Abstract
Diabetes mellitus is the leading cause of end stage renal disease and is responsible for more than 40% of all cases in the United States. Current therapy directed at delaying the progression of diabetic nephropathy includes intensive glycemic and optimal blood pressure control, proteinuria/albuminuria reduction, interruption of the renin-angiotensin-aldosterone system through the use of angiotensin converting enzyme inhibitors and angiotensin type-1 receptor blockers, along with dietary modification and cholesterol lowering agents. However, the renal protection provided by these therapeutic modalities is incomplete. More effective approaches are urgently needed. This review highlights the available standard therapeutic approaches to manage progressive diabetic nephropathy, including markers for early diagnosis of diabetic nephropathy. Furthermore, we will discuss emerging strategies such as PPAR-gamma agonists, Endothelin blockers, vitamin D activation and inflammation modulation. Finally, we will summarize the recommendations of these interventions for the primary care practitioner.
KEY WORDS: diabetes mellitus, nephropathy, disease management, measurement, therapeutic strategies
INTRODUCTION
A large source of morbidity and premature mortality in diabetes mellitus (DM) relates to the development of late complications affecting multiple organ systems. One of these complications, diabetic nephropathy (DN), has become the leading cause of end stage renal disease (ESRD) in the United States.1
DN is defined by persistent pathological albuminuria; 300 mg of urinary albumin excretion in a 24-hour collection and abnormal renal function as recognized by an abnormal plasma creatinine (PCr) level, glomerular filtration rate (GFR) or calculated creatinine clearance.2
Although both DM type-1(DMT1) and type-2 (DMT2) lead to DN, the course of DN has been better identified in DMT1. The earliest renal manifestation of diabetes is glomerular hyperfiltration, followed by a decline in GFR and increased albuminuria usually 5 or more years after the onset of DM. Finally, overt albuminuria develops and GFR continues to fall often, in association with the development of hypertension.3
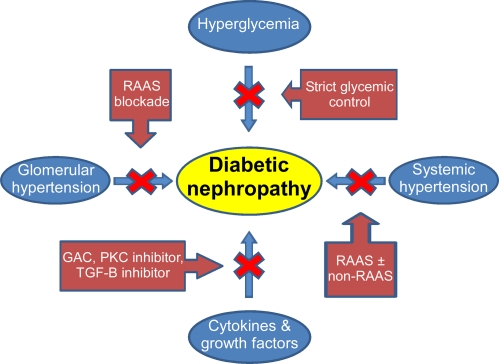
The exact pathogenesis of DN is complex and not completely understood. Among the pathogenic factors are: hyperglycemia, increased systemic and glomerular pressure, increased activity of the renin-angiotensin-aldosterone-system (RAAS) and stimulation of several cytokines and growth factors by metabolic and hemodynamic factors. Several therapeutic interventions targeting these mechanisms have been developed and implemented with various degrees of success (Fig. 1).
Figure 1.
Pathogenesis of diabetic nephropathy and steps to slow its onset and progression. Abbreviations: RAAS, Renin-Angiotensin-Aldosterone System; GAC, Sulodexide; PKC, Protein Kinase C; TGF-β, Transforming growth Factor-beta.
Diabetic Nephropathy Markers
The early diagnosis of DN is imperative for adequate management of the disease. For years, measurement of urine albumin has been the mainstay for the detection of early DN.4 Although early reports indicated that as many as 80% of patients with elevated rates of microalbuminuria would progress to develop overt DN, recent studies suggest that the rate of progression from microalbuminuria to nephropathy is lower, in the range of 25-30%.5,6 Perhaps more worrisome is the realization that some diabetic patients develop DN in the absence of microalbuminuria.7 In newly diagnosed diabetics, Zerbini et al.8 found that GFR began to decrease prior to the appearance of microalbuminuria. Thus, urinary albumin lacks both sensitivity and specificity to detect early DN. Measurements of cytokines such as connective tissue growth factor (CTGF), transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α) and urinary podocytes have emerged as potential markers of progressive DN.9–13 These markers merit ongoing study but are not yet available for clinical practice.
Additional promising markers are kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL). In a recent cohort study,14 all patients with DMT1 and DMT2 had elevated urinary and serum levels of NGAL compared with matched control groups. More significantly, high levels of NGAL preceded the development of pathological albuminuria and reached higher levels in patients with overt DN. Additional studies demonstrated that NGAL represents a novel and independent risk predictor for progression and severity of renal disease. Furthermore, a recent study by Vaidya et al. showed that decreased urinary levels of KIM-1 and NAGL were associated with microalbuminuria regression in patients with DMT1.15 These data raise the possibility that NGAL may become a useful noninvasive tool for the early detection of incipient nephropathy and for estimating the severity of kidney involvement. However, until further results are available, periodic measurements of microalbuminuria and serum creatinine (for estimated GFR calculations) remain the standard of care for DN screening.
THERAPEUTIC STRATEGIES FOR DN
Current Therapeutic Strategies for Diabetic Nephropathy
Available therapeutic options directed at delaying the progression of DN include intensive blood glucose (BG) control, improved blood pressure (BP) control, interruption of the RAAS using angiotensin-converting enzyme inhibitors (ACEi) and/or angiotensin type-1 (AT1) receptor blockers (ARBs) along with dietary modification and cholesterol-lowering agents (Table 1).
Table 1.
Available Therapeutic Modalities in DN
| Current therapy | Emerging therapy |
|---|---|
| 1-Intensive glucose control | 1-TZDs/PPAR-gamma agonists |
| a-Medication | 2-ACE-2 |
| b-Pancreatic transplantation | 3-Endothelin blockers |
| 2-Blood Pressure Control | 4-AGEs inhibitors |
| a-Affecting RAAS: | 5-Vitamin D activation |
| i. ACEi | 6-Inflammation modulation |
| ii. ARBs | |
| iii. Renin inhibitors | |
| iv. Aldosterone inhibitors | |
| b-Not affecting RAAS: | |
| i. CCB | |
| ii. Beta blockers | |
| iii. Diuretics | |
| 3-Dyslipidemia and lipid-lowering drugs | |
| 4-Multifactorial intervention |
Intensive Glucose Control
The Diabetes Control and Complications Trial (DCCT) performed more than 15 years ago was a milestone in our approach to delaying the onset and slowing the progression of DN.16 That study of 1441 patients with DMT1 over an 8-year period found that strict glycemic control reduced the incidence of albuminuria by 50% compared with standard therapy. This protective effect persisted for more than a decade after the completion of the trial.17,18 The benefits of intensive glycemic control were not limited to delaying the onset and slowing the progression of DN but extended to decreasing the incidence of the cardiovascular diseases (CVD); the main cause of mortality in these patients.19
Likewise, the United Kingdom Prospective Diabetes Study (UKPDS) was designed as a randomized clinical trial comparing the effects of intensive diabetes treatment with four pharmacological mono-therapies, versus a diet control group on the complications of diabetes in about 4000 patients with DMT2 followed over 10 years.20,21 It showed that intensive BG control by either sulphonylureas or insulin reduced the risk of microvascular complications.22 Each 1% reduction in HbA1c was associated with 21% reduction in the risk of any diabetes-related endpoints and 37% decrease in microvascular complications.20,21
More recent randomized controlled studies in patients with DMT2 have yielded mixed results. The ADVANCE (Action in Diabetes and Vascular Disease)23 trial showed that intensive glycemic control reduced albuminuria, nephropathy and the need for dialysis. Likewise, the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial, showed significantly lower rates of albuminuria (but not of more advanced nephropathy) in the intensive glycemic therapy group.24 Contrary, the VADT (Veterans Affair Diabetic Trial)25 did not show improvements in either nephropathy or retinopathy with intensive glycemic control. The lack of benefit in VADT may be explained by the longer duration of diabetes and the short follow-up time. However, the enthusiasm for strict glycemic control must be tempered by the observations in the ACCORD, ADVANCE and VADT trials that strict glycemic control had either a deleterious or no beneficial impact on major cardiovascular outcomes.
Another strategy in the management of DM is pancreas transplantation. Pancreas transplantation is usually performed in patients who have DMT1, although 6% of recipients are reported to have DMT2. The specific criteria for defining a candidate as having DMT1 or DMT2 are dependent on the transplant institution.26,27 Pancreatic transplantation to achieve normal glycemic control has yielded promising results; reduction in proteinuria28 and histologic improvements in diabetic glomerulopathy.29–31
In summary, intensive glycemic treatment in both DMT1 and DMT2 reduces the risk and progression of early DN. The specific goal for HbA1c should be individualized considering the potential benefits and harms of different levels of HbA1c. A goal HbA1c of 7% appears reasonable with a target level set somewhat higher in older patients.32
Blood Pressure Control
Both systolic and diastolic hypertension markedly accelerate the progression of DN. Normotensive patients with advanced DN show slower progression compared with hypertensive patients.33–42
Non-pharmacologic approaches (dietary modifications especially and increased physical activity) are effective in reducing BP in non-diabetic individuals43 and may have similar benefits for diabetic patients. However, pharmacologic approaches remain the mainstay for controlling BP in patients with DM.44,45
Several randomized controlled trials indicate that multiple antihypertensive agents, often more than three, are commonly required to achieve optimal BP control.35,46–49 The optimal target BP for patients with DN has been long debated.50 Early studies by the UKPDS group20–22 suggesting that each 10 mmHg decrease in SBP is associated with a 13% reduction in microvascular complications, led investigators to believe that lower BP is better. The ADVANCE trial randomized hypertensive diabetic patients to a fixed combination of perindopril and indapamide vs. placebo when added to usual anti-hypertensive care.37 Treatment with active agent was associated with a 5 mmHg reduction in SBP (135 vs. 140 mmHg) and a 14% reduction in mortality. Based on these and other findings, the American Diabetes Association51 and the Joint National Committee 752 recommend a target BP of <130/80 mmHg for patients with diabetes. Several studies have investigated the benefit of even lower BP targets. The recent ACCORD trial failed to show a reduction in cardiovascular events but increased rates of hyperkalemia and renal dysfunction when targeting a SBP < 120 mmHg as compared with <140 mmHg.53 A subgroup analysis of 6400 patients with diabetes in the INVEST study54 and a cross-sectional analysis of patients in the Swedish National Diabetes Registry55 also failed to show a reduction in mortality in patients with SBP < 130 vs. 130–139 mmHg. These recent observations do not support BP goals of <130/80 and even bring into question the need to reduce SBP below 140 mmHg.
Summary: The ADA and JNC 7 recommend reducing BP to <130/80 in patients with DM. However, given the difficulty in achieving this goal and the lack of strong evidence of benefit from reducing SBP to <130 vs. 140 mmHg, we believe that physicians should strive to achieve a target SBP of less than 140 mmHg in diabetic patients. A target of <130/80 mmHg can be pursued in younger patients, patients who tolerate their antihypertensive regimens well, patients with significant proteinuria (over 500 mg/day) and patients at particularly high risk of stroke.
Agents Affecting Renin-angiotensin-aldosterone-system
ACEi/ ARBs
Activation of the RAAS system plays a crucial role in the pathophysiology of DN. Several trials have established the efficacy of ACEi and ARBs in reducing the progression of DN.56,57 The beneficial effects of RAAS blockade go beyond a reduction in systemic BP and include a reduction of intraglomerular pressure and proteinuria, thereby slowing progression of CKD.56,57
A head to head comparison of ACEi (enalapril) and ARB (telmisartan) in patients with DMT2, hypertension and early-stage DN, did not reveal any differences between the two agents with respect to BP control, proteinuria or changes in GFR.58 Thus, ACEi and ARBs appear to be equally effective in slowing the progression of DN. Other factors, such as cost or side-effects, should dictate the selection of either class of agents.
Two issues relating to the management of DN with ACEi and/or ARBs remain to be clarified; the roles of high-dose monotherapy and those of the combination of ACEi/ARBs. Higher doses of valsartan59 (320–640 vs. 160 mg/day) or candesartan60 (128 vs. 16 mg/day) produced significantly greater reductions in albuminuria as compared to conventional doses independent of BP effect. Although these results are encouraging, the long-term effects of high-dose ARB remain uncertain.
A second unresolved issue centers on the efficacy of combined therapy with both ACEi and ARBs in slowing DN. Small trials had reported greater reductions in proteinuria and even slowing of renal dysfunction in patients treated with combined therapy as compared to monotherapy.61 The enthusiasm for this approach was dampened by the ONTARGET (Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) in which the combination therapy arm showed worse renal outcomes (doubling of the SCr and the need for dialysis), as compared with either of the monotherapy arms.62 Thus, unless new data emerge supporting the use of dual therapy with ACEi and ARBs, monotherapy with either agent will remain the first-line for patients with DN. Even so, combination ACEi and ARB seems reasonable for selected patients, such as those with no history of hypotension or major cardiac disease, who have persistent proteinuria on ACEi or ARB monotherapy. In such circumstances, referral to a nephrologist is appropriate.
Renin Inhibitors
The benefit of blocking the RAAS with ACEi and ARBs in a variety of kidney diseases, including DN, is now well established.63–70 However, such treatment does not completely abrogate the progression of kidney disease.69–71 This partial response has been attributed in part to feedback effects, such as angiotensin-escape and aldosterone-escape.72,73
In light of these phenomena, alternative approaches to optimize the RAAS-blockade are being sought. The activity of renin, the rate-limiting-step in the RAAS cascade, is increased when either ACEi or ARBS are used for prolonged periods. Thus, direct inhibition of renin activity has potential advantages over ACEi and ARBs. Not only does renin inhibition lower BP through its action on the RAAS, it has also additional direct actions mediated through a renin receptor.74 Aliskiren, an orally active non-peptidic renin inhibitor, decreases plasma renin activity, although renin concentration is increased.75–77 Therefore, aliskiren may cause more complete RAAS-blockade and reduce the compensatory feedback as compared to ACEi/ARBs. Several short-term studies showed that aliskiren effectively lowers BP in non-diabetic78,79 and diabetic80 patients.
Parving et al.81 performed a prospective randomized study of aliskiren in 599 patients with DMT2, hypertension and proteinuria. They found that dual RAAS-blockade with aliskiren and losartan reduced albuminuria 20% more than losartan alone despite a very small difference in BP81. Thus, renin inhibitors may be effective in delaying the progression of DN. However, better outcome data on renal function itself, rather than surrogate end points as proteinuria, are needed.
Aldosterone Antagonists
The mineralocorticoid receptor antagonists’ spironolactone and eplerenone reduce proteinuria when administered alone with additional antiproteinuric benefits when given with ACEi or ARBs in patients with DMT1 and DMT2.82–93 This additive antiproteinuric benefit is independent of further BP reduction.86,87,93 The lack of sexual side-effects of eplerenone makes it a good alternative to spironolactone, particularly in men. These results, if supported by long-term outcomes, indicate that mineralocorticoid antagonists may be a valuable addition to our armamentarium to delay progression of DN with close monitoring of serum potassium and creatinine.
Agents that do not Interrupt Renin-Angiotensin-Aldosterone-System
As discussed, agents which interrupt the RAAS are recommended as first line treatment of hypertension in individuals with diabetes. However, the reduction in BP per se, rather than the choice of specific BP agents, is of paramount importance in delaying the progression of renal disease in DN.44 Therefore, patients who are intolerant to ACEi and/or ARB, or who need further reduction of BP beyond what can be achieved with these drugs, will require treatment with other classes of antihypertensive agents.
Calcium Channel Blockers (CCB)
Parving and colleagues reviewed studies published from 1989–1996 comparing CCB to ACEi in patients with incipient and overt DN.94 Eight studies examined a total of 160 (DMT1) and 54 (DMT2) patients treated with either dihydropyridine calcium-channel-blockers (DHPCCB) or ACEi.95–102 All patients had microalbuminuric DN. While both groups demonstrated identical reductions in the mean arterial BP and beneficial effects on GFR, the groups receiving ACEi showed superior reductions in microalbuminuria. Similar results were observed in evaluating another eleven studies of patients with macroalbuminuric DN with a total of 177 patients (DMT1) and 76 patients (DMT2).103–113 However, the ACCOMPLISH trial showed that the combination of amlodipine, a DHP CCB, with benazepril (ACEi), was superior to benazepril plus thiazide diuretic in reducing cardiovascular events114 and the progression of kidney disease115 in diabetes.
Data obtained from animal and human studies confirm that non-dihydropyridine calcium-channel-blockers (NDHP CCBs) can reduce proteinuria and slow the progression of kidney disease in diabetics.116–119 Bakris and colleagues showed that NDHP CCBs were comparable to ACEi and superior to beta-blockers in reducing proteinuria and delaying progression of renal disease in patients with DN.120 This superiority of NDHP CCBs over beta-blockers was independent of BP control.121 These findings have been confirmed by other investigators.122,123
Beta-Blockers and Diuretics
Relatively few studies have examined the effects of beta-blockers and diuretics in patients with DN. Nielson and colleagues showed that though ACEi were superior to beta-blockers in reducing proteinuria; both drugs equally reduced the decline in kidney function.124 Likewise, the UKPDS study showed that ACEi and beta-blockers were equally effective in reducing both macrovascular and microvascular complications in DMT2.125 Less is known regarding the effects of diuretics in DN. In one study, the diuretic agent indapamide was equivalent to enalapril in reducing microalbuminuria in hypertensive diabetic patients.126 Although the GUARD study127 showed that a combination of ACEi with hydrochlorothiazide resulted in a greater reduction in albuminuria than did the combination of ACEi and DHP CCB, the larger ACCOMPLISH study115 showed that the same combination of ACEi/hydrochlorothiazide was less effective than the ACEi/CCB combination in reducing the decline in GFR in high risk, mainly diabetic, hypertensive patients. These studies suggest that while beta-blockers and diuretics may be helpful in the management of DN, they should probably be used in combination with ACEi or ARBs.
Dyslipidemia and Lipid-Lowering Drugs
CVD is the leading cause of death in patients with advanced CKD.128 Both DMT1129 and DMT2130 are associated with dyslipidemia; mainly hypertriglyceridemia. Several studies showed marked cardiovascular benefits for treating dyslipidemia in patients with DM.131–138 Unfortunately, most of these studies excluded patients with CKD. Thus, direct data showing a benefit of lipid-lowering therapy on CVD in patients with CKD are limited. Post hoc analysis of the “Heart Protection Study” (HPS)134 showed marginally significant reductions in the relative-risk of cardiovascular events in diabetic patients with CKD. Similar results were obtained from a post hoc analysis of the CARE (Cholesterol and Recurrent Events) study.139 Although animal studies indicate that statins may slow the progression of DN; evidence in human trials is lacking.
Multifactorial Intervention
A recent Danish study140 examined the impact of a multifactorial intervention on the risk of cardiac and renal outcomes in patients with DMT2 and microalbuminuria. Patients were treated with either conventional therapy or an intensive regimen consisting of tight glucose control, RAAS-blockers, aspirin, and lipid-lowering agents. The mean treatment period was 7.8 years followed by a mean of 5.5 year observation period. During the entire 13.3 years follow-up, 30% vs. 50% died in the intensive-therapy compared to the conventional therapy groups with an absolute risk-reduction of 20% death. Additionally, DN developed in 20 vs. 37 patients in the intensive-therapy group compared to the conventional therapy group (relative-risk: 0.44; 95% CI: 0.25-0.77; p = 0.004), with one patient in the intensive therapy group progressing to ESRD requiring dialysis as compared with six patients in the conventional therapy group (p = 0.04). This study highlights the need for targeting multiple pathways in order to reduce the burden of diabetic complications.
Early Referral to Nephrologist
Primary care providers are on the front line in our battle against DN. The availability of several guidelines from professional societies, such as the American Diabetes Association, National Kidney Foundation and the American Society of Hypertension has helped a great deal in this fight. The coordinated effort among the different specialties; primary care provider, endocrinologist and nephrologist remains crucial for optimal patient outcomes. Early referral to nephrologists has been found to be associated with decreased rates of decline in GFR141 and mortality.142 In spite of that, it is still not uncommon for CKD patients to be seen by a nephrologist for the first time only one month before starting dialysis.143,144 Similar trends were also noted in Europe, Australia, New Zealand and Canada. Ghossein et al. suggested that the greatest benefit to patients occurs when referral to nephrologist is initiated before the plasma creatinine concentration exceeds 1.5 to 2 mg/dL or GFR is less than 60 mL/min per 1.73 m2.145
Summary: Strategies for the early identification and treatment of DN have evolved based on new clinical trials. The current evidenced-based approaches place emphasis on individualizing optimal glycemic control, BP control with RAAS-blockade as first-line agents, and optimization of traditional cardiovascular risk factors. Successful outcomes can be achieved with treatment that incorporates the aforementioned multifaceted interventions to slow the progression of diabetic renal disease and its associated complications (Table 2).
Table 2.
Antihypertensive Agents and Proteinuria Effect
| Agents affecting RAAS | Proteinuric Effect |
| ACEI/ARB | Equally effective in reducing proteinuria and slowing DN progression56–58 |
| Direct renin inhibit (aliskiren) | Comparable anti-proteinuria reduction to ACEI/ARB81 |
| ACEI/ARB+direct renin inhibitor | Lower proteinuria by 20% more than monotherapy81 |
| Aldosterone antagonists | Reduce proteinuria effectively82–93 |
| ACEI/ARB+Aldosterone antagonists | Additive anti-proteinuric effect independent of BP control86,87,93 |
| Agents not affecting RAAS | Proteinuric Effect |
| DHP CCB | Very effective BP control but inferior to RAAS blockade in reducing proteinuria95–102 |
| NDHP CCB | Can reduce proteinuria. Some studies showing comparable anti-proteinuric effect to ACEI/ARB116–119 |
| β-Blockers and diuretics | Inferior to RAAS and NDHP in reducing proteinuria but comparable in reducing decline in GFR124,126 |
| Combination of RAAS±non-RAAS agents | Proteinuric Effect |
| ACEI+Diuretics | Greater proteinuric reduction but lesser BP reduction than ACEI+DHP CCB127 |
| ACEI+DHP CCB | Better BP control but inferior in proteinuric control than ACEi+diuretics127 |
EMERGING THERAPEUTIC AGENTS FOR DIABETIC NEPHROPATHY
Thiazolidinediones / PPAR-Gamma Agonists
Thiazolidinediones (TZDs) exert their hypoglycemic activity by reducing insulin resistance.146 Beyond their hypoglycemic actions, PPARЎ agonists exert a number of beneficial effects in diabetes including improvement in endothelial function,147,148 reduction in pro-atherogenic inflammatory markers149 and angiotensin-I and –II,150 down regulation of AT1 mRNA and protein in vascular smooth muscle cells,151,152 decrease in urine endothelin-1 secretion,153 attenuated lipid accumulation and its related injury in mesangial cells,154,155 and inhibition of glomerular and tubular cell proliferation156,157. Several animal158–163 and human studies153,164–175 using various TZDs have demonstrated a reduction in proteinuria and BP. Unfortunately; most of these studies were of short duration averaging 1–9 months in the animal studies158–163 and 3–12 months in human studies.153,164–175
The use of TZDs has become less frequent due to higher rates of cardiovascular complications. A recent observational, retrospective, inception cohort of 227,571 Medicare patients who were treated with rosiglitazone or pioglitazone for a 12-month period and followed for up to 3 years after initiation of therapy showed rosiglitazone was associated with a higher risk of cardiovascular complications and all-cause mortality in patients 65 years or older compared with pioglitazone.176 Rosiglitazone is currently restricted by the FDA to patients already benefiting from rosiglitazone or patients who cannot be controlled with other medications and are unwilling to use pioglitazone (http://www.fda.gov/Drugs/DrugSafety/ucm255005.htm; accession date: September 21, 2011).
Angiotensin-Converting Enzyme-2 (ACE2)
ACE2 is a recently discovered homologue of ACE. ACE2 is abundantly expressed in the kidney where it may counter-balance the classical RAAS.177 Whereas ACE promotes formation of angiotensin-II, ACE2 metabolizes angiotensin-II to angiotensin 1–7, and angiotensin-I to angiotensin 1-9.178 Angiotensin 1–7 has vasodilatory effects on the kidney,179 suggesting a possible protective role of ACE2 in kidney diseases.
Few studies have addressed the potential role of ACE2 in the pathogenesis of DN. Ye et al. showed that while ACE expression was increased in diabetic mouse glomeruli, the glomerular immunostaining for ACE2 was attenuated.180 Furthermore, the administration of MLN-4760, a specific ACE2-inhibitor, resulted in worsening albuminuria in a diabetic mouse model.180 These findings suggest a protective role for ACE2 in early DN and that maneuvers aimed at upregulation of ACE2 activity may have a therapeutic potential.
Endothelin (ET) Blockers
First described by Yanagisawa et al.181 more than 20 years ago, the ET system is a family of 21-amino acid peptides with powerful vasoconstrictor and pressor properties. The renal ET system is activated in patients with DN as well as in rat models of diabetes-induced damage182–184 leading to exacerbation of proteinuria, glomerular capillary hypertension, an increase in glomerular permeability, and excessive protein filtration.185 Moreover, it was shown that altered ET-1 production may contribute to hypertension.186,187
Several human studies demonstrated a correlation between plasma or urinary levels of ET-1 and markers of DN, such as hyperfiltration, mesangial expansion, macro- and/or microalbuminuria, and uremia.188–192 In addition, interventional studies using endothelin-receptor-blockers have yielded encouraging results193–198 in rodent models of DMT1195,198 or DMT2.196,197 Honing et al.199 reported reversal of proteinuria in 10 patients with DMT1 after treatment with the ET-antagonist; atrasentan for 12 weeks. The antiproteinuric effect of ET-antagonism was confirmed in a study of avosentan.200 When the avosentan-treated patients were followed up after 3- and 6-months in the ASCEND (Avosentan on Doubling of Serum Creatinine, End-stage Renal Disease and Death in Diabetic Nephropathy) phase III clinical trial, substantial reductions in albuminuria were seen.201 Unfortunately, drug-related adverse events including fluid retention, led to early termination of the ASCEND trial.201 Much more work will be needed to determine the safety and effectiveness of ET antagonists in preventing or treating DN in humans.
Advanced Glycation Endproducts (AGEs) Inhibitors
AGEs are a heterogeneous group of compounds that can alter the structure and function of tissue proteins and stimulate cellular responses associated with diabetic complications. AGEs are excreted by the kidneys and tend to increase in the setting of renal impairment.202 Receptors for AGE (RAGE) specifically recognize proteins that bound AGE, and enable macrophages to stimulate the removal and replacement of senescent macromolecules that have been cross-linked and denatured by long-term exposure to glucose.203 This AGE-RAGE interaction may play a role in the pathologic process associated with the AGE accumulation. Thus, interventions aiming at inhibiting the formation of AGE, reversing the cross-linking of already formed AGE, and/or interfering with AGE/RAGE interactions, may have beneficial effects in delaying, preventing, or reversing long-term diabetic complications.
Preclinical studies evaluated the effect of the AGE inhibitor; Pimagedine (PG) on renal function in diabetic animals yield promising results.204 Therefore, two major multicenter clinical trials were initiated to evaluate the use of PG in patients with DMT2204 (ACTION II) and DMT1205 (ACTION I). Unfortunately, due to PG side-effects, its clinical development has been suspended.206
Other AGE inhibitors (Pyridoxamine) and cross-link breaker (Alagebrium) have been studied. Both agents are well-tolerated in man,207–209 but their efficacy in preventing or slowing of DN remains to be established.
Selective Vitamin D Activation
Paricalcitol, a vitamin D receptor activator, reduced albuminuria and slowed progression of kidney disease in mice, so de Zeeuw et al., assessed the role of paricalcitol in reducing albuminuria in 281 patients with DN.210 They found that paricalcitol, when given in a dose of 2 μg/day, reduced albuminuria by −18% to −28%, with comparable side effects to the placebo arm of the study. This was only a short 24-week study leaving questions about the long-term cost of the drug as well as the safety of using paricalcitol which may aggravate adynamic bone disease.
Inflammation Modulation
Recently, an orally available synthetic triterpenoid, Bardoxolone methyl, has shown promising results in DN. Bardoxolone methyl exerts potent anti-oxidant and anti-inflammatory activity via induction of the Nrf2 transcription factor. A Phase 2 trial of bardoloxone methyl treatment for 8 weeks in 20 patients with moderate-severe CKD and DMT2 demonstrated improved renal function as evidenced by increased eGFR paralleled by a significant reduction in serum creatinine and BUN.211 A subsequent trial examined the effect of bardoloxone methyl (25–150 mg/d) administered for 52 weeks to 227 patients with moderate to severe CKD and DMT2.212 Bardoloxone methyl produced a significant increase in GFR of 8–11 ml/min/1.73 m2. The improvement in GFR was evident by 8–12 weeks of treatment and persisted for the entire 52-week treatment period. Likewise, bardoloxone treatment reduced the proportion of patients who experienced a 25% fall in GFR from 13% in the placebo group to only 2% in treatment group. Although hard outcomes, such as dialysis dependency and death, were not evaluated, these results are very encouraging and justify further study of bardoxolone methyl and related compounds.
Summary: Although none of these recent interventions are ready for use in patients, it is encouraging that active research is ongoing to prevent DN. It is not clear whether these emerging strategies will be more effective than, or additive to, current therapeutic approaches in improving the renal and CVD outcomes in diabetes.
SUMMARY AND CONCLUSIONS
Primary care physicians (PCP) remain the front line defenders in our fight against diabetic nephropathy. There are several important roles that the PCP can play before consulting a nephrologist. First, the PCP should strive for optimal glycemic and BP control as a means to prevent or delay the development of diabetic nephropathy. Second, the PCP should periodically screen patients with diabetes for signs of early renal involvement. The ADA213 currently recommends annual screening for the presence of microalbuminuria in type 1 diabetic patients with diabetes duration of 5 years and in all type 2 diabetic patients, starting at diagnosis and during pregnancy. Due to the inadequacies of microalbuminuria, it is also recommended that the serum creatinine should be measured at least annually for the estimation of glomerular filtration rate (eGFR) in all adults with diabetes regardless of the degree of urine albumin excretion. Third, once nephropathy is detected, a team approach should be adopted between the PCP, nephrologist, dietitian and endocrinologist to ensure optimal management focused on multiple risk factor interventions. Optimum individualized glucose control in both DMT1 and DMT2 remains a crucial target for DM therapy. A goal of HbA1c of 7% appears acceptable for most patients. Optimal BP management is associated with reduced microvascular complications. The ADA and JNC 7 recommend reducing BP to <130/80 in patients with DM, which seems a reasonable goal for young patients and for patients who can tolerate this goal well, as well as patients with evidence of microvascular disease. Agents which block the RAAS system have a superior reno-protective effect in patients with DN. Currently; there are tremendous ongoing efforts by laboratory and clinical researchers to gain a better understanding of the pathophysiology of DN, to identify better markers for early diagnosis of the disease, and to develop better therapeutic approaches to combat this devastating disease.
Acknowledgment
This work was supported by NIH Grant DK077444.
Conflict of Interest
None disclosed.
References
- 1.Annual Data Report. Bethesda: The National Institutes of Diabetes and Digestive and Kidney Diseases; 2005. [Google Scholar]
- 2.Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK) Am J Kidney Dis. 2003;42:617–22. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 3.Adler S. Diabetic nephropathy: linking histology, cell biology, and genetics. Kidney Int. 2004;66:2095–106. doi: 10.1111/j.1523-1755.2004.00988.x. [DOI] [PubMed] [Google Scholar]
- 4.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- 5.Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes. 2000;49:1399–408. doi: 10.2337/diabetes.49.9.1399. [DOI] [PubMed] [Google Scholar]
- 6.Caramori ML, Fioretto P, Mauer M. Enhancing the predictive value of urinary albumin for diabetic nephropathy. J Am Soc Nephrol. 2006;17:339–52. doi: 10.1681/ASN.2005101075. [DOI] [PubMed] [Google Scholar]
- 7.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52:1036–40. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 8.Zerbini G, Bonfanti R, Meschi F, et al. Persistent renal hypertrophy and faster decline of glomerular filtration rate precede the development of microalbuminuria in type 1 diabetes. Diabetes. 2006;55:2620–5. doi: 10.2337/db06-0592. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y, Aten J, Bende RJ, et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53:853–61. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TQ, Tarnow L, Andersen S, et al. Urinary connective tissue growth factor excretion correlates with clinical markers of renal disease in a large population of type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29:83–8. doi: 10.2337/diacare.29.1.83. [DOI] [PubMed] [Google Scholar]
- 11.Langham RG, Kelly DJ, Gow RM, et al. Transforming growth factor-beta in human diabetic nephropathy: effects of ACE inhibition. Diabetes Care. 2006;29:2670–5. doi: 10.2337/dc06-0911. [DOI] [PubMed] [Google Scholar]
- 12.Kalantarinia K, Awad AS, Siragy HM. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 2003;64:1208–13. doi: 10.1046/j.1523-1755.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Ushiyama C, Suzuki S, et al. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15:1379–83. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 14.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–44. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaidya VS, Niewczas MA, Ficociello LH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Int. 2011;79:464–70. doi: 10.1038/ki.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. [DOI] [PubMed]
- 17.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–389. [DOI] [PMC free article] [PubMed]
- 18.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. [DOI] [PMC free article] [PubMed]
- 19.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 22.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed]
- 23.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 24.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–30. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 26.Kelly WD, Lillehei RC, Merkel FK, et al. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61:827–37. [PubMed] [Google Scholar]
- 27.Gruessner AC, Sutherland DE. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of October 2002. Clin Transplant. 2002;2002:41–77. [PubMed] [Google Scholar]
- 28.Coppelli A, Giannarelli R, Vistoli F, et al. The beneficial effects of pancreas transplant alone on diabetic nephropathy. Diabetes Care. 2005;28:1366–70. doi: 10.2337/diacare.28.6.1366. [DOI] [PubMed] [Google Scholar]
- 29.Bilous RW, Mauer SM, Sutherland DE, et al. The effects of pancreas transplantation on the glomerular structure of renal allografts in patients with insulin-dependent diabetes. N Engl J Med. 1989;321:80–5. doi: 10.1056/NEJM198907133210204. [DOI] [PubMed] [Google Scholar]
- 30.Fioretto P, Mauer SM, Bilous RW, et al. Effects of pancreas transplantation on glomerular structure in insulin-dependent diabetic patients with their own kidneys. Lancet. 1993;342:1193–6. doi: 10.1016/0140-6736(93)92183-t. [DOI] [PubMed] [Google Scholar]
- 31.Fioretto P, Steffes MW, Sutherland DE, et al. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 32.Huang ES, Zhang Q, Gandra N, et al. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann Intern Med. 2008;149:11–9. doi: 10.7326/0003-4819-149-1-200807010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mogensen CE. Long-term antihypertensive treatment inhibiting progression of diabetic nephropathy. Br Med J (Clin Res Ed) 1982;285:685–8. doi: 10.1136/bmj.285.6343.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrier RW, Estacio RO, Esler A, et al. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–97. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 35.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–713. [PMC free article] [PubMed]
- 36.Cost effectiveness analysis of improved blood pressure control in hypertensive patients with type 2 diabetes: UKPDS 40. UK Prospective Diabetes Study Group. BMJ. 1998;317:720–726. [PMC free article] [PubMed]
- 37.Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–40. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 38.Galan BE, Perkovic V, Ninomiya T, et al. Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol. 2009;20:883–92. doi: 10.1681/ASN.2008070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 40.Standards of medical care in diabetes. Diabetes Care. 2005;28(Suppl 1):S4-S36. [PubMed]
- 41.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1-290. [PubMed]
- 42.Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010. [DOI] [PMC free article] [PubMed]
- 43.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 44.Mogensen CE, Keane WF, Bennett PH, et al. Prevention of diabetic renal disease with special reference to microalbuminuria. Lancet. 1995;346:1080–4. doi: 10.1016/s0140-6736(95)91747-0. [DOI] [PubMed] [Google Scholar]
- 45.Kasiske BL, Kalil RS, Ma JZ, et al. Effect of antihypertensive therapy on the kidney in patients with diabetes: a meta-regression analysis. Ann Intern Med. 1993;118:129–38. doi: 10.7326/0003-4819-118-2-199301150-00009. [DOI] [PubMed] [Google Scholar]
- 46.Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) trial. Diabetologia. 1996;39:1646–54. doi: 10.1007/s001250050629. [DOI] [PubMed] [Google Scholar]
- 47.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 48.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control and the progression of chronic renal disease. N Engl J Med. 1994;330:877–84. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 49.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–31. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 50.Nilsson PM, Cederholm J. Diabetes, hypertension, and outcome studies: overview 2010. Diabetes Care. 2011;34(Suppl 2):S109–13. doi: 10.2337/dc11-s204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arauz-Pacheco C, Parrott MA, Raskin P. Treatment of hypertension in adults with diabetes. Diabetes Care. 2003;26(Suppl 1):S80–2. doi: 10.2337/diacare.26.2007.s80. [DOI] [PubMed] [Google Scholar]
- 52.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 53.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–8. doi: 10.1001/jama.2010.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cederholm J, Gudbjornsdottir S, Eliasson B, et al. Systolic blood pressure and risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish national diabetes register. J Hypertens. 2010;28:2026–35. doi: 10.1097/HJH.0b013e32833c8b75. [DOI] [PubMed] [Google Scholar]
- 56.Dunn MJ. Prostaglandins, angiotension II, and proteinuria. Nephron. 1990;55(Suppl 1):30–7. doi: 10.1159/000186032. [DOI] [PubMed] [Google Scholar]
- 57.Melchior WR, Bindlish V, Jaber LA. Angiotensin-converting enzyme inhibitors in diabetic nephropathy. Ann Pharmacother. 1993;27:344–50. doi: 10.1177/106002809302700318. [DOI] [PubMed] [Google Scholar]
- 58.Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–61. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 59.Hollenberg NK, Parving HH, Viberti G, et al. Albuminuria response to very high-dose valsartan in type 2 diabetes mellitus. J Hypertens. 2007;25:1921–6. doi: 10.1097/HJH.0b013e328277596e. [DOI] [PubMed] [Google Scholar]
- 60.Burgess E, Muirhead N, Rene de Cotret P, et al. Supramaximal dose of candesartan in proteinuric renal disease. J Am Soc Nephrol. 2009;20:893–900. doi: 10.1681/ASN.2008040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kunz R, Friedrich C, Wolbers M, et al. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]
- 62.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–53. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 63.Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet. 1997;349:1787–1792. [PubMed]
- 64.Ravid M, Brosh D, Levi Z, et al. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;128:982–8. doi: 10.7326/0003-4819-128-12_part_1-199806150-00004. [DOI] [PubMed] [Google Scholar]
- 65.Estacio RO, Jeffers BW, Gifford N, et al. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23(Suppl 2):B54–64. [PubMed] [Google Scholar]
- 66.Ravid M, Savin H, Jutrin I, et al. Long-term stabilization of angiotensin-converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Intern Med. 1993;118:577–81. doi: 10.7326/0003-4819-118-8-199304150-00001. [DOI] [PubMed] [Google Scholar]
- 67.Andersen S, Tarnow L, Rossing P, et al. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int. 2000;57:601–6. doi: 10.1046/j.1523-1755.2000.00880.x. [DOI] [PubMed] [Google Scholar]
- 68.Brenner BM, Cooper ME, Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 69.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 70.Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 71.Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 72.Meiracker AH, AJ Man in 't Veld, Admiraal PJ, et al. Partial escape of angiotensin converting enzyme (ACE) inhibition during prolonged ACE inhibitor treatment: does it exist and does it affect the antihypertensive response? J Hypertens. 1992;10:803–12. [PubMed] [Google Scholar]
- 73.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–92. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 74.Ingelfinger JR. Aliskiren and dual therapy in type 2 diabetes mellitus. N Engl J Med. 2008;358:2503–5. doi: 10.1056/NEJMe0803375. [DOI] [PubMed] [Google Scholar]
- 75.Nussberger J, Wuerzner G, Jensen C, et al. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39:E1–8. doi: 10.1161/hy0102.102293. [DOI] [PubMed] [Google Scholar]
- 76.Azizi M, Menard J, Bissery A, et al. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol. 2004;15:3126–33. doi: 10.1097/01.ASN.0000146686.35541.29. [DOI] [PubMed] [Google Scholar]
- 77.Azizi M, Menard J, Bissery A, et al. Hormonal and hemodynamic effects of aliskiren and valsartan and their combination in sodium-replete normotensive individuals. Clin J Am Soc Nephrol. 2007;2:947–55. doi: 10.2215/CJN.00360107. [DOI] [PubMed] [Google Scholar]
- 78.Oparil S, Yarows SA, Patel S, et al. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007;370:221–9. doi: 10.1016/S0140-6736(07)61124-6. [DOI] [PubMed] [Google Scholar]
- 79.Pool JL, Schmieder RE, Azizi M, et al. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens. 2007;20:11–20. doi: 10.1016/j.amjhyper.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 80.Uresin Y, Taylor AA, Kilo C, et al. Efficacy and safety of the direct renin inhibitor aliskiren and ramipril alone or in combination in patients with diabetes and hypertension. J Renin Angiotensin Aldosterone Syst. 2007;8:190–8. doi: 10.3317/jraas.2007.028. [DOI] [PubMed] [Google Scholar]
- 81.Parving HH, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–46. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 82.Ustundag A, Tugrul A, Ustundag S, et al. The effects of spironolactone on nephron function in patients with diabetic nephropathy. Ren Fail. 2008;30:982–91. doi: 10.1080/08860220802389342. [DOI] [PubMed] [Google Scholar]
- 83.Saklayen MG, Gyebi LK, Tasosa J, et al. Effects of additive therapy with spironolactone on proteinuria in diabetic patients already on ACE inhibitor or ARB therapy: results of a randomized, placebo-controlled, double-blind, crossover trial. J Investig Med. 2008;56:714–9. doi: 10.2310/JIM.0b013e31816d78e9. [DOI] [PubMed] [Google Scholar]
- 84.Kang YS, Ko GJ, Lee MH, et al. Effect of eplerenone, enalapril and their combination treatment on diabetic nephropathy in type II diabetic rats. Nephrol Dial Transplant. 2009;24:73–84. doi: 10.1093/ndt/gfn448. [DOI] [PubMed] [Google Scholar]
- 85.Epstein M, Buckalew VJ, Martinez F, Altamirano J, Roniker B, Kleiman J, Krause S, Eplerenone 021 Investigators Antiproteinuric efficacy of eplerenone, enalapril, and eplerenone/enalapril combination therapy in diabetic hypertensives with microalbuminuria Am J Hypertens 20021524A11824855 [Google Scholar]
- 86.Rachmani R, Slavachevsky I, Amit M, et al. The effect of spironolactone, cilazapril and their combination on albuminuria in patients with hypertension and diabetic nephropathy is independent of blood pressure reduction: a randomized controlled study. Diabet Med. 2004;21:471–5. doi: 10.1111/j.1464-5491.2004.01194.x. [DOI] [PubMed] [Google Scholar]
- 87.Sato A, Hayashi K, Naruse M, et al. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41:64–8. doi: 10.1161/01.hyp.0000044937.95080.e9. [DOI] [PubMed] [Google Scholar]
- 88.Rossing K, Schjoedt KJ, Smidt UM, et al. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care. 2005;28:2106–12. doi: 10.2337/diacare.28.9.2106. [DOI] [PubMed] [Google Scholar]
- 89.Schjoedt KJ, Rossing K, Juhl TR, et al. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2006;70:536–42. doi: 10.1038/sj.ki.5001580. [DOI] [PubMed] [Google Scholar]
- 90.Ogawa S, Takeuchi K, Mori T, et al. Spironolactone further reduces urinary albumin excretion and plasma B-type natriuretic peptide levels in hypertensive type II diabetes treated with angiotensin-converting enzyme inhibitor. Clin Exp Pharmacol Physiol. 2006;33:477–9. doi: 10.1111/j.1440-1681.2006.04390.x. [DOI] [PubMed] [Google Scholar]
- 91.Schjoedt KJ, Rossing K, Juhl TR, et al. Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int. 2005;68:2829–36. doi: 10.1111/j.1523-1755.2005.00756.x. [DOI] [PubMed] [Google Scholar]
- 92.Bianchi S, Bigazzi R, Campese VM. Antagonists of aldosterone and proteinuria in patients with CKD: an uncontrolled pilot study. Am J Kidney Dis. 2005;46:45–51. doi: 10.1053/j.ajkd.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Mehdi UF, Adams-Huet B, Raskin P, et al. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20:2641–50. doi: 10.1681/ASN.2009070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parving HH, Tarnow L, Rossing P. Renal protection in diabetes: an emerging role for calcium antagonists. J Hypertens Suppl. 1996;14:S21–5. doi: 10.1097/00004872-199606234-00005. [DOI] [PubMed] [Google Scholar]
- 95.Mimran A, Insua A, Ribstein J, et al. Comparative effect of captopril and nifedipine in normotensive patients with incipient diabetic nephropathy. Diabetes Care. 1988;11:850–3. doi: 10.2337/diacare.11.10.850. [DOI] [PubMed] [Google Scholar]
- 96.Baba T, Murabayashi S, Takebe K. Comparison of the renal effects of angiotensin converting enzyme inhibitor and calcium antagonist in hypertensive type 2 (non-insulin-dependent) diabetic patients with microalbuminuria: a randomised controlled trial. Diabetologia. 1989;32:40–4. doi: 10.1007/BF00265402. [DOI] [PubMed] [Google Scholar]
- 97.Comparison between perindopril and nifedipine in hypertensive and normotensive diabetic patients with microalbuminuria. Melbourne Diabetic Nephropathy Study Group. BMJ. 1991;302:210–216. [DOI] [PMC free article] [PubMed]
- 98.Mosconi L, Ruggenenti P, Perna A, et al. Nitrendipine and enalapril improve albuminuria and glomerular filtration rate in non-insulin dependent diabetes. Kidney Int Suppl. 1996;55:S91–3. [PubMed] [Google Scholar]
- 99.Crepaldi G, Carraro A, Brocco E, et al. Hypertension and non-insulin-dependent diabetes. A comparison between an angiotensin-converting enzyme inhibitor and a calcium antagonist. Acta Diabetol. 1995;32:203–8. doi: 10.1007/BF00838494. [DOI] [PubMed] [Google Scholar]
- 100.Velussi M, Brocco E, Frigato F, et al. Effects of cilazapril and amlodipine on kidney function in hypertensive NIDDM patients. Diabetes. 1996;45:216–22. doi: 10.2337/diab.45.2.216. [DOI] [PubMed] [Google Scholar]
- 101.Corradi LLP, Pasotti C, Zoppi A, Preti P, Lazzari P, et al. Effect of amlopidine vs. fosinopril on microalbuminuria in elderly hypertensive patients with type II diabetes. Am J Hypertens. 1996;9:152A. [Google Scholar]
- 102.Jungmann EHT, Malanyn M, Mortasawi N, Schererich J, Usadel KH. Comparative study on renal effects of nitrendipine vs. enalapril in microalbuminuric patients with type 1 diabetes mellitus. Diabetologia. 1992;35:A149. [PubMed] [Google Scholar]
- 103.Stornello M, Valvo EV, Scapellato L. Hemodynamic, renal, and humoral effects of the calcium entry blocker nicardipine and converting enzyme inhibitor captopril in hypertensive type II diabetic patients with nephropathy. J Cardiovasc Pharmacol. 1989;14:851–5. doi: 10.1097/00005344-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Bakris GL. Effects of diltiazem or lisinopril on massive proteinuria associated with diabetes mellitus. Ann Intern Med. 1990;112:707–8. doi: 10.7326/0003-4819-112-9-707. [DOI] [PubMed] [Google Scholar]
- 105.Romero R, Salinas I, Lucas A, et al. Comparative effects of captopril versus nifedipine on proteinuria and renal function of type 2 diabetic patients. Diabetes Res Clin Pract. 1992;17:191–8. doi: 10.1016/0168-8227(92)90094-8. [DOI] [PubMed] [Google Scholar]
- 106.Ferder L, Daccordi H, Martello M, et al. Angiotensin converting enzyme inhibitors versus calcium antagonists in the treatment of diabetic hypertensive patients. Hypertension. 1992;19:II237–42. doi: 10.1161/01.hyp.19.2_suppl.ii237. [DOI] [PubMed] [Google Scholar]
- 107.Bakris GL, Barnhill BW, Sadler R. Treatment of arterial hypertension in diabetic humans: importance of therapeutic selection. Kidney Int. 1992;41:912–9. doi: 10.1038/ki.1992.139. [DOI] [PubMed] [Google Scholar]
- 108.Norgaard K, Jensen T, Christensen P, et al. A comparison of spirapril and isradipine in patients with diabetic nephropathy and hypertension. Blood Press. 1993;2:301–8. doi: 10.3109/08037059309077172. [DOI] [PubMed] [Google Scholar]
- 109.Rossing P, Tarnow L, Boelskifte S, et al. Differences between nisoldipine and lisinopril on glomerular filtration rates and albuminuria in hypertensive IDDM patients with diabetic nephropathy during the first year of treatment. Diabetes. 1997;46:481–7. doi: 10.2337/diab.46.3.481. [DOI] [PubMed] [Google Scholar]
- 110.Corradi LFR, Zoppi A, Lusardi P, Preti P, Lazzari P, et al. Long term effects of ramipril and nitrendipine on albuminuria in diabetic hypertensive patients with impaired renal function. Am J Hypertens. 1996;9:151A. doi: 10.1038/sj.jhh.1000732. [DOI] [PubMed] [Google Scholar]
- 111.Bakris GL CJ, Vicknair N, Leurgans S. Effect of nondihydropyridine calcium antagonists (NDCAs) on progression of nephropathy from noninsulin dependent diabetes (NIDDM) J Am Soc Nephrol. 1995;6:446. [Google Scholar]
- 112.O'Donnell MJ, Rowe B, Lawson N, Horton A, Gide OHV, Barnett AH. Comparative study of lisinopril and nifedipine in treatment of diabetic patients with hypertension and macroproteinuria. Diabetes. 1991;40:505A. [Google Scholar]
- 113.Holdaas H, Hartmann A, Lien MG, Nielsen L, Fauchald T, Jervell J, et al. Lisinopril but not nifedipine reduces urinary albumin excretion in diabetic nephropathy. Kidney Int. 1990;37:239. [Google Scholar]
- 114.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–28. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 115.Bakris GL, Sarafidis PA, Weir MR, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010;375:1173–81. doi: 10.1016/S0140-6736(09)62100-0. [DOI] [PubMed] [Google Scholar]
- 116.Bakris GL, Weir MR, Secic M, et al. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int. 2004;65:1991–2002. doi: 10.1111/j.1523-1755.2004.00620.x. [DOI] [PubMed] [Google Scholar]
- 117.Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int. 1997;51:2–15. doi: 10.1038/ki.1997.2. [DOI] [PubMed] [Google Scholar]
- 118.Kloke HJ, Branten AJ, Huysmans FT, et al. Antihypertensive treatment of patients with proteinuric renal diseases: risks or benefits of calcium channel blockers? Kidney Int. 1998;53:1559–73. doi: 10.1046/j.1523-1755.1998.00912.x. [DOI] [PubMed] [Google Scholar]
- 119.Gansevoort RT, Sluiter WJ, Hemmelder MH, et al. Antiproteinuric effect of blood-pressure-lowering agents: a meta-analysis of comparative trials. Nephrol Dial Transplant. 1995;10:1963–74. [PubMed] [Google Scholar]
- 120.Bakris GL, Copley JB, Vicknair N, et al. Calcium channel blockers versus other antihypertensive therapies on progression of NIDDM associated nephropathy. Kidney Int. 1996;50:1641–50. doi: 10.1038/ki.1996.480. [DOI] [PubMed] [Google Scholar]
- 121.Bakris GL, Mangrum A, Copley JB, et al. Effect of calcium channel or beta-blockade on the progression of diabetic nephropathy in African Americans. Hypertension. 1997;29:744–50. doi: 10.1161/01.hyp.29.3.744. [DOI] [PubMed] [Google Scholar]
- 122.Nakao N, Yoshimura A, Morita H, et al. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003;361:117–24. doi: 10.1016/S0140-6736(03)12229-5. [DOI] [PubMed] [Google Scholar]
- 123.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–10. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 124.Nielsen FS, Rossing P, Gall MA, et al. Long-term effect of lisinopril and atenolol on kidney function in hypertensive NIDDM subjects with diabetic nephropathy. Diabetes. 1997;46:1182–8. doi: 10.2337/diab.46.7.1182. [DOI] [PubMed] [Google Scholar]
- 125.Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ. 1998;317:713–720. [PMC free article] [PubMed]
- 126.Marre M, Puig JG, Kokot F, et al. Equivalence of indapamide SR and enalapril on microalbuminuria reduction in hypertensive patients with type 2 diabetes: the NESTOR Study. J Hypertens. 2004;22:1613–22. doi: 10.1097/01.hjh.0000133733.32125.09. [DOI] [PubMed] [Google Scholar]
- 127.Bakris GL, Toto RD, McCullough PA, et al. Effects of different ACE inhibitor combinations on albuminuria: results of the GUARD study. Kidney Int. 2008;73:1303–9. doi: 10.1038/ki.2008.102. [DOI] [PubMed] [Google Scholar]
- 128.National Kidney Foundation K/DOQI clinical practice guidelines for managing dyslipidemias in chronic kidney disease. Am J Kidney Dis. 2003;41:S1–92. [PubMed] [Google Scholar]
- 129.O'Brien T, Nguyen TT, Zimmerman BR. Hyperlipidemia and diabetes mellitus. Mayo Clin Proc. 1998;73:969–76. doi: 10.4065/73.10.969. [DOI] [PubMed] [Google Scholar]
- 130.Ginsberg HN. REVIEW: efficacy and mechanisms of action of statins in the treatment of diabetic dyslipidemia. J Clin Endocrinol Metab. 2006;91:383–92. doi: 10.1210/jc.2005-2084. [DOI] [PubMed] [Google Scholar]
- 131.Koskinen P, Manttari M, Manninen V, et al. Coronary heart disease incidence in NIDDM patients in the Helsinki Heart Study. Diabetes Care. 1992;15:820–5. doi: 10.2337/diacare.15.7.820. [DOI] [PubMed] [Google Scholar]
- 132.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 133.Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs. usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288:2998–3007. [DOI] [PubMed]
- 134.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [DOI] [PubMed]
- 135.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 136.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 137.Sever PS, Poulter NR, Dahlof B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial–lipid-lowering arm (ASCOT-LLA) Diabetes Care. 2005;28:1151–7. doi: 10.2337/diacare.28.5.1151. [DOI] [PubMed] [Google Scholar]
- 138.Vijan S, Hayward RA. Pharmacologic lipid-lowering therapy in type 2 diabetes mellitus: background paper for the American College of Physicians. Ann Intern Med. 2004;140:650–8. doi: 10.7326/0003-4819-140-8-200404200-00013. [DOI] [PubMed] [Google Scholar]
- 139.Tonelli M, Moye L, Sacks FM, et al. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med. 2003;138:98–104. doi: 10.7326/0003-4819-138-2-200301210-00010. [DOI] [PubMed] [Google Scholar]
- 140.Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 141.Jones C, Roderick P, Harris S, et al. Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transplant. 2006;21:2133–43. doi: 10.1093/ndt/gfl198. [DOI] [PubMed] [Google Scholar]
- 142.Chan MR, Dall AT, Fletcher KE, et al. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med. 2007;120:1063–70. doi: 10.1016/j.amjmed.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 143.Avorn J, Bohn RL, Levy E, et al. Nephrologist care and mortality in patients with chronic renal insufficiency. Arch Intern Med. 2002;162:2002–6. doi: 10.1001/archinte.162.17.2002. [DOI] [PubMed] [Google Scholar]
- 144.Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137:479–86. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 145.Ghossein C, Serrano A, Rammohan M, et al. The role of comprehensive renal clinic in chronic kidney disease stabilization and management: the Northwestern experience. Semin Nephrol. 2002;22:526–32. doi: 10.1053/snep.2002.35970. [DOI] [PubMed] [Google Scholar]
- 146.Lebovitz HE, Banerji MA. Insulin resistance and its treatment by thiazolidinediones. Recent Prog Horm Res. 2001;56:265–94. doi: 10.1210/rp.56.1.265. [DOI] [PubMed] [Google Scholar]
- 147.Natali A, Baldeweg S, Toschi E, et al. Vascular effects of improving metabolic control with metformin or rosiglitazone in type 2 diabetes. Diabetes Care. 2004;27:1349–57. doi: 10.2337/diacare.27.6.1349. [DOI] [PubMed] [Google Scholar]
- 148.Pistrosch F, Passauer J, Fischer S, et al. In type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care. 2004;27:484–90. doi: 10.2337/diacare.27.2.484. [DOI] [PubMed] [Google Scholar]
- 149.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 150.Harte A, McTernan P, Chetty R, et al. Insulin-mediated upregulation of the renin angiotensin system in human subcutaneous adipocytes is reduced by rosiglitazone. Circulation. 2005;111:1954–61. doi: 10.1161/01.CIR.0000161954.17870.5D. [DOI] [PubMed] [Google Scholar]
- 151.Takeda K, Ichiki T, Tokunou T, et al. Peroxisome proliferator-activated receptor gamma activators downregulate angiotensin II type 1 receptor in vascular smooth muscle cells. Circulation. 2000;102:1834–9. doi: 10.1161/01.cir.102.15.1834. [DOI] [PubMed] [Google Scholar]
- 152.Sugawara A, Takeuchi K, Uruno A, et al. Differential effects among thiazolidinediones on the transcription of thromboxane receptor and angiotensin II type 1 receptor genes. Hypertens Res. 2001;24:229–33. doi: 10.1291/hypres.24.229. [DOI] [PubMed] [Google Scholar]
- 153.Nakamura T, Ushiyama C, Shimada N, et al. Comparative effects of pioglitazone, glibenclamide, and voglibose on urinary endothelin-1 and albumin excretion in diabetes patients. J Diabetes Complications. 2000;14:250–4. doi: 10.1016/s1056-8727(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 154.Ruan XZ, Moorhead JF, Fernando R, et al. PPAR agonists protect mesangial cells from interleukin 1beta-induced intracellular lipid accumulation by activating the ABCA1 cholesterol efflux pathway. J Am Soc Nephrol. 2003;14:593–600. doi: 10.1097/01.asn.0000050414.52908.da. [DOI] [PubMed] [Google Scholar]
- 155.Wu J, Zhang Y, Wang N, et al. Liver X receptor-alpha mediates cholesterol efflux in glomerular mesangial cells. Am J Physiol Renal Physiol. 2004;287:F886–95. doi: 10.1152/ajprenal.00123.2004. [DOI] [PubMed] [Google Scholar]
- 156.Chana RS, Lewington AJ, Brunskill NJ. Differential effects of peroxisome proliferator activated receptor-gamma (PPAR gamma) ligands in proximal tubular cells: thiazolidinediones are partial PPAR gamma agonists. Kidney Int. 2004;65:2081–90. doi: 10.1111/j.1523-1755.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 157.Panchapakesan U, Pollock CA, Chen XM. The effect of high glucose and PPAR-gamma agonists on PPAR-gamma expression and function in HK-2 cells. Am J Physiol Renal Physiol. 2004;287:F528–34. doi: 10.1152/ajprenal.00445.2003. [DOI] [PubMed] [Google Scholar]
- 158.Yoshimoto T, Naruse M, Nishikawa M, et al. Antihypertensive and vasculo- and renoprotective effects of pioglitazone in genetically obese diabetic rats. Am J Physiol. 1997;272:E989–96. doi: 10.1152/ajpendo.1997.272.6.E989. [DOI] [PubMed] [Google Scholar]
- 159.Buckingham RE, Al-Barazanji KA, Toseland CD, et al. Peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, protects against nephropathy and pancreatic islet abnormalities in Zucker fatty rats. Diabetes. 1998;47:1326–34. doi: 10.2337/diab.47.8.1326. [DOI] [PubMed] [Google Scholar]
- 160.Yamashita H, Nagai Y, Takamura T, et al. Thiazolidinedione derivatives ameliorate albuminuria in streptozotocin-induced diabetic spontaneous hypertensive rat. Metabolism. 2002;51:403–8. doi: 10.1053/meta.2002.30953. [DOI] [PubMed] [Google Scholar]
- 161.Isshiki K, Haneda M, Koya D, et al. Thiazolidinedione compounds ameliorate glomerular dysfunction independent of their insulin-sensitizing action in diabetic rats. Diabetes. 2000;49:1022–32. doi: 10.2337/diabetes.49.6.1022. [DOI] [PubMed] [Google Scholar]
- 162.Baylis C, Atzpodien EA, Freshour G, et al. Peroxisome proliferator-activated receptor [gamma] agonist provides superior renal protection versus angiotensin-converting enzyme inhibition in a rat model of type 2 diabetes with obesity. J Pharmacol Exp Ther. 2003;307:854–60. doi: 10.1124/jpet.103.055616. [DOI] [PubMed] [Google Scholar]
- 163.Yoshida K, Kohzuki M, Xu HL, et al. Effects of troglitazone and temocapril in spontaneously hypertensive rats with chronic renal failure. J Hypertens. 2001;19:503–10. doi: 10.1097/00004872-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 164.Imano E, Kanda T, Nakatani Y, et al. Effect of troglitazone on microalbuminuria in patients with incipient diabetic nephropathy. Diabetes Care. 1998;21:2135–9. doi: 10.2337/diacare.21.12.2135. [DOI] [PubMed] [Google Scholar]
- 165.Nakamura T, Ushiyama C, Suzuki S, et al. Effect of troglitazone on urinary albumin excretion and serum type IV collagen concentrations in Type 2 diabetic patients with microalbuminuria or macroalbuminuria. Diabet Med. 2001;18:308–13. doi: 10.1046/j.1464-5491.2001.00463.x. [DOI] [PubMed] [Google Scholar]
- 166.Nakamura T, Ushiyama C, Osada S, et al. Pioglitazone reduces urinary podocyte excretion in type 2 diabetes patients with microalbuminuria. Metabolism. 2001;50:1193–6. doi: 10.1053/meta.2001.26703. [DOI] [PubMed] [Google Scholar]
- 167.Aljabri K, Kozak SE, Thompson DM. Addition of pioglitazone or bedtime insulin to maximal doses of sulfonylurea and metformin in type 2 diabetes patients with poor glucose control: a prospective, randomized trial. Am J Med. 2004;116:230–5. doi: 10.1016/j.amjmed.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 168.Yanagawa T, Araki A, Sasamoto K, et al. Effect of antidiabetic medications on microalbuminuria in patients with type 2 diabetes. Metabolism. 2004;53:353–7. doi: 10.1016/j.metabol.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 169.Hanefeld M, Brunetti P, Schernthaner GH, et al. One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care. 2004;27:141–7. doi: 10.2337/diacare.27.1.141. [DOI] [PubMed] [Google Scholar]
- 170.Schernthaner G, Matthews DR, Charbonnel B, et al. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab. 2004;89:6068–76. doi: 10.1210/jc.2003-030861. [DOI] [PubMed] [Google Scholar]
- 171.Agarwal R, Saha C, Battiwala M, et al. A pilot randomized controlled trial of renal protection with pioglitazone in diabetic nephropathy. Kidney Int. 2005;68:285–92. doi: 10.1111/j.1523-1755.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 172.Lebovitz HE, Dole JF, Patwardhan R, et al. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab. 2001;86:280–8. doi: 10.1210/jcem.86.1.7157. [DOI] [PubMed] [Google Scholar]
- 173.Sarafidis PA, Lasaridis AN, Nilsson PM, et al. The effect of rosiglitazone on urine albumin excretion in patients with type 2 diabetes mellitus and hypertension. Am J Hypertens. 2005;18:227–34. doi: 10.1016/j.amjhyper.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 174.Pistrosch F, Herbrig K, Kindel B, et al. Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes. 2005;54:2206–11. doi: 10.2337/diabetes.54.7.2206. [DOI] [PubMed] [Google Scholar]
- 175.Bakris GL, Ruilope LM, McMorn SO, et al. Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J Hypertens. 2006;24:2047–55. doi: 10.1097/01.hjh.0000244955.39491.88. [DOI] [PubMed] [Google Scholar]
- 176.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–8. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 177.Burns KD. The emerging role of angiotensin-converting enzyme-2 in the kidney. Curr Opin Nephrol Hypertens. 2007;16:116–21. doi: 10.1097/MNH.0b013e3280123c0e. [DOI] [PubMed] [Google Scholar]
- 178.Rice GI, Thomas DA, Grant PJ, et al. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 180.Ye M, Wysocki J, William J, et al. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–75. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 181.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–5. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 182.Minchenko AG, Stevens MJ, White L, et al. Diabetes-induced overexpression of endothelin-1 and endothelin receptors in the rat renal cortex is mediated via poly(ADP-ribose) polymerase activation. FASEB J. 2003;17:1514–6. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- 183.Hocher B, Lun A, Priem F, et al. Renal endothelin system in diabetes: comparison of angiotensin-converting enzyme inhibition and endothelin-A antagonism. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S492–5. doi: 10.1097/00005344-199800001-00141. [DOI] [PubMed] [Google Scholar]
- 184.Klahr S, Morrissey J. Progression of chronic renal disease. Am J Kidney Dis. 2003;41:S3–7. doi: 10.1053/ajkd.2003.50074. [DOI] [PubMed] [Google Scholar]
- 185.Benigni A. Defining the role of endothelins in renal pathophysiology on the basis of selective and unselective endothelin receptor antagonist studies. Curr Opin Nephrol Hypertens. 1995;4:349–53. doi: 10.1097/00041552-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 186.Kohan DE. Autocrine role of endothelin in rat inner medullary collecting duct: inhibition of AVP-induced cAMP accumulation. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S174–9. doi: 10.1097/00005344-199322008-00047. [DOI] [PubMed] [Google Scholar]
- 187.Ahn D, Ge Y, Stricklett PK, et al. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114:504–11. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee YJ, Shin SJ, Tsai JH. Increased urinary endothelin-1-like immunoreactivity excretion in NIDDM patients with albuminuria. Diabetes Care. 1994;17:263–6. doi: 10.2337/diacare.17.4.263. [DOI] [PubMed] [Google Scholar]
- 189.Mattia G, Cassone-Faldetta M, Bellini C, et al. Role of plasma and urinary endothelin-1 in early diabetic and hypertensive nephropathy. Am J Hypertens. 1998;11:983–8. doi: 10.1016/s0895-7061(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 190.Ak G, Buyukberber S, Sevinc A, et al. The relation between plasma endothelin-1 levels and metabolic control, risk factors, treatment modalities, and diabetic microangiopathy in patients with Type 2 diabetes mellitus. J Diabetes Complications. 2001;15:150–7. doi: 10.1016/s1056-8727(01)00137-4. [DOI] [PubMed] [Google Scholar]
- 191.Candido R, Allen TJ. Haemodynamics in microvascular complications in type 1 diabetes. Diabetes Metab Res Rev. 2002;18:286–304. doi: 10.1002/dmrr.313. [DOI] [PubMed] [Google Scholar]
- 192.Zanatta CM, Gerchman F, Burttet L, et al. Endothelin-1 levels and albuminuria in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;80:299–304. doi: 10.1016/j.diabres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 193.Chade AR, Krier JD, Textor SC, et al. Endothelin-a receptor blockade improves renal microvascular architecture and function in experimental hypercholesterolemia. J Am Soc Nephrol. 2006;17:3394–403. doi: 10.1681/ASN.2006060635. [DOI] [PubMed] [Google Scholar]
- 194.Sasser JM, Sullivan JC, Hobbs JL, et al. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol. 2007;18:143–54. doi: 10.1681/ASN.2006030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thone-Reinke C, Simon K, Richter CM, et al. Inhibition of both neutral endopeptidase and endothelin-converting enzyme by SLV306 reduces proteinuria and urinary albumin excretion in diabetic rats. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S76–9. doi: 10.1097/01.fjc.0000166208.12297.8d. [DOI] [PubMed] [Google Scholar]
- 196.Sugimoto K, Fujimori A, Yuyama H, et al. Renal protective effect of YM598, a selective endothelin type A receptor antagonist. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S451–4. doi: 10.1097/01.fjc.0000166320.68753.e6. [DOI] [PubMed] [Google Scholar]
- 197.Gross ML, Ritz E, Schoof A, et al. Renal damage in the SHR/N-cp type 2 diabetes model: comparison of an angiotensin-converting enzyme inhibitor and endothelin receptor blocker. Lab Investig. 2003;83:1267–77. doi: 10.1097/01.lab.0000085188.23709.29. [DOI] [PubMed] [Google Scholar]
- 198.Cosenzi A, Bernobich E, Trevisan R, et al. Nephroprotective effect of bosentan in diabetic rats. J Cardiovasc Pharmacol. 2003;42:752–6. doi: 10.1097/00005344-200312000-00009. [DOI] [PubMed] [Google Scholar]
- 199.Honing ML, Hijmering ML, Ballard DE, et al. ABT-627, a selective eta-receptor antagonist, reduces proteinuria in patients with diabetes mellitus. In: Regulation of Vascular Tone in Humans by Endothelium-Derived Mediators. Utrecht, Netherlands: Elinkwijk BV; 2000;89–102
- 200.Wenzel RR et al. The ETA-selective antagonist SPP301 on top of standard treatment reduces urinary albumin excretion rate in patients with diabetic nephropathy. ASN Renal Week 2005.
- 201.Mann JF, Green D, Jamerson K, et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527–35. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Glomb MA, Pfahler C. Amides are novel protein modifications formed by physiological sugars. J Biol Chem. 2001;276:41638–47. doi: 10.1074/jbc.M103557200. [DOI] [PubMed] [Google Scholar]
- 203.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–21. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 204.Abdel-Rahman E, Bolton WK. Pimagedine: a novel therapy for diabetic nephropathy. Expert Opin Investig Drugs. 2002;11:565–74. doi: 10.1517/13543784.11.4.565. [DOI] [PubMed] [Google Scholar]
- 205.Freedman BI, Wuerth JP, Cartwright K, et al. Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II) Control Clin Trials. 1999;20:493–510. doi: 10.1016/s0197-2456(99)00024-0. [DOI] [PubMed] [Google Scholar]
- 206.Williams ME. Clinical studies of advanced glycation end product inhibitors and diabetic kidney disease. Curr Diab Rep. 2004;4:441–6. doi: 10.1007/s11892-004-0054-0. [DOI] [PubMed] [Google Scholar]
- 207.Williams ME. A phase 2 clinical trial of pyridoxamine (Pyridorin) in type 1 and type 2 diabetic patients with overt nephropathy (PYR-206) J Am Soc Nephrol. 2003;2003:7A. [Google Scholar]
- 208.Kass DA, Shapiro EP, Kawaguchi M, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–70. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 209.Vasan S, Foiles P, Founds H. Therapeutic potential of breakers of advanced glycation end product-protein crosslinks. Arch Biochem Biophys. 2003;419:89–96. doi: 10.1016/j.abb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 210.Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–51. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 211.Pergola PE, Krauth M, Huff JW, et al. Effect of bardoxolone methyl on kidney function in patients with T2D and Stage 3b-4 CKD. Am J Nephrol. 2011;33:469–76. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 212.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011. [DOI] [PubMed]
- 213.Standards of medical care in diabetes--2007. Diabetes Care. 2007;30(Suppl 1):S4-S41. [DOI] [PubMed]