Abstract
Elevated plasma homocysteine (Hcy) is an independent risk factor for vascular disease and stroke in part by causing generalized endothelial dysfunction. A receptor that is sensitive to Hcy and its intracellular signaling systems has not been identified. β-catenin is a pleiotropic regulator of transcription and cell function. Using a brain microvascular endothelial cell line (bEnd.3), we tested the hypothesis that Hcy causes receptor-dependent nuclear translocation of β-catenin. Hcy increased phosphorylation of Y731 on vascular endothelial cadherin (VE-cadherin), a site involved in coupling β-catenin to VE-cadherin. This was blocked by inhibition of either metabotropic glutamate receptor 5 (mGluR5) or ionotropic glutamate receptor (NMDAr) and by shRNA knockdown of mGluR5. Expression of these receptors was confirmed by flow cytometry, immunohistochemistry, and western blotting. Directed pharmacology with specific agonists elucidated a signaling cascade where Hcy activates mGluR5 which activates NMDAr with subsequent PKC activation and uncoupling of the VE-cadherin/ β-catenin complex. Moreover, Hcy caused a shift in localization of β-catenin from membrane-bound VE-cadherin to the cell nucleus, where it bound DNA, including a regulatory region of the gene for claudin-5, leading to reduced expression of claudin-5. Nuclear localization, DNA binding of β-catenin, and reduced claudin-5 expression were blocked by inhibition of mGluR5. Knockdown of mGluR5 expression with shRNA also rescued claudin-5 expression from the effects of Hcy treatment. These data uniquely identify mGluR5 as a master switch that drives β-catenin nuclear localization in vascular endothelium and regulates cell-cell coupling in response to elevated Hcy levels. These studies dissect a pharmacological opportunity for developing new therapeutic targets in HHcy.
Keywords: homocysteine, endothelial, cadherin, catenin
1. INTRODUCTION
Homocysteine (Hcy) is an intermediate aminothiol derived from methionine demethylation. Hyperhomocysteinemia (HHcy) is an elevated concentration of Hcy in plasma and is categorized as mild (15-30 μM), moderate (31-100 μM), or severe (>100 μM). Mild to moderate HHcy occurs in 5-10% of the general population, 40% of patients with peripheral vascular disease, and may reach 91% in hemodialysis patients (Carmel and Jacobsen, 2001; Moustapha and Robinson, 1999; Ueland and Refsum, 1989). Several large scale studies have established Hcy as a significant, independent risk factor for venous thromboembolism, hypercoagulability, cardiovascular disease, stroke, and dementia (Boushey et al., 1995; Seshadri et al., 2002; Wald et al., 2002; Zhang et al., 2010). Current therapies for elevated Hcy are limited to vitamin supplements, which serve as co-factors in the pathways of Hcy metabolism (remethylation and transsulfuration), but these therapies have shown low efficacy in remediating disease risk or progression unless the patients have severe HHcy (Joseph et al., 2009; Loscalzo, 2006; Maron and Loscalzo, 2009). Without a better understanding of the mechanisms by which Hcy alters vascular function, therapeutic options will remain limited.
HHcy impairs vascular function in part by disrupting endothelial cell redox balance, angiogenesis, and cell-cell adhesion. The broad range of effects of Hcy on endothelial cells is most readily explained by the concomitant induction of numerous pathways rather than by the independent activation of a unique pathway for every phenotype. β-catenin is such a signaling molecule with pleiotropic effects; it is best known for regulating genes in the canonical Wnt pathway, including genes involved in cell differentiation, proliferation, and survival (Clevers, 2006; Grigoryan et al., 2008). Endothelial cell specific knockout of β-catenin is lethal in utero at day E12.5 (Cattelino et al., 2003). However, endothelial-specific gain-of-function mutation in β-catenin is also lethal in utero (Corada et al., 2010), emphasizing the fundamental importance of maintaining homeostatic control over β-catenin signaling in the vascular endothelium. Under basal conditions, β-catenin is sequestered to the plasma membrane in a complex with vascular endothelial cadherin (VE-cadherin). If association with the VE-cadherin complex is prevented, β-catenin is either degraded in the cytosol or accumulates in the cell nucleus where it participates in regulation of transcription factors. We recently reported that Hcy reduces expression of the tight junction protein claudin-5 (Beard et al., 2011; Bearden et al., 2010), which is regulated by the association between VE-cadherin and β-catenin (Taddei et al., 2008). Hence, data support the idea that β-catenin signaling would be an efficient and logical mechanism for the effects of Hcy on vascular endothelium. The role of this pathway in HHcy remains unknown.
Elevations in extracellular glutamate cause redox imbalance (Sharp et al., 2005) and formation of peroxynitrite in endothelial cells, which involves the NMDAr (Scott et al., 2007). Glutamate receptors, both metabotropic and ionotropic, are also implicated in regulating the ‘tightness’ of endothelial cell junctions (Liu et al., 2004; Liu et al., 2010). In the nervous system, these receptors cooperate in long-term potentiation (Rosenbrock et al., 2010), which may require nuclear translocation of β-catenin (Chen et al., 2006). Recent studies show that metabotropic and ionotropic glutamate receptor-dependent signaling are not necessarily independent. In fact, a growing body of literature supports the presence of a signaling cascade where activation of metabotropic glutamate receptors (mGluRs) subsequently potentiates activation of NMDAr. However, it has also been shown that potentiation of NMDAr can modulate mGluR activity (Perroy et al., 2008). This interplay between metabotropic and ionotropic glutamate receptors is established in neurons (Anwyl, 2009; Chen et al., 2011; Jia et al., 2001) but has not been shown in the endothelium. Hcy is capable of activating both metabotropic and ionotropic glutamate receptors (Boldyrev and Johnson, 2007; Kingston et al., 1998; Lipton et al., 1997; Shi et al., 2003; Zieminska et al., 2003)}, leaving unanswered the question of whether the pathway is functional in the endothelium. Subtype 5 of the mGluRs (mGluR5) is expressed on endothelium in vivo (Gillard et al., 2003) and activated by Hcy derivatives (Kingston et al., 1998; Shi et al., 2003). We recently found that Hcy drives mGluR5-dependent increase in peroxynitrite production in endothelium (Mayo et al., 2011). We have also demonstrated an NMDAr-dependent opening of endothelial cell-junctions at the blood-brain barrier in response to Hcy in vitro and to HHcy in vivo (Beard et al., 2011). Building from this knowledge base, we propose that mGluR5 and NMDAr cooperate in mediating Hcy-induced disruption in the vascular endothelium. The purpose of this project was two-fold: 1) to dissect the signaling cascade involving mGluR5 and NMDAr and 2) to elucidate their potential mechanistic roles in Hcy-mediated regulation of the VE-cadherin/β-catenin complex and nuclear localization of β-catenin.
2. MATERIALS AND METHODS
2.1 Materials
All reagents used were purchased from Fisher Scientific or Sigma-Aldrich. For all experiments, the Hcy used was D/L-homocysteine purchased from Sigma-Aldrich.
2.2 Brain microvascular endothelial cell culture
Cerebral microvascular endothelial (bEnd.3) cells were purchased from ATCC ((Montesano et al., 1990); Manassas, VA, USA). The bEnd.3 cells were cultured at 37°C in a humidified incubator with 5% CO2 and balance room air in Dulbecco’s Modified Eagle’s Medium high glucose (DMEM-H; Invitrogen), 10% bovine calf serum (Hyclone; Fisher Scientific), and 1X gentamicin or penicillin/streptomycin and amphotericin B (Invitrogen) in plastic culture flasks. All experiments were performed with cells from the 3rd-6th passages. For conditioned media experiments, RAW 264.7 cells (ATCC) were grown to ~70% confluence in the same media and treated with 1ng/mL lipopolysaccharide to induce secretion of inflammatory cytokines; 12 hours later media was harvested and used for treating bEnd.3 cells.
2.3 Brain microvascular endothelial cell treatments
At 4 days post-confluence, culture media was changed to 5% bovine calf serum for 24 hours and then serum-free for treatment, which began on the fifth day post-confluence. D,L-homocysteine (Sigma), N-methyl-D-aspartic acid (NMDA; Tocris), 3,5-dimethyl-tricyclo[3.3.1.13,7]decan-1-amine hydrochloride (Memantine; Tocris) were dissolved in phosphate-buffer saline (PBS) as a stock solution and diluted in DMEM upon treatment. 2-methyl-6-(phenylethynyl) pyridine hydrochloride (MPEP; Tocris), (RS)-2-chloro-5-hydroxyphenylglycine (CHPG; Tocris), bisindolylmaleimide I (BIM-I; Calbiochem), and chelerythrine (Acros) were dissolved in DMSO as stock solutions and diluted in culture media upon treatment. An equal volume of PBS and/or DMSO in DMEM were used as vehicle controls for respective experiments. Table 1 lists antibodies used in these experiments.
Table 1
| Antibody | cat # |
|---|---|
| polyclonal goat anti-VEC | C-19 |
| monoclonal mouse anti-phosphotyrosine | sc-7020 |
| polyclonal rabbit anti-VEC pY731 | 441145G |
| monoclonal mouse anti-NMDA R1 | 32-0500 |
| polyclonal rabbit anti-mGluR5 | ab76561 |
| HRP conjugated monoclonal anti-β-actin | C4 |
| anti-rabbit conjugated to phycoerythrin | 558416 |
| anti-mouse conjugated to phycoerythrin | P852 |
| polyclonal rabbit anti-β-catenin | CP1061 |
2.4 Western blotting
bEnd.3 cells were lysed by collecting in PBS with 1% Triton X-100, protease inhibitors (Complete Mini; Roche), and 1mM sodium vanadate. Samples were denatured in 5X Laemmli sample buffer with 10% β-mercaptoethanol, separated by SDS-PAGE, blotted to PVDF membranes, and probed using antibodies at 1:1000 overnight at 4°C. Host species appropriate and alkaline phosphatase conjugated secondary antibodies were applied for one hour and immunopositive bands were quantified (Versadoc, BioRad) following chemiluminescent detection (Lumiphos, Pierce). For immunoprecipitation experiments, samples were incubated with Protein G Dynabeads (Invitrogen) pre-charged with anti-VE-cadherin antibodies; precipitates were washed and eluted, and samples were handled as above for Western blotting.
2.5 Flow cytometry
bEnd.3 cells were detached from culture flasks with trypsin, centrifuged to remove trypsin, blocked in PBS with 3% bovine serum albumin and Fc Block (BD Biosciences) for 10 minutes at room temperature, followed by primary and secondary antibody incubations separated by rinses with PBS. Cells were washed thoroughly with cold PBS and analyzed immediately using a FACS Calibur flow cytometer (BD Biosciences), with gates set on live cells stained with secondary antibody alone. Data analysis was performed using Cell Quest software (BD Biosciences).
2.6 In-cell ELISA and immunochemical analyses
After respective treatments, bEnd.3 cells were washed twice in Tris-buffered saline (TBS) with 2mM Ca2+, 2mM Mg2+, and 1mM sodium vanadate. TBS was completely removed and cells were incubated in 4% paraformaldehyde for 15 minutes, rinsed twice in TBS, permeabilized with 1% Triton X-100 in TBS, and blocked with 3% BSA in TBS with 0.01% Tween-20 (TBST) for 2 hours at room temperature. Samples were then incubated with primary antibodies diluted 1:500 in TBST with 3% bovine serum albumin at 4°C overnight. Samples were then washed 3 times for 10 minutes each using TBST. For in-cell ELISA experiments, samples were incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG for 30 minutes, washed as above, and detected using a colorimetric ELISA substrate (BluePhos; KPL). For ELISAs of cell-surface expression (cell-surface ELISA), permeabilizing detergents were omitted from all steps and solutions in the above protocol. Absorbance was measured at 610nm using a plate reader (Synergy HT, BioTek). For immunocytochemistry, species-specific secondary antibodies conjugated to Alexa Fluor-488 (Invitrogen) were used to detect primary antibodies. DNA was co-labeled with Hoescht-33258 (Sigma). Images were acquired by fluorescence microscopy (Leica, DMLFS) with a digital camera (MicroPublisher 3.3 RTV; QImaging; QCapture software).
2.8 Protein co-immunoprecipitation
Cells were grown in 12-well plates as above, lysed first with 25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40 and 5% glycerol. The remaining insoluble cytoskeletal fraction was then lysed with 0.5% SDS and 1% Triton-X100 in 10 mm Tris-HCl and 10 mM NaCl. All of the cytoskeletal lysate protein (20μg) from each well was immunoprecipitated with Protein G Dynabeads (Invitrogen) charged with anti-VE-Cadherin antibody (sc-6458, Santa Cruz Biotechnology). Precipitates were washed with TBS with 0.02% Tween-20, 2mM Ca2+ and eluted with Laemmli sample buffer and handled as above for western blotting for β-catenin. Internal controls included non-charged beads from which no VE-cadherin or β-catenin was recovered.
2.9 Cell fractionation
All steps were performed at 0-4 °C and all lysis solutions contained protease inhibitors (Complete Mini; Roche). Cells (bEnd.3) were: 1) lysed with 1% NP-40 for 15 seconds, 2) rinsed 3X with PBS, 3) incubated in 0.5% NP-40 with 0.25% deoxycholate for 1-2 minutes (until >90% of nuclei were liberated and floating by visual inspection using a dissection microscope), 4) rinsed 3X with PBS, and 5) lysed with 0.5% SDS for 10 minutes then 6) the nuclei from step 3 were lysed with 0.5% SDS vortexed on highest setting for 5 seconds, supplemented with 1/10th volume of 6M NaCl, vortexed on lowest setting for 15 seconds. The lysates from steps 1, 5, and 6 were centrifuged at 19,000 g for 30 min. Supernatants from steps 1 and 5 were designated as cytosolic and cytoskeletal protein fractions, respectively. Pellet from step 6 was designated as the nuclear protein fraction.
2.10 Chromatin immunoprecipitation (ChIP) assay
Cells were cross-linked with 1% paraformaldehyde for 20 minutes at 21°C. Cells were scraped and resuspended in sonication buffer (20mM Tris, pH 8.1, 1% SDS, 1% Triton X-100, 10mM EDTA), and sonicated (Cell Disruptor W-10, Heat Systems) at power 6-7 for 15 minutes using a duty cycle of 15 seconds on with 45 seconds off while submerged in an ice-water slurry. DNA fragments were precipitated using β-catenin antibody pre-conjugated to protein G Plus agarose beads (sc-2002, Santa Cruz Biotech), then rinsed in high and low salt buffers (20mM Tris, pH 8.1, 0.1% SDS, 1% Triton X-100, 2mM EDTA, 500mM NaCl, and 20mM Tris, pH 8.1, 0.1% SDS, 1% Triton X-100, 2mM EDTA, 150mM NaCl, respectively) and eluted with 1% SDS + 100mM NaHCO3. Eluates were incubated with proteinase K at 45°C for 1 hour and then at 65°C for 4 hours. DNA was precipitated with 70% ethanol at -20°C and resuspended in TE (10mM Tris, 1mM EDTA, pH 8.1). DNA (8μl per well) was loaded into ethidium bromide gels and run following standard procedures. DNA concentrations were determined using nanodrop ND-1000 spectrophotometer (Nanodrop). PCR was performed at: 95 °C for 15 minutes (94°C for 30 seconds; 54 °C for 30 seconds, 72°C for 1 minute) for 35 cycles; 72 °C for 7 minutes using: 5′-CTGCTGAACTTGGGGAAGAC-3′ and 5′-AAGGGAGTGAGGGAAGGAAA-3′ at 1mM final concentration in 25μl of total reaction mixture. This region was recently identified as the primary regulator of claudin-5 expression under the control of β-catenin (Taddei et al., 2008). Total PCR product was loaded into ethidium bromide gels and run following standard procedures. Gels were imaged and density quantified with Bio-Rad VersaDoc software.
2.11 shRNA
mGluR5 knockdown was achieved using the lentiviral vector GIPZ containing the shRNAmir sequences V3LMM_455250 (shRNA-mGluR5(1) and V3LMM_455252 (shRNA-mGluR5(2) from Open Biosystems (Huntsville, AL, USA), and the non-silencing (NS) shRNAmir (catalog # RHS4348) was used as a negative control. Optimal multiplicity of infection (MOI) was determined following the manufacturer’s instructions. A stable culture was generated by growing these cells in the presence of 10 μg/mL puromycin for 7 days. Percentage of mGluR5 knockdown was quantified with cell-surface ELISA and Western blot by immunolabeling for the extracellular domain of mGluR5 (sc-47147; Santa Cruz Biotechnology).
2.12 Statistics
All data are presented as the mean ± s.e.m. (n=6-32/group). Pair-wise comparisons were performed with student t-test and group-wise data were compared using Analysis of Variance with Tukey or Dunnett post-hoc analyses. Alpha was set at 0.05 prior to experiments for statistical significance.
3. RESULTS
3.1 Hcy increases phosphorylation of VE-cadherin at Y731 in a dose- and time-dependent manner
Based on the importance of phosphotyrosine modifications in VE-cadherin function and complexing with other proteins, we investigated the effect of Hcy on phosphorylation of VE-cadherin tyrosine residues. Treatment of brain microvascular endothelial cells with Hcy concentrations in the physiologic range (2 and 20μM) for 2 hours increased total tyrosine phospholabeling on VE-cadherin compared to control (Fig. 1A). Previous work has identified nine potential tyrosine residues on the intracellular domain of VE-cadherin which can be phosphorylated. Of these, Y658 & Y731 have been extensively studied and site-specific and validated antibodies were commercially available. Moreover, β-catenin binds in the Y731 region but not the Y658 region, providing a site-specific comparitor for further study of the effects of Hcy on the β-catenin/VE-cadherin complex. This difference has special significance for the present studies because phosphorylation of Y731 inhibits β-catenin binding to VE-cadherin (Potter et al., 2005). Therefore, we tested the effect of Hcy on phosphorylating these sites on VE-cadherin. After two hours treatment, western blot analysis revealed that Hcy dose-dependently increased phosphorylation of VE-cadherin at Y731, but not Y658 (Fig. 1B). Having established the specificity of the pY731 antibody in western blotting (single band at expected size) we investigated the potential of using in-cell ELISAs for subsequent experiments. Macrophages are known to release inflammatory cytokines in response to lipopolysaccharide that are potent stimuli for phosphorylation of VE-cadherin at Y731 (Xiong et al., 1998). Therefore, we incubated bEnd.3 cells with increasing dilutions of media removed from RAW 264.7 cells treated with lipopolysaccharide and determined that the in-cell ELISA was effective at measuring changes in VE-cadherin pY731 (Fig. 1C). We then established the time course for VE-cadherin phosphorylation at Y731 in response to Hcy treatment. Within 15 minutes of Hcy treatment, pY731 was significantly increased from control, which continued in a time-dependent manner that reached a plateau beyond 1 hour (Fig. 1C).
Figure 1. Homocysteine increases phosphorylation of VE-cadherin at Y731in a dose- and time- dependent manner in brain microvascular endothelial cells.
A) Treating bEnd.3 cells with Hcy for 2 hours elicited a dose-dependent increase in total tyrosine phosphorylation of VE-cadherin (upper panel) and site specific phosphorylation at Y731 but not Y658 (lower panel) by western blotting. B) Quantification of western blot band densities demonstrates a dose dependent increase in pY731/VE-cadherin following 2 hours treatment with Hcy. C) In-cell ELISA for pY731 VE-cadherin demonstrates that Hcy (20μM) elicited a time-dependent increase in phosphorylation of VE-cadherin at Y731 but no change in total VE-cadherin expression. Positive control (Pos Ctrl) for pY731 is media conditioned by activated macrophages (RAW 264.7 macrophages activated by 24 hours incubation with lipopolysaccharide). * p < 0.05 compared with 0 μM (B) or time 0 (C).
3.2 NMDAr and mGluR5 antagonists reduce Hcy-mediated phosphorylation of VE-cadherin at Y731
Next, we investigated the role of NMDAr and mGluR5 expressed on bEnd.3 cells in Hcy-mediated phosphorylation of VE-cadherin at Y731. We confirmed expression of both NMDAr and mGluR5 receptors in brain microvascular endothelial cells by western blotting, immunocytochemistry, flow cytometry, and cell-surface ELISA (Fig. 2). Cell-surface expression did not change over the course of experiments [99.6 ± 0.7% of control (time 0) at 15, 30, 60, and 120 mins (p>0.05)]. Antagonists of mGluR5 (MPEP) and NMDAr (memantine) produced dose-dependent inhibition of Hcy-mediated phosphorylation of VE-cadherin at Y731, in combination and individually (Fig. 3). We conclude that both receptors are required for the effects of Hcy on VE-cadherin pY731.
Figure 2. Endothelial cells express mGluR5.
A-B) Immunodetection of mGluR5 in bEnd.3 cells by flow cytometry (A) and western blotting (B). C-D) Immunodetection of NMDA NR1 in bEnd.3 cells by flow cytometry (C) and western blotting (D). For flow cytometry, antibody control is secondary antibody only (omission of primary antibody). E) Immunohistochemistry shows punctate expression of mGluR5 and NMDA NR1 across the cell surface (red); nuclei labeled with Hoescht 33528. Secondary ctrl is the control image of cell labeled with secondary antibody and Hoescht 33528 (omission of primary antibody).
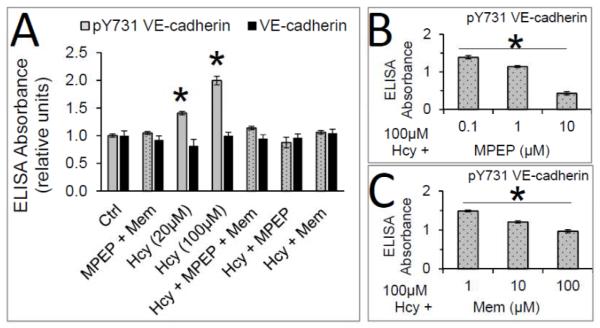
Figure 3. Memantine and MPEP inhibit Hcy-mediated phosphorylation of VE-cadherin at Y731.
A) Hcy (20 or 100μM) for 2 hours increased pY731 on VE-cadherin, which was inhibited by the addition of the NMDAr and/or mGluR5 specific antagonists, memantine (100 μM) and MPEP (10 μM) respectively. * p<0.05 compared with control. No changes in total VE-cadherin expression were observed (p>0.05) B) MPEP inhibited the effect of 100 μM Hcy in a dose-dependent manner. C) Memantine (Mem) inhibited the effect of 100 μM Hcy in a dose-dependent manner. For panels B-C, * indicates significant differences for all pairwise comparisons, p < 0.05.
3.3 Hcy increases phosphorylation of VE-cadherin at Y731 by activating an mGluR5-to-NMDAr cascade
Given that both MPEP and memantine were effective in inhibiting Hcy-mediated phosphorylation of VE-cadherin at Y731, we used receptor-specific agonists to investigate the relationship between these receptors in triggering the phosphorylation of VE-cadherin at Y731. Activating mGluR5 with CHPG increased phosphorylation of VE-cadherin at Y731, while simultaneously inhibiting the NMDAr with memantine blocked the phosphorylation (Fig. 4A). Activation of NMDAr with NMDA also increased phosphorylation of Y731 of VE-cadherin; however, concomitant inhibition of mGluR5 with MPEP did not affect the phosphorylation (Fig. 4A). Both CHPG and NMDA increase phosphorylation of Y731on VE-cadherin in a dose-dependent manner (Fig. 4B, C). We conclude that activation of either receptor is sufficient to drive the response but that NMDAr is necessarily downstream of mGluR5 activation. Because mGluR5 activation was required for Hcy-mediated phosphorylation of VE-cadherin (Figs 3-4), we conclude that Hcy did not directly activate NMDAr in this model system.
Figure 4. Homocysteine increases phosphorylation of VE-cadherin at Y731by triggering an mGluR5- to-NMDAr cascade.
Homocysteine (20μM), an mGluR5 agonist (CHPG; 25μM), an NMDAr agonist (NMDA; 25μM), and both agonists together all significantly increased pY731 on VE-cadherin (p<0.05). A) Activating mGluR5 (CHPG; 25μM) with concomitant inhibition of NMDAr (Mem; memantine; 100μM) blocked phosphorylation at Y731. However, activating NMDAr (NMDA; 25μM) with concomitant inhibition of mGluR5 (MPEP; 10μM) did not block phosphorylation at Y731. * p < 0.05 compared with control. B) Two hours treatment with CHPG (mGluR5-specific agonist) elicited a dose- dependent increase of pY731. C) Two hours treatment with NMDA (NMDAr-specific agonist) elicited a dose-dependent increase of pY731. * indicates that all means are statistically significant from Ctrl, p < 0.05. No significant changes in total VE-cadherin expression were observed (p>0.05).
3.4 PKC is involved in Hcy activation of glutamate receptors and pY731 on VE-cadherin
PKC is a downstream target of both NMDAr and mGluR5 activation in other models. We questioned whether PKC is involved in this pathway and, if so, at which steps. A broad-spectrum PKC inhibitor (1μM BIM-I inhibits all PKC isoforms except PKCζ (Coultrap et al., 1999)) blocked Hcy, NMDA, and CHPG mediated phosphorylation of VE-cadherin at Y731 (Fig. 5A). Dose-dependent responses for BIM-I and for another broad-spectrum PKC inhibitor, chelerythrine, provide further evidence for the role of PKC in Hcy-mediated phosphorylation of VE-cadherin at Y731 (Fig. 5B). These findings demonstrate that PKC is required downstream of each step in the pathway. While this does not establish whether PKC is involved in the mGluR5-dependent activation of NMDAr, it does show that PKC is required at least downstream of the NMDAr in this signaling cascade.
Figure 5. Hcy-mediated phosphorylation of VE-cadherin at Y731 requires PKC activation.
A) The PKC inhibitor BIM-I (1 μM) inhibited Hcy (20μM), NMDA (25 μM), and CHPG (25 μM) mediated phosphorylation of VE-cadherin at Y731. * p < 0.05 compared with control. # p < 0.05 compared with Hcy alone. † p < 0.05 compared with CHPG alone. ‡ p < 0.05 compared with NMDA alone. B) Dose response effects of BIM-I and another PKC inhibitor, chelerythrine, further confirm the role of PKC in phosphorylation of VE-cadherin at Y731 in response to Hcy.
3.5 Hcy dissociates the VE-cadherin/β-catenin complex
Immunohistochemistry and subcellular fractionation showed that Hcy treatment reduced membrane localization of β-catenin while increasing its nuclear expression (Fig. 6 A-D). To explore regulation of the VE-cadherin/β-catenin complex with chronic Hcy treatment, we immunoprecipitated VE-cadherin from the cytoskeleton-associated fraction (non-ionic detergent, NP-40, insoluble fraction) after 3 days of 20 μM Hcy treatment and quantified the amount of β-catenin that co-immunoprecipitated. Hcy significantly reduced the amount of β-catenin that was co-immunoprecipitated with VE-cadherin (Fig. 6E). Therefore, β-catenin dissociates from VE-cadherin following Hcy treatment.
Figure 6. Hcy dissociates β-catenin from VE-cadherin.
A) Immunohistochemistry for β-catenin (green) and nuclei (pseudo-color of Hoescht DNA stain) in untreated brain microvascular endothelial cells. B) Chronic treatment with Hcy (20μM; 3 days) reduced cell membrane expression of β-catenin but increased its nuclear localization (yellow shows localization through combination of green and red). C) Representative western blots of respective cell fractions isolated from untreated (control) and Hcy-treated cells (20μM; 3 days). D) Quantification of β-catenin expression in cell fractions by western blot. E) Co-immunoprecipitation of VE-cadherin demonstrates significant loss of β-catenin from the VE-cadherin/β-catenin complex in response to 3 days Hcy treatment (20 μM). * p < 0.05 compared with control.
3.6 mGluR5 activation by Hcy mediates nuclear localization of β-catenin
Hcy treatment increased β- catenin nuclear accumulation and binding to DNA (Fig. 7A,B), which was prevented by mGluR5 inhibition with MPEP. This finding establishes mGluR5 as a Hcy-induced trigger for β-catenin nuclear localization. A comprehensive screening of all the genes regulated by this pathway was beyond the scope of this study. However, to explore the precision of this regulatory pathway, we performed qPCR for the claudin-5 gene. Specifically, we screened for a region of the claudin-5 promoter that is regulated through dissociation of the VE-cadherin/β-catenin complex (Taddei et al., 2008) because we have reported that Hcy decreases expression of claudin-5 by endothelial cells (Beard et al., 2011; Bearden et al., 2010). Three days of Hcy treatment increased the binding of β-catenin with this regulatory region of the claudin- 5 promoter (Fig. 8A) and decreased expression of claudin-5 protein (Fig. 8B), both of which were prevented by mGluR5 inhibition with MPEP.
Figure 7. β-catenin binds with DNA in response to Hcy.
A) Representative image and B) quantification of DNA precipitated during chromatin immunoprecipitation (ChIP) assays for β-catenin. Results demonstrate significant increase in binding with DNA in response to Hcy treatment (20 μM; 3 days), which is blocked by concomitant inhibition of mGluR5 (MPEP). * p<0.05 compared with all other groups; Hcy + MPEP group was not significantly different (p>0.05) from control (Ctrl) or MPEP alone.
Figure 8. Specificity of β-catenin signaling in response to Hcy.
A) Image and densitometry of qPCR for the promoter region of the claudin-5 gene (cldn5) following ChIP for β-catenin demonstrating precision of β-catenin localization to a specific gene region known to be regulated through VE-cadherin/β-catenin nuclear signaling. * p<0.05 compared with all other groups; Hcy (20μM) + MPEP (10μM) group was not significantly different (p>0.05) from control (Ctrl) or MPEP alone B) Expression of claudin-5 protein at the cell surface. * p<0.05 compared with all other groups.
3.7 shRNA
Lentiviral knockdown of mGluR5 reduced protein expression by approximately 55%. This knockdown was sufficient to block the effects of Hcy on phosphorylation of VE-cadherin at pY731 and on expression of claudin-5 (Fig. 9).
Figure 9. Lentiviral knockdown (shRNA) of mGluR5 in brain microvascular endothelial cells blocks effects of Hcy.
A) Phosphorylation of VE-cadherin at Y731 in response to Hcy treatment is rescued in cells with shRNA for mGluR5 using two different shRNA sequences. † p<0.05 compared with non-silencing and compared with control; * p<0.05 compared with non-silencing and not different from control. B) Reduced expression of claudin-5 in response to Hcy treatment is rescued in cells with shRNA for mGluR5. Results presented compared with control (Control = 0 μM Hcy). * p<0.05 compared with non-silencing and not different from control. A-B) Results presented compared with control (horizontal bar; control = 0 μM Hcy treatment) for reference.
4. DISCUSSION
In this study, we dissect a novel receptor-dependent signaling cascade by which Hcy may effect pleiotropic regulation of gene expression in vascular endothelium. From a pharmacological perspective, perhaps the most significant finding is that mGluR5 is a Hcy detector in vascular endothelium and its activation by Hcy drives a signaling cascade that leads to impaired sequestering of β-catenin by VE-cadherin, with subsequent accumulation in the nucleus and interaction with genomic targets. Hence, these findings provide evidence that endothelial mGluR5 may be a novel target for therapeutic intervention in diseases associated with HHcy.
Many years of effort have developed an understanding of the signaling that leads to EC-EC junction disassembly in angiogenesis, inflammation, and leukocyte extravasation. Tyrosine phosphorylation of VE-cadherin is an important part of the signaling involved in EC-EC barrier modulation (Nwariaku et al., 2002). Specifically, phosphorylation of Y731 on VE-cadherin impairs binding of β-catenin (Potter et al., 2005), which is consistent with the Hcy-mediated loss of β-catenin from VE-cadherin at the plasma membrane/cytoskeletal complex in the present study (Fig. 6). This site is also a target of several permeability inducing factors and the process of leukocyte extravasation (Gavard, 2009). Neutrophils may use a similar mechanism as it has been shown that glutamate release by neutrophils is an important step in cerebral endothelial transmigration (Collard et al., 2002). Our results elucidate a mechanism that couples post-translational modification (phosphorylation of VE-cadherin) with transcription-level control of protein expression. Transcription-level control of tight junction protein is likely to mediate effects that are longer lasting than those arising from solely from post-translational modification of junctional proteins (e.g., nitration or phosphorylation). For example, down-regulation of claudin-5 expression by this pathway may produce more profound cerebrovascular barrier impairment than phosphorylation of VE-cadherin alone.
Because antagonists of either mGluR5 or NMDAr inhibited Hcy-mediated phosphorylation of VE-cadherin at Y731, we determined how these receptors cooperate in our model. We explored three possibilities to explain our results: 1) Hcy requires both receptors to exert its effects on VE-cadherin, 2) Hcy activates NMDAr, which activates mGluR5 or 3) Hcy activates mGluR5, which activates NMDAr. Because activation of either receptor independently leads to phosphorylation, we were able to eliminate the first possibility. Activation of mGluR5 while inhibiting NMDAr over-activity blocked the phosphorylation of VE-cadherin Y731 while the reverse had no effect. These results lead us to conclude that Hcy triggers mGluR5 receptors, which leads to a downstream activation of the NMDAr and subsequent pY731 of VE-cadherin. To be relevant for the human condition of HHcy, it was important to explore chronic effects of Hcy on cell signaling. Uncoupling of VE-cadherin/β-catenin and increased binding of β-catenin to DNA with three days of Hcy treatment at physiologic concentrations is consistent with a tonic transcriptional regulation in HHcy.
The process may not, however, be as simple as phosphorylation of VE-cadherin because β-catenin did not dissociate from VE-cadherin despite phosphorylation at Y731 in a previous study (Adam et al., 2010). Hence, it is possible that the pool of β-catenin that accumulates in the nucleus is newly formed protein (not previously bound to VE-cadherin), which is unable to bind VE-cadherin after phosphorylation of Y731. Indeed, Potter et al. (Potter et al., 2005) showed that β-catenin is unable to bind VE-cadherin previously phosphorylated at Y731. Pulse-chase experiments would be required to empirically determine whether the nuclear β-catenin in HHcy derives from the VE-cadherin-bound pool.
The cerebral endothelium expresses functional mGluR5 and NMDAr in vitro and in vivo as shown by several groups (Betzen et al., 2009; Collard et al., 2002; Daneman et al., 2010; Gillard et al., 2003; Krizbai et al., 1998; Lin and Maiese, 2001; Parfenova et al., 2003; Reijerkerk et al., 2010; Sharp et al., 2003a). Glutamate decreased EC barrier integrity in vitro by activating NMDAr (Sharp et al., 2003b) and mGluR (Collard et al., 2002). Our study is consistent with these reports and unifies the observations in the pathological setting of HHcy by providing the first evidence that a metabotropic-to-ionotropic signaling cascade may regulate microvascular endothelium in response to elevated Hcy levels. In a recent publication, we showed that Hcy increased β-catenin binding to the claudin-5 promoter and led to a decrease in claudin-5 expression with concomitant increases in permeability of an endothelial monolayer {Beard, 2011 #16}. We further demonstrated decreased expression of claudin-5 in the cerebrovasculature of HHcy mice that was associated with increased permeability of the blood-brain barrier. Both of these effects were rescued with the NMDAr antagonist memantine. The current study extends this work by establishing the role of mGluR5 in initiating this cascade.
PKC is a known target of both mGluR5 (Pin and Duvoisin, 1995) and NMDAr activation (Koles et al., 2001) by a Gq-PLC [barb2right] IP3/DAG pathway for mGluR5and by inward calcium current for NMDAr. Several studies have shown that Hcy-mediated effects in the endothelium and other cell types involve PKC signaling (Beauchamp and Renier, 2002; Signorello et al., 2009; Siow et al., 2006). There are limitations in our understanding of the pathway presented. In the present study we did not dissect the steps coupling PKC, a serine/threonine kinase, with phosphorylation of tyrosine 731 of VE-cadherin. For example, PKC can activate Src (a tyrosine kinase) and Src is known to phosphorylate VE-cadherin at Y731 (Adam et al., 2010). Because PKC activity was required for the response to each treatment (Hcy, CHPG, NMDA; Fig. 5), we do not know whether PKC is also involved in the potentiation of the NMDAr channel by mGluR5. Answering this question would require electrophysiology experiments though others have demonstrated that PKC modulates NMDAr (Chen et al., 2011; Tingley et al., 1993). However, the data do show that PKC activation is downstream of Hcy-induced glutamate receptor activation and upstream of VE-cadherin phosphorylation.
This study elucidates a receptor-dependent mechanism that may be involved in the many damaging effects of HHcy in the vascular endothelium. Current therapies for HHcy are limited to vitamin supplements, which serve as co-factors in Hcy metabolism but may not produce sufficient therapeutic efficacy for recommended use in disease prevention. Here, we demonstrate that Hcy-mediated signaling through β-catenin nuclear localization can be inhibited by blocking endothelial mGluR5. These data provide a novel set of targets for future attempts at pharmacologic remediation of vascular dysfunction in HHcy.
ACKNOWLEDGMENTS
Thanks to Dr. Jean Pfau, Director of the ISU Flow Cytometry Core Facility for assistance with flow cytometry experiments.
GRANTS This work was supported by ISU FRC4019 and NIH P20 RR-016454.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES None
REFERENCES
- Adam AP, Sharenko AL, Pumiglia K, Vincent PA. Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. J Biol Chem. 2010;285:7045–7055. doi: 10.1074/jbc.M109.079277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Beard RS, Jr., Reynolds JJ, Bearden SE. Hyperhomocysteinemia increases permeability of the blood-brain barrier by NMDA receptor-dependent regulation of adherens and tight junctions. Blood. 2011;118:2007–2014. doi: 10.1182/blood-2011-02-338269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden SE, Beard RS, Jr., Pfau JC. Extracellular transsulfuration generates hydrogen sulfide from homocysteine and protects endothelium from redox stress. Am J Physiol Heart Circ Physiol. 2010;299:H1568–1576. doi: 10.1152/ajpheart.00555.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MC, Renier G. Homocysteine induces protein kinase C activation and stimulates c-Fos and lipoprotein lipase expression in macrophages. Diabetes. 2002;51:1180–1187. doi: 10.2337/diabetes.51.4.1180. [DOI] [PubMed] [Google Scholar]
- Betzen C, White R, Zehendner CM, Pietrowski E, Bender B, Luhmann HJ, Kuhlmann CR. Oxidative stress upregulates the NMDA receptor on cerebrovascular endothelium. Free Radic Biol Med. 2009;47:1212–1220. doi: 10.1016/j.freeradbiomed.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Boldyrev AA, Johnson P. Homocysteine and its derivatives as possible modulators of neuronal and non-neuronal cell glutamate receptors in Alzheimer’s disease. J Alzheimers Dis. 2007;11:219–228. doi: 10.3233/jad-2007-11209. [DOI] [PubMed] [Google Scholar]
- Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- Carmel R, Jacobsen DW. Homocysteine in health and disease. Cambridge University Press; Cambridge, UK; New York: 2001. [Google Scholar]
- Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Liao PF, Chan MH. mGluR5 positive modulators both potentiate activation and restore inhibition in NMDA receptors by PKC dependent pathway. J Biomed Sci. 2011;18:19. doi: 10.1186/1423-0127-18-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Collard CD, Park KA, Montalto MC, Alapati S, Buras JA, Stahl GL, Colgan SP. Neutrophil-derived glutamate regulates vascular endothelial barrier function. J Biol Chem. 2002;277:14801–14811. doi: 10.1074/jbc.M110557200. [DOI] [PubMed] [Google Scholar]
- Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010;18:938–949. doi: 10.1016/j.devcel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Sun H, Tenner TE, Jr., Machu TK. Competitive antagonism of the mouse 5-hydroxytryptamine3 receptor by bisindolylmaleimide I, a “selective” protein kinase C inhibitor. J Pharmacol Exp Ther. 1999;290:76–82. [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J. Breaking the VE-cadherin bonds. FEBS Lett. 2009;583:1–6. doi: 10.1016/j.febslet.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Gillard SE, Tzaferis J, Tsui HC, Kingston AE. Expression of metabotropic glutamate receptors in rat meningeal and brain microvasculature and choroid plexus. J Comp Neurol. 2003;461:317–332. doi: 10.1002/cne.10671. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Lu YM, Agopyan N, Roder J. Gene targeting reveals a role for the glutamate receptors mGluR5 and GluR2 in learning and memory. Physiol Behav. 2001;73:793–802. doi: 10.1016/s0031-9384(01)00516-9. [DOI] [PubMed] [Google Scholar]
- Joseph J, Handy DE, Loscalzo J. Quo vadis: whither homocysteine research? Cardiovasc Toxicol. 2009;9:53–63. doi: 10.1007/s12012-009-9042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston AE, Lowndes J, Evans N, Clark B, Tomlinson R, Burnett JP, Mayne NG, Cockerham SL, Lodge D. Sulphur-containing amino acids are agonists for group 1 metabotropic receptors expressed in clonal RGT cell lines. Neuropharmacology. 1998;37:277–287. doi: 10.1016/s0028-3908(98)00018-5. [DOI] [PubMed] [Google Scholar]
- Koles L, Wirkner K, Illes P. Modulation of ionotropic glutamate receptor channels. Neurochem Res. 2001;26:925–932. doi: 10.1023/a:1012380416876. [DOI] [PubMed] [Google Scholar]
- Krizbai IA, Deli MA, Pestenacz A, Siklos L, Szabo CA, Andras I, Joo F. Expression of glutamate receptors on cultured cerebral endothelial cells. J Neurosci Res. 1998;54:814–819. doi: 10.1002/(SICI)1097-4547(19981215)54:6<814::AID-JNR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21:262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chi OZ, Weiss HR. Effects of metabotropic glutamate receptor stimulation on blood-brain barrier permeability during focal cerebral ischemia. Neurochem Res. 2004;29:1857–1862. doi: 10.1023/b:nere.0000042212.14137.6f. [DOI] [PubMed] [Google Scholar]
- Liu X, Hunter C, Weiss HR, Chi OZ. Effects of blockade of ionotropic glutamate receptors on blood-brain barrier disruption in focal cerebral ischemia. Neurol Sci. 2010;31:699–703. doi: 10.1007/s10072-010-0241-5. [DOI] [PubMed] [Google Scholar]
- Loscalzo J. Homocysteine trials--clear outcomes for complex reasons. N Engl J Med. 2006;354:1629–1632. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39–54. doi: 10.1146/annurev.med.60.041807.123308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo JN, Beard RS, Jr., Price TO, Chen CH, Erickson MA, Ercal N, Banks WA, Bearden SE. Nitrative stress in cerebral endothelium is mediated by mGluR5 in hyperhomocysteinemia. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Pepper MS, Mohle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Moustapha A, Robinson K. Homocysteine: an emerging age-related cardiovascular risk factor. Geriatrics. 1999;54:41, 44–46, 49–51. [PubMed] [Google Scholar]
- Nwariaku FE, Liu Z, Zhu X, Turnage RH, Sarosi GA, Terada LS. Tyrosine phosphorylation of vascular endothelial cadherin and the regulation of microvascular permeability. Surgery. 2002;132:180–185. doi: 10.1067/msy.2002.125305. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Fedinec A, Leffler CW. Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metab. 2003;23:190–197. doi: 10.1097/01.WCB.000004823561824.C4. [DOI] [PubMed] [Google Scholar]
- Perroy J, Raynaud F, Homburger V, Rousset MC, Telley L, Bockaert J, Fagni L. Direct interaction enables cross-talk between ionotropic and group I metabotropic glutamate receptors. J Biol Chem. 2008;283:6799–6805. doi: 10.1074/jbc.M705661200. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–31912. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- Reijerkerk A, Kooij G, van der Pol SM, Leyen T, Lakeman K, van Het Hof B, Vivien D, de Vries HE. The NR1 subunit of NMDA receptor regulates monocyte transmigration through the brain endothelial cell barrier. J Neurochem. 2010;113:447–453. doi: 10.1111/j.1471-4159.2010.06598.x. [DOI] [PubMed] [Google Scholar]
- Rosenbrock H, Kramer G, Hobson S, Koros E, Grundl M, Grauert M, Reymann KG, Schroder UH. Functional interaction of metabotropic glutamate receptor 5 and NMDA-receptor by a metabotropic glutamate receptor 5 positive allosteric modulator. Eur J Pharmacol. 2010;639:40–46. doi: 10.1016/j.ejphar.2010.02.057. [DOI] [PubMed] [Google Scholar]
- Scott GS, Bowman SR, Smith T, Flower RJ, Bolton C. Glutamate-stimulated peroxynitrite production in a brain-derived endothelial cell line is dependent on N-methyl-D-aspartate (NMDA) receptor activation. Biochem Pharmacol. 2007;73:228–236. doi: 10.1016/j.bcp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Sharp CD, Fowler M, Jackson T.H.t., Houghton J, Warren A, Nanda A, Chandler I, Cappell B, Long A, Minagar A, Alexander JS. Human neuroepithelial cells express NMDA receptors. BMC Neurosci. 2003a;4:28. doi: 10.1186/1471-2202-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp CD, Hines I, Houghton J, Warren A, Jackson T.H.t., Jawahar A, Nanda A, Elrod JW, Long A, Chi A, Minagar A, Alexander JS. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am J Physiol Heart Circ Physiol. 2003b;285:H2592–2598. doi: 10.1152/ajpheart.00520.2003. [DOI] [PubMed] [Google Scholar]
- Sharp CD, Houghton J, Elrod JW, Warren A, Jackson T.H.t., Jawahar A, Nanda A, Minagar A, Alexander JS. N-methyl-D-aspartate receptor activation in human cerebral endothelium promotes intracellular oxidant stress. Am J Physiol Heart Circ Physiol. 2005;288:H1893–1899. doi: 10.1152/ajpheart.01110.2003. [DOI] [PubMed] [Google Scholar]
- Shi Q, Savage JE, Hufeisen SJ, Rauser L, Grajkowska E, Ernsberger P, Wroblewski JT, Nadeau JH, Roth BL. L-homocysteine sulfinic acid and other acidic homocysteine derivatives are potent and selective metabotropic glutamate receptor agonists. J Pharmacol Exp Ther. 2003;305:131–142. doi: 10.1124/jpet.102.047092. [DOI] [PubMed] [Google Scholar]
- Signorello MG, Segantin A, Passalacqua M, Leoncini G. Homocysteine decreases platelet NO level via protein kinase C activation. Nitric Oxide. 2009;20:104–113. doi: 10.1016/j.niox.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Siow YL, Au-Yeung KK, Woo CW, O K. Homocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cbeta activation. Biochem J. 2006;398:73–82. doi: 10.1042/BJ20051810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Roche KW, Thompson AK, Huganir RL. Regulation of Nmda Receptor Phosphorylation by Alternative Splicing of the C-Terminal Domain. Nature. 1993;364:70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- Ueland PM, Refsum H. Plasma homocysteine, a risk factor for vascular disease: plasma levels in health, disease, and drug therapy. J Lab Clin Med. 1989;114:473–501. [PubMed] [Google Scholar]
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, Elson G, Legarda D, Leibovich SJ. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol. 1998;153:587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sun K, Chen J, Liao Y, Qin Q, Ma A, Wang D, Zhu Z, Wang Y, Hui R. High plasma homocysteine levels contribute to the risk of stroke recurrence and all-cause mortality in a large prospective stroke population. Clin Sci (Lond) 2010;118:187–194. doi: 10.1042/CS20090142. [DOI] [PubMed] [Google Scholar]
- Zieminska E, Stafiej A, Lazarewicz JW. Role of group I metabotropic glutamate receptors and NMDA receptors in homocysteine-evoked acute neurodegeneration of cultured cerebellar granule neurones. Neurochem Int. 2003;43:481–492. doi: 10.1016/s0197-0186(03)00038-x. [DOI] [PubMed] [Google Scholar]