Abstract
Significant safety issues have emerged concerning the general use of DRYVAX® vaccine. Vaccination with replication-defective recombinant adenovirus (rAd) vaccines may offer a safer and effective alternative to live vaccinia virus (VV) vaccination. Six individual poxvirus glycoproteins: A33R, A34R, A36R, B5R, A27L or L1R that are normally expressed on the surface of infectious vaccinia virus were encoded in rAd vaccines and tested in mice in this study. A single-shot intramuscular injection of rAd encoding A27L protected mice against a lethal intranasal challenge with VV at 4 weeks post vaccination. By 10 weeks post vaccination, a significant decrease in post-challenge morbidity was observed that correlated with potent neutralizing antibody responses and the emergence of specific polyfunctional T cell responses. The immunogenicity and protective efficacy of rAd-A27L immunization persisted for at least 35 weeks post-vaccination. This study is the first demonstration that a single-shot subunit vaccine encoding a poxvirus protein confers protection against the mortality and morbidity associated with poxvirus infection.
Keywords: A27L, smallpox, adenovirus, vaccine
1. Introduction
In the late 1980’s, smallpox was eradicated from its natural environment as a result of a global campaign coordinated by the World Health organization (WHO) that used the live vaccinia virus vaccine [1]. DRYVAX® (Wyeth, PA), is a derivative of a vaccinia virus strain used during the eradication campaign, is usually administered via superficial scarification of the skin. This vaccine may now be inappropriate for use in the general population due to documented side effects, both in immune-deficient individuals and in the immune competent population [2, 3]. ACAM 2000, a cell culture-adapted derivative of DRYVAX®, was approved by the FDA in 2008 for stockpiling, although not for general use [4, 5]. Considered to be less neurovirulent than DRYVAX®, cardiac complications were nevertheless evident following vaccination [5, 6]. These findings, together with recent threats posed by bio-terrorism and by the emergence of zoonotic poxvirus infections such as monkeypox, have focused increased research attention on the development of an easily administered, safer vaccine that rapidly generates protective immune responses.
The proven clinical efficacy of the DRYVAX® vaccine during the smallpox eradication era, and the high sequence homology between different members of the genus Orthopoxvirus (family Poxviridae), indicate that vaccines that protect against vaccinia virus should be protective against variola, monkeypox, and potentially other poxviruses [7-9]. During poxvirus replication, multiple virus forms can be differentiated in the infected cell by the number of envelopes that enclose the virion core. The intracellular mature virion (IMV) is produced by membrane wrapping of the virion core and contains multiple proteins, including the A27L and L1R glycoproteins involved in formation and transport of IMV particles from virus factories in the infected cell cytoplasm to the intracellular membranous organelles (trans-golgi apparatus) [8, 10-12]. Some virions are released from cells as extracellular enveloped virions (EEV) by fusion of the outermost membrane with the cell membrane. EEV contain many proteins with multiple functions, including A33R, A34R, A36R and B5R, that have roles in actin polymerization and EEV release [13, 14], in membrane wrapping and EEV spread [15, 16] and in release of EEV particles [16, 17].
The need for a safe, single-dose vaccine that could be administered easily and generate protective immune responses in the event of a smallpox outbreak prompted us to evaluate the immunogenicity and protective efficacy of different glycoprotein subunits, particularly against infection by the natural, respiratory route. Because of their critical role in poxvirus morphogenesis and transmission, immune responses against these molecules might not only limit virus replication but also prevent host-to-host transmission. Previous studies have indicated that either multiple immunizations with DNA or protein-based vaccines encoding single IMV or EEV poxvirus glycoproteins, or with a mixture of vaccine vectors encoding multiple glycoproteins, may be required for protection against poxvirus infections via the respiratory route [8, 18-27].
Genetic strategies using replication-deficient virus vectors are currently at the forefront of vaccine research [28]. Here, we used recombinant adenovirus (rAd) vectors to deliver poxvirus glycoprotein subunits, due to their relatively large capacity for inserted foreign genes, their ability to infect multiple cell types, their demonstrated capacity to induce TH1 type immune responses, and their relative safety in immune competent and immune-deficient individuals following deletion of their E1 and E3 genes necessary for viral gene expression upon entry and immune evasion [29-34]. We demonstrate that single-shot, intramuscular immunization with rAd encoding the A27L IMV glycoprotein induced potent cellular and antibody immune responses and was sufficient to provide long-term protection in mice against a lethal intranasal challenge with vaccinia virus. Partial protection was observed following immunization with rAd encoding A33R, A34R, B5R, or L1R. Our findings suggest that rAd-based subunits may represent an effective strategy for the generation of protective immunity against poxvirus infections. They also provide the initial demonstration that a single poxvirus glycoprotein, namely A27L, can be used to generate effective immune responses against virulent poxvirus infections by the respiratory route.
2. Materials and methods
2.1. Viruses
A33R, A34R, A36R, B5R, A27L and L1R genes were amplified by PCR from genomic DNA of vaccinia virus Western Reserve (VV-WR) using the primers described in Table 1. Adenovirus vaccine vectors encoding these PCR products as inserts were then generated using the Gateway system (ViraPower™ Adenoviral Gateway Expression System, Invitrogen.Inc, Carlsbad, CA). Briefly, “CACC” sequences at the 5′ end of the PCR product are recognized by topoisomerase in the entry vector (pENTR™/D-TOPO®, Invitrogen.Inc), facilitating directional cloning of the gene sequence. Genes were transferred into the destination vector (pAd/CMV/V5-DEST®, Invitrogen.Inc) containing genes necessary for formation of the adenovirus capsid via homologous recombination. The destination vector was linearized and transfected into competent 293A helper cells (Invitrogen.Inc) for production of rAd virus particles encoding individual poxvirus genes. A control rAd without inserted foreign genes (rAd-Empty) was also derived via this process. rAd vaccine vectors were then propagated, purified and concentrated to vaccine grade stocks via CsCl2 gradient high-speed ultra-centrifugation and anion exchange filters (Acrodisc® Units with Mustang® Q Membranes, Pall Corporation). Plaque titration and HPLC were used to establish the titers of purified adenovirus vaccine stocks. Vaccinia virus Western Reserve (VV-WR, ATCC VR-119) strain used for challenge studies was kindly provided by Bernard Moss, LVD, NIH. VV-WR was grown on monolayers of CV-1 cells (African green monkey kidney cell line, ATCC CCL-70) and titrated on 143B cells (Human osteosarcoma cell line, ATCC CRL-8303) using the plaque assay.
Table 1. A single immunization with recombinant adenovirus encoding vaccinia virus glycoprotein A27L elicits complete protection against a virulent respiratory poxvirus infection.
| A33R | Forward 5′- CACCGGATCCATGATGACACCAGAAAACGACGAAG -3′ (BamHI) Reverse 5′- GAATTCTTAGTTCATTGTTTTAACAC -3′ (EcoRI) |
| A34R | Forward 5′- CACCGGATCCATGAAATCGCTTAATAGACAAACTG -3′ (BamHI) Reverse 5′- GAATTCTCACTTGTAGAATTTTTTAACAC -3′ (EcoRI) |
| A36R | Forward 5′- CACCGGATCCATGATGCTGGTACCTCTTATCACG -3′ (BamHI) Reverse 5′- GAATTCTCACACCAATGATACGACCGATG -3′ (EcoRI) |
| B5R | Forward 5′- CACCAGATCTATGAAAACGATTTCCGTTGTTACG -3′ (BgIII) Reverse 5′- CTGCAGTTACGGTAGCAATTTATGGAAC -3′ (PstI) |
| A27L | Forward 5′- CACCATGGGATCCATGGACGGAACTC -3′ (BamHI) Reverse 5′- GAATTCTTACTCATATGGACGCCGTC -3′ (EcoRI) |
| L1R | Forward 5′- CACCATGGGATCCATGGGTGCCGCAC -3′ (BamHI) Reverse 5′- GAATTCTCAGTTTTGCATATCCGTGG -3′ (EcoRI) |
Primer sequences for the vaccinia virus proteins used for PCR amplification with highlighted “CACC” sequences and underlined restriction enzyme sites
2.2. Mice and immunization
Six to eight week old female Balb/c mice (Charles River, Wilmington, MA) were used in this study. All animal studies were conducted under the guidelines of the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center. Adenovirus vaccines were administered via the intramuscular route by injecting 1×109PFU/100μl/mouse, with 50μl injected into each of the hind legs. Tail scarification with VV was performed by placing a 10μl drop of PBS containg 1×107PFU of VV-NYCBH approximately 1 cm from the base of the tail. The virus suspension was then scratched into the tail using a 28 gauge needle approximately 15 times taking care not to draw blood. Scab formation at the site of inoculation approximately 2 weeks later was taken as an indicator of successful vaccination. For challenge studies, mice were given intranasal (i.n.) inocula of VV-WR selected on the basis of preliminary in vivo titration experiments showing that these doses resulted in 100% mortality in unvaccinated mice. Mice that sustained more than 30% loss of body mass were euthanized according to IACUC guidelines. LD50 via the i.n. route was determined by the classical method of Reed and Muench (1LD50 = 1.33 × 104 pfu) [35]. Mice were weighed on the day of challenge and every day thereafter for 2 weeks. Loss of body mass was used as a measure of morbidity.
2.3. ELISA
Antibody responses were measured by ELISA using 96 well MaxiSorp (NUNC™, Roskilde, Denmark) plates coated with 10 ng of recombinant A27L protein per well and incubated overnight at 4°C. Recombinant A27L vaccinia virus protein was provided by NIH Biodefense and Emerging Infections Research Resource Repository (BEI Resources, Manassas, VA). Plates were washed four times with PBS/0.05%Tween, blocked with skim milk (1 gram per 20 ml PBS) for 2 hours at 37°C, washed four times again, and treated with 50 μl diluted serum samples diluted in PBS/0.5%BSA/0.05%Tween for 1 h at 37°C. After six further washes, 50μl of biotinylated goat anti-mouse IgG antibody (Southern Biotech, Birmingham, AL) was added to each well at a final concentration of 0.5 μg/ml in PBS and incubated at room temperature for 45 minutes. Plate were then washed six times, treated with 50 μl of diluted streptavidin conjugated alkaline phosphatase (diluted 1:2000, Amersham Biosciences, Piscataway, NJ) and incubated at room temperature for 30 minutes. After eight further washes, plates were developed by adding 200 μl of p-nitrophenyl phosphate of (AP-Yellow One Component Microwell Substrate, BioFx laboratories, Owings Mills, MD). The reaction was stopped using Alkaline Phosphatase Stop Reagent (BioFx laboratories, Owings Mills, MD) and plates were analyzed at 405nm using an ELISA plate reader (Synergy HT Multi-Mode Microplate Reader, BioTek Instruments, Inc. Winooski, VT). The highest dilution of serum with an optical density greater than twice that of the naïve sera was taken as the endpoint titer.
2.4. Plaque reduction neutralization test (PRNT50)
To measure neutralizing antibody responses, VV-WR (100 PFU/100 μl) was incubated with equal volumes of serial two-fold dilutions of serum samples in Dulbecco’s minimal essential medium (DMEM) containing 2% FBS for 1h at 37°C. Monolayers of 143B (ATCC) cells in 6-well plates (Corning Inc, Corning, NY) were infected with 200μl of the mixture of the VV-WR and diluted serum samples (final dilutions starting at 1:100) for 1 h at room temperature. 3 ml of complete medium (DMEM with10% fetal bovine serum, 10mM HEPES, 50U/ml penicillin, 50μg/ml streptomycin, 2mM L-glutamine, 10mM Sodium pyruvate) was then added to each well and plates were incubated for 2 days at 37°C, then stained and fixed by replacing the media with 1 ml of 1% crystal violet in 70% methanol for 30 seconds. Plates were then gently washed with tap water and air-dried. The highest dilution of serum resulting in more than 50% inhibition of plaque formation was taken as the PRNT50 titer.
2.5. Isolation of lymphocytes
After sacrifice, spleens were harvested and cells gently dispersed with a 5 ml syringe plunger in 10 ml of complete medium per spleen (RPMI 1640 with 2mM L-glutamine, 10 mM HEPES, 50 ug/ml streptomycin, 50 U/ml penicillin, 50 mM 2-mercaptoethanol, 10mM Sodium pyruvate and 10% fetal bovine serum). Cell suspensions were passed through a 100 μm sterile nylon cell strainer and washed three times with complete medium. Red blood cell lysis buffer (Sigma-Aldrich Co, St. Louis, MO) was used to lyse and remove red blood cells.
2.6. Interferon-γ ELISPOT assay
Briefly, 96-well Multiscreen™-IP plates (Millipore Corporation, Billerica, MA) were coated with 100 μl of anti-mouse interferon-γ (IFNγ) (Mabtech, Inc, Nackastrand, Sweden) at 10 μl in PBS and incubated overnight at 4°C. The plates were washed five times with PBS and blocked with 200 μl of RPMI 1640 containing 10% FBS for 2 h at room temperature. The media was then removed and 2×105 murine spleen cells in triplicate were incubated for 18 h at 37°C with 2 μg/ml of peptide. Peptides included the published CD4+ T cell P10 epitope AAMISLAKKIDVQTGRRPYE [24] and the putative CD8+ T cell epitopes A27L 92-100 (AMISLAKKI), A27L 6-14 (FPGDDDLAI), and A27L 27-35 (KPEAKREAI) based on peptide prediction algorithms SYFPEITHI (http://www.syfpeithi.de) and BioInformatics and Molecular Analysis Section (BIMAS, http://www-bimas.cit.nih.gov/molbio/hla_bind/). All peptides used in the study were synthesized by GenScript Corporation (Piscataway, NJ). Some cultures were incubated in the presence of recombinant A27L protein (BEI Resources). The plates were then washed twice with PBS/0.05%Tween and three times with PBS, then incubated with 100 μl of biotinylated anti-mouse IFNγ (Mabtech, Inc, Nackastrand, Sweden) at 1 μg/ml in PBS/0.5%FBS for 2h at room temperature and washed 5 times with PBS prior to incubation with 100 μl of streptavidin-alkaline phosphatase (Mabtech) at 1 μg/ml in PBS for 1 h at room temperature. The plates were then washed five times with PBS, developed with 100 μl of 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Moss, Inc, Pasadena, MD) and the reaction was stopped by washing with tap water. Plates were read after air drying using an ELISPOT reader (AID Autoimmun Diagnostika GmbH, Straßberg, Germany). Data are presented as spot-forming cells (SFC) per million cells.
2.7. Intracellular cytokine staining (ICS) assay
One million mouse spleen cells were stimulated in a 100 μl volume containing 10 μg/ml of peptide in 96-well round-bottom plates (Corning Inc, Corning, NY) at 37°C under 5% CO2 for 2 h. 10 μl (1 μg/ml final concentration) of 1:100 dilution of Brefeldin A (BD Biosciences, San Jose, CA) was added to each well and the plates were incubated for a further 4 h at 37°C under 5% CO2. Cells were then washed and stained with Pacific Blue-conjugated anti-CD3e, phycoerythrin-Cy5-conjugated anti-CD8 and fluorescein isothiocynate-conjugated anti-CD4 for 30 minutes at 4°C. Cells were then washed, permeabilized with cytofix/cytoperm (BD Biosciences, San Jose, CA) for 20 minutes at 4°C, and stained for intracellular cytokines using phycoerythrin-conjugated anti-mouse IL-2, phycoerythrin-Cy7-conjugated anti-mouse TNFα (tumor necrosis factor) and allophycocyanin-conjugated anti-mouse IFNγ for 30 minutes at 4°C. Stained cells were washed and resuspended in 1% formalin. Data were acquired using a BD LSR II system (Beckman Coulter, Fullerton, CA) and FlowJo software (Tree star, Inc, Ashland, OR) was used for analysis.
2.8. Statistical analyses
Comparison of weight loss following challenge between groups was performed using the unpaired Student’s t-test. Immune response data are presented as means with SEM. Comparison of ELISA and PRNT50 antibody titers between groups were performed using the Mann-Whitney U-test. Comparison of cellular immune responses between groups was performed using the unpaired Student’s t-test. P values of <0.05 were considered statistically significant.
Results
3.1. Intramuscular immunization with recombinant adenovirus encoding A27L protects mice against virulent respiratory poxvirus infection
To test whether immunization with recombinant adenovirus (rAd) vaccine vectors encoding poxvirus glycoproteins was protective against lethal poxvirus challenge, mice were immunized intramuscularly (i.m.) with a single dose of rAd encoding A33R, A34R, A36R, B5R, A27L, or L1R glycoproteins. Control mice were immunized with rAd-Empty. Four weeks after immunization, mice were challenged intranasally (i.n.) with 5LD50 of vaccinia virus, Western Reserve strain (VV-WR) and observed for maintenance of pre-challenge body weight (morbidity) and survival, along with other indicators of sickness including ruffling of fur and swelling of the forehead, indicative of the development of encephalitis.
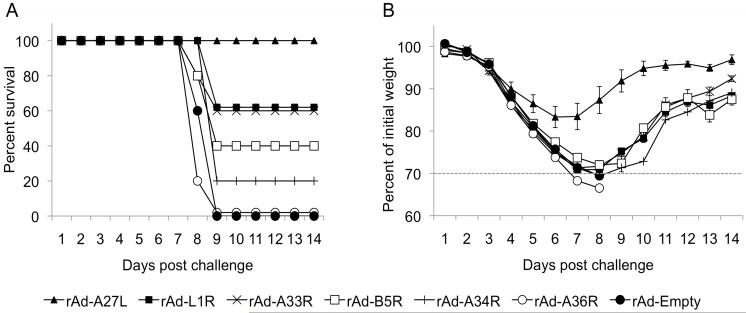
As shown in Fig 1A, all of the mice vaccinated with empty control vector succumbed to challenge by day 9. These mice had generally developed severe ruffling of the fur and signs of encephalitis by day 6 post challenge. In stark contrast, immunization with rAd-A27L resulted in complete protection against lethal i.n. challenge, while partial protection was observed following immunization with rAd encoding A33R, A34R, B5R or L1R, with surviving mice developing severe ruffling of the fur and symptoms similar to those described above in control mice. None of the mice given rAd-A36R survived poxvirus challenge. Mice given rAd-A27L lost, on average, only 17% of their body mass with no other apparent symptoms, clearly indicating significantly reduced morbidity compared to vaccination with the other glycoproteins (Fig. 1B). These data clearly demonstrate that A27L is highly protective against lethal poxvirus challenge in mice when given as a single-shot vaccine in an rAd virus vector. Enhanced rAd-A27L mediated protective efficacy at 10 weeks post vaccination
Fig. 1. Immunization with rAd-A27L IM is protective against lethal vaccinia virus challenge in mice.
6 – 8 week old Balb/c mice were immunized with 1×109 pfu of rAd. Vaccines encoding poxvirus glycoproteins were tested individually. Immunized mice were challenged i.n. with 5LD50 VV-WR at 4 weeks post vaccination and weighed at challenge and daily thereafter. Loss of body mass was used as a measure of morbidity. Mice that sustained more than 30% loss of body mass as shown by data points below the dotted line (B) were euthanized. Survival (A) and weight loss (B) are shown as mean values within each group ± SEM.
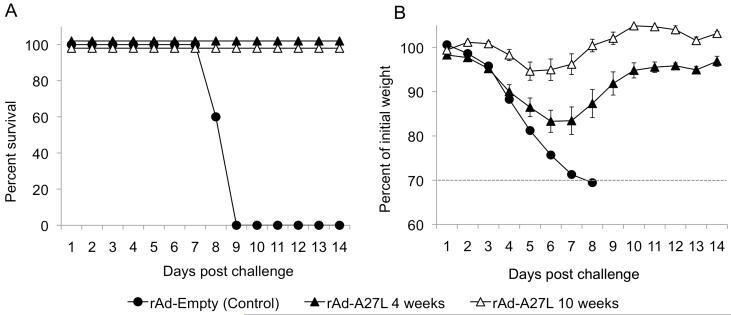
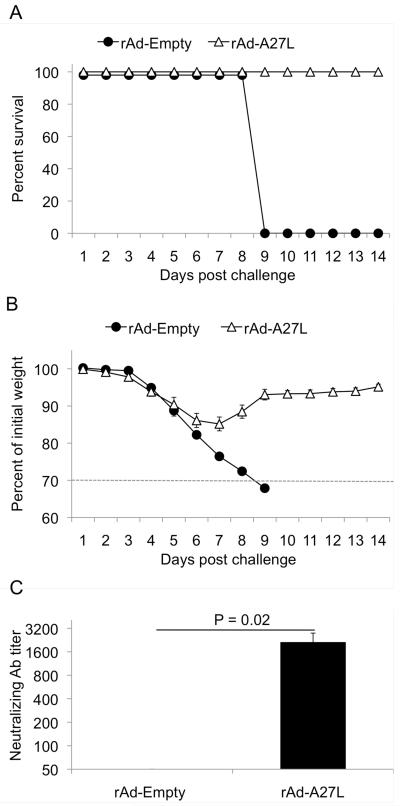
To test whether the protective efficacy of a single-shot rAd-A27L vaccine persisted beyond 4 weeks, mice given rAd-A27L were challenged at 10 weeks after immunization. As shown in Fig 2A, all vaccinated mice survived challenge at this time point (Fig. 2A). Interestingly, weight loss was less pronounced and the mice recovered their starting weight sooner than mice that had been challenged at 4 weeks post vaccination (Fig. 2B). Indeed, maximum weight loss averaged less than 6%, indicative of low morbidity following otherwise lethal challenge.
Fig. 2. Immunization with rAd-A27L protects mice against lethal vaccinia virus challenge for up to 10 weeks post vaccination.
6 – 8 week old Balb/c mice were immunized i.m. with 1×109 pfu rAd-A27L and challenged i.n. with 5LD50 VV-WR at 10 weeks post vaccination. Mice that sustained more than 30% loss of body mass as shown by data points below the dotted line (B) were euthanized. Survival (A) and weight loss (B) are shown as mean values for each group ± SEM.
3.2. Intramuscular immunization with recombinant adenovirus encoding A27L elicits potent cellular and antibody responses
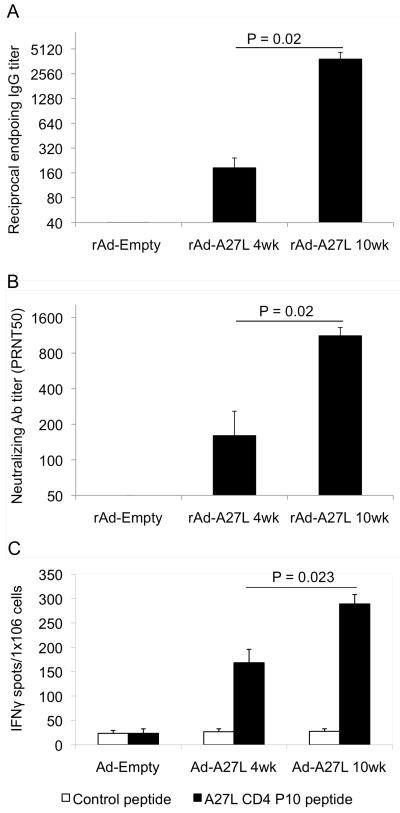
Next, we attempted to correlate the protective efficacy of rAd-A27L with vaccine-induced cellular and humoral immune responses. To measure A27L-specific antibody responses, we tested immune sera against recombinant A27L protein by ELISA. A27L-specific antibody responses were detected in rAd-A27L immunized mice at 4 weeks post vaccination, albeit at low titers, while significantly greater (P<0.02) responses were seen at 10 weeks post vaccination (Fig. 3A). A similar pattern was seen in terms of specific neutralizing antibody levels measured by PRNT50 assay, with significantly greater (P<0.02) responses also seen at 10 weeks post vaccination (Fig. 3B).
Fig. 3. Immunization with rAd-A27L generates potent cellular and antibody responses.
6 – 8 week old Balb/c mice were immunized i.m. with 1×109 pfu rAd-A27L or rAd-empty. Antibody and cellular immune responses were evaluated at 4 weeks and 10 weeks post vaccination. Pre-challenge sera were collected at one day prior to challenge. Serum IgG antibody titers (A) were evaluated against recombinant A27L protein by ELISA and neutralizing antibody titers (B) by PRNT50 assay. Endpoint antibody titers for the IgG ELISA were defined as the greatest serum dilutions having more than double the O.D. of pre-immune sera. Endpoint titers for the PRNT50 assay were defined as the greatest serum dilutions that caused a 50% reduction in numbers of plaques compared to the virus control wells. Spleen cells were harvested from immunized mice and used for analysis of cellular responses against the A27L CD4 P10 peptide by IFNγ-ELISPOT assay (C). Data are presented as numbers of IFNγ-positive spot forming cells (SFC) per million cells.
The IFNγ-ELISPOT assay was used to evaluate the induction of antigen-specific cellular responses in rAd-A27L immunized mice. It is thought that cellular responses may be required for control of virus replication and delay in disease progression until antibody responses are generated. High-level CD4+ T cell responses against P10, the immune dominant CD4+ T cell-epitope in A27L, were observed at 4 weeks post vaccination with rAd-A27L, and these had increased significantly by week 10 (Fig. 3C). In addition, ELISPOT responses were tested against peptides representing a number of putative CD8+ T cell epitopes in A27L derived from prediction algorithms, however none proved to be reactive in these assays (data not shown). It is possible that no CD8+ T cell epitopes for BALB/c mice are present in the relatively small A27L glycoprotein and that the majority of host T cell responses are mounted against the dominant P10 CD4+ T cell epitope. Studies are underway to clarify this point.
3.3. Multifunctional T cell responses following rAd-A27L immunization
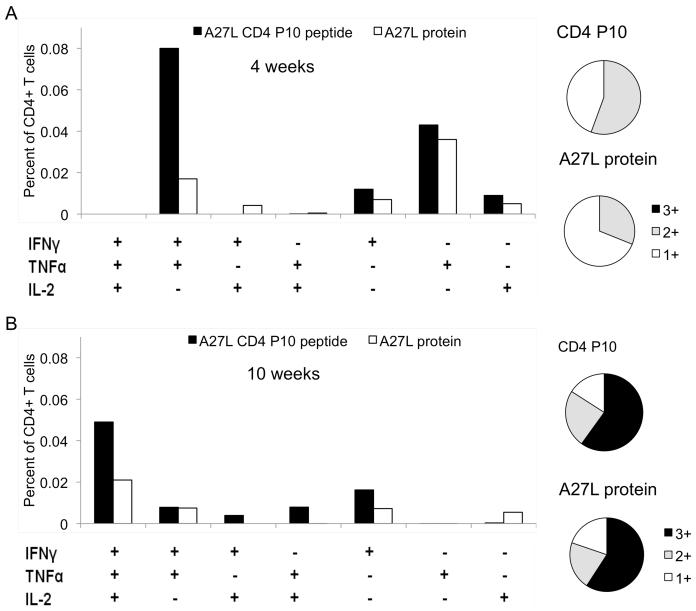
Vaccination strategies that induce antigen-specific T cells with the capacity to simultaneously produce IFNγ,TNFα, and IL-2 or combinations and of these cytokines (multifunctional T cells), mediate enhanced protection against different intracellular pathogens in a number of disease models [36, 37]. Concomitant production of IFNγ and TNFα may lead to synergistic enhancement of the killing capacity of a cell-mediated immune response against intracellular pathogens, while IL-2 mediates expansion and maintenance of antigen-specific T cells. We therefore evaluated multifunctional CD4+ T cell responses in rAd-A27L immunized mice using multiparameter flowcytometry, using both recombinant A27L protein and P10 peptide as recall antigens.
In general, CD4+ T cell responses against recombinant A27L were detected at lower levels than P10-specific responses, probably reflecting the differential antigen processing and presentation requirements for the protein and the immune dominant peptide. By 4 weeks post vaccination, most of the antigen specific CD4+ T cells appeared to be producing IFNγ and/or TNFα (Fig. 4A), however by 10 weeks, a population of A27L/P10-specific cells capable of producing all three cytokines had emerged as the dominant reactive population (Fig. 4B). The latter population was not detected at the 4 week time point, suggestive of the development of a qualitatively superior CD4+ T cell response with time, that correlates with the enhanced protective efficacy of rAd-A27L vaccination that was demonstrated above.
Fig. 4. Ad-A27L generates specific, cytokine expressing multifunctional T cells.
Multiparameter flow cytometry was used to estimate the frequency of CD4+ T cells expressing IFNγ, TNFα or IL-2 at 4 weeks (A) and 10 weeks (B) post vaccination. Boolean gating analysis [26, 27] was used to estimate fractions of the total cell populations that express different combinations of one (1+), two (2+) or three (3+) of these cytokines.
3.4. Long-term persistence of protection mediated by rAd-A27L vaccine
To test for long term persistence of the protective effects of rAd-A27L immunization, mice were given rAd-A27L, challenged i.n. with a lethal dose of VV-WR at 35 weeks post vaccination, and observed for maintenance of pre-challenge body weight (morbidity) and survival. Each of the mice immunized with rAd-A27L survived lethal i.n. challenge at this time point (Fig 5A). None lost greater than 20% of their initial body mass and all quickly regained lost weight (Fig 5B). The mice developed only slight ruffling of the fur and there were no obvious signs of encephalitis. In contrast, all of the mice given control vaccine succumbed to challenge by day 9 and had developed severe ruffling of the fur, with obvious signs of encephalitis by day 6 post challenge. Sera from these mice were collected at one day prior to challenge and were evaluated for the presence of neutralizing antibody responses. Solid neutralizing antibody titers were detected in protected, rAd-A27L-immunized mice (P<0.02; Fig 5C). These data clearly show that the protective efficacy of rAd-A27L vaccine may persist for many months and suggest that A27L represents an effective target for long-term protection against lethal poxvirus infections.
Fig. 5. Long term persistence of rAd-A27L mediated protective efficacy and immunogenicity.
6 – 8 week old Balb/c mice were immunized i.m. with 1×109 pfu of rAd-A27L or rAd-empty control vaccine and challenged i.n. with 5LD50 VV-WR at 35 weeks post vaccination. Mice that sustained more than 30% loss of body mass as shown by data points below the dotted line (B) were euthanized. Survival (A) and weight loss (B) are shown as mean values for each group ± SEM. Sera were harvested one day prior to challenge and tested for neutralizing antibody responses by PRNT50 assay. The endpoint titer for the PRNT50 assay was defined as the greatest serum dilution that caused a 50% reduction in the number of plaques compared to the virus control wells.
4. Discussion
DRYVAX®, the live vaccine used for the eradication of smallpox from its natural environment is contraindicated in individuals with immune deficiencies and has been associated with serious adverse events in otherwise healthy people [2, 3]. Infections due to emerging zoonotic pathogens such as monkeypox virus, or the intentional release of pathogenic poxviruses, are viewed as potentially serious threats to public health, and effective new vaccine strategies with reduced side effects are needed.
In this study, we have shown that a single-shot intramuscular dose of an Ad-based vaccine encoding the IMV glycoprotein A27L confers protection against intranasal challenge with a lethal dose of VV-WR. Protection against lethal challenge was associated with solid A27L-specific antibody responses, including neutralizing antibodies, and the development of polyfunctional CD4+ T cell responses. The emergence of the latter, together with significantly elevated neutralizing antibody responses, were associated with significantly reduced morbidity following lethal challenge at 10 weeks after vaccination, compared to challenge at 4 weeks. The intranasal route of challenge with a highly virulent strain of vaccinia virus VV-WR was used to mimic natural exposure to smallpox via the respiratory tract and it is likely to be important to identify approaches capable of protecting against infection via this route. The other poxvirus proteins tested in this study were of variable protective efficacy. The EEV proteins A33R, A34R or B5R provided partial protection, with 60%, 20% or 40% survival, respectively, while A36R was not protective. L1R, the other IMV protein tested in this study, protected 60% of mice against challenge.
Previous studies have described gene or protein-based subunit vaccine approaches to immunization against a variety of poxvirus glycoproteins [8, 9, 18, 20-27, 38-41]. In most cases, multiple shots were required to generate protective immunity. Interestingly, DNA-based vaccination studies in mice showed minimal protection against poxvirus challenge via the intraperitoneal route following up to three shots of A27L-vaccine, and this was attributed to the generation of only low levels of neutralizing antibodies [8]. Another study tested six different poxvirus glycoproteins in DNA vaccines in mice, demonstrating partial protection against intranasal poxvirus challenge in A27L-vaccinated mice, but, once again, only after at least 3 shots and in the absence of measurable specific antibody responses [20]. Finally, intramuscular administration of rAd encoding A27L gave only partial protection against systemic (i.p.) poxvirus challenge at 4 weeks after vaccination, although its efficacy against respiratory challenge was not tested [22]. Here, we extend these studies to show that single-shot systemic delivery of rAd-A27L was fully protective against the lethal effects of respiratory poxvirus challenge at 4 weeks, and up to 35 weeks, after immunization, and that protection correlated with strong and sustained neutralizing antibody titers. We were also able to demonstrate qualitative differences in cellular immune responses by 10 weeks post vaccination, with the development of a small but distinct population of A27L-specific polyfunctional CD4+ T cells. These cells display rapid recall responses and appear to correlate with enhanced protection in other disease models [36, 37]. Our future studies will include a detailed characterization of local and circulating reactive T cell populations around 35 weeks post vaccination, including CD8+ T cell populations, although it is as yet unclear whether A27L contains reactive sites for CD8+ T cells in this model. Studies with overlapping peptide pools and in class I-deficient mice should help to clarify this issue. We also plan to evaluate host immune responses at time points earlier than 4 weeks post-vaccination that might contribute to the protective efficacy of this approach.
In summary, our data show that a single-shot rAd-A27L immunogen can serve as a potential subunit smallpox vaccine. This construct, alone or in combination with other poxvirus subunits, could be used to induce protective immune responses in the event of an outbreak and, conceivably, might represent a candidate for a first line of defense via rapid ring vaccination (the vaccination of all susceptible contacts in an area around infectious outbreak), particularly given its ability to provide protection following respiratory exposure to poxvirus. Recombinant Ad-27L vaccine could also be used as a component of heterologous prime-boost immunization strategies that may generate protective host immune responses of even greater efficacy and/or longevity, including co-delivery with additional poxvirus subunits. Protective immunity due to the rAd-A27L vaccine is long lasting and the vector appears to be safe, probably due, in part, to its inability to replicate well in mammalian cells. Studies in mice suggest that mucosal delivery of replication-defective adenovirus vectors does not result in spread to neural tissues [34], while no serious vaccine-related toxicity has been reported following widespread use of these vectors.
Further studies are required to evaluate the immunogenicity and protective efficacy of this approach in non-human primates. It will also be of interest to study the protective efficacy of A27L subunit vaccination in the context of different Ad vector serotypes that may be even more effective for use in humans. Finally, direct application of poxvirus subunit vaccines to pulmonary mucosa may generate local responses that further enhance their efficacy against virulent poxvirus infection via the natural respiratory route.
Acknowledgments
We acknowledge the help of Robert Kutner in the propagation and purification of recombinant adenovirus vaccine vectors and Constance Porretta for assistance with flow cytometry. The following reagent was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Vaccinia Virus (WR) A27L Protein with C-terminal Histidine Tag, Recombinant from baculovirus, NR-2622. We also acknowledge Bernard Moss, LVD, NIAID, NIH for providing the Vaccinia virus (WR). This study was supported by NIH grant 5R21AI054172-02 (AJR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].The global eradication of smallpox . Final report of the global commission for the certification of smallpox eradication. World Health Organization; Geneva: 1980. [Google Scholar]
- [2].Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clin Infect Dis. 2003 Jul 15;37(2):251–71. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- [3].Casey CG, Iskander JK, Roper MH, Mast EE, Wen XJ, Torok TJ, et al. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA. 2005 Dec 7;294(21):2734–43. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- [4].Kennedy RB, Ovsyannikova IG, Jacobson RM, Poland GA. The immunology of smallpox vaccines. Curr Opin Immunol. 2009 Jun;21(3):314–20. doi: 10.1016/j.coi.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].US Food and Drug Administration 2010 Feb 23; www.fda.gov/BiologicsBloodVaccines/Vaccines/QuestionsaboutVaccines/ucm078041.htm.
- [6].Artenstein AW. New generation smallpox vaccines: a review of preclinical and clinical data. Rev Med Virol. 2008 Jul-Aug;18(4):217–31. doi: 10.1002/rmv.571. [DOI] [PubMed] [Google Scholar]
- [7].Massung RF, Liu LI, Qi J, Knight JC, Yuran TE, Kerlavage AR, et al. Analysis of the complete genome of smallpox variola major virus strain Bangladesh-1975. Virology. 1994 Jun;201(2):215–40. doi: 10.1006/viro.1994.1288. [DOI] [PubMed] [Google Scholar]
- [8].Hooper JW, Custer DM, Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003 Feb 1;306(1):181–95. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sakhatskyy P, Wang S, Zhang C, Chou TH, Kishko M, Lu S. Immunogenicity and protection efficacy of subunit-based smallpox vaccines using variola major antigens. Virology. 2008 Feb 5;371(1):98–107. doi: 10.1016/j.virol.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chung CS, Chen CH, Ho MY, Huang CY, Liao CL, Chang W. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J Virol. 2006 Mar;80(5):2127–40. doi: 10.1128/JVI.80.5.2127-2140.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ravanello MP, Hruby DE. Conditional lethal expression of the vaccinia virus L1R myristylated protein reveals a role in virion assembly. J Virol. 1994 Oct;68(10):6401–10. doi: 10.1128/jvi.68.10.6401-6410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sanderson CM, Hollinshead M, Smith GL. The vaccinia virus A27L protein is needed for the microtubule-dependent transport of intracellular mature virus particles. J Gen Virol. 2000 Jan;81(Pt 1):47–58. doi: 10.1099/0022-1317-81-1-47. [DOI] [PubMed] [Google Scholar]
- [13].Wolffe EJ, Weisberg AS, Moss B. The vaccinia virus A33R protein provides a chaperone function for viral membrane localization and tyrosine phosphorylation of the A36R protein. J Virol. 2001 Jan;75(1):303–10. doi: 10.1128/JVI.75.1.303-310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van Eijl H, Hollinshead M, Smith GL. The vaccinia virus A36R protein is a type Ib membrane protein present on intracellular but not extracellular enveloped virus particles. Virology. 2000 May 25;271(1):26–36. doi: 10.1006/viro.2000.0260. [DOI] [PubMed] [Google Scholar]
- [15].Engelstad M, Smith GL. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology. 1993 Jun;194(2):627–37. doi: 10.1006/viro.1993.1302. [DOI] [PubMed] [Google Scholar]
- [16].Wolffe EJ, Katz E, Weisberg A, Moss B. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J Virol. 1997 May;71(5):3904–15. doi: 10.1128/jvi.71.5.3904-3915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wolffe EJ, Isaacs SN, Moss B. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J Virol. 1993 Aug;67(8):4732–41. doi: 10.1128/jvi.67.8.4732-4741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hooper JW, Thompson E, Wilhelmsen C, Zimmerman M, Ichou MA, Steffen SE, et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004 May;78(9):4433–43. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sakhatskyy P, Wang S, Chou TH, Lu S. Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen. Virology. 2006 Nov 25;355(2):164–74. doi: 10.1016/j.virol.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pulford DJ, Gates A, Bridge SH, Robinson JH, Ulaeto D. Differential efficacy of vaccinia virus envelope proteins administered by DNA immunisation in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine. 2004 Sep 3;22(25-26):3358–66. doi: 10.1016/j.vaccine.2004.02.034. [DOI] [PubMed] [Google Scholar]
- [21].Fogg C, Lustig S, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004 Oct;78(19):10230–7. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kaufman DR, Goudsmit J, Holterman L, Ewald BA, Denholtz M, Devoy C, et al. Differential antigen requirements for protection against systemic and intranasal vaccinia virus challenges in mice. J Virol. 2008 Jul;82(14):6829–37. doi: 10.1128/JVI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lai CF, Gong SC, Esteban M. The purified 14-kilodalton envelope protein of vaccinia virus produced in Escherichia coli induces virus immunity in animals. J Virol. 1991 Oct;65(10):5631–5. doi: 10.1128/jvi.65.10.5631-5635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Berhanu A, Wilson RL, Kirkwood-Watts DL, King DS, Warren TK, Lund SA, et al. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J Virol. 2008 Apr;82(7):3517–29. doi: 10.1128/JVI.01854-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Heraud JM, Edghill-Smith Y, Ayala V, Kalisz I, Parrino J, Kalyanaraman VS, et al. Subunit recombinant vaccine protects against monkeypox. J Immunol. 2006 Aug 15;177(4):2552–64. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]
- [26].Hooper JW, Ferro AM, Golden JW, Silvera P, Dudek J, Alterson K, et al. Molecular smallpox vaccine delivered by alphavirus replicons elicits protective immunity in mice and non-human primates. Vaccine. 2009 Dec 11;28(2):494–511. doi: 10.1016/j.vaccine.2009.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, et al. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol. 2005 Sep;79(18):11724–33. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ramsay AJ, Kent SJ, Strugnell RA, Suhrbier A, Thomson SA, Ramshaw IA. Genetic vaccination strategies for enhanced cellular, humoral and mucosal immunity. Immunol Rev. 1999 Oct;171:27–44. doi: 10.1111/j.1600-065x.1999.tb01341.x. [DOI] [PubMed] [Google Scholar]
- [29].Amalfitano A. Utilization of adenovirus vectors for multiple gene transfer applications. Methods. 2004 Jun;33(2):173–8. doi: 10.1016/j.ymeth.2003.11.006. [DOI] [PubMed] [Google Scholar]
- [30].Hilgendorf A, Lindberg J, Ruzsics Z, Honing S, Elsing A, Lofqvist M, et al. Two distinct transport motifs in the adenovirus E3/10.4-14.5 proteins act in concert to down-modulate apoptosis receptors and the epidermal growth factor receptor. J Biol Chem. 2003 Dec 19;278(51):51872–84. doi: 10.1074/jbc.M310038200. [DOI] [PubMed] [Google Scholar]
- [31].Ada GLRA. Vaccines, Vaccination and the Immune Response. Raven Lippincott; Philadelphia: 1997. [Google Scholar]
- [32].Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. 2001 Dec;1(3):209–19. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- [33].Ramsay AJ, Ramshaw IA, Ada GL. DNA immunization. Immunol Cell Biol. 1997 Aug;75(4):360–3. doi: 10.1038/icb.1997.56. [DOI] [PubMed] [Google Scholar]
- [34].Damjanovic D, Zhang X, Mu J, Fe Medina M, Xing Z. Organ distribution of transgene expression following intranasal mucosal delivery of recombinant replication-defective adenovirus gene transfer vector. Genet Vaccines Ther. 2008;6:5. doi: 10.1186/1479-0556-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Reed LJ, Muench J. A simple method of estimating fifty percent endpoints. The American Journal of Hygiene. 1938;27:493–7. [Google Scholar]
- [36].Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007 Jul;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- [37].Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008 Apr;8(4):247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- [38].Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007 Feb 26;25(10):1814–23. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Galmiche MC, Goenaga J, Wittek R, Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999 Feb 1;254(1):71–80. doi: 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- [40].He YMC, Vassell RA, Merchlinsky M, Weir JP, Weiss CD. Recombinant A27 protein synergizes with modified vaccinia Ankara in conferring protection against a lethal vaccinia virus challenge. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.10.078. doi:10.1016/j.vaccine.2009.10.078. [DOI] [PubMed] [Google Scholar]
- [41].Demkowicz WE, Maa JS, Esteban M. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J Virol. 1992 Jan;66(1):386–98. doi: 10.1128/jvi.66.1.386-398.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]