Abstract
Fecal indicator microbes such as enterococci are often used to assess potential health risks caused by pathogens at recreational beaches. Microbe levels often vary based on collection time and sampling location. The primary goal of this study was to assess how spatial and temporal variations in sample collection which are driven by environmental parameters impact enterococci measurements and beach management decisions. A secondary goal was to assess whether enterococci levels can be predictive of the presence of Staphylococcus aureus a skin pathogen. Over a ten day period hydrometeorologic data hydrodynamic data bather densities enterococci levels and S. aureus levels including methicillin-resistant S. aureus (MRSA) were measured in both water and sand. Samples were collected hourly for both water and sediment at knee-depth and every 6 hours for water at waist-depth supratidal sand intertidal sand and waterline sand. Results showed that solar radiation tides and rainfall events were major environmental factors that impacted enterococci levels. S. aureus levels were associated with bathing load but did not correlate with enterococci levels or any other measured parameters. The results imply that frequencies of advisories depend heavily upon sample collection policies due to spatial and temporal variation of enterococci levels in response to environmental parameters. Thus sampling at different times of the day and at different depths can significantly impact beach management decisions. Additionally the lack of correlation between S. aureus and enterococci suggests that use of fecal indicators may not accurately assess risk for some pathogens.
Keywords: Enterococci, S. aureus, Beaches, Management, Variability
1. Introduction
Exposure to microbial pathogens poses a risk to swimmers in recreational waters through routes such as ingestion inhalation and skin contact (Boehm et al. 2009a). In order to reduce the risk of exposure water quality at recreational beaches is assessed by regulatory agencies using indicator microbes. Enterococci is used as a fecal indicator at recreational marine beaches due to its historical associations with adverse health effects in humans bathing in point source (e.g. human sewage) impacted recreational waters (Cabelli et al. 1982; Fleisher et al. 2010). According to U.S. EPA guidelines enterococci levels in marine waters should not exceed a monthly geometric mean of 35 colony forming units (CFU)/100 mL and a single sample value of 104 CFU/100 mL. States develop their own standards and sampling procedures based on these EPA guidelines (U.S. EPA 1986). Many states (Table 1) issue advisories immediately after the single sample guideline is exceeded. Other states collect a follow-up sample thus requiring two consecutive samples before an advisory is issued.
Table 1.
Fraction of days advisories would have been issued based on given sampling times and criteria
| Standard One | Standard Two | Standard Three | Standard Four | |
|---|---|---|---|---|
| Description | Knee depth, no consecutive samples |
Knee depth, consecutive samples |
Waist depth, no consecutive samples |
Waist depth, consecutive samples |
| Statesa | AL, HI, MD, TX, VA |
MS,WA | CT, GA, LA, ME, MA, NY, RI |
FL |
| Time | ||||
| 12:00 AM | 9/9b (100%) | 7/7b (100%) | 1/10 (10%) | 0/9 (0%) |
| 1:00 AM | 7/10 (70%) | 4/9 (44%) | ||
| 2:00 AM | 7/10 (70%) | 5/9 (56%) | ||
| 3:00 AM | 4/10 (40%) | 1/9 (11%) | ||
| 4:00 AM | 4/10 (40%) | 1/9 (11%) | ||
| 5:00 AM | 6/10 (60%) | 3/9 (33%) | ||
| 6:00 AM | 3/10 (30%) | 0/9 (0%) | 0/10 (0%) | 0/9 (0%) |
| 7:00 AM | 2/10 (20%) | 1/9 (11%) | ||
| 8:00 AM | 2/10 (20%) | 1/9 (11%) | ||
| 9:00 AM | 2/10 (20%) | 1/9 (11%) | ||
| 10:00 AM | 3/10 (30%) | 0/9 (0%) | ||
| 11:00 AM | 2/10 (20%) | 0/9 (0%) | ||
| 12:00 PM | 0/10 (0%) | 0/9 (0%) | 0/9 (0%) | 0/9 (0%) |
| 1:00 PM | 3/10 (30%) | 1/9 (11%) | ||
| 2:00 PM | 4/10 (40%) | 0/9 (0%) | ||
| 3:00 PM | 1/10 (10%) | 0/9 (0%) | ||
| 4:00 PM | 4/10 (40%) | 2/9 (22%) | ||
| 5:00 PM | 5/10 (50%) | 3/9 (33%) | ||
| 6:00 PM | 5/10 (50%) | 3/9 (33%) | 1/10 (10%) | 0/9 (0%) |
| 7:00 PM | 6/10 (60%) | 5/9 (56%) | ||
| 8:00 PM | 5/9c (56%) | 2/8b (25%) | ||
| 9:00 PM | 5/10 (50%) | 3/9 (33%) | ||
| 10:00 PM | 6/10 (60%) | 4/9 (44%) | ||
| 11:00 PM | 6/10 (60%) | 4/9 (44%) | ||
States with marine beaches not included in this analysis have management criteria that differ from the four standard types listed (rainfall advisories, different enterococci levels, ankle-depth sampling, etc.)
The sample on Day 3 was lost and therefore affected consecutive measurements for Days 2-3 and Days 3-4. Only 9 samples were available for this hour.
The sample on Day 5 was not collected due to a storm.
Differences in sample collection times and locations may affect enterococci measurements and resulting beach management decisions. Water quality samples in many states including Florida are generally collected in approximately waist-depth water and are often collected in the morning. Water samples in other states may be collected in knee-depth water or in ankle-depth water (Dorfman and Rosselot 2010). Samples collected at different times of the day can result in significantly different enterococci levels due to a variety of environmental factors such as solar radiation rainfall tide and the presence of bathers (Wright et al. 2011; Whitman et al. 2004; Boehm et al. 2002). Prior research has also indicated that enterococci levels in water samples collected approximately 100 meters offshore can be lower than those collected 10 meters offshore at beaches not impacted by offshore point sources of pollution (Wright et al. 2011).
One of the limitations in using enterococci as the sole human health indicator at marine beaches is that it may not be able to account for non-gastrointestinal illnesses (Boehm et al. 2009a) caused by pathogens such as Staphylococcus aureus a usual commensal colonizing bacteria that is capable of causing skin infections. Studies have indicated that waters with high S. aureus densities may be associated with higher risks of skin eye and ear infections (Charoenca and Fujioka 1995; Gabutti et al. 2000). Recent studies have also indicated that methicillin-resistant S. aureus (MRSA) an antibiotic-resistant strain of S. aureus that can lead to serious infections is found in recreational beach sands (Goodwin and Pobuda 2009; Soge et al. 2009; Tice et al. 2010; Shah et al. 2011; Plano et al. 2011).
In order to properly understand if enterococci levels are an accurate indicator of health effects both environmental influences and pathogen presence in relation to enterococci levels should be thoroughly understood. The first objective of this study was to understand how spatial and temporal variance in sample collection may affect beach management decisions based on enterococci measurements. Numerous other studies have characterized spatial and temporal environmental variables that impact enterococci measurements and these factors vary in importance at each study beach (Wymer et al. 2005). This study however specifically examines relationships between these variables and beach advisory scenarios that result from different methodologies in sample collection. The second objective of this study was to determine whether presence of enterococci can be predictive of a skin pathogen such as S. aureus including MRSA. This study presents an analysis of 238 sampling events over a 10-day period providing a high-resolution analysis unique to this study.
2. Materials and Methods
2.1. Site Description
This study was conducted over a ten-day period at Hobe Cat Beach in Miami Florida USA. This beachhas been the subject of extensive long-term study (Fleming et al. 2004; Shibata et al. 2004; Elmir et al. 2007; Wright et al. 2009; Abdelzaher et al. 2010; Sinigalliano et al. 2010; Fleisher et al. 2010; Abdelzaher et al. 2011; Wright et al. 2011; Shah et al. 2011) and has been characterized as a non-point source beach. The beach faces central Biscayne Bay a semi-enclosed subtropical lagoon and is characterized by poor water circulation. The water at the beach is relatively shallow and the beach itself is narrow with a mean distance from the water line to the inshore edge of the sand of 5 meters. The length of the beach is approximately 1.6 kilometers (Shibata et al. 2004). It is the only beach in Miami-Dade County that allows visitors to bring dogs. Twenty percent of samples from this beach exceeded microbial water quality guidelines in 2009 and the beach was placed under an advisory for 7 days during that year (Dorfman and Rosselot 2010). The area surrounding the beach has been extensively evaluated for point sources of pollution such as sewage outfalls and septic tanks but no point sources have been found (Shibata et al. 2004).
2.2. Water and sediment sampling
Temporal differences in water quality were evaluated by collecting samples hourly over a 10-day period. Spatial differences were evaluated by collecting samples in both knee-deep and waist-deep water. Sampling was conducted from June 1st to June 10th 2010 along 10 transects marked by poles located at the upper edge of the wrack line. All samples were collected aseptically into Whirlpak® bags. Every hour over the 10-day period water and subtidal sediment samples were collected at knee-depth (0.3 meters). Knee-deep water samples were collected from the water surface (5-10 centimeters underwater). The sediment samples were collected aseptically into Whirl-Pak® bags using sterilized spoons from the upper 5 centimeters of the submerged sand in an area of about 20 x 20 centimeters.
Every 6 hours starting at 6:00 AM on June 1st a surface water sample (5-10 cm deep) was collected from waist-deep water (1 m deep). Three additional shoreline sediment samples were also collected every 6 hours in order to evaluate spatial variation in sediment enterococci levels. Supratidal sand samples representing sand above the high tide line were collected from the upper 5 cm of sand in a location 0.15 meters shoreward from the transect poles. Fixed-location intertidal sands representing sand between the high and low tide lines were collected 2.4 meters toward the water from the corresponding transect pole. Waterline sediment samples were collected 0.08 meters above the water’s edge and moved due to tidal action.
2.3. Environmental Parameters
Every hour salinity pH and water temperature were measured near the knee-depth sample site (YSI model 650-01m environmental monitoring systems; YSI Yellow Springs OH). Turbidity of the collected water samples was measured in the lab using a nephelometer (TD-40; Turner Designs Sunnyvale CA). The presence of humans dogs and birds on both water and sand 65 meters to the right and to the left of the middle sampling transect was also recorded every hour. Rainfall solar shortwave radiation and wind data were recorded every two minutes at a measurement station less than 1 km from the sampling site (NSF NIEHS OHH Center Remote Sensing Facilities Core: http://yyy.rsmas.miami.edu/etc/download-weatherpak.cgi). Tidal data were recorded by a wave and tide recorder every 32 seconds 160 meters offshore (TWR 2050 RBR Ottawa Ontario). More details about data processing are available in the supplemental text.
2.4. Microbial Analysis
Upon collection samples were transported in coolers with freezer packs immediately to a lab located within 1 km for analysis of microbes. Enterococci were analyzed using EPA Method 1600 (U.S. EPA 2006). S. aureus were extracted from samples via membrane filtration. Filters were placed on Baird Parker agar with Egg Yolk Tellurite Enrichment (Becton Dickinson Sparks MD) and incubated at 37°C for 24 hours. Colonies that were identified as presumptive S. aureus colonies were subjected to further confirmation testing as described by Plano et al. (2011). More details about S. aureus analysis methods are provided in the supplemental text. Processing of sediment samples required extraction of enterococci and S. aureus from sediment into water by adding a measured amount of sediment (approximately 10 g) into a sterile plastic bottle with 100 mL of sterile phosphate buffered dilution water. These samples were shaken vigorously for 2 minutes to promote the transfer of bacteria into the water (Boehm et al. 2009b). Sediment was allowed to settle for 2 minutes and then 3 and 25 mL of the supernatants were filtered. Any remaining sediment from each sample that was not used for microbe quantification was used to analyze water content grain size and volatile organic compound percentage for each of the samples (Shah et al. 2011). This is further described in the supplemental text.
2.5 Statistical Analysis
One-way analysis of variance (ANOVA) was used to determine significant differences among the means with alpha set at 0.05 (i.e. 95% confidence limit). Pearson’s χ2 contingency test was used to test associations between categorical variables with alpha also set at 0.05. Pearson correlation analysis was also performed in order to compare physical-chemical parameters enterococci levels and S. aureus levels. Pearson correlation coefficients (r) greater than an absolute value of 0.45 and a p-value less than 0.05 were considered significant for this study. Averages are reported with standard deviations.
3. Results and Discussion
3.1. Environmental Parameters
Over the 10-day sampling period the physical-chemical characteristics of the water samples were typical of subtropical marine water (average pH 8.04 salinity 33.5 practical salinity units (psu) water temperature ranging from 27°C to 37°C). Solar radiation levels ranged from <10 to 890 watts per square meter (W/m2) between the hours of 7:00AM and 8:00PM with near zero values outside this time. Tides were semidiurnal and although the vertical tidal range was relatively small (0.7 meters) the horizontal range was relatively large (7.3 meters) due to the mild beach slope. Out of the 238 samples collected 35 were collected during rainfall. The total rainfall for the 10-day study duration was 63.3 millimeters. The number of bathers (present only during the beach operating hours from 7:00AM until 8:00PM) was also typical for this beach site (Wang et al. 2010) with weekends showing the maximum number of bathers. Within 65 m of the sampling transect the maximum numbers of humans in the water and on shore during a sampling event were 72 and 53 respectively and the maximum numbers of dogs in the water and on the shore were 8 and 5 respectively.
3.2. Spatial Variation
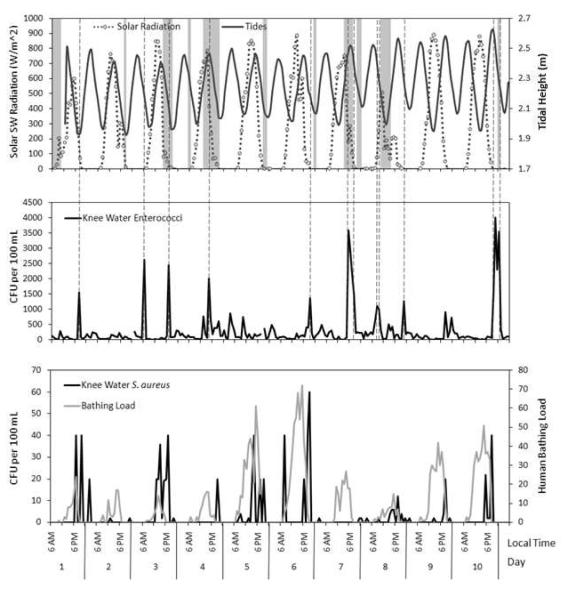
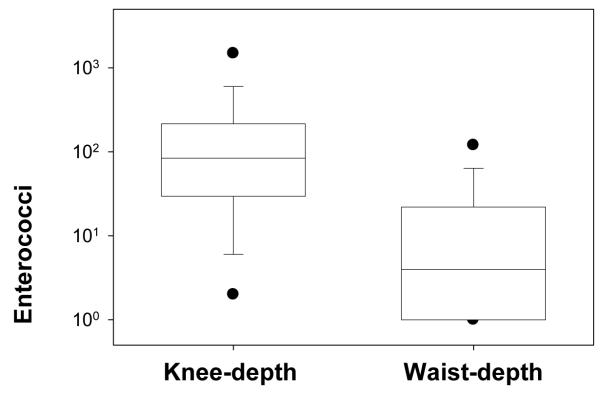
Enterococci measurements were affected by sampling location as observed from knee-depth versus waist-depth samples. Knee-depth water samples had enterococci levels ranging from below detection (2 CFU/100 mL) to above the detection limit (4000 CFU/100 mL) with an average of 270 CFU/100 mL and standard deviation of ±590 CFU/100 mL. Enterococci levels exceeded 1000 CFU/100mL a value that is nearly 10 times greater than the regulatory standard in 16.5% of the knee-depth water samples. These exceedances occurred as 9 separate events during the hourly 10-day sampling program. These exceedances ranged in duration from 1 to 3 hours in length (Figure 1). Waist-depth samples ranged from below detection to 640 CFU/100 mL with an average of 32±100 CFU/100mL. The enterococci levels in the knee-depth samples were significantly higher than those of the waist-depth samples (p = 0.02). A comparison of median values and ranges of knee-depth and waist-depth enterococci levels is presented in Figure 2. Forty-three percent (43%) of knee-depth water samples were above the enterococci regulatory guideline for single samples of 104 CFU/100mL while 5% of waist-depth samples exceeded the guideline. Retrieval of a water sample from waist-depth water resulted in a lower enterococci measurement than taking a sample at the same time from the knee-depth location in 89.5% of the samples. Thus collecting samples from knee-depth would result in a higher number of advisories than collecting samples from waist-depth.
Figure 1.
Comparison of physical parameters knee depth water enterococci knee depth water S. aureus and bathing load. The top graph displays tidal height (dark grey line) solar shortwave (SW) radiation averaged over the previous hour (dotted line with data points) and times of rainfall (grey rectangles). The middle graph displays knee depth enterococci levels with dashed lines aligning peak enterococci events (>1000 CFU/100mL) to corresponding physical parameters. The bottom graph displays knee depth water S. aureus levels and number of bathers present at each hour.
Figure 2.
Box plot of knee-depth and waist-depth water enterococci levels (n = 238 for knee-depth samples n = 40 for waist-depth samples). The center line in the boxes indicates the median value. Whiskers indicate 10th and 90th percentiles. Outliers (dots) indicate 5th and 95th percentiles. Ninety percent of the waist-depth samples had lower values than 50 percent of knee depth values.
The likely source of spatial variation in water samples is wash-in of enterococci from the intertidal zone caused by tides and rainfall. Prior studies have indicated that shoreline sediment can be a non-point source of bacteria (Desmarais et al. 2002; Rogerson et al. 2003; Whitman and Nevers 2003; Alm et al. 2006) including shoreline sediment at this study site (Shibata et al. 2004; Wright et al. 2011; Phillips et al. 2011a; Phillips et al. 2011b). Although tidal height and knee-depth water enterococci levels did not correlate (r = 0.21) during non-rain conditions knee-depth samples collected during outgoing tides had significantly higher enterococci levels than samples collected during incoming tides (p = 0.04). Additionally 7 out of the 9 peak events occurred during outgoing tides which is similar to observations in past studies (Abdelzaher et al. 2011). In addition to outgoing tides rain events (defined in the supplemental text) were associated with an increase in knee-water enterococci levels. Knee-depth samples collected during rain events had higher enterococci levels than samples collected during non-rain events (p = 0.004). Sixty two percent (62%) of samples collected during rain events had enterococci levels above 104 CFU/100 mL while 39% of non-rain event samples had enterococci levels above 104 CFU/100 mL. These results indicate that enterococci may be washing in from the shoreline sediments into the water through rainfall runoff and tidal action.
Analysis of sediment samples further support tidal wash-in as a source of spatial variation. Out of the four different types of sediment samples supratidal sand had the highest enterococci levels with an average of 131±210 CFU/g. The average levels for the fixed-location intertidal waterline and subtidal samples were 18±42 CFU/g 19±18 CFU/g and 14±23 CFU/g respectively. Supratidal samples had significantly higher enterococci levels than fixed-location intertidal samples (p = 0.001) waterline samples (p = 0.002) and subtidal samples (p = <0.0001). No significant differences in enterococci levels were noted between the remaining sediment samples (p > 0.1). Possible causes of higher enterococci levels in supratidal sand include lack of tidal washing which allows enterococci to accumulate (Bonilla et al. 2007) and lower moisture content in which predators such as protozoa cannot survive (Boehm et al. 2005; Desmarais et al. 2002; Solo-Gabriele et al. 2000; Wright et al. 2011). Other sediment characteristics such as water content VOC percentage and grain sizes were found not to correlate with water or sediment enterococci measurements.
3.3. Temporal Variation
Time of day during which samples are taken can also impact results used for management purposes. Analysis of samples showed that elevated solar radiation was likely a major contributor to decreases in enterococci levels in water samples. This affected the results seen at different sampling hours in a manner consistent with other studies (Boehm et al. 2002).
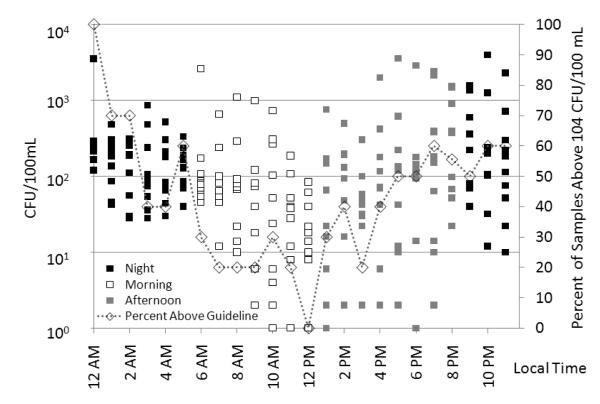
Samples were grouped into morning (6AM – 12PM) afternoon (1PM – 8PM) and night (9PM – 5AM) samples based on sunset and sunrise times during the 10-day sampling program. Morning knee-depth enterococci levels were significantly lower than night enterococci levels (p = 0.04). Variation is also shown between early morning and late morning samples (Figure 3). No significant difference in enterococci levels was observed between night and afternoon (p = 0.98) or between morning and afternoon (p = 0.06) samples . Additionally none of the 9 enterococci peak events (> 1000 CFU/100 ml) occurred when solar radiation levels were at peak values. All enterococci peaks occurred when solar radiation levels were below 415 W/m2 which is an approximate midrange value for the solar radiation data (Figure 1). Log-normalized enterococci levels in all knee-depth water samples inversely correlated with solar radiation (r = − 0.47 p < 0.0001). Although humans and dogs are sources of enterococci (Elmir et al. 2007; Elmir et al. 2009; Wright et al. 2009) and these sources are usually most abundant during times of high solar radiation the effects of solar inactivation likely outweighed the contribution from humans and dogs during times of elevated solar radiation.
Figure 3.
Knee-depth water enterococci levels grouped by hour. Black squares indicate night samples (9PM-5AM) white squares indicate morning samples (6AM-12PM) and grey squares indicate afternoon samples (1PM-8PM). The dotted line indicates the percentage of samples each hour above the swim advisory single sample guideline of 104 CFU/100 mL.
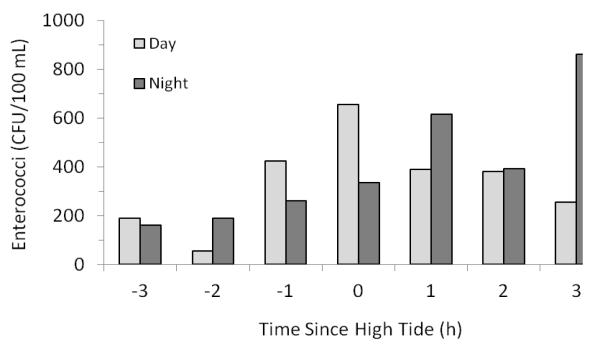
Patterns were also identified when daytime and nighttime enterococci levels were grouped by hours before and after high tide. Enterococci levels during the day generally peaked at high tide (Figure 4). Enterococci levels at night however would continue rising after high tide occurred. We hypothesize that as the tide rises enterococci is released from the sand. At night after high tide solar radiation is not present to inactivate the enterococci allowing levels to remain high as the tidal height decreases. After high tide during the day however solar inactivation causes die-off of released enterococci causing levels to decrease after high tide.
Figure 4.
Daytime and nighttime mean enterococci levels grouped by number of hours before and after high tide.
Due to the influence of solar radiation on water enterococci levels in combination with other factors that vary temporally different management decisions could result from collecting samples at different hours of the day. One hundred percent of the samples taken at noon for example had enterococci levels below the single sample standard of 104 CFU/100 mL. The geometric mean of all knee-depth noon samples was 16 CFU/100 mL which is below the geometric mean used for swim advisories of 35 CFU/100 mL (U.S. EPA 1986). Thus no beach advisories would have been issued based upon the 12:00 noon samples. Samples taken at a later or earlier time however could have led to the issuance of swim advisories if collected at knee-depth. Samples collected at 6:00 AM exceeded this guideline on June 1st 3rd and 8th. Fifty-percent of the 6:00PM samples exceeded the single sample standard and all 12:00 midnight samples exceeded the single sample standard (Figure 3). As a result beach advisories could have been issued during all consecutive days of this study based upon the 12:00 midnight knee-depth samples. For samples taken at 6:00 AM 6:00 PM and 12:00 midnight each set exceeded the recommended geometric mean level with geometric means of 112 83 and 299 CFU/100 mL respectively.
3.4. S. aureus
For the water samples 45 out of 238 (19%) knee-depth samples and 5 out of 40 (13%) waist-depth samples were positive for S. aureus. In sediment 4 out of 40 (10%) supratidal 1 out of 40 (2.5%) intertidal 1 out of 40 (2.5%) waterline and 1 out of 239 (0.42%) subtidal samples were positive for presence of S. aureus. Two of the 238 (0.84%) knee-depth water samples tested positive for MRSA. None of the other samples (waist-depth or sediment samples) tested positive for MRSA.
Results show that S. aureus levels in the water were elevated following times characterized by high bather load (Figure 1) with S. aureus levels higher when bathers were present than when bathers were not present (p = 0.02). These results support that bathers may be a source of S. aureus at beaches which has been suggested by other studies (Gabutti et al. 2000; Elmir et al. 2007; Plano et al. 2011; Plano et al. in review). S. aureus levels were also found to not correlate with enterococci levels in the knee-depth water samples (r = 0.07). The lack of correlation between S. aureus and enterococci is not unexpected. Although both S. aureus and enterococci can originate from human bather shedding the numbers of organisms shed may be very different as shown by Elmir et al. (2007) where the number of S. aureus shed per bather was 10 fold greater than the number of enterococci shed. Additionally the two organisms are associated with humans in very different ways. S. aureus are primarily skin colonizing organisms found in only 30-40% of all people while enterococci species are associated with the gastrointestinal tract of all people. Therefore the differences between S. aureus and enterococci levels are likely a combination of the different levels shed by humans coupled with possible higher sensitivity to solar radiation and a stronger shoreline source for enterococci. Therefore both bacteria were observed at the study beach but each are subject to different inputs and different responses to environmental conditions resulting in a lack of association between the two bacteria.
The two knee-depth water samples from which MRSA were isolated were collected on June 5th at 1:00 PM and June 7th at 8:00 PM. Consequentially these two sampling times had considerably different bathing loads (41 and 0 respectively) and solar radiation levels (841 W/m2 and 11 W/m2). Of interest was that enterococci levels for both of the MRSA samples were above the single sample guideline (171 and 1360 CFU/100mL) and thus a beach advisory could have been issued for both of these cases if such decisions were based upon knee-depth samples without confirmatory analyses. Due to the small sample size positive for the presence of MRSA the correspondence between MRSA and enterococci could be entirely coincidental. More research is needed to further evaluate a possible relation between MRSA and enterococci at this beach.
3.5. Comparison of Different Beach Management Standards
Enterococci levels during this study were analyzed based on sampling procedures applied by different states. The majority of beach sampling procedures can be split into 4 standard types depending upon sampling depth and number of exceedances that trigger an advisory (Table 1). All states with marine beaches not included in Table 1 base their standards on different criteria such as different enterococci levels different sampling depths and issuance of advisories based on rainfall. This analysis shows that issuing advisories based on one-time samples collected at knee-depth (Standard 1) results in the highest percentage of advisories. Requiring consecutive knee-depth samples to exceed regulatory guidelines (Standard 2) results in considerably less advisories with no advisories issued for samples collected between 10:00 AM and 12:00 PM. Standard 3 results in very few advisories (only 10% of the time for samples collected at 6:00 PM and at 12:00 AM). No advisories would have been issued at the study beach site based on Standard 4 (waist depth requiring confirmatory results). As described above the data set shows that sample collection time affects percentages of advisories issued. Collection of samples during times of high solar radiation for example results in a lower percentage of advisories issued than collecting samples during times of lower solar radiation.
S. aureus presence was also compared to beach closure scenarios. Under Standard 1 48% of water samples positive for S. aureus were collected during times that the beach would have been under an advisory (Table 2). Considering only the 137 sampling hours the beach would have been open under Standard 1 S. aureus was present in water samples during 26 of those hours (19%). Considering the 101 sampling hours the beach would have been under an advisory according to Standard 1 24 of those hours were positive for S. aureus (24%). Given the relatively even distribution of S. aureus between times the beach would have been open or closed there was no significant association found between S. aureus presence and beach advisories (Pearson’s χ2 test p = 0.37). Under the Standard 2 criteria which relies on consecutive enterococci exceedances 12 of the 50 (24%) positive water samples for S. aureus were collected during times that the beach would have been under an advisory. Under Standards 3 and 4 no advisories would have been in effect when S. aureus was present in knee or waist-depth water. Thus management strategies based on shallower sampling locations and instant advisories provide more protection for exposure to S. aureus than other management strategies simply because more advisories would be issued. Results also suggest that the detection of enterococci does not necessarily indicate risks from potential S. aureus exposures.
Table 2.
Comparison of S. aureus presence and advisory issuance based on given sampling criteria.
| Standard One | Standard Two | Standard Three | Standard Four | |
|---|---|---|---|---|
| Advisory when S. aureus is present |
24/50 (48%) | 12/50 (24%) | 0/50 (0%) | 0/50 (0%) |
|
| ||||
| No advisory when S. aureus is present |
26/50 (52%) | 38/50 (76%) | 50/50 (100%) | 50/50 (100%) |
Time lags in advisory issuance due to sample processing were also considered. In 52 of 98 knee-depth water samples with greater than 104 enterococci CFU/100 ml exceedances were observed exactly 24 hours later as well (5 exceedances on day 10 were removed due to no data being available the next day). Under a scenario in which beach advisories are issued exactly 24 hours after sampling occurs exposure to elevated enterococci levels the next day would have been prevented in these instances. A significant association was found for enterococci between exceedance status of one sample and exceedance status of the sample collected 24 hours later (Pearson’s χ2 test p = 0.006). This association was influenced by the effects of solar radiation on enterococci levels and the fact that solar radiation varies in cycles of 24 hours. Also with a 24 hour lag in advisory issuance exposure to S. aureus would have been prevented in 20 cases. In 22 other instances however enterococci levels were too low to prevent exposure to S. aureus 24 hours later. No significant association was found between enterococci exceedances and S. aureus presence 24 hours later (Pearson’s χ2 test p = 0.79).
Faster same day qPCR-based technologies have been proposed to decrease inaccuracies associated with time lags. The use of same-day qPCR may require sampling earlier in the morning in order to obtain results in time for the peak bathing period. However because of the influence of solar radiation on enterococci adjusting the sampling time earlier to facilitate same-day results may result in a shift in the baseline enterococci measures. Such potential shifts should be considered when making beach management decisions based upon same-day samples.
4. Conclusion
The results of this study showed that a fecal indicator such as enterococci may not be predictive of the presence of some pathogens such as S. aureus. Thus additional human health risks may be present even when indicator levels are low and additional indicators may be needed to protect bathers from pathogens that do not correlate with enterococci. At this beach site an epidemiologic study (Fleisher et al. 2010; Sinigalliano et al. 2010) found that bathers were at higher risk than non-bathers in contracting skin illness and the level of risk for skin illness was related to enterococci levels. The results of these prior studies in combination with this study suggest that enterococci may be indicative of a skin pathogen other than S. aureus. Further research should be conducted to identify other potential skin pathogens or other sources of skin irritation and infection at recreational beaches. Associations between MRSA and enterococci should also continue to be evaluated due to both MRSA-positive samples being collected at times when enterococci exceeded regulatory levels.
Ultimately enterococci levels are influenced by the interplay of several environmental factors including solar radiation levels and release from suspected sand sources via tide and rainfall. The time and location at which a water sample is taken may greatly influence the measured levels of enterococci and the resulting beach management decision. To remove bias due to sampling location and time observed levels can be scaled by the known temporal and spatial variability and thereby provide an approach to issuing beach advisories that more closely correlates with actual health risks to beachgoers.
Supplementary Material
Acknowledgements
This research was funded by the NSF-NIEHS Oceans and Human Health Program (NIEHS # 1 P50 ES12736 and NSF #OCE0432368/0911373). Support was also provided by the NSF REU program in Oceans and Human Health. We would like to thank the numerous students at University of Miami and volunteers from the Miami-Dade County Department of Health who participated in this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting typesetting and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content and all legal disclaimers that apply to the journal pertain.
References
- Abdelzaher AM, Wright ME, Ortega C, Solo-Gabriele HM, Miller G, Elmir S, Newman X, Shih P, Bonilla JA, Bonilla TD, Palmer CJ, Scott T, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano C, Gidley M, Plano LWR, Zhu X, Wang JD, Fleming LE. Presence of Pathogens and Indicator Microbes at a Non-Point Source Subtropical Recreational Marine Beach. Applied & Environmental Microbiology. 2010;76(3):724–732. doi: 10.1128/AEM.02127-09. doi:10.1128/AEM.02127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelzaher AM, Wright ME, Ortega C, Hasan AR, Shibata T, Solo-Gabriele HM, Kish J, Withum K, He G, Elmir SM, Bonilla JA, Bonilla TD, Palmer CJ, Scott TM, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano CD, Gidley ML, Wanless D, Plano LRW, Garza AC, Zhu X, Stewart JR, Dickerson JW, Yampara-Iquise H, Carson C, Fleisher JM, Fleming LE. Daily measures of microbes and human health at a non-point source marine beach. Journal of Water Health. 2011;9(3):443–457. doi: 10.2166/wh.2011.146. doi:10.2166/wh.2011.146. [DOI] [PubMed] [Google Scholar]
- Alm EW, Burke J, Hagan E. Persistence and potential growth of the fecal indicator bacteria, Escherichia coli, in shoreline sand at Lake Huron. Journal of Great Lakes Research. 2006;32(2):401–405. doi:10.3394/0380-1330. [Google Scholar]
- Boehm AB, Grant SB, Kim JH, Mowbray SL, McGee CD, Clark CD, Foley DM, Wellman DE. Decadal and shorter period variability of surf-zone water quality at Huntington beach, California. Environmental Science and Technology. 2002;36(18):3885–3892. doi: 10.1021/es020524u. doi:10.1021/es020524u. [DOI] [PubMed] [Google Scholar]
- Boehm AB, Weisberg SB. Tidal forcing of enterococci at marine recreational beaches at fortnighly and semidiurnal frequencies. Environmental Science and Technology. 2005;39(15):5575–5583. doi: 10.1021/es048175m. doi:10.1021/es048175m. [DOI] [PubMed] [Google Scholar]
- Boehm AB, Ashbolt NJ, Colford JM, Dunbar LE, Fleming LE, Gold MA, Hansel J, Hunter PR, Ichida AM, McGee CD, Soller JA, Weisberg SB. A sea change ahead for recreational water quality criteria. Journal of Water Health. 2009a;7(1):9–20. doi: 10.2166/wh.2009.122. doi:10.2166/wh.2009.122. [DOI] [PubMed] [Google Scholar]
- Boehm AB, Griffith J, McGee C, Edge TA, Solo-Gabriele HM, Whitman R, Cao Y, Getrich M, Jay JA, Ferguson D, Goodwin KD, Lee C, Madison M, Weisberg SB. Faecal indicator bacteria enumeration in beach sand: a comparison study of extraction methods in medium to coarse sands. Journal of Applied Microbiology. 2009b;107(5):1740–1750. doi: 10.1111/j.1365-2672.2009.04440.x. doi:10.1111/j.1365-2672.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla TD, Nowosielski K, Cuvelier M, Hartza A, Greenb M, Esiobub N, McCorquodalea DS, Fleisher JM, Rogerson A. Prevalence and distribution of fecal indicator 21 organisms in South Florida beach sand and preliminary assessment of health effects associated with beach sand exposure. Marine Pollution Bulletin. 2007;54(9):1472–1482. doi: 10.1016/j.marpolbul.2007.04.016. doi:10.1016/j.marpolbul.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Cabelli VJ, Dufour AP, McCabe LJ, Levin MA. Swimming associated gastroenteritis and water quality. American Journal of Epidemiology. 1982;115(4):606–616. doi: 10.1093/oxfordjournals.aje.a113342. [DOI] [PubMed] [Google Scholar]
- Charoenca N, Fujioka RS. Association of staphylococcal skin infections and swimming. Water Science and Technology. 1995;31(5-6):11–17. [Google Scholar]
- Desmarais TR, Solo-Gabriele HM, Palmer CJ. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Applied and Environmental Microbiology. 2002;68(3):1165–1172. doi: 10.1128/AEM.68.3.1165-1172.2002. doi:10.1128/AEM.68.3.1165-1172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman M, Rosselot KS. Testing the Waters: A Guide to Water Quality at Vacation Beaches. National Resources Defense Council (NRDC); 2010. [Google Scholar]
- Elmir SM, Wright ME, Abdelzaher A, Solo-Gabriele HM, Fleming LE, Miller G, Rybolowik M, Shih MP, Pillai SP, Cooper JA, Quaye EA. Quantitative evaluation of bacteria released by bathers in a marine water. Water Research. 2007;41(1):3–10. doi: 10.1016/j.watres.2006.10.005. doi:10.1016/j.watres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmir SM, Shibata T, Solo-Gabriele HM, Sinigalliano CD, Gidley ML, Miller G, Plano L, Kish J, Withum K, Fleming L. Quantitative evaluation of enterococci and bacteroidales released by adults and toddlers in marine water. Water Research. 2009;43(18):4610–4616. doi: 10.1016/j.watres.2009.07.006. doi:10.1016/j.watres.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher JM, Fleming LE, Solo-Gabriele HM, Jonathan KK, Sinigalliano CD, Plano LRW, Elmir SM, Wang JD, Withum K, Shibata T, Gidley ML, Abdelzaher AM, He G, Ortega C, Zhu X, Wright M, Hollenbeck J, Backer LC. The BEACHES Study: health effects and exposures from non-point source microbial contaminants in subtropical recreational marine waters. International Journal of Epidemiology. 2010;39(5):1291–1298. doi: 10.1093/ije/dyq084. doi:10.1093/ije/dyq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Solo-Gabriele HM, Elmir SM, Shibata T, Squicciarini D, Quirino W, Arguello M, Van de Bogart G. A Pilot Study of Microbial Contamination of Subtropical Recreational Waters. Florida Journal of Environmental Health. 2004;184(29):29–33. [PMC free article] [PubMed] [Google Scholar]
- Gabutti G, De Donno A, Bagordo F, Montangna MT. Comparative survival of faecal and human contaminants and use of Staphylococcus aureus as an effective indicator of human pollution. Marine Pollution Bulletin. 2000;40(8):697–700. doi:10.1016/S0025-326X(00)00007-2. [Google Scholar]
- Goodwin KD, Pobuda M. Performance of CHROMagarTM Staph aureus and CHROMagarTM MRSA for detection of Staphylococcus aureus in seawater and beach sand-Comparison of culture, agglutination, and molecular analyses. Water Research. 2009;43(19):4802–4811. doi: 10.1016/j.watres.2009.06.025. doi:10.1016/j.watres.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Phillips MC, Solo-Gabriele HM, Piggot AM, Klaus JS, Zhang Y. Relationships between sand and water quality at recreational beaches. Water Research. 2011a;45:6763–6769. doi: 10.1016/j.watres.2011.10.028. doi:10.1016/j.watres.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MC, Solo-Gabriele HM, Reniers AJHM, Wang JD, Kiger RT, Abdel-Mottaleb N. Pore water transport of enterococci out of beach sediments. Marine Pollution Bulletin. 2011b;62:2293–2298. doi: 10.1016/j.marpolbul.2011.08.049. doi:10.1016/j.marpolbul.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plano LRW, Garza AC, Elmir SM, Shibata T, Solo-Gabriele HM, Kish J, Sinigalliano CD, Gidley ML, Miller G, Withum K, Fleming LE. Shedding of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus from adult and pediatric bathers in marine waters. BMC Microbiology. 2011;11(1):5. doi: 10.1186/1471-2180-11-5. doi:10.1186/1471-2180-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plano LRW, Shibata T, Garza AC, Kish J, Fleisher J, Sinigalliano CD, Gidley ML, Withum K, Elmir SM, Cleary T, Fleming LE, Solo-Gabriele HM. Characterization of Staphylococcus aureus and community associated MRSA at a recreational marine beach in South Florida. (201X) In review.
- Rogerson A, McCorquodale D, Esiobu N. Prevalence and survival of microorganisms in shoreline interstitial waters: a search for indicators of health risks. EPA 828830. U.S. EPA; Washington, DC: 2003. [Google Scholar]
- Sinigalliano CD, Fleisher JM, Gidley ML, Solo-Gabriele HM, Shibata T, Plano L, Elmir SM, Wang JD, Wanless D, Bartowiak J, Boiteau R, Withum K, Abdelzaher A, He G, Ortega C, Zhu X, Wright M, Kish J, Hollenbeck J, Backer LC, Fleming LE. Traditional and molecular analyses for fecal indicator bacteria in non-point source subtropical recreational marine waters. Water Research. 2010;44(13):3763–3772. doi: 10.1016/j.watres.2010.04.026. doi:10.1016/j.watres.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Abdelzaher AM, Phillips M, Hernandez R, Solo-Gabriele HM, Kish J, Scorzetti G, Fell JW, Diaz MR, Scott TM, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano CD, Gidley ML, Wanless D, Agar A, Lui J, Stewart JR, Plano LRW, Fleming LE. Indicator microbes correlate with pathogenic bacteria, yeast, and helminthes in sand at a subtropical recreational beach site. Journal of Applied Microbiology. 2011;110:1571–1583. doi: 10.1111/j.1365-2672.2011.05013.x. doi:10.1111/j.1365-2672.2011.05013.x. [DOI] [PubMed] [Google Scholar]
- Shibata T, Solo-Gabriele HM, Fleming LE, Elmir SM. Monitoring marine recreational water quality using multiple microbial indicators in an urban tropical environment. Water Research. 2004;38:3119–3131. doi: 10.1016/j.watres.2004.04.044. doi:10.1016/j.watres.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soge OO, Meschke JS, No DB, Roberts MC. Characterization of methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative Staphylococcus spp. isolated from US West Coast public marine beaches. Journal of Antimicrobial Chemotherapy. 2009;64(6):1148–1155. doi: 10.1093/jac/dkp368. doi:10.1093/jac/dkp368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solo-Gabriele HM, Wolfert MA, Desmarais TR, Palmer CJ. Sources of Escherichia coli in a Coastal Subtropical Environment. Applied and Environmental Microbiology. 2000;66(1):230–237. doi: 10.1128/aem.66.1.230-237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice AD, Pombo D, Hui J, Kurano M, Bankowski MJ, Seifried SE. Quantitation of Staphylococcus aureus in seawater using CHROMagar SA. Hawaii Medical Journal. 2010;69(1):8–12. [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Ambient water quality criteria for bacteria. EPA A440/5-84-002. U.S. EPA; Washington, DC: 1986. [Google Scholar]
- U.S. Environmental Protection Agency . Enterococci in Water by Membrane Filtration Using membrane-Enterococcus Indoxyl-fi-D-Glucoside Agar (mEI) EPA-821-R-06-009. U.S. EPA; Washington, DC: 2006. [Google Scholar]
- Wang JD, Solo-Gabriele HM, Abdelzaher AM, Fleming LE. Estimation of enterococcus input from bathers and animals on a recreational beach using camera images. Marine Pollution Bulletin. 2010;60(8):1270–1278. doi: 10.1016/j.marpolbul.2010.03.016. doi:10.1016/j.marpolbul.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman RL, Nevers MB. Foreshore sand as a source of Escherichia coli in nearshore water of a Lake Michigan beach. Applied and Environmental Microbiology. 2003;69(9):5555–5562. doi: 10.1128/AEM.69.9.5555-5562.2003. doi:10.1128/AEM.69.9.5555-5562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman RL, Nevers MB, Korinek GC, Byappanahalli MN. Solar and temporal effects on Escherichia coli concentration at a Lake Michigan swimming beach. Applied and Environmental Microbiology. 2004;70(7):4276–4285. doi: 10.1128/AEM.70.7.4276-4285.2004. doi:10.1128/AEM.70.7.4276-4285.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ME, Solo-Gabriele HM, Elmir S, Fleming LE. Microbial load from animal feces at a recreational beach. Marine Pollution Bulletin. 2009;58(11):1649–1656. doi: 10.1016/j.marpolbul.2009.07.003. doi:10.1016/j.marpolbul.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ME, Abdelzaher AM, Solo-Gabriele HM, Elmir S, Fleming LE. The inter-tidal zone is the pathway of input of enterococci to a subtropical recreational marine beach. Water Science and Technology. 2011;63(3):542–549. doi: 10.2166/wst.2011.255. doi:10.2166/wst.2011.255. [DOI] [PubMed] [Google Scholar]
- Wymer LJ, Brenner KP, Martinson JW, Stutts WR, Schaub SA, Dufour AP. Result from a Study on Microbiological Monitoring in Recreational Waters. EPA600/R-04/023. U.S. EPA; Washington, DC: 2005. The EMPACT Beaches Project. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.