more problematic is that any change in apparent diffusion coefficient, is likely to be an order of magnitude more subtle than what was detectible as demonstrated by Kwong et al (Kwong et al., 1991b). Cerebral spinal fluid also tends to dominate the signal at these low b values. Nevertheless, this line of research, inspired by Le Bihan’s work, was extremely interesting to me. From this point on, I was intent on measuring or mapping brain activation with any MRI-based method possible. At about this time, I started working with Wong, who was both mentor and co-conspirator in these efforts.
Also at this this time, Eric was working on developing the EPI pulse sequence and reconstruction. For performing EPI on the GE 1.5 tesla scanner, he was using a local wrist gradient coil that he designed and constructed previously.
Aug 3 – 5, 1991
Eric Wong designed the three-axis, balanced-torque head gradient coil using his conjugate gradient descent optimization method (Wong et al., 1991a) running on his NeXT computer. With a bit of my help and the help of his wife, Denise, Wong built this coil out of sewer pipe, wire, and epoxy in the BRI machine shop. This gradient coil was the latest in a series of several that had built since starting graduate school. The first were wrist or small animal local gradient coils (Wong et al., 1991b). For this head coil, the inner diameter was 26.5 cm. On the standard GE gradients at the time (100 Amp), the gradient strength was about 2 G/cm for all three axes with a rise time of 50 microseconds from zero to full scale. Because the gradient coil was balanced-torque it did not require any reinforced stabilization while being used for scanning. It was simply lifted into place (weighing less than 30 lbs) on the patient table and somewhat superficially strapped down with a patient restraint band. Given how local gradient coils (even torque-balanced) are stabilized inside scanners today, this procedure that was used seems a bit cavalier in retrospect.
This first head gradient coil, described in more detail in the paper by Eric Wong in this special issue is shown in Figure 1. Figure 1 A shows Eric and myself during the initial stages of building the coil. We had traced out and wound the z-axis wires. We repeated, three times, the steps of tracing, gouging out grooves for the wires, placing wires, adding epoxy, and smoothing out the dried epoxy. In all, the process took an entire weekend of continuous work and required just about every piece of equipment in the machine shop to be used – not always in a conventional manner (e.g. we had a lathe hooked up to rotate the gradient coil so that the epoxy dried evenly). Figure 1 B shows Eric, his wife Denise, and myself with the completed coil and with our first image – we hadn’t slept in two days as we were pressed for time to complete it (more on that below). On the following Monday morning, the machine shop manager, Dick Johnson, who took great pride in keeping an orderly shop, saw a single drop of dried epoxy on the floor and expressed his dismay. All other evidence of our completely taking over and reconfiguring the machine shop was gone.
Figure 1.
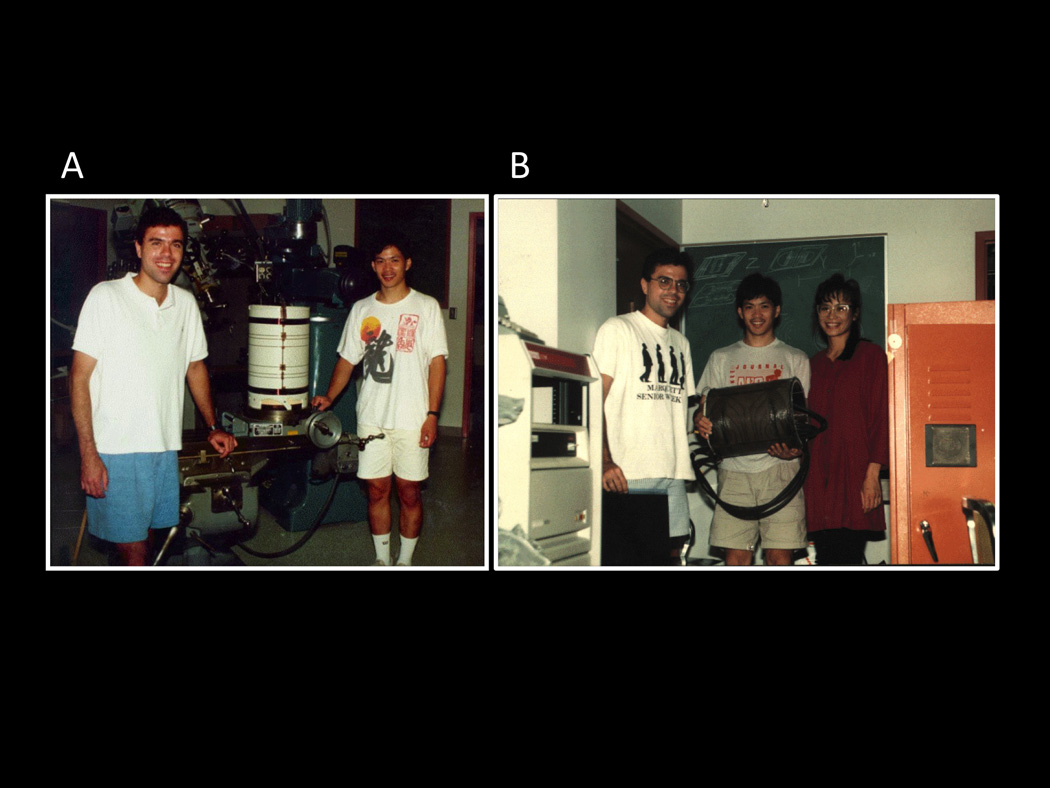
These were taken during the first week of August, 1991. They show: A. Eric Wong and myself during the early stages of the construction of the three-axis balanced torque human head gradient coil that was used for the first collection of fMRI data at MCW. Here, the coil has the first layer of wires (z-axis) applied. B. Here, Eric, his wife Denise, and I show the coil after collecting the first image (an apple) with it. It was interfaced to a GE 1.5T Signa scanner. This picture was taken after we’ve been working almost continuously for about 36 hours to complete it.
This gradient / RF coil configuration was decommissioned in 1992 yet performed well enough during this crucially important time. The motivation to quickly build the coil prior to SMRM stemmed from the fact that Eric had written an abstract to be presented at the meeting on a perfusion measurement sequence that he had developed (Wong and Hyde, 1991). Implementation of this sequence required single-shot spin-echo echo planar imaging (EPI). He had preliminary results using his smaller gradient coil on a small animal, but wanted human results to further demonstrate the technique. This sequence, involving two spatially interleaved comb pulses – 90 degree excite then a 180 degree refocus - ultimately turned out to be too sensitive to motion. Nevertheless, the coil was built and the sequences, including EPI, worked well with the use of the human head gradient coil.
Aug 7, 1991
The first object (an apple) and human head (Eric’s wife) were imaged using the gradient coil. The pulse sequence was a basic fast low angle shot (FLASH) imaging sequence. The first echo planar images of a human head at MCW were also collected on this day. At this point in time Eric and I still had no idea that imaging brain activation with time series collection of gradient-echo images and using endogenous blood oxygenation contrast was possible, yet we were perfectly prepared, only days prior to our learning of this, to hit the ground running in this direction. At this moment, we were one of the few groups in the world that had EPI capability. This capability greatly improved time series signal stability, thus proving important if not critical to detecting very small activation-related changes.
Aug 12, 8:40 AM, 1991
Tom Brady (MGH NMR Center) gave a plenary lecture on “Future Prospects for MR Imaging” in the Tenth Anniversary Session of the 10th Annual SMRM meeting in San Francisco (Brady, 1991). Here he showed Ken Kwong’s movie of brain activation obtained using MRI but with no exogenous contrast. The movie was shown as a time series of subtraction images. This is the point where one might say that the light bulbs appeared above Eric’s and my heads as we sat in the audience. A key statement by Brady, perhaps more open-ended than what he intended was, to paraphrase: “…and we don’t really know what’s causing this.” This one phrase was inspirational in that I saw a huge opportunity for a thesis project, but still had no idea it would turn into a career.
Neither Eric nor I were able to determine from Kwong’s movie if the signal went up or down with activation (i.e. which way the subtraction was performed). I assumed, incorrectly, that during brain activation, the increase in cerebral metabolic rate would outweigh any abundance of oxygen delivered with a flow increase, thus leading to a decrease in oxygenation - and signal. Therefore, I was only looking for signal decreases in functionally active regions. In the MCW group’s first successful results – described below - we only saw increases that were localized in the appropriate places (motor cortex). For a short time this puzzled me. While investigating the literature of metabolic and hemodynamic changes with brain activation (Fox and Raichle, 1986; Frostig et al., 1990), I realized that my initial hypothesis was wrong and that there was ample evidence from other modalities that the blood oxygenation level dependent (BOLD) signal should, in fact, increase during activation.
Aug 10–16, 1991
Bernice Hoppel (MGH NMR Center) in her abstract presentation at SMRM, 1991: “Measurement of Regional Brain Oxygenation State Using Echo Planar Line-width Mapping” (Hoppel et al., 1991) showed Ken Kwong’s movie again.
Aug 10–16, 1991
Ken Kwong’s SMRM abstract (Kwong et al., 1991a) and later his paper (Kwong et al., 1991b) helped to demonstrate that Intravoxel Incoherent Motion (IVIM) was in fact less selective and sensitive to perfusion than previously thought. This was important in that it contributed to redirecting the focus of the field to blood oxygenation level dependent contrast for imaging of brain function.
Aug 10–16, 1991
Jack (John) Belliveau (MGH NMR Center) presented the first MRI-based brain activation maps. He compared two gadolinium-injection derived blood volume maps obtained using EPI data collection – one before and one during visual stimulation (Belliveau et al., 1991a). Jack received the Young Investigator Award at this meeting for this pioneering work.
Aug 20, 1991
Once back from the SMRM meeting, Wong modified his spin-echo EPI sequence, taking out the 180-degree pulse, to create gradient-echo EPI. During this time, I contacted Robert Weisskoff from MGH, asking about some essential parameters such as echo time (50 ms was about optimal for 1.5 Tesla since the T2* of gray matter was in this range.), and the temporal signal to noise (Robert mentioned a value of 120/1 which, interestingly, has never really improved in twenty years due to the predominance of physiologic noise in the time series.)
Sept 2, 1991
Eric and I started performing experiments using gradient-echo EPI to detect brain activation. Likely, because of RF coil issues and motion effects, these initial experiments were unsuccessful. In addition, it probably didn’t help that I was still looking for activation-induced signal decreases.
Sept 14, 1991
The first successful fMRI experiment at MCW was carried out on the 1.5T GE Signa scanner at Froedtert Memorial Hospital (Bandettini et al., 1992). I was the volunteer. Eric was operating the scanner. Finger tapping was performed since we were both familiar with motor cortex organization and, with the help of a textbook homunculus map, knew where to look. Since the entire collection and reconstruction process was extremely time consuming and cumbersome – as we scanned “blind” (without seeing any images or raw data during the scan session) and had to reconstruct all data the following day, and since our early EPI sequence only allowed single-plane (i.e. no multi-slice), we had to prospectively make very good choices of where to image. We decided to hedge our bets and choose a very thick axial slice. Imaging parameters were as follows: Field strength = 1.5T, TE = 50 ms, TR 3 sec, FOV = 24, matrix size = 64 × 64, number of imaging planes = 1, slice thickness = 25 mm, number of images in time series = 72, timing was rest = 75 sec, active = 75 sec, rest = 75 sec. Post processing was performed on a Sun SPARCstation 1+ and two Tektronix XD88 workstations. Eric yelled through the intercom at the appropriate times for the subject to “go” or “stop”. A simple linear drift removal method was used as well as the initial elements of correlation analysis (vector product with boxcar function) to create the functional maps. The complete depiction of the first successful experiment is shown in Figures 2 – 4, which are pages out of my coffee-stained lab notebook dating back to September 14 and September 16. We performed the experiment on September 14 and finally looked carefully at the data on September 16.
Figure 2.
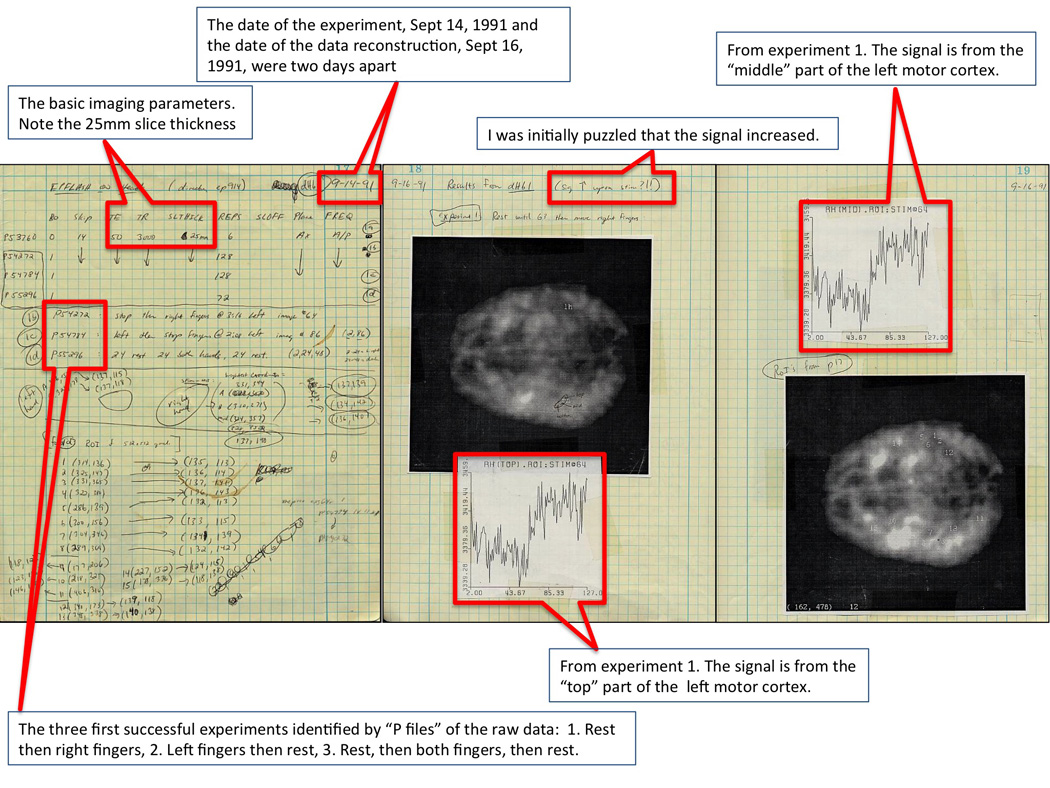
These are the first three of nine pages from my lab notebook, showing our first successful results. The experiments were performed Sept 14, 1991 and the data were finally reconstructed and analyzed Sept 16, 1991. The first three experiments were of a very thick (25 mm) axial slice through the motor cortex of my brain. The signal clearly goes up in the contralateral motor cortex during finger tapping. I was initially puzzled that the signal should increase, but then, on a review of PET and optical imaging literature, giving evidence that with activation, oxygen extraction fraction went down, it all made sense.
Figure 4.
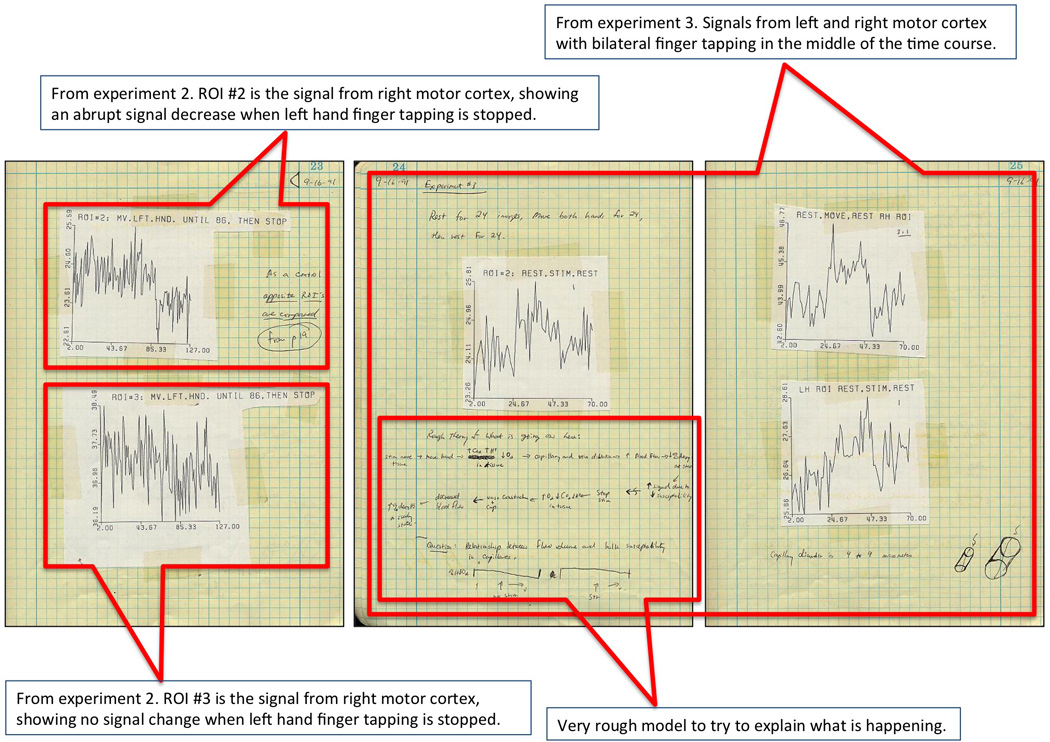
These are the last three pages of the nine from my lab notebook, showing our first successful results. They include more time series, and, specifically, a time series showing our first true block design: rest, bilateral finger tapping, and then rest again. I also started doodling a rough model for what I thought was going on.
It should also be noted that these are the first fMRI results obtained with a whole brain RF coil, which helped to provide convincing evidence that fMRI was a localized effect. If one used a surface coil, and an entire region became bright, it would not be immediately clear if it were a global and/or artifactual effect. The use of a whole brain coil, and localized motor cortex activity on one side and then the other, allowed us to differentiate focal activation (i.e. one motor cortex) from global artifact.
Oct, 1991
I used the prototype GE z-axis gradient coil for functional MRI for the first time. Since the GE bays at the plant in Waukesha (at least the Applied Science Lab Bay 2) had no intercom, the subject was notified to move his fingers by my pulling a string that was attached to the subject’s fingers. Since the gradient coil was only z-axis, collection of sagittal or coronal echo planar images was required. The first results with this coil are shown in Figure 5 - reproduced from Figure 2a and 3 of Bandettini et al (Bandettini et al., 1992) with permission from Wiley. Note the activation in the appropriate place on the homunculus yet the strong pulsation artifact at the base of the brain. The first volunteer for this study was a new graduate student, Alan Song, who later went on to characterize the effects of diffusion weighting on activation-induced BOLD signal changes (Song et al., 1996), and is now professor and director of the Brain Imaging and Analysis Center at Duke University.
Figure 5.
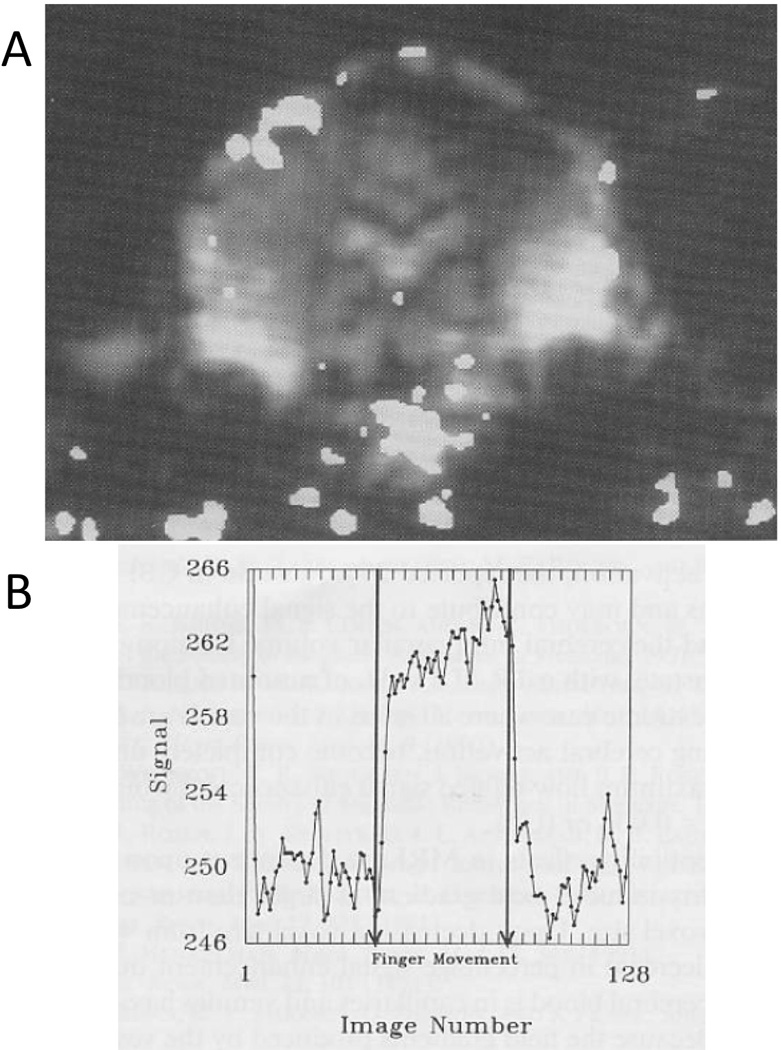
These are the results from the GE prototype z-axis head gradient coil. During this experiment (Alan Song was the volunteer), I had to pull a string attached to his hand to get him to tap his fingers. A. This is a functional map that was “thresholded” or “threshheld” (these really should become words since they are used so much in fMRI) and superimposed on an EPI anatomic image from the time series. Because the gradient coil was z-axis, we had to collect either sagittal or coronal EPI slices. Note the large pulsation artifact at the base of the brain. B. The signal to noise of the GE body RF coil was a better than that of the first generation RF coil inside the gradient coil. The time series signal from a region of interest in the motor cortex looks less contaminated by noise. (Reproduced with permission from Wiley)
Figure 3.
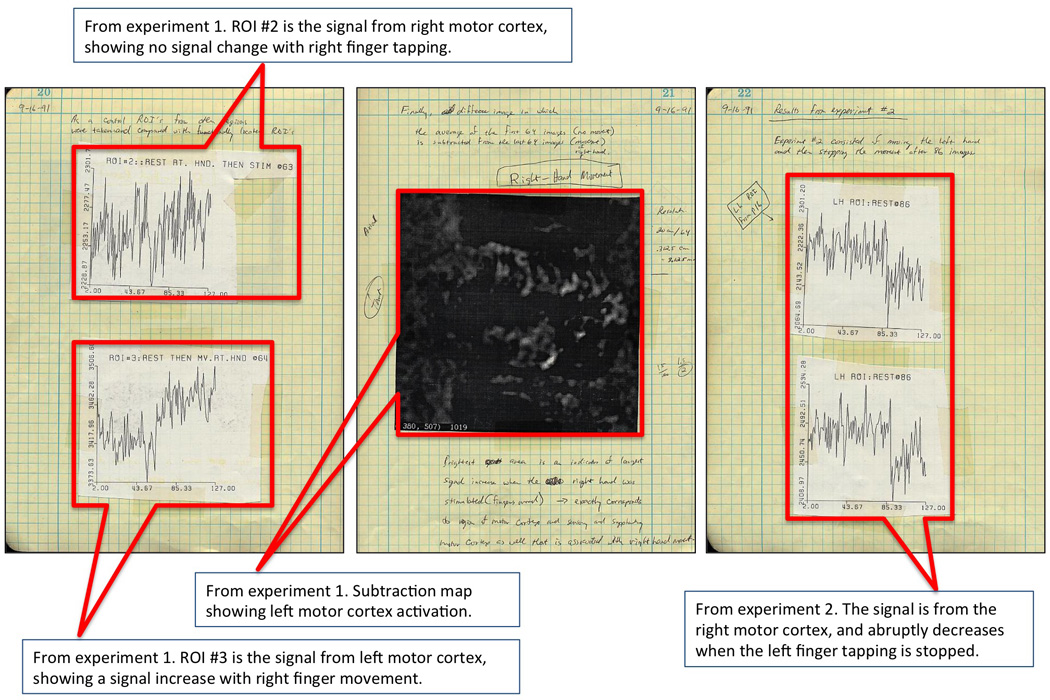
These are the second three of nine pages from my lab notebook, showing our first successful results. I show control data showing no signal change from the ipsalateral motor cortex and also show a subtraction map, revealing a signal increase in left motor cortex during right finger tapping.
Nov, 1991
Jack Belliveau’s paper showing the first gadolinium-based, functional MRI results was published in Science (Belliveau et al., 1991b). The results, as they were depicted on the cover of Science, quickly became iconic. Ironically, while this method (dynamic susceptibility contrast) would prove to be clinically useful for mapping baseline blood volume and perfusion, it was obsolete for the purpose of functional brain imaging before the ink on that Science article dried. This paper was important, however, as it opened everyone’s eyes to the new potential of MRI and permanently changed the human functional brain imaging world.
Nov, 1991 – Feb 1992
MCW’s first paper on fMRI was being written up. I had referred to PET research by Fox et al (Fox and Raichle, 1986) and optical imaging results of Frostig et al (Frostig et al., 1990) to explain the neurovascular coupling mechanism behind the observed signal increases – and specifically, to give evidence that blood oxygenation increased in these activated regions due flow increases apparently exceeding the metabolic demand. Other literature that I cited in our paper what was critical to explaining this effect was Thulborn’s early study of blood oxygenation effects on T2 (Thulborn et al., 1982), the work of Brindle et al (Brindle et al., 1979), Brooks et al (Brooks and Di Chiro, 1987), Gomori et al (Gomori et al., 1987), Hayman et al (Hayman et al., 1988) and Wright et al (Wright et al., 1991) in further explaining blood susceptibility and the effect on MR signal. Not referenced in my initial paper but fundamentally important in elucidating the unique qualities of Hemoglobin is the work of Coryell and Pauling (Pauling and Coryell, 1936). Also, unknown to me as I was trying to understand these effects, was a clear description by Fabry and San George in 1983, of the varying susceptibility effects of hemoglobin (Fabry and San George, 1983).
Ogawa’s pioneering studies helped to complete the picture, fully explaining the observed MR signal changes in vivo (Ogawa and Lee, 1990; Ogawa et al., 1990a; Ogawa et al., 1990b). Ogawa also coined the term “BOLD.” Lastly, the details of susceptibility contrast at the “intermediate exchange regime” were beautifully explained and simulated in the manuscripts by Ogawa et al (Ogawa and Lee, 1990), Fisel et al (Fisel et al., 1991), Villringer et al (Vilringer et al., 1988), and Majumdar et al (Majumdar, 1991). Corroborating in vivo data, were also shown by Turner et al (Turner et al., 1991).
A paper that was apparently overlooked by everyone was another in vivo study by Terrier et al in which they observed BOLD effects in the rat kidney during renal ischemia (Terrier et al., 1989).
One other side note, it appears that many, including Kwong, Turner, Ogawa, and Koretsky were beginning to realize in the late 80’s and very early 90’s that blood was a potentially useful endogenous contrast, and were all working towards brain activation imaging. I jumped in relatively late, having just entered graduate school in 1989 and, for a few months, having travelled down the unsuccessful diffusion gradient sensitization path for imaging perfusion changes. We made a quick alteration in our approach, based on Brady’s presentation, and then went back to the literature to explain our rapidly obtained results. We obtained these quickly since we had EPI capability – something that several other of these leading groups lacked at the time.
Feb 5, 1992
MCW’s first paper on fMRI, “Time course EPI of human brain function during task activation” was submitted to Magnetic Resonance in Medicine (MRM) as a Communication. Kwong’s paper was submitted on March 26 to PNAS, and Ogawa’s paper was submitted on March 31 to PNAS. From talking with the other authors from MGH and MN, both of these papers were apparently second submissions after initial rejections from higher tier journals.
February to April, 1992
Wong built a second, higher performance and more robust three axis, balanced torque head gradient coil and an insertable end-cap quadrature coil. Other coils – for 0.5T and 3T – were also built for this gradient coil. Andrzej Jesmanowicz spearheaded the Bruker 3T EPI pulse sequence and recon development that was starting about this time.
The method of optimization of Wong’s new gradient coil, shown in Figure 6 A, was again conjugate gradient descent. It had an inner diameter of 30 cm and a length of 37 cm. The maximum gradient strengths (G/cm@100 Amps) were 2.272 for X, 2.336 for Y, and 2.487 for Z. As a result of the extremely low coil inductance (0.149 mH for X, 0.174 mH for Y, and 0.076 mH for Z), the minimum rise time from zero amplitude to full scale was approximately 50 µs. This coil itself was used at MCW until about 1999. Medical Advances, a local company with relations with MCW Biophysics, subsequently marketed this basic coil design (the NIH and University of Wisconsin – Madison each bought one). The key advantage to this and the previous gradient coil was that it allowed rapid gradient switching required for EPI without any modification to the, at the time, standard (100 Amp), gradient amplifiers.
Figure 6.
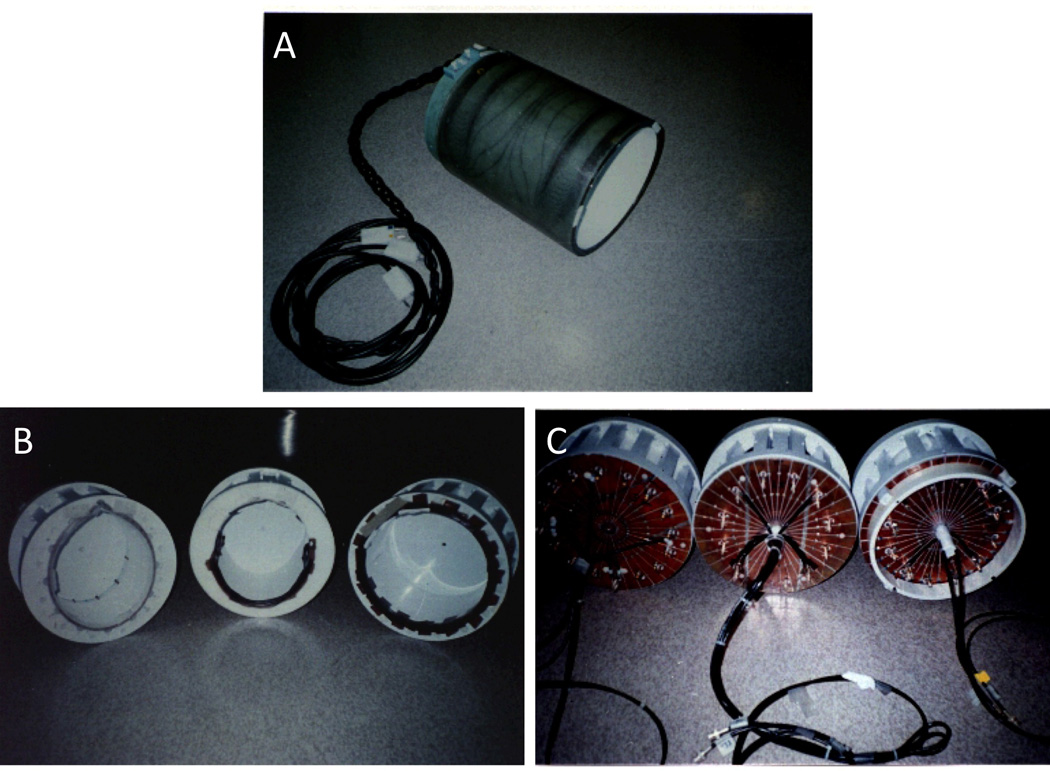
A. Our second-generation gradient coil. It was a bit larger and had the option to switch the z-axis windings from parallel to series to double the gradient strength in z for diffusion weighting – at the expense of slew rate. One advantage was that it allowed the insertion of different RF coils. The 0.5T, 1.5T, and 3T coils are shown in B and C. C shows that they were all end-capped with slits in the copper end-caps placed to minimize eddy currents.
The RF coil used at 1.5 Tesla was a low pass elliptical 12 element coil. The S/N improvement over a standard GE birdcage coil was measured to be approximately 1.5. The RF coils used at 0.5 T, 1.5 T, and 3 T are shown in Figure 6 B and C.
Figure 7A shows the second-generation gradient coil as it looked when it was about to be used for scanning. It was strapped to the table with a patient restraint band, and then the table, coil, and subject were pushed into the scanner bore. Figure 7B shows the two coils, modeled by me, in a side-by-side comparison.
Figure 7.
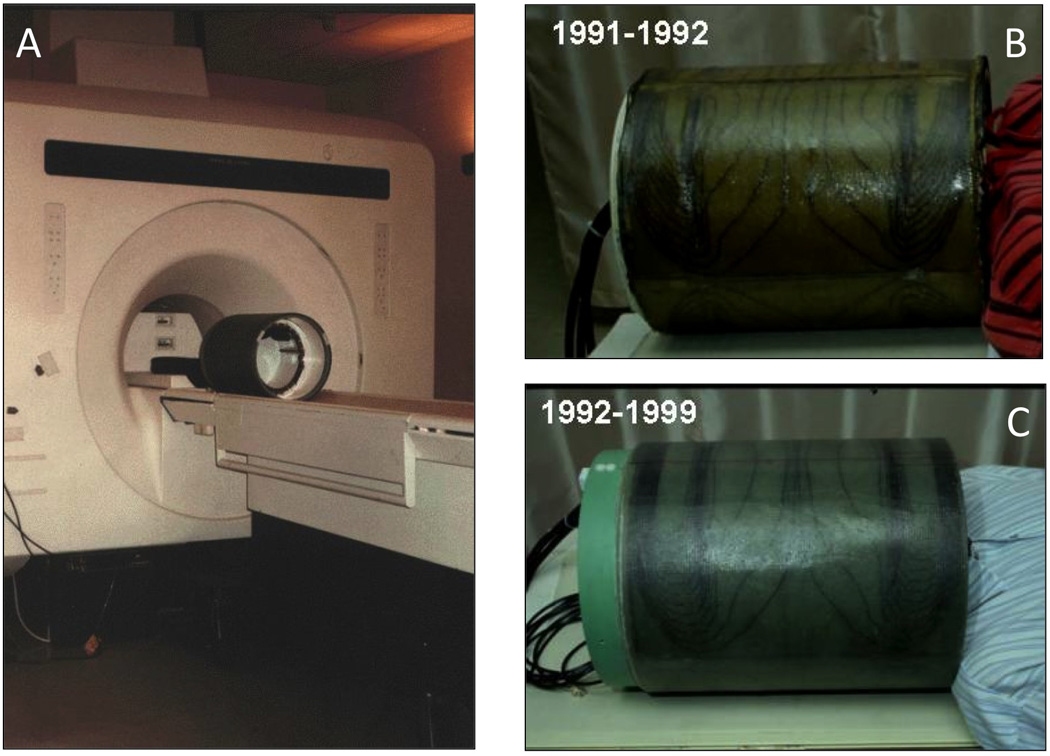
A. This is a picture of the balanced-torque gradient coil as it looked when it was about to be used. We only added a patient restraint band when using it. B and C show the two gradient coils being modeled by me – quite comfortable. The years that they were used are shown in the upper left of each picture.
April 28, 1992
The simultaneous, back-to-back publications of abstracts from MCW and MGH at the Society of Magnetic Resonance Imaging (SMRI) meeting in New York were the first abstract-form written documents on fMRI.
This more-clinical MRI meeting was held 6 months out of phase of the more-methods-based Society of Magnetic Resonance in Medicine (SMRM) meeting. It was essential to present the result as soon as possible, so I attended this meeting by myself for the sole purpose of giving this presentation. It was my first public presentation of any kind of my research. As mentioned, the MGH group – specifically Ken Kwong – also gave a presentation (Kwong et al., 1992b), showing their flow related and blood oxygenation related brain activation results.
At this SMRI meeting, I demonstrated the TE-dependence of the activation-induced gradient-echo and spin-echo signal, thus proving that the mechanism was based in R2* and R2 changes respectively. I showed that the change in R2* was larger than R2, thus helping to prove that the contrast mechanism was bulk susceptibility changes in the intermediate exchange regime (small vessels to red blood cells). I also presented a movie of left then right finger tapping - demonstrating clear delineation of function. In these early days we were mostly trying to prove that what we were seeing was not an artifact (i.e. motion, scanner instabilities, etc.) and had a mechanism based in brain activation. Years after this meeting, Ken Kwong mentioned to me that this movie of left, then right, then left hand finger tapping was one of the most clear demonstrations that fMRI was really looking at brain activation. The alternating left then right motor cortex activation data, collected with our unique whole brain RF coil (both MGH and UM had surface coils only), was helpful in showing that fMRI was “real” and not a global effect/artifact. The movie that was shown at this oral presentation is shown in Figure 8.
Figure 8.
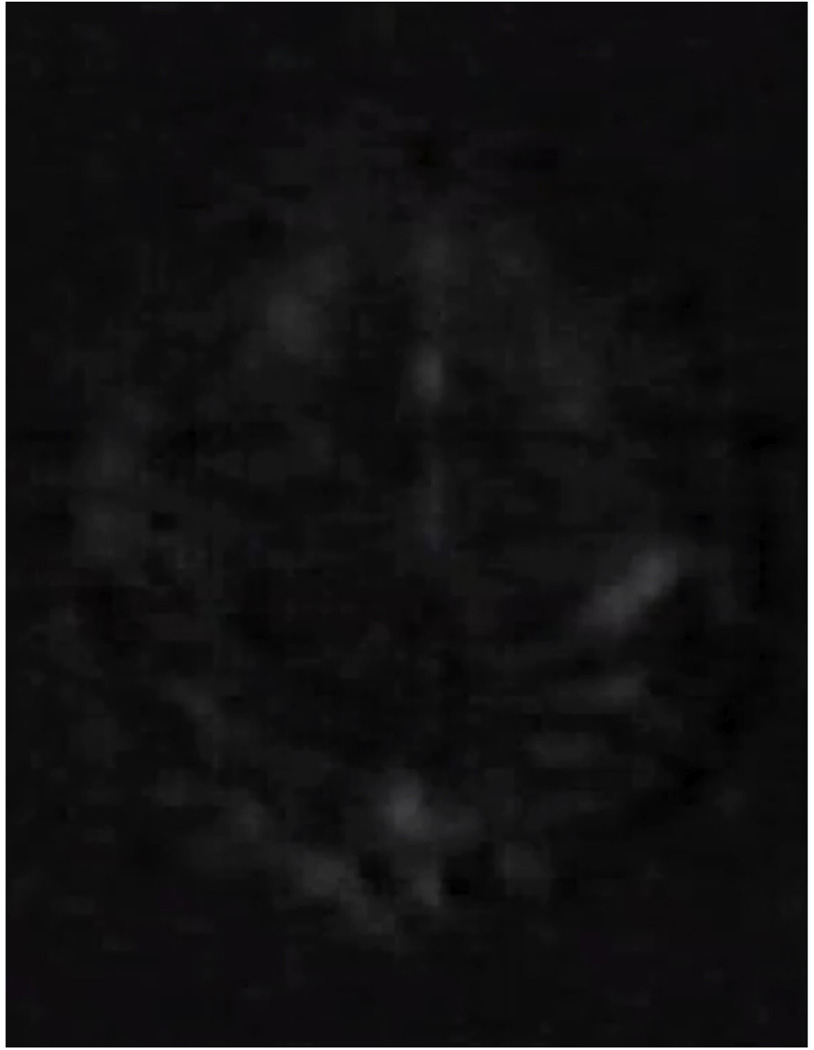
This fMRI-based brain activation movie of alternating left and right finger tapping was first presented at the SMRI meeting in New York in April of 1992. It is an axial slice and consists of a series of images that were subtracted from a baseline image. It is approximately 5 × faster than real time and clearly shows right then left motor cortex becoming active associated with right then left finger tapping.
Lastly, it’s worth mentioning that Michael Stehling (Stehling et al., 1992) also presented his breath-hold results at 1.0 Tesla during this same session. Siemens had just developed an EPI sequence, and this was their first demonstration of BOLD effects using EPI.
Two years following this meeting, SMRM and SMRI were merged into one larger meeting that was known as the Society of Magnetic Resonance (SMR). In 1996 SMR became the International Society of Magnetic Resonance in Medicine (ISMRM) that we have now.
June, 1992
“Time course EPI of human brain function during task activation” was published by Magnetic Resonance in Medicine (Bandettini et al., 1992).
June 17–19. 1993
One of the first conferences on fMRI, called “Functional MRI of the Brain” in the Ritz Carlton Hotel in Arlington, VA. For a fascinating snapshot into the fMRI world when it was only one year old, read the meeting report from this workshop (Le Bihan et al., 1993). I recall many of the luminaries past and present attending this - including Arno Villringer, Bob Shulman, Denis Le Bihan, Peter Fox, and Louis Sokoloff. I specifically remember a continuation of a classic debate between Fox and Sokoloff on whether brain activation causes hemodynamic “uncoupling” or not.
Also, I recall the first public discussions of resting state “physiologic fluctuations.” Jim Hyde, as chairman of a session, was allowed to show three slides at the start. They showed how the noise was clearly not Gaussian or white – therefore “physiologic” in origin. Robert Weisskopf and Peter Jezzard immediately came to their feet and said they had seen the same thing. It wouldn’t be until about 1994 when Bharat Biswal, also from MCW, made the jump to calculate the spatial correlation of this noise, thereby discovering “resting state” time series correlations across functional regions in the brain.
Feb 1, 1993
The second MCW paper: “Processing strategies for time-course data sets in functional MRI of the human brain,” was submitted to Magnetic Resonance in Medicine. It was published in August of 1993 (Bandettini et al., 1993). This concept for the paper came about from the merging of the kernel of an idea originated by Eric Wong during one late-night scan session (reference function vector product mapping) with the expertise of Andrzej Jesmanowicz who brought in a substantial mathematical expertise to the basic concept of correlation analysis. From this collaboration emerged a set of analysis tools that are still ubiquitous today. While this paper has turned out to be the most cited paper from this group - likely to be passed up soon by Biswal’s resting state correlation paper (Biswal et al., 1995) - several ideas contained in it never reached wide use. These ideas included Fourier harmonic mapping (mapping the faster changing areas vs slower changing areas using block designs and looking at the power spectrum maps at the higher harmonics) and (one that I’m particularly fond of) multiplexed task designs. The demonstration of multiplexed tasks (left vs. right finger tapping at alternating on/off frequencies) was great fun and extremely challenging to carry out.
Oct 31, 1994
I defended my thesis “MRI of Brain Function using Endogenous Susceptibility Contrast,” and was honored to have Seiji Ogawa, Andy Greene, Victor Haughton, Scott Hinks, Jim Hyde, Andrzej Jesmanowicz, Ronald Tikofsky on my thesis committee.
Nov, 1994
I left MCW to start my post doc at Massachusetts General Hospital under the guidance of Bruce Rosen. I stayed there until July of 1996, when I returned to MCW as an assistant professor. I left MCW again in March of 1999 to become director of the fMRI Core Facility and chief of the Unit on Functional Imaging Methods at the National Institutes of Health.
January, 1995
Eric Wong moved to the University of California, San Diego, where he joined the Departments of Radiology and Psychiatry. He continued to help out with fMRI research at UCSD, but mostly focused on arterial spin labeling (ASL) method development.
Post 1995
Following Eric and I, MCW Biophysics graduates have included several individuals who are still active and successful in fMRI methods development. These include: Rasmus Birn (UW Madison), Bharat Biswal (New Jersey Medical School), Jack Knight-Scott (Children’s Healthcare of Atlanta), Hanbing Lu (National Institute of Drug Abuse), Wen-Ming Luh (National Institute of Mental Health), Beth Meyerand (UW Madison), Christopher Pawella (Medical College of Wisconsin), Vinai Roopchansingh (National Institute of Mental Health), Ziad Saad (National Institute of Mental Health), and Alan Song (Duke University). In addition, MCW Biophysics has had several highly successful post docs pass through who did fMRI related work, including Jerzy Bodurka (Laureate Institute for Brain Research, Tulsa), Steve Tan (GE Medical Systems), and David Soltysik (Fedral Drug Administration).
Lastly, the MCW Biophysics had outstanding pre-fMRI graduates: One in particular was Tom Grist (UW Madison) who currently has a wide focus that touches a bit on fMRI. As a medical student, he rotated through the BRI, helping to develop RF coils.
Key Elements
Aside from what happened at MCW, I would like to try to convey the elements that were important in the early and rapid inception of fMRI at MCW. Why did a Midwest medical college succeed in rapidly starting a productive, multidisciplinary fMRI methods and application program? There were many serendipitous “right place, right time” elements that were essential, but aside from these, there were a few aspects to the success of MCW that were not accidental. I attempt to put these in perspective below.
The Biophysics Research Institute
Jim Hyde set up a unique, interdisciplinary center that has the feel of a place that brims with potential. His strong presence (patrolling the halls with his cup of coffee – intent to share his latest ideas) minimized any cancerously destructive internal politics. His political skill gave the BRI access to all the expertise and resources that MCW offered. This atmosphere along with the outstanding physical infrastructure opened up considerable freedom for graduate students and professors alike. For the most part, graduate students in MRI were not plugged into projects and rigid labs but, rather, had to find their own way with some input by their mentors and colleagues. Wong and I formed a research and development team that was essentially “flying under the radar” with no significant barriers to essential resources. Our mentors, while offering sound direction and insight during the mid to later stages of fMRI advancement, were, in the early stages, relatively unaware of the rapid developments in fMRI at MCW that were going on in the late summer and fall of 1991.
The Institute (now Department) was and is fundamentally based on hardware development. This was essential for successful EPI and fMRI in the early days since, until about 1996, no vendor (other than Advanced NMR who sold a “retrofitted” EPI capability on GE scanners) had EPI capability as a product on their scanners. The BRI had a well-appointed machine shop and coil shop. It also had a computer room, which consisted of two Tektronix XD88 workstations. Andrzej Jesmanowicz continuously occupied one and the other was open for use. In summary, the important elements of the BRI were the available machine and coil building and testing equipment, some powerful computational tools, truly open access for professors and students alike, and a highly interdisciplinary, and scientifically relaxed yet stimulating setting.
Scanner Access
The 1.5 Tesla clinical GE scanner was accessible by BRI researchers from about 9:00 PM to 5:00 AM on weekdays and in the afternoon on weekends. These time slots were when all the research scanning was performed. A dedicated scanner technologist, Lloyd Estowski, who understood the quirks of research and how it differed from the day-to-day clinical grind, manned this scanner. He was important in many of the early experiments – either running the scanner as I jumped in or being a volunteer while I scanned. On one memorable late night, I recall Lloyd actually falling asleep while tapping his fingers with his arm raised. During these years, we had an informal umbrella protocol that allowed a high level of flexibility, and we were not required to pay for scanner time out of grant money. Jim Hyde negotiated for this time with the Radiology Department of Froedtert Memorial Lutheran Hospital. In fact, he recalls threatening to charge them for our use of the time rather than pay for it because of the clear benefit to the Radiology Department.
One drawback was that the scanner was located a relatively long walk away. The basic procedure, repeated hundreds of times, was to load up the gradient coil, RF coil, notebooks, graffiti boards (for adjusting the gradient-amplifier output to gradient coil inductance), and storage tapes on to a rubber push cart at about 8:30 PM in the BRI, then walk through the quarter to half mile of basement hallways through campus to arrive at the scanner. Setup, requiring powering down of the gradient amplifiers, took about 30 minutes. When scanning was completed, usually between midnight at 2 AM, the setup was disassembled as the data (the still used GE format “P-files”) was saved. We would then walk back to the BRI, go to the computer room, upload the data, and reconstruct it. Sometimes, if things didn’t work out, we would go back that same evening for additional experiments.
Jim Hyde
Jim already had a well-respected international reputation in EPR. When he arrived at MCW from Varian, he set up an open, resource-rich, and intellectually stimulating environment that attracted talented and unconventional people. As mentioned, the center had generally open scanner access, a wide array of freely available hardware and computational tools, and a healthy interaction with departments and individuals throughout the school. He also personally encouraged all forms of scientific creativity, and most enjoyed hallway conversations regarding the use of “first principles” to advance a method or to deepen an understanding of a concept or a finding. He was not a micromanager, giving all students “just enough rope to hang themselves with.” At the same time, he clearly was the leader. Turf wars, petty disputes, etc. were minimized simply by his presence and extreme impatience with a loss of focus on what was important – the creation of new ideas and tools.
Eric Wong
Of all the people at MCW, Eric was perhaps the most important for the first success of fMRI at MCW. Eric wrote all the pulse sequences and almost all of the image reconstruction code in the first few years of fMRI at MCW. He designed, built, and interfaced the gradient coil and RF coil for performing EPI and fMRI on the standard GE scanner. Without his contributions (all while finishing his own PhD and entering the final stages of medical school), the fMRI effort at MCW would simply not have emerged with the speed that it did – if at all. The only other person capable of doing what Eric did at MCW was Andrzej Jesmanowicz who had his focus elsewhere during this time. The amount of high-level creative work that Eric performed to get fMRI off the ground was prodigious.
Peter Bandettini
My desire to image human brain function with MRI perhaps helped focus the highly talented group at MCW. I like to think that I brought to the table an instinct on what approaches and what experiments would work best – what the most interesting and relevant questions and approaches were. I had the time and freedom to work pretty much around the clock. My research involved (and still involves) characterizing the BOLD response, determining the best way to process fMRI data, and generally finding the most effective ways to extract as much information as possible from the fMRI time series.
Andrzej Jesmanowicz
Andrzej is perhaps the most colorful character in Biophysics at MCW. He sky dives, makes skydiving films, rides a unicycle from his parked car to the door of MCW, and was an important player in the Polish resistance in the late 70’s, using his technical expertise to enhance communications. He arrived at MCW – referred by a friend who collaborated with MCW Biophysics - after doing a stint as a VCR repairman in the US. Andrzej is an expert in MRI physics, processing, hardware, and computation. He became active in fMRI shortly after the first success, and among other things, greatly increased the sophistication of the developing correlation analysis approaches. He advanced EPI reconstruction methods, implementing several phase correction strategies. When the 3T Bruker scanner arrived at MCW in 1993, he completely re-engineered many of the components and re-wrote many of the pulse sequences such that it was to become, in 1994, one of the first routinely used 3 T fMRI scanners in the world. On this scanner he implemented multiplexed four channel acquisition in the early 90s. He was essential in implementing the first “real-time” fMRI scanner. He continues to advance basic MRI and fMRI speed and resolution. While no one fully understands Andrzej in conversation at MCW, they all reap the benefits of his creations. He continues to create.
Ron Tikofsky
Nearing retirement after an active career at nearby Froedtert Hospital in Computed Tomography, he had one decisive contribution with regard to fMRI at MCW. When I came to Hyde with my preliminary results and suggested that they formed a basis for a thesis project, Jim was correctly skeptical, mentioning that the idea of imaging thinking seemed soft. I recall him saying something to the effect of: “You have a thought…you don’t have a thought. It seems very fuzzy.” Hyde suggested that I talk with this highly esteemed friend of his at MCW, Dr. Ron Tikofsky, who had experience in this world of brain function imaging. Ron would “wizen you up.” On talking with me and seeing my preliminary data, Tikofsky expressed the highest enthusiasm for this project (to put it mildly), which then received Hyde’s blessing.
Scott Hinks
Initially, my GE-based co-advisor was Dr. Carl Crawford, an engineer/physicist who had a background in Computed Tomography. Once I chose MRI of brain function as a topic, Carl asked that I find another advisor as he felt that his expertise was not sufficient for proper guidance. I was grateful that Scott stepped in at this critical time and became an important catalyst for my experiments determining spin-echo, gradient-echo TE dependence of the activation-induced signal change. Scott also maintained the very important link to GE medical systems.
GE Medical Systems
The world headquarters of GE Medical is in Waukesha Wisconsin, 15 miles from the Medical College of Wisconsin. A good interaction existed between the two groups (we even had a journal club at GE for awhile). GE’s vast computational resources, scanner availability, and considerable expertise were very important for several aspects of the fMRI project. For example, we had to use a GE-based filter tool to generate a full pass EPI filter to perform all fMRI at MCW. For several years after this creation, this filter that we created for our purposes alone was accidentally included in the available filters that GE supplied on the scanners. In the very early stages of fMRI, the data was saved on 20 MByte reel-to-reel tapes. GE had the all-important tape reader for reading the data off of these. Not many people at GE really knew what I was doing – coming in at 4 PM and working past midnight in my office in the Applied Science Lab. Other physicists of the Applied Science Lab: Physicists Rich Kinsinger (director), Matt Bernstein, Kevin King, Erika Schneider, Tom Foo, IT guy Don Birzer, and computer engineer as well as Ultimate Frisbee buddy Jim Kohli were all very supportive and helpful. An example of this helpfulness: a week before the 1992 SMRM meeting in Berlin, I accidentally deleted all the fMRI data – stored on GE computers - that I was going to present for my several talks there. Over a weekend, Kohli found the backup tape and saved me! I still owe him.
Robert Cox
Bob started at MCW in 1993. While he was not present during the initial inception of fMRI at MCW, once he arrived, he picked up where Andrzej left off and started writing an open fMRI analysis and display platform (AFNI) (Cox, 1996) for the growing number of users who were also becoming more demanding. He has a framed picture on his wall of an email informing Jim Hyde on Nov 27, 1994 that he has decided to stop developing AFNI (about a year after he started). This picture of the letter is shown in Figure 9. He obviously changed his mind, and continued to code! Seventeen years later, he’s still coding AFNI.
Figure 9.

The picture (from Bob Cox’s current NIH office wall) of the email sent from Bob to Jim Hyde telling him that he was stopping development of AFNI – back in November 1994, and about a year after he started…he had barely just begun.
The early adopters: Jeff Binder / Ted DeYoe / Andy Greene / Tom Prieto / Steve Rao / Victor Haughton / Elliot Stein / Zerrin Yetkin
Functional MRI for this small group of established clinicians and neuroscientists was a risk and an adventure. A key component in the development of fMRI is that they worked well with others, were energetic, and were very good at implementing a new and relatively raw technology to their specific models of brain function as well as their specific applications. An early picture of some of these enthusiastic early adopters at MCW is shown in Figure 10 at the first OHBM meeting in Paris in June of 1995.
Figure 10.

A picture of the “early adopters” of fMRI at MCW with Jim Hyde, taken at the first Organization for Human Brain Mapping (OHBM) meeting in Paris in June, 1995. From left are Ted DeYoe (focus was vision), Elliot Stein (focus was drug effects), Jim Hyde, Steve Rao (focus was motor activation), and Jeff Binder (focus was language and auditory processing).
In these early days, Jeff focused on language processing, Ted focused on visual processing, Andy was among the first to use fMRI on animal models, Tom developed subject interface devices, Steve focused on motor function, Victor and Zerrin focused on clinical applications, Elliot focused on drug - specifically nicotine - effects as measured by the fMRI signal. These individuals were all essential in taking fMRI at MCW from the pure development stage to the early application stages. In retrospect, it was surprising that these researchers were able to alter the allocation of their focus and resources so rapidly and effectively. In these early days, this collaborative effort was further galvanized by the success of an NIH program project grant devoted to the development of fMRI.
Conclusion
Several highly fortunate circumstances came together to allow fMRI to start rapidly in the Biophysics Research Institute at the Medical College of Wisconsin. First, the center was set up to allow innovation and rapid development in RF and gradient coil hardware that would allow scanning ability well beyond what was then possible with clinical scanners. At this time, EPI capability could not be purchased. It had to be developed in-house. Scanner access was completely open, and the environment was encouraging of innovation. Collaboration with GE was important for efficient solutions to some specific issues. This great environment coupled with the motivation of Eric Wong and myself, resulted in fMRI results within a month of us hearing of the idea. Of course motivation, the right environment, and the excitement in the field were not enough in themselves. Specifically, the work of Eric Wong leading up to MCW’s first fMRI results was critical. Before we knew that fMRI existed, Eric designed, built, and interfaced a three-axis head gradient and RF coil… in about a week for his own research purposes. Prior to building a head gradient coil, while working with local wrist/small animal gradient coil that he had previously made, he programed the GE scanners to perform EPI, wrote the reconstruction software, and performed subsequent highly challenging phase correction tweaks…all in about a month. I generally assisted Eric, but, for the most part, provided the enthusiasm and energy to help make fMRI effort at MCW come together during the early years.
The MRI and fMRI researchers in MCW Biophysics have continued to produce method advancements, creations, and discoveries that have helped define and push the field. This MCW Biophysics fMRI-related work started with the local gradient coil creation by Wong (Wong et al., 1991a, b) the early fMRI findings (Bandettini et al., 1992) and development of correlation analysis (Bandettini et al., 1993). It has continued, including early analyses of spin-echo and gradient-echo and TE dependence (Bandettini et al., 1994), early simulations of BOLD contrast using a novel “deterministic diffusion” method (Bandettini and Wong, 1995), a report on the novel utility of hypercapnia normalization (Bandettini and Wong, 1997) and the mapping of scanner noise induced brain activation (Bandettini et al., 1998) by me. Eric Wong produced an early paper on optimized isotropic diffusion weighting (Wong et al., 1995). Alan Song had a very early report on parallel multi-channel acquisition, echo-volume imaging (Song et al., 1994), and the attenuation effect on BOLD signal changes by diffusion weighting (Song et al., 1996). Bharat Biswal had perhaps most famous paper coming from MCW Biophysics – that first report of resting state correlations between functionally related regions (Biswal et al., 1995). The results shown in this paper were produced using the second-generation head gradient coil described above. For quite some time, most thought that this observation was artifactual or due to physiologic processes – or even scanner instabilities - not related to synchronously fluctuating brain activity. Now the field of connectivity is explosively growing - spawning a new journal, called “Brain Connectivity,” started by Chris Pawella – another former MCW Biophysics graduate student - and Biswal. Last to be mentioned but certainly not the last of the MCW Biophysics innovations is the first report of real-time fMRI (Cox et al., 1995), and the introduction of the processing platform, AFNI (Cox, 1996) by Bob Cox. These continuing accomplishments from this department are of course, in part, due to the fortune of having talented people working together with outstanding resources. These resources, and perhaps the dynamics among the people in the department, are partly due to Jim Hyde’s vision and leadership in setting up and fostering an unconventional yet stimulating and effective environment.
MCW Biophysics and now hundreds of centers around the world continue to innovate and advance fMRI. Those of us in this incredible field are extremely lucky, as it continues to show rapid growth and a positive impact on the many areas that use neuroimaging.
Supplementary Material
Acknowledgements
I would like to thank Patricia Bandettini, Scott Hinks, Jim Hyde, and Eric Wong for help in the proofreading of this document. The NIH intramural programs, NINDS and NIMH, support Peter Bandettini.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Bandettini PA, Jesmanowicz A, Van Kylen J, Birn RM, Hyde JS. Functional MRI of brain activation induced by scanner acoustic noise. Magnetic Resonance in Medicine. 1998;39:410–416. doi: 10.1002/mrm.1910390311. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing Strategies for Time-Course Data Sets in Functional Mri of the Human Brain. Magnetic Resonance in Medicine. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC. Effects of Biophysical and Physiological- Parameters on Brain Activation-Induced R(2)Asterisk and R(2) Changes - Simulations Using a Deterministic Diffusion-Model. International Journal of Imaging Systems and Technology. 1995;6:133–152. [Google Scholar]
- Bandettini PA, Wong EC. A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. Nmr in Biomedicine. 1997;10:197–203. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<197::aid-nbm466>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Jesmanowicz A, Hinks RS, Hyde JS. Spin-Echo and Gradient-Echo Epi of Human Brain Activation Using Bold Contrast - a Comparative-Study at 1.5 T. Nmr in Biomedicine. 1994;7:12–20. doi: 10.1002/nbm.1940070104. [DOI] [PubMed] [Google Scholar]
- Belliveau JW, Kennedy DN, McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS, Vevea JM, Brady TJ, Rosen BR. Functional mapping of the human visual cortex by nuclear magnetic resonance imaging. San Francisco: 1991a. p. 115. [DOI] [PubMed] [Google Scholar]
- Belliveau JW, Kennedy DN, McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS, Vevea JM, Brady TJ, Rosen BR. Functional Mapping of the Human Visual-Cortex by Magnetic-Resonance-Imaging. Science. 1991b;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar Mri. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blamire AM, Ogawa S, Ugurbil K, Rothman D, McCarthy G, Ellermann JM, Hyder F, Rattner Z, Shulman RG. Dynamic Mapping of the Human Visual-Cortex by High-Speed Magnetic-Resonance-Imaging. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:11069–11073. doi: 10.1073/pnas.89.22.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady TJ. Future prospects for MR imaging; 10th Annual Meeting of the Society of Magnetic Resonance in Medicine; San Francisco. 1991. p. 2. [Google Scholar]
- Brindle KM, Brown FF, Campbell ID, Grathwohl C, Kuchel PW. Application of spin-echo nuclear magnetic resonance to whole-cell systems. Membrane transport. Biochemical Journal. 1979;180:37–44. doi: 10.1042/bj1800037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks RA, Di Chiro G. Magnetic resonance imaging of stationary blood: A review. Medical Physics. 1987;14:903–913. doi: 10.1118/1.595994. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A, Hyde JS. Real-Time Functional Magnetic- Resonance-Imaging. Magnetic Resonance in Medicine. 1995;33:230–236. doi: 10.1002/mrm.1910330213. [DOI] [PubMed] [Google Scholar]
- Fabry ME, San George RC. Effect of magnetic susceptibility on nuclear magnetic resonance signals arising from red cells: A warning. Biochemistry. 1983;22:4119–4125. doi: 10.1021/bi00286a020. [DOI] [PubMed] [Google Scholar]
- Fisel CR, Ackerman JL, Buxton RB, Garrido L, Belliveau JW, Rosen BR, Brady TJ. MR contrast due to microscopically heterogeneous magnetic susceptibility: Numerical simulations and applications to cerebral physiology. Magnetic Resonance in Medicine. 1991;17:336–347. doi: 10.1002/mrm.1910170206. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm J, Bruhn H, Merboldt KD, Hanicke W. Dynamic Mr Imaging of Human Brain Oxygenation During Rest and Photic-Stimulation. Jmri-Journal of Magnetic Resonance Imaging. 1992;2:501–505. doi: 10.1002/jmri.1880020505. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomori JM, Grossman RI, Yu-Ip C, Asakura T. NMR relaxation times of blood: Dependence on field strength oxidation state and cell integrity. Journal of Computer Assisted Tomography. 1987;11:684–690. [PubMed] [Google Scholar]
- Hayman LA, Ford JJ, Taber KH, Saleem A, Round ME, Bryan RN. T2 effect of hemoglobin concentration: Assessment with in vitro MR spectroscopy. Radiology. 1988;168:489–491. doi: 10.1148/radiology.168.2.3393669. [DOI] [PubMed] [Google Scholar]
- Hoppel BE, Weisskoff RM, Thulborn KR, Moore J, Rosen BR. Measurement of regional brain oxygenation state using echo planar linewidth mapping; 10th Annual Meeting of the Society of Magnetic Resonance in Medicine; San Francisco. 1991. p. 308. [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, Brady TJ, Rosen BR. Dynamic Magnetic-Resonance-Imaging of Human Brain Activity During Primary Sensory Stimulation. Proceedings of the National Academy of Sciences of the United States of America. 1992a;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Stern CE, Chesler DA, Goldgerg IE, Poncelet BP, Kennedy DN, Weisskoff RM, Cohen MS, Turner R, Cheng H-M, Brady TJ, Rosen BR. Functional MR Imaging of the Primary Visual and Motor Cortex; 10'th Annual Society for Magnetic Resonance Imaging (SMRI) Conference; Ney York, NY. 1992b. [Google Scholar]
- Kwong KK, McKinstry RC, Chien D, Crawley AP, Pearlman JD, Brady TJ, Rosen BR. CSF Suppressed quantitative single shot diffusion imaging; Tenth Annual Society of Magnetic Resonance in Medicine Meeting; San Francisco. 1991a. p. 215. [DOI] [PubMed] [Google Scholar]
- Kwong KK, McKinstry RC, Chien D, Crawley AP, Pearlman JD, Rosen BR. CSF-suppressed quantitative single-shot diffusion imaging. Magnetic Resonance in Medicine. 1991b;21:157–163. doi: 10.1002/mrm.1910210120. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Breton E, Lallemand D. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Turner R. The capillary network: A link between IVIM and classical perfusion. Magnetic Resonance in Medicine. 1992;27:171–178. doi: 10.1002/mrm.1910270116. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Turner R, Moseley M, Hyde JS. Functional MRI of the Brain. Magnetic Resonance in Medicine. 1993;30:405–408. doi: 10.1002/mrm.1910300402. [DOI] [PubMed] [Google Scholar]
- Majumdar S. Quantitative study of the susceptibility difference between trabecular bone and bone marrow: Computer simulations. Magnetic Resonance in Medicine. 1991;22:101–110. doi: 10.1002/mrm.1910220111. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM. Magnetic-Resonance-Imaging of Blood-Vessels at High Fields - Invivo and Invitro Measurements and Image Simulation. Magnetic Resonance in Medicine. 1990;16:9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain Magnetic-Resonance-Imaging with Contrast Dependent on Blood Oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1990a;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-Sensitive Contrast in Magnetic-Resonance Image of Rodent Brain at High Magnetic-Fields. Magnetic Resonance in Medicine. 1990b;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic Signal Changes Accompanying Sensory Stimulation - Functional Brain Mapping with Magnetic-Resonance-Imaging. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L, Coryell CD. The magnetic properties and structure of hemoglobin, oxyhemoglobin, and carbonmonoxyhemoglobin. Proceedings of the National Academy of Sciences. 1936;22:210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AW, Wong EC, Hyde JS. Echo-Volume Imaging. Magnetic Resonance in Medicine. 1994;32:668–671. doi: 10.1002/mrm.1910320518. [DOI] [PubMed] [Google Scholar]
- Song AW, Wong EC, Tan SG, Hyde JS. Diffusion weighted fMRI at 1.5 T. Magnetic Resonance in Medicine. 1996;35:155–158. doi: 10.1002/mrm.1910350204. [DOI] [PubMed] [Google Scholar]
- Stehling M, Fang M, Ladebeck R, Schmitt F. Functional Echo-Planar MR Imaging at 1 T; 10'th Annual Society for Magnetic Resonance Imaging (SMRI) Conference; New York, NY. 1992. [Google Scholar]
- Terrier F, Lazeyras F, Posse S, Aue WP, Zimmermann A, Frey BM, Frey FJ. Study of acute renal ischemia in the rat using magnetic resonance imaging and spectroscopy. Magnetic Resonance in Medicine. 1989;12:114–136. doi: 10.1002/mrm.1910120114. [DOI] [PubMed] [Google Scholar]
- Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochimica et Biophysica Acta. 1982;714:265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- Turner R, Jezzard P, Wen H, Kwong KK, Lebihan D, Zeffiro T, Balaban RS. Functional Mapping of the Human Visual-Cortex at 4 and 1.5 Tesla Using Deoxygenation Contrast Epi. Magnetic Resonance in Medicine. 1993;29:277–279. doi: 10.1002/mrm.1910290221. [DOI] [PubMed] [Google Scholar]
- Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J. Echo-planar imaging of intravoxel incoherent motion. Radiology. 1990;177:407–414. doi: 10.1148/radiology.177.2.2217777. [DOI] [PubMed] [Google Scholar]
- Turner R, Lebihan D, Moonen CTW, Despres D, Frank J. Echo-Planar Time Course Mri of Cat Brain Oxygenation Changes. Magnetic Resonance in Medicine. 1991;22:159–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]
- Vilringer A, Rosen BR, Belliveau JW, Ackerman JL, Lauffer RB, Buxton RB, Yong-Sheng C, Wedeen VJ, Brady TJ. Dynamic imaging with lanthanide chelates in normal brain: Contrast due to magnetic susceptibility effects. Magnetic Resonance in Medicine. 1988;6:164–174. doi: 10.1002/mrm.1910060205. [DOI] [PubMed] [Google Scholar]
- Wong EC, Cox RW, Song AW. Optimized isotropic diffusion weighting. Magnetic Resonance in Medicine. 1995;34:139–143. doi: 10.1002/mrm.1910340202. [DOI] [PubMed] [Google Scholar]
- Wong EC, Hyde JS. Perfusion Imaging by Interleaved Excitation; 10th Annual Meeting of the Society of Magnetic Resonance in Medicine; San Francisco. 1991. p. 791. [Google Scholar]
- Wong EC, Jesmanowicz A, Hyde JS. Coil optimization for MRI by conjugate gradient descent. Magnetic Resonance in Medicine. 1991a;21:39–48. doi: 10.1002/mrm.1910210107. [DOI] [PubMed] [Google Scholar]
- Wong EC, Jesmanowicz A, Hyde JS. High-resolution, short echo time MR imaging of the fingers and wrist with a local gradient coil. Radiology. 1991b;181:393–397. doi: 10.1148/radiology.181.2.1924778. [DOI] [PubMed] [Google Scholar]
- Wright GA, Hu BS, Macovski A. 1991 I.I. Rabi Award. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5 T. Journal of magnetic resonance imaging : JMRI. 1991;1:275–283. doi: 10.1002/jmri.1880010303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


