Abstract
Preeclampsia is a hypertensive disorder of pregnancy that affects many organs including the brain. Neurological complications occur during preeclampsia, the most serious of which is seizure known as eclampsia. Although preeclampsia can precede the eclamptic seizure, it often occurs during normal pregnancy, suggesting that processes associated with normal pregnancy can promote neuronal excitability. Here we investigated whether circulating inflammatory mediators that are elevated late in gestation when seizure also occurs are hyperexcitable to neuronal tissue. Evoked field potentials were measured in hippocampal slices in which control horse serum that slices are normally grown in, was replaced with serum from nonpregnant or late-pregnant Wistar rats for 48 hours. We found that serum from pregnant, but not nonpregnant rats, caused hyperexcitability to hippocampal neurons and seizure activity that was abrogated by inhibition of tumor necrosis factor alpha (TNFα) signaling. Additionally, application of TNFα mimicked this increased excitability. Pregnant serum also caused morphological changes in microglia characteristic of activation, and increased TNFα mRNA expression that was not seen with exposure to nonpregnant serum. However, TNFα protein was not found to be elevated in pregnant serum itself, suggesting that other circulating factors during pregnancy caused activation of hippocampal slice cells to produce a TNFα-mediated increase in neuronal excitability. Lastly, although pregnant serum caused neuroinflammation and hyperexcitability of hippocampal slices, it did not increase blood-brain barrier permeability, nor were pregnant rats from which the serum was taken undergoing seizure. Thus, the BBB has an important role in protecting the brain from circulating neuroinflammatory mediators that are hyperexcitable to the brain during pregnancy. These studies provide novel insight into the underlying cause of eclampsia without elevated blood pressure and the protective role of the BBB that prevents exposure of the brain to hyperexcitable factors.
Keywords: pregnancy, seizure, hippocampal slice, neuroinflammation, TNFα, microglia, blood-brain barrier
Introduction
The occurrence of unexplained seizure during pregnancy, known as eclampsia, has plagued women and physicians for centuries. It is one of the most serious complications of pregnancy and is life-threatening for both mother and fetus (Sabai, 2005; Duley, 2009). Although seizure during pregnancy is often associated with preeclampsia - the appearance of hypertension and proteinuria during the last half of gestation - it also occurs in women with seemingly uncomplicated pregnancy who have normal blood pressures. In fact, studies have consistently found that there is little correlation between blood pressure and seizure in pregnancy (Sibai, et al., 1986; Sabai, 1990; Douglas and Redman, 1994; Katz et al., 2000). These findings have established that preeclampsia is not necessarily a prodrome for eclampsia and imply that factors or processes associated with normal pregnancy may provoke the eclamptic seizure.
Normal pregnancy is a state of considerable biological adaptation. Almost every physiological system is known to adapt for successful pregnancy to occur. Changes in cardiovascular, renal, immune, and endocrine systems take place to allow for the fetal allograft to survive early in pregnancy and to meet the demands of the growing fetus later in gestation (Liu, 2004; Monga, 2004; Aagaard-Tillery et al., 2006). To this end, large amounts of hormones, including growth factors, cytokines and chemokines are produced, mostly by the fetal-placental unit, and released into the circulation (Liu, 2004; Aagaard-Tillery et al., 2006; Rusterholz et al., 2007). Hormone production rises considerably throughout pregnancy, reaching a peak late in gestation when seizure also occurs (Liu, 2004; Aagaard-Tillery et al., 2006; Rusterholz et al., 2007). In fact, eclamptic seizures occur exclusively in the last half of pregnancy, and most often during late-gestation when levels of circulating hormones are highest (Sibai, et al., 1986; Sabai, 1990; Douglas and Redman, 1994; Katz et al., 2000). In addition, there is a high propensity for eclampsia to occur in multi-fetal gestations when the levels of hormones are significantly elevated, and in developing countries where peripheral inflammation is high, further suggesting a role for these circulating pro-inflammatory mediators in eclampsia (Sabai, 2005; Duley, 2009; van den Broek, 2000).
The molecular mechanisms that lead to seizure activity are not completely understood, but can be provoked by a number of conditions, including acute metabolic derangements, inflammation and injury (Palladino and Stanley, 2010; Riazi et al., 2010; Shlosberg et al., 2010). Central to these states in evoking seizure activity is the activation of microglia. Microglia are resident macrophages that are ubiquitously distributed throughout the brain parenchyma and react swiftly to pathological stimuli (Ladeby et al., 2005; Vezzani et al., 2008). Microglial activation is characterized by a number of features including morphologic transformation and production of pro-inflammatory cytokines such as tumor necrosis factor-α (TNFα) (Hulse et al., 2008) that then act on neurons to change their excitability state (Stellwagen et al., 2005; Stellwagen and Malenka, 2006). In particular, microglia-produced TNFα can change neuronal excitability and promote seizure activity through increasing cell-surface AMPA receptors and decreasing GABAA receptors, thus decreasing inhibitory synaptic activity (Stellwagen et al., 2005). Recently, peripheral inflammation has been shown to increase seizure susceptibility through microglial activation and TNFα production (Riazi et al., 2008), demonstrating that circulating inflammatory mediators can affect brain excitability to promote seizure.
Pregnancy is also a state of peripheral inflammation in which there are elevated levels of circulating pro-inflammatory cytokines and oxidative stress (Sacks et al., 1998; D’Mello et al., 2009; Szarka et al., 2010). Because some of these factors can activate microglia, we hypothesized that circulating pro-inflammatory mediators produced during normal pregnancy would be hyperexcitable to the brain (D’Mello et al., 2009). Thus, we investigated the effect of circulating factors in the serum of late-pregnant (LP) rats on microglial activation and excitability of hippocampal neurons. In addition, because the blood-brain barrier (BBB) has an important role in limiting transport of damaging circulating factors from the blood into the brain that could promote seizure activity (Cipolla, 2006), we also investigated how serum from late-pregnant animals affected BBB permeability.
Materials and methods
Animal model of pregnancy and serum samples
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont. A rat model was used to study the effects of pregnancy on brain inflammation and neuronal excitability. The rat is a useful model of pregnancy because it has similar placentation (hemochorial) as well as physiological, cardiovascular and immune adaptations as humans (Gilson et al, 1992), and a short gestation (22 days). Timed-pregnant Wistar rats were purchased from Harlan on day 5–6 of gestation and kept in the animal care facility at the University of Vermont. Age-matched nonpregnant virgin female Wistar rats were used as controls. Animals were kept in a 12 hour light, 12 hour dark cycle and allowed food and water ad libitum.
On the day of an experiment, animals were anesthetized with isoflurane in oxygen and decapitated. Trunk blood was collected in serum separator tubes, centrifuged and serum obtained. Samples were frozen at −80°C until use.
Hippocampal slice cultures and measurement of neuronal excitability
Slice cultures were prepared as previously described (Kunkler and Kraig, 1997). Briefly, hippocampi were collected from 8–10 day old CO2-anesthetised rat pups, sectioned to 350 μm and placed on Millicell-CM tissue culture inserts (Millipore) in 6-well Falcon trays (Becton Dickinson). Cultures were maintained at 36°C with 5% CO2-balance air and 95% humidity, with culture media changed biweekly and containing: 50 ml Basal Medium Eagle, 25 ml Earle’s Balanced Salt Solution, 23 ml horse serum, 0.5 ml GlutaMax (at 200 mM), 0.1 ml Gentamicin (at 10 mg/ml), 0.4 ml Fungizone (at 250 μg/ml), 1.45 ml D-Glucose (42 mM). All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Chicago.
At 18 days in vitro (DIV) slice cultures were screened for cell death using 5 μM Sytox Green (504/523 nm fluorescence excitation/emission when bound to DNA; #S7020; Invitrogen) (Hulse et al., 2008). Cultures with pyramidal neuron layer injury were not used. Cultures were used between 21–24 DIV, a time range well within the period associated with mature synaptic function and quiescent microglia consistent with those seen normally in vivo (Hulse et al., 2008; Kunkler and Kraig, 1998; Kunkler et al., 2005).
1–3 days after Sytox screening, slice culture media was replaced with media where horse serum (23% of total volume) was substituted with serum from nonpregnant (NP) or late-pregnant (LP) rats. Control slices were kept in the horse serum-based media. All groups were incubated under normal incubation conditions for 48 hours before experiments.
For electrophysiological recordings, slice culture inserts were placed in 35 mm culture dishes with two 1 mm glass rods at the bottom to stabilize the insert membrane which otherwise might move up and down. In addition, 3 compressible sections of tubing (#95809; Pharmed) 2 mm long were positioned equally around inserts to further hold the inserts in place. A ~10 mm wide and 3–4 cm long piece of cotton was soaked in ~1 ml of media and placed around the inside perimeter of the insert flush with the walls and away from the slices to help maintain a humidified environment. The top of the culture dish was then covered with polyvinyl chloride film (Fisher Scientific) to prevent fluid loss and allow CO2 diffusion. Cultures were placed in a microincubator (PDMI-2; Medical Systems) set at 36°C and aerated with 5% CO2-balance air and media maintained at pH 7.3 as tested with a micro-combination pH electrode (M-413; Microelectrodes) to create a “static” recording environment. A battery-operated cautery tool (Gemini Cautery System; Braintree Scientific Instruments) was used to burn an elliptical hole in the polyvinyl chloride film directly above the inserts for microelectrode placement.
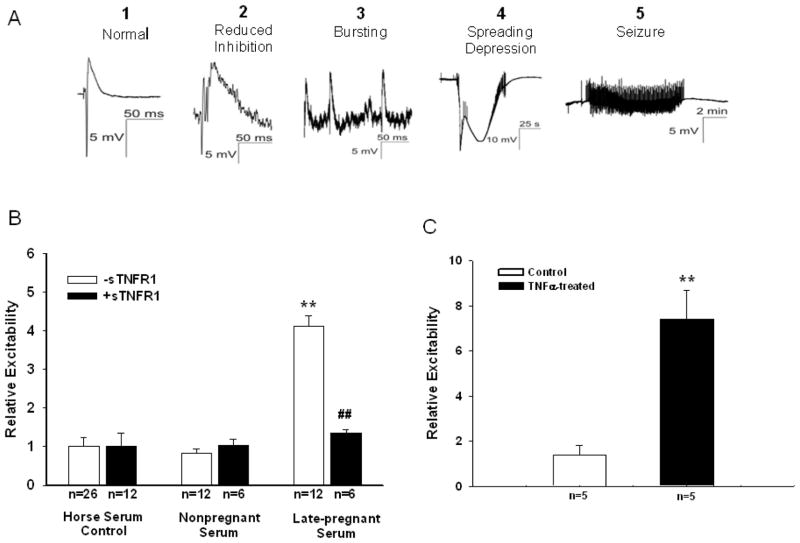
Culture inserts were allowed to equilibrate in their recording environment for ~10 minutes. Then, a sharp (2–4 μm tip diameter) microelectrode was placed at the CA3 pyramidal neuron layer to record responses evoked by a dentate gyrus bipolar stimulating electrode, using pulses of 10–50 μA at 100 μs (Kunkler and Kraig, 1998; Kunkler et al., 2005; Grinberg et al., 2011; Pusic et al., 2011). The electrical stimulus was repeated after minutes with recordings made from each of three slices for approximately 20 minutes. Typical electrical responses are shown in Figure 1A. Evoked responses were graded on a weighted scale (normal field potential, 1; reduced synaptic inhibition, 2; spontaneous bursting, 3; spreading depression, 4; and electrographic seizures, 5).
Figure 1. Effect of pregnant serum and TNFα on hippocampal slice excitability.
(A) Representative evoked potentials measured after replacement of control horse serum with serum from nonpregnant or late-pregnant rats using a graded scale (1–5) to compare excitability between groups. (B) Late-pregnant serum caused significant neuronal hyperexcitability that was not seen with nonpregnant or control serum. Addition of soluble tumor necrosis factor receptor 1 (sTNFR1) to late-pregnant serum prevented the increase in neuronal excitability without affecting the responses to control or nonpregnant serum. **p<0.01 vs. control and nonpregnant serum; ## p<0.01 vs. late-pregnant serum –sTNFR1. (C) Addition of 200 ng/ml TNFα to slices caused significantly (p=0.002) increased neuronal excitability. **p<0.01 vs. control.
Measurement of microglial activation by CD11b immunostaining density
Immunostaining procedures for CD11b followed standard protocols previously reported for hippocampal slice cultures (Hulse et al., 2008) that were adapted from work involving whole animal brains and digital imaging strategies (Caggiano and Kraig, 1996). Briefly, three random CA3 area of interest replicates were photographed per biological replicate with the examiner blinded to experimental conditions. Resultant microglial immunostaining density technical replicates were averaged to yield a single value per slice culture. Control levels were set to 1.00 to allow for inter-experiment comparisons (Hulse et al., 2008).
Measurement of TNFα by ELISA
The level of TNFα in NP and LP serum was determined using an enzyme-linked immunosorbent assay (ELISA, R&D Systems, DY293B, Minneapolis, MN) and used according to manufacturer’s instructions. After microplate coating with capture antibody, sample incubation (4 h), biotinylated detection antibody addition (3 h) and streptavidin-horseradish peroxidase processing for tetramethylbenzidine substrate development, the optical density in each well was measured at 450 nm with background correction.
Measurement of TNFα mRNA from hippocampal slice cultures
Quantitative real-time RT-PCR (qPCR) was used to measure changes in TNFα in hippocampal slices. Detection of cytokine mRNA by qPCR is a sensitive technique for analyzing cytokine levels in tissues since cytokine mRNA and protein levels are highly correlated (Hein et al., 2001; Blaschke V et al, 2000). qPCR procedures followed previously described methods (Pusic et al., 2011). Following treatment, slice culture inserts were submerged in 3 ml RNAlater (Ambion) and stored at 4°C for up to 3 days until further processing. The hippocampus proper was dissected away from slices using a glass knife (#10100-00, Fine Scientific) and then gently lifted off the insert with a fine tip paint brush (Reed Sable, size 5/0, Ted Pella) and harvested in 1 ml of cold sterile PBS in RNase/DNase, DNA free 1.5 ml microcentrifuge tubes. After a brief centrifugation, PBS supernatant was removed and slices were resuspended in 500 μl of cold TRIzol reagent. Samples were incubated for 5 min at room temperature, then used immediately or stored at −80°C for later use. Total RNA isolation was performed using a combination of TRIzol and Qiagen’s RNeasy micro kit columns.
Quantification of RNA samples was performed using Ribogreen according to the manufacturer’s protocol (Invitrogen), with yeast tRNA standards as a reference. 60 ng of total RNA was reverse transcribed into cDNA using Bio-Rad’s iScript cDNA synthesis kit, as per the manufacturer’s instructions. Resultant cDNA was diluted 1:4 and 1 μl was used for each reaction. TNFα mRNA expression levels were determined by quantitative PCR (qPCR) using the real-time iCycler™ PCR platform (Bio-Rad). cDNA was amplified in duplicate wells using 200 nM of gene specific primers (see below) in a total reaction volume of 25 μl. All experiments were performed using iQ SYBR Green Supermix with fluorescein (Bio-Rad) in accordance with the manufacturer’s instructions. Thermal cycling conditions were as follows: an initial incubation at 95°C for 10 min to activate the polymerase followed by 40 cycles of 95°C for 10 s, 60°C for 30 min and 72°C for 20 s, and a melt curve (55°C, 1 min, 80 cycles of 0.5°C increments for 10 s each starting at 25°C). Relative gene expression normalized to ribosomal protein L13a (Rpl13a) was calculated using the delta-delta Ct method (Yuan et al., 2008). Rpl13a was chosen as the best housekeeping gene for our experiments, since stable expression has been shown in ischemia studies, and expression levels are similar to that of TNFα (Hulse et al., 2008). Primer sequences were as follows: Rpl13a forward, 5′-TTG CTT ACC TGG GGC GTC T- 3′; Rpl13a reverse, 5′-CTT TTT CCT TCC GTT TCT CCT C- 3′; TNFα forward, 5′-ACC ACG CTC TTC TGT CTA CTG A- 3′; TNFα reverse, 5′-CTG ATG AGA GGG AGC CCA TTT G- 3′.
BBB permeability in response to serum exposure
The effect of LP and NP serum on permeability of the BBB was determined as previously described with slight modifications (Roberts et al., 1989; Amburgey et al., 2010). Briefly, a modified Landis technique was used with cerebral veins perfused with serum (20% in physiological buffer) from NP or LP rats. Permeability measurement of veins was performed within an arteriograph chamber that was superfused with physiological saline solution (PSS) at pH 7.4±0.05 and kept at 37°C. The proximal cannula of the arteriograph chamber was connected to an in-line pressure transducer and servo system that allowed for measurement and adjustment of intravascular pressure. Veins were equilibrated at an intravascular pressure of 10 mmHg for 2 hours with plasma in the lumen. Intravascular pressure was then increased to 25 mmHg and the servo controlling pressure was disconnected from the pressure transducer. This allowed measurement of the pressure drop without compensation by the servo system. The measured pressure drop due to filtration of water through the vessel wall in response to hydrostatic pressure was used as a measure of BBB permeability, as previously described (Roberts et al., 1989; Amburgey et al., 2010). The drop in pressure was then converted to filtration (Jv/S) and hydraulic conductivity (Lp) using a conversion curve that relates the volume of water flowing out of the cannula per mmHg. Cerebral veins were used for these experiments because these vessels have BBB properties (Butt and Jones, 1992), can be exposed to plasma, and are kept in their physiological, pressurized state. This method of measuring BBB permeability has been used successfully in previous studies (Roberts et al., 1989; Amburgey et al., 2010) and provides a measure of both transcellular and paracellular permeability.
Statistical analysis and data presentation
Values are mean ± SEM. Differences in relative excitability between the different serums was determined using one-way analysis of variance with post hoc Holm-Sidak test for multiple comparisons. Differences in excitability within groups with and without sTNFR1 were determined using one-way analysis of variance. Differences in optical density of microglia and TNFα mRNA between groups were determined using one-way analysis of variance with a post hoc Holm-Sidak test for multiple comparisons. Differences in Lp and Jv/S after exposure to NP or LP serum were determined using one-way analysis of variance.
Results
Late-pregnant serum is hyperexcitable to hippocampal slice cultures via TNFα
Hippocampal slice cultures were used to study the effect of female rat serum on neuronal excitability because slices closely parallel their adult in vivo counterpart and allow for controlled microenvironmental conditions. Rat hippocampal slices are normally cultured in horse serum (HS)–based media where astrocytes are non-reactive (Kunkler and Kraig, 1997), microglia are quiescent (Hulse et al., 2008), and electrophysiological function is similar to that seen in vivo (Kunkler and Kraig, 1998; Kunkler et al., 2005). To determine if circulating factors during pregnancy affected neuronal excitability, we replaced control HS (n=26) with rat serum from virgin nonpregnant (n=12) or LP (day 20 of a 22 day gestation; n=12) Wistar rats for 48 hours and measured evoked potentials. Figure 1A shows representative evoked potentials measured from hippocampal slices with serum exposure. Evoked potentials were compared from each group using a graded scale, also shown in Figure 1A. We found that where NP serum was similar to control HS in that evoked field potentials were normal, LP serum caused the slices to be hyperexcitable and provoked seizure activity (Figure 1B). When slice cultures were incubated in serum with soluble TNFα receptor 1 [(sTNFR1) 200 ng/ml, #425-R1-050; R&D Systems, Minneapolis, MN; n=6–12] to inhibit TNFα signaling (Mitchell et al., 2010), evoked seizure activity in response to the LP serum was prevented and slice evoked responses were similar to control and NP serum (Figure 1B). To ascertain the involvement of TNFα in hyperexcitability, a separate set of hippocampal slices were incubated in normal horse serum media (n=5/group) containing TNFα (200ng/ml, #510-RT, R&D Systems) added immediately prior to measuring evoked potentials as described above. Since serum can dampen cytokine signaling, we increased TNFα from that used with serum-free media (i.e., 100 ng/ml; Mitchell et al., 2011). Figure 1C shows that TNFα alone caused a significant (P =0.002) increase in neuronal hyperexcitability. Together, these results demonstrate that there are circulating factors in pregnant serum that cause neuronal hyperexcitability and seizure activity through a mechanism involving TNFα.
Late-pregnant serum causes microglial activation in hippocampal slice cultures
All brain cell types and related endothelial cells can produce TNFα, however, microglia are the predominant source of this cytokine under pathophysiological (Hopkins and Rothwell, 1995; Allen and Rothwell, 2001) and physiological (Hulse et al., 2008) conditions within brain tissue. TNFα production by activated microglia is important for synaptic transmission and may have a role in seizure activity (Ladeby et al., 2005; Stellwagen et al., 2005; Stellwagen and Malenka, 2006; Riazi et al., 2008; Vezzani et al., 2008). We therefore measured the microglial reactive state in the slice cultures after exposure to female rat serum by immunohistochemical staining with the specific microglial marker CD11b in which enhanced CD11b reactivity is indicative of activation (Caggiano and Kraig, 1996; Hulse et al., 2008). Activation of microglia was measured using a highly sensitive optical density technique, used in previous studies (Caggiano and Kraig, 1996; Hulse et al., 2008). We found that microglia exposed to NP rat serum (n=6) were quiescent with similar appearance to those exposed to control HS (n=12). However, LP serum exposure (n=6) to the slices caused significant activation of microglia as shown by the increased optical density (Figure 2). Thus, LP serum causes neuronal hyperexcitability and microglial activation in hippocampal slices.
Figure 2. Microglial immunostaining density of hippocampal slices after exposure to pregnant serum.
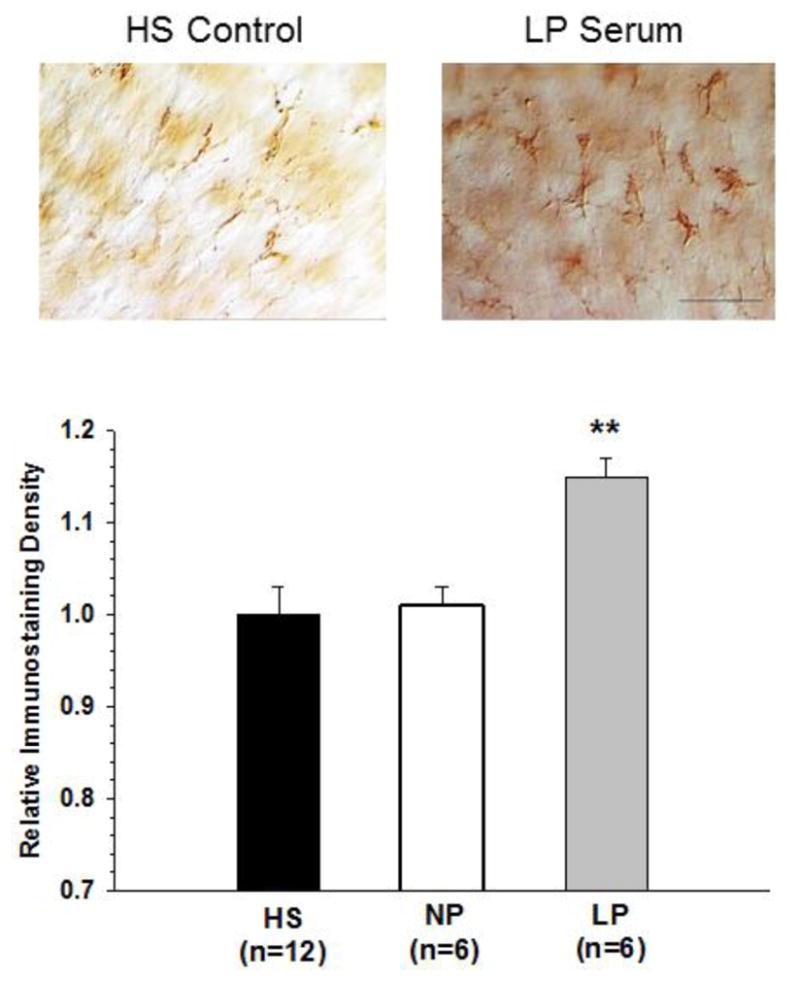
Replacement of control horse serum (HS) with serum from late-pregnant (LP) rats caused microglial activation that was not seen with nonpregnant (NP) serum. **p<0.01 vs. HS and NP. Scale bar = 25 μm.
TNFα is increased in hippocampal slice cultures but not serum
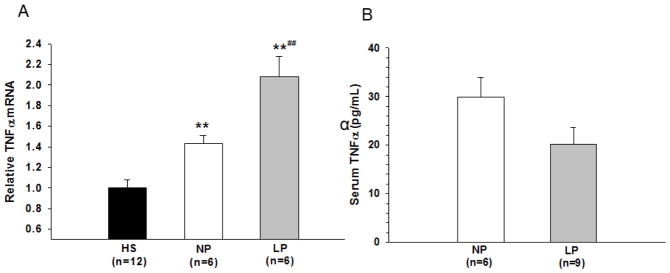
Our results above suggested that LP serum causes hyperexcitability of hippocampal slices due to microglial activation and involves TNFα. We next wanted to know if there was increased TNFα in LP serum that was activating microglia and causing neuronal excitability or if the microglia or other cells within the slice (neurons, astrocytes) were being activated by an unknown circulating factor in the serum that then stimulated the cells to produce TNFα, thus making the slices hyperexcitable. We therefore measured TNFα mRNA by qPCR in slices after exposure to the different serums and in the serum itself by ELISA. We found that TNFα mRNA was significantly increased in slices exposed to both NP (n=6) and LP (n=6) rat serums compared to control (n=12), however, the level of TNFα mRNA was considerably greater in the hippocampal slices after application of the LP serum (Figure 3A). When measured in the NP and LP serum itself, TNFα levels were low and not different between groups (Figure 3B), as has been shown in human pregnancy (Szarka et al., 2010). This result suggests that TNFα produced within the slice is the primary source of TNFα-induced seizure activity with LP serum.
Figure 3. TNFα expression in slices and serum.
(A) Replacement of control horse serum (HS) with serum from nonpregnant (NP) or late-pregnant (LP) rats significantly increased mRNA expression of TNFα in hippocampal slice cultures. LP serum caused a significantly greater increase in TNFα expression compared to NP. ** p<0.01 vs. HS; ## p<0.01 vs. NP. (B) There was no significant difference in the amount of TNFα protein in NP and LP serum as measured by ELISA.
Serum did not affect permeability of the BBB
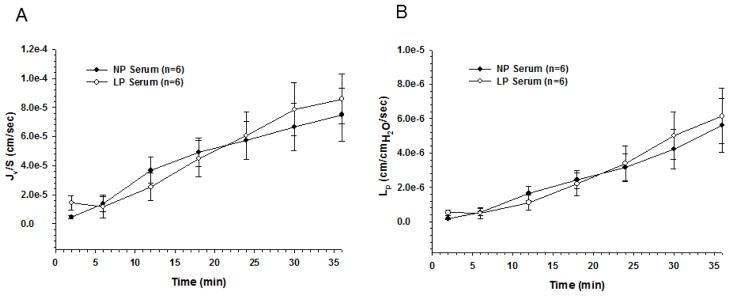
An important consideration of these findings is that under normal conditions microglia within the brain do not encounter serum because of the unique properties of the BBB that limits passage of proteins, ions and other serum constituents from entering the brain (Cipolla, 2006). Therefore, for LP serum to promote seizure activity, activate microglia, and increase slice TNFα, it would also have to increase BBB permeability. To determine if serum from LP animals increased BBB permeability, we perfused isolated and pressurized cerebral veins with NP (n=6) or LP (n=6) serum and measured fluid filtration (Jv/S) and hydraulic conductivity (Lp), as measures of BBB permeability (Roberts et al., 2009). Unlike measures of solute or tracer permeability, Lp is the critical transport parameter that relates water flux to hydrostatic pressure (Michel, 1984), an important consideration for the brain during preeclampsia and eclampsia (Amburgey et al., 2010). Cerebral veins were used because these vessels have BBB properties (Butt and Jones, 1992) and are a primary site of BBB disruption (Mayhan and Heistad, 1986). We found that exposure to either LP or NP serum increased Jv/S and Lp over the 36 minutes of measurement (Figures 4A and 4B, respectively), but there was no difference in the degree of BBB permeability between the two types of serum. These results demonstrate that despite considerable effects of LP serum on microglial activation and neuronal excitability, it did not increase BBB permeability compared to NP serum. These results also importantly demonstrate a protective role of the BBB that appears to limit the seizure potential from circulating peripheral factors during pregnancy.
Figure 4. Blood-brain barrier permeability in response to pregnant serum.
(A) Fluid filtration (Jv/S) in response to hydrostatic pressure measured for 36 minutes showed no difference when cerebral veins were exposed to late-pregnant (LP) vs. nonpregnant (NP) serum. (B) Hydraulic conductivity (Lp) measured over 36 minutes also showed no effect of LP serum compared to NP.
Discussion
Our results here demonstrate for the first time that there are circulating factors produced during normal pregnancy that are hyperexcitable to the brain. This hyperexcitability appears to be due to serum-induced TNFα production as the effect on neuronal evoked potentials was prevented by inhibition of TNFα signaling by the addition of sTNFR1 to LP serum. In addition, TNFα caused significant hyperexcitability of hippocampal slices, further demonstrating an important role for TNFα in neuronal excitability. However, the level of TNFα in the different serums was similar, suggesting that TNFα-induced excitability was not due elevated TNFα in LP serum, but another factor that activated cells within the slice to produce TNFα and affected neuronal excitability. Although LP serum produced hyperexcitability when applied directly to isolated hippocampal slices, the animals themselves displayed no seizure activity. This was likely due to the protective role of the BBB that limits passage of damaging proteins and other serum constituents into the brain parenchyma. Despite considerable differences in the ability of the serums to provoke neuronal excitability, there was no difference in their ability to affect BBB permeability. Thus, although pregnancy may be a pro-inflammatory state peripherally that is capable of promoting hyperexcitability of brain tissue, the CNS is protected from these factors by an intact BBB.
The present study showed that serum from normal pregnant rats caused microglial activation and increased neuronal excitability in a TNFα-dependent manner (i.e., sTNFR1 abrogated LP-induced serum effects). Because TNFα was not increased in LP serum, it appears that TNFα produced within the slice in response to LP serum caused neuronal hyperexcitability. Increased TNFα in hippocampal slices after exposure to LP serum was confirmed by qPCR, a highly sensitive and standard technique for measuring changes in cytokine protein levels because of the close correlation between cytokine mRNA and protein (Hein et al., 2001; Blaschke et al., 2000). Although microglia were activated in response to the LP serum and likely producing TNFα (Riazi et al., 2008), there may be other cell types producing TNFα and involved in inducing seizure activity, including astrocytes and neurons that were also exposed to LP serum. It is therefore possible that factors present in LP serum (eg., other cytokines, growth factors or electrolytes) affected neurons or astrocytes which caused hyperexcitability of the hippocampal slices and production of TNFα from these cell types which then activated microglia. Thus, while the results from this study cannot ascertain the exact role of the different cell types contained within the slice in producing hyperexcitability in response to LP serum, we have shown that it involves TNFα from within the slice. Future studies will be needed to dissect the exact pathway that elicits hyperexcitability and cell types involved.
The ability of LP serum to activate microglia and provoke seizure activity, but not increased BBB permeability or cause seizure in the intact animal, suggests that the BBB is not responsive to those factors and prevents the passage of these neuroinflammatory substances into the brain. While specialized tight junctions of the BBB limit passive diffusion of blood-borne solutes (Cipolla, 2006; Cipolla, 2009), brain endothelium also contains both active and carrier-mediated influx and efflux transporters that actively control passage of neuroactive peptides, cytokines and chemokines into the brain from the blood (Cipolla, 2006; Pan et al., 2006). Thus, neuroinflammatory substances could gain access to the brain either through an increase in permeability of the BBB or through the activity of specific transporters that are either active or carrier-mediated. One limitation of this study is that we measured the ability of LP serum to affect BBB permeability, but not how pregnancy affects the expression or activity of transporters. However, the fact that the LP animals did not display overt neurological dysfunction or seizure activity suggests that seizure-provoking serum is not gaining access to the brain tissue in the intact animal, but is excluded by the BBB.
Our findings here also suggest that conditions that increase BBB permeability during pregnancy would provoke microglial activation and seizure through exposure to serum-derived neuroinflammatory factors. In a previous study, we found that plasma from preeclamptic women, but not normal pregnant women, caused a significant increase in BBB permeability (Amburgey et al., 2010). In addition, preeclampsia is a state of oxidative stress, endothelial dysfunction, and systemic edema (Roberts et al., 1989; Hubel, 1995). If similar effects occur in cerebral endothelium, it could expose the brain to serum-derived factors that we show here are neuroinflammatory and hyperexcitable to neurons. Thus, increased BBB permeability and exposure to neuroinflammatory substances may explain the high propensity of preeclamptic women to have neurological symptoms, including seizure (Roberts and Redman, 1993). Moreover, these results also may explain why magnesium sulfate is effective at preventing eclampsia in preeclamptic women as it is protective of the BBB (Euser et al., 2008).
Clinical implications
Eclampsia remains a significant life-threatening complication of pregnancy, yet there are no reliable tests or symptoms for predicting the development of seizure. Our findings here suggest that circulating factors produced during normal pregnancy can cause neuroinflammation and seizure activity. Our results also point to the important role of the BBB in limiting the seizure potential from the periphery because the animals from which the LP serum was obtained were not undergoing seizure. Thus, other factors that disrupt the BBB during pregnancy and preeclampsia are likely involved in promoting eclampsia. Further studies are needed, however, to understand the adaptation of the BBB to pregnancy and what factors might influence the passage of neuroinflammatory components into the brain to cause seizure.
Highlights.
Pregnant serum caused microglial activation and hyperexcitability of hippocampal neurons.
Neuronal hyperexcitability with pregnant serum was prevented by inhibition of TNFα signaling.
Addition of TNFα to hippocampal slices caused similar hyperexcitability as pregnant serum.
The BBB appears to protect the brain from neuroinflammatory and hyperexcitable pregnant serum.
These results provide novel insight into the mechanisms by which seizure occurs during pregnancy.
Acknowledgments
We gratefully acknowledge our funding sources as follows. MJC is supported by NINDS grant RO1 NS045940, a supplement from the Neural Environment Cluster at the NINDS, 3RO1 NS045940-06S1, ARRA supplement 3RO1 NS045940-05S1, and NHLBI grant PO1 HL095488. RPK is supported by the NINDS grant RO1 NS19108, NICHD grant PO1 HD09402, ARRA supplement 3-R01NS019108-23S1, the Migraine Research Foundation and the White Foundation. ADP and YYG are supported by the NIH training grant T32-GM07839. MEP is supported by NHLBI grant R01 HL089177 and NCRR CoBRE P20RR15557. We would like to thank Victor May, Ph.D. for his help with the TNFα ELISA and Marcia P. Kraig for maintenance of hippocampal slice cultures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aagaard-Tillery KM, Silver R, Dalton J. Immunology of normal pregnancy. Semin Fetal Neonatal Med. 2006;11:279–295. doi: 10.1016/j.siny.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Amburgey O, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood-brain barrier permeability: Role of VEGF Signaling. Hypertension. 2010;56:1003–1008. doi: 10.1161/HYPERTENSIONAHA.110.158931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke V, Reich K, Blaschke S, Zipprich S, Neumann C. Rapid quantitation of proinflammatory and chemoattractant cytokine expression in small tissue samples and monocyte-derived dendritic cells: validation of a new real-time RT-PCR technology. J Immunol Methods. 2000;246:79–90. doi: 10.1016/s0022-1759(00)00304-5. [DOI] [PubMed] [Google Scholar]
- Butt AM, Jones HC. Effect of histamine and antagonists on electrical resistance across the blood-brain barrier in rat brain-surface microvessels. Brain Res. 1992;569:100–105. doi: 10.1016/0006-8993(92)90374-i. [DOI] [PubMed] [Google Scholar]
- Caggiano AO, Kraig RP. Eicosanoids and nitric oxide influence induction of reactive gliosis from spreading depression in microglia but not astrocytes. J Comp Neurol. 1996;369:93–108. doi: 10.1002/(SICI)1096-9861(19960520)369:1<93::AID-CNE7>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ. Stroke and the Blood-Brain Interface. In: Dermietzel R, Spray D, Nedergaard M, editors. Blood-brain Barrier Interfaces. Weinheim, Germany: Wiley Press; 2006. pp. 631–634. [Google Scholar]
- Cipolla MJ. The Cerebral Circulation. In: Granger N, Granger J, editors. Integrated Systems Physiology – from Molecule to Function. San Rafael (CA): Morgan & Claypool Publishers; 2009. pp. 37–40. [Google Scholar]
- D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor alpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas KA, Redman CWG. Eclampsia in the United Kingdom. BMJ. 1994;309:1395–1400. doi: 10.1136/bmj.309.6966.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Euser AG, Bullinger LV, Cipolla MJ. Magnesium sulfate decreases blood-brain barrier permeability during acute hypertension in pregnant rats. Exp Physiol. 2008;93:254–261. doi: 10.1113/expphysiol.2007.039966. [DOI] [PubMed] [Google Scholar]
- Gilson GJ, Mosher MD, Conrad KP. Systemic hemodynamics and oxygen transport during pregnancy in chronically instrumented, conscious rats. Am J Physiol. 1992;263:H1911–H1918. doi: 10.1152/ajpheart.1992.263.6.H1911. [DOI] [PubMed] [Google Scholar]
- Grinberg YY, Milton JG, Kraig RP. Spreading depression sends microglia on Lévy flights. PLoS ONE. 2011;6:e19294. doi: 10.1371/journal.pone.0019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein J, Schellenberg U, Bein G, Hackstein H. Quantification of murine IFN-gamma mRNA and protein expression: impact of real-time kinetic RT-PCR using SYBR green I dye. Scand J Immunol. 2001;54:285–291. doi: 10.1046/j.1365-3083.2001.00928.x. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system I: Expression and recognition. Trends in Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- Hubel CA. Dyslipidemia, iron, and oxidative stress in preeclampsia: assessment of maternal and feto-placental interactions. Semin Reprod Endocrinol. 1998;16:75–92. doi: 10.1055/s-2007-1016255. [DOI] [PubMed] [Google Scholar]
- Hulse RE, Swenson WG, Kunkler PE, White DM, Kraig RP. Monomeric IgG is neuroprotective via enhancing microglial recycling endocytosis and TNF-alpha. J Neurosci. 2008;28:12199–12211. doi: 10.1523/JNEUROSCI.3856-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz VL, Farmer R, Kuller JA. Preeclampsia into eclampsia: toward a new paradigm. Am J Obstet Gynecol. 2000;182:1389–1396. doi: 10.1067/mob.2000.106178. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Schmitt MW, Nicholson C, Kraig RP. Optical current source density analysis in hippocampal organotypic culture shows that spreading depression occurs with uniquely reversing currents. J Neurosci. 2005;25:3952–3961. doi: 10.1523/JNEUROSCI.0491-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Reactive astrocytosis from excitotoxic injury in hippocampal organ culture parallels that seen in vivo. J Cereb Blood Flow Metab. 1997;17:26–43. doi: 10.1097/00004647-199701000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Calcium waves precede electrophysiological changes of spreading depression in hippocampal organ cultures. J Neurosci. 1998;18:3416–3425. doi: 10.1523/JNEUROSCI.18-09-03416.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, et al. Microglial cell population dynamics in the injured adult central nervous system. Brain Res Brain Res Rev. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Liu JH. Endocrinology of Pregnancy. In: Creasy R, Resnik R, editors. Maternal-Fetal Medicine, Principles and Practice. Philadelphia, USA: Saunders; 2004. pp. 121–134. [Google Scholar]
- Luppi P, Tse H, Lain KY, Markovic N, Piganelli JD, DeLoia JA. Preeclampsia activates circulating immune cells with engagement of the NF-kappaB pathway. Am J Reprod Immunol. 2006;56:135–144. doi: 10.1111/j.1600-0897.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Heistad DD. Role of veins and cerebral venous pressure in disruption of the blood-brain barrier. Circ Res. 1986;59:216–220. doi: 10.1161/01.res.59.2.216. [DOI] [PubMed] [Google Scholar]
- Michel CC. Fluid movements through capillary walls. In: Renkin EM, Michel CC, editors. Handbook of Physiology, section 2, vol IV, Microcirculation. American Physiological Society; Washington: 1984. pp. 375–409. [Google Scholar]
- Mitchell HM, Levasseur V, Kraig RP. TNF-α increases spreading depression susceptibility via reduced GABAergic inhibition - implications for the transformation of episodic to chronic migraine. Soc Neurosci. 2010;36 Prog #346.3. [Google Scholar]
- Mitchell HM, White DM, Domowicz MS, Kraig RP. Cold pre-conditioning neuroprotection depends on TNF-α and is enhanced by blockade of interleukin-11. J Neurochem. 2011;117:187–196. doi: 10.1111/j.1471-4159.2010.07103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga M. Maternal Cardiovascular and Renal Adaptation to pregnancy. In: Creasy R, Resnik R, editors. Maternal-Fetal Medicine, Principles and Practice. Philadelphia, USA: Saunders; 2004. pp. 111–120. [Google Scholar]
- Palladino AA, Stanley CA. The hyperinsulinism/hyperammonemia syndrome. Rev Endocr Metab Disord. 2010;11:171–178. doi: 10.1007/s11154-010-9146-0. [DOI] [PubMed] [Google Scholar]
- Pan Q, Xiang S, Tu H, Kastin J. Cytokines interact with the blood-brain barrier. In: Dermietzel R, Spray D, Nedergaard M, editors. Blood-brain Barrier Interfaces. Weinheim, Germany: Wiley Press; 2006. pp. 247–264. [Google Scholar]
- Pusic AD, Grinberg YY, Mitchel HM, Kraig RP. Modeling neural immune signaling of episodic and chronic migraine using spreading depression in vitro. JoVE. 2011 doi: 10.3791/2910. http://www.jove.com/index/Details.stp?ID=2910. [DOI] [PMC free article] [PubMed]
- Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci USA. 2008;105:17151–17156. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Pittman QJ. Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res. 2010;89:34–42. doi: 10.1016/j.eplepsyres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Redman CWG. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447–1454. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- Roberts TM, Chapman AC, Cipolla MJ. PPARγ activation with rosiglitazone reverses increased hydraulic conductivity induced by chronic hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H1347–H1353. doi: 10.1152/ajpheart.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol. 2007;29:151–162. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- Sabai BM. Eclampsia. VI. Maternal-perinatal outcome in 254 consecutive cases. Am J Obstet Gynecol. 1990;163:1049–1055. doi: 10.1016/0002-9378(90)91123-t. [DOI] [PubMed] [Google Scholar]
- Sabai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402–410. doi: 10.1097/01.AOG.0000152351.13671.99. [DOI] [PubMed] [Google Scholar]
- Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibai BM, Abdella TN, Spinnato JA, Anderson GD. Eclampsia. V. The incidence of nonpreventable eclampsia. Am J Obstet Gynecol. 1986;154:581–586. doi: 10.1016/0002-9378(86)90605-8. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Szarka A, Rigó J, Jr, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek NR, Letsky EA. Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr. 2000;72(1 Suppl):247S–256S. doi: 10.1093/ajcn/72.1.247S. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Wang D, Stewart CN., Jr Statistical methods for efficiency adjusted real-time PCR quantification. Biotechnol J. 2008;3:112–123. doi: 10.1002/biot.200700169. [DOI] [PubMed] [Google Scholar]