Abstract
Background
Electronic personal health records (PHRs) have the potential to empower patients in self-management of chronic diseases, which should lead to improved outcomes.
Objective
To measure the association between use of an advanced electronic medical record-linked PHR and diabetes quality measures in adults with diabetes mellitus (DM).
Design
Retrospective audit of PHR use and multivariable regression analyses.
Patients
10,746 adults 18–75-years of age with DM seen at least twice at the office of their primary care physician at the Cleveland Clinic from July 2008 through June 2009.
Main Measures
PHR use was measured as number of use days. Diabetes quality measures were: hemoglobin A1c (HbA1c), LDL cholesterol, blood pressure, body mass index (BMI), HbA1c testing, ACEi/ARB use and/or microalbumin testing, pneumococcal vaccination, foot and dilated eye examination, and smoking status.
Key Results
Compared to non-users, PHR users were younger, had higher incomes and educational attainment, were more likely to identify as Caucasian, and had better unadjusted and adjusted diabetes quality measure profiles. Adjusted odds ratio of HbA1c testing was 2.06 (p < 0.01) and most recent HbA1c was 0.29% lower (p < 0.01). Among PHR users, increasing number of login days was generally not associated with more favorable diabetes quality measure profiles.
Conclusions
PHR use, but not intensity of use, was associated with improved diabetes quality measure profiles. It is likely that better diabetes profiles among PHR users is due to higher level of engagement with their health among those registered for the PHR rather than PHR use itself. PHR use was infrequent. To maximize value, next-generation PHRs must be designed to engage patients in everyday diabetes self-management.
KEY WORDS: personal health record, electronic personal health record, PHR, chronic disease
INTRODUCTION
The cost of chronic disease management places a tremendous strain on the U.S. health care system. The total estimated cost of diabetes care alone in 2007 was $174 billion, or roughly 10%of total healthcare spending1. The electronic personal health record (PHR), defined as an “application through which individuals can access, manage and share their health information…in a private, secure, and confidential environment,”2 has great potential for addressing cost and quality of chronic disease management. Access to effective and tailored patient education, electronic patient–provider communication, and the wealth of clinical information and web-based resources contained within modern PHRs could lead to improvements in chronic disease outcomes through improved patient-centered care and self-management.
Few research findings have been published on the value of PHRs in chronic disease management3. Three prospective randomized trials evaluated the use of PHRs in the management of type 2 diabetes mellitus (DM)4–6. These studies demonstrated modest but inconsistent improvements in diabetes quality measures when comparing PHR users to non-users. However, because all three studies included additional interventions, such as regular contact with a diabetes care manager,6 the independent effect of PHR use cannot be determined. Further, small sample sizes in these trials may have limited the ability to detect clinically meaningful differences between study groups.
In this study, we explored the actual use of Cleveland Clinic’s electronic medical record (EMR)-linked PHR by a large primary care cohort of patients with DM to determine if use of the PHR was associated with improved diabetes quality measures.
METHODS
Participants
We included all primary care patients actively managed DM aged 18–75-years seen in Cleveland Clinic departments of internal medicine and family medicine from July 2008 through June 2009. Diagnosis of diabetes was defined by the presence of appropriate ICD-9 codes (250–250.93, 357.2, 362.0, and 366.41) within the EMR-based longitudinal problem list. Patients with a diagnosis of only “gestational diabetes” or “steroid induced diabetes” were excluded. Patients were considered actively managed if they were seen at least twice in the primary site of their assigned primary care physician during the 12-month period.
PHR Registration and Use
The Cleveland Clinic PHR, MyChart (Epic Inc., Verona, WI), was made available to the general patient population in 2006. As of July 2010, approximately 20–40% of primary care patients utilized the PHR, depending on practice site and physician. Patients were able to register for free access to the internet-based PHR at each office visit, through their primary care provider, or directly through the Cleveland Clinic website. Multiple direct media campaigns to the community efforts were made prior to and throughout the study period (although unrelated to study). Patient access is granted through an identity verification process either in person or via the internet. Once activated, a message is sent to a patient’s primary physician informing them that their patient has been granted access to the PHR, at which time the primary care provider can initiate secure PHR communications with the patient, verify medical issues and current medications, and share laboratory results with the PHR-enabled patient.
Once registered, patients require only a secure web browser and internet access to log in and engage in a variety of activities within the PHR. Upon release of patient information by a primary provider, patients with diabetes can access their EMR-based diagnoses and co-morbidities, laboratory and other test results, along with secure messaging through the PHR with their provider. In addition, diabetic patients can access glucometer readings, if entered, a set of diabetes-related health and wellness links, and diabetes specific health reminders (including recommended glycated hemoglobin, urine albumin, and cholesterol testing due dates, recommendation for pneumococcal vaccination, and due dates for diabetic foot and dilated retinal eye exams).
Data Collection
We queried our institutional EMR clinical data repository (Clarity, Epic Inc., Verona, WI) for demographic and clinical data on eligible patients. We queried the PHR usage log to determine number of PHR use days over the study period, along with patient access data for various PHR functions (e.g. number of times a patient reviewed laboratory results, number of messages received from or sent to a patient’s primary care physician). All data was de-identified upon extraction and stored in a secure Oracle 9i database (Oracle Corp., Redwood City, CA) for subsequent statistical analysis.
Quality measures
Table 1 provides a list of the diabetes quality process and outcome measures. We chose to select the same measures as those used for public reporting in the Better Health Greater Cleveland chronic disease improvement collaborative, as they had been vetted through a multi-organizational approval process and were based on nationally accepted measures.
Table 1.
Diabetes Quality Care Measures Used in this Study
| Variable | Categorical Measure | Continuous Measure |
|---|---|---|
| Dilated retinal eye exam | Eye exam recorded within study period | |
| Pneumococcal vaccination | Documented lifetime vaccination | |
| Attention to kidneys | Use of ACEi/ARB and/or test for microalbuminuria within study period | |
| Attention to feet | Documented foot exam within study period | |
| Smoking cessation | Documented non-smoker | |
| HbA1c | HbA1c value measured within study period | Last documented value within study period |
| Blood pressure | Last documented systolic and diastolic blood pressure values within study period | |
| LDL cholesterol | Last documented value within study period | |
| Body mass index (BMI) | Last documented value within study period |
Data Analysis
Bivariable analyses investigated the crude association between PHR users and non-users with respect to baseline demographic characteristics and diabetes quality measure values.
Categorical variables were analyzed using Fisher’s exact test or Pearson’s chi-square test. Continuous variables were analyzed using two-sample t-tests or Welch’s t-test. Multivariable logistic and linear regression modeling investigated the association between PHR use and diabetes quality measure values. Locally weighted scatterplot smoothing (LOESS) curves were used to assess linearity in the relationship between number of PHR use days and quality measures. Other than the large effect of becoming a PHR user (0 use days to 1 use day), linearity appeared reasonable among users. Thus, the analyses of PHR use has two components: the effect of being a PHR user (non-users versus patients with ≥1 use day) and among PHR users, the incremental benefit of increasing use days. Adjustment was performed for patient demographic characteristics (age and income as continuous variables and gender, racial/ethnic group [Caucasian versus other], and insurance type [commercial insurance versus other] as categorical variables). As physician use of the PHR is likely to affect patient engagement, adjustment was also made for level of physician engagement (the percentage of the physician’s patients from the study sample who used the PHR ≥1 day during the study period).
RESULTS
Patient PHR Use
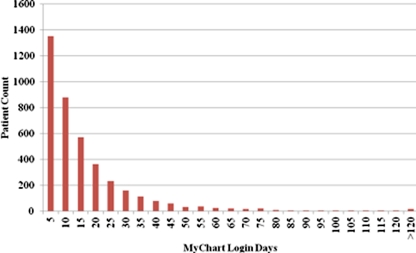
Of 10,746 eligible patients with DM, 4,036 (37.6%) were enrolled in the PHR by July 2008. The mean number of login days during the 12-month study period was 15 (SD 18) and the median number 9. A histogram of number of login days for PHR users is shown in Figure 1. Among the most commonly used features, 96% of PHR users reviewed laboratory tests ordered by their providers (mean 7.8 times [SD 8.5]), 94% read messages from their providers (mean 8.3 times [SD 8.6]), and 91% reviewed laboratory results (mean 5.7 times [SD 5.3]).
Figure 1.
Histogram of number of login days for PHR users.
Demographic Differences between PHR Users and Non-users
Personal health record users were younger, more likely to have commercial insurance, identify as Caucasian, have higher household incomes, and live in a region with higher rates of high school completion compared to MyChart non-users (Table 2). Among PHR users, demographic data varied little between users with many (use >5% of days within year) and few PHR use days (data not shown).
Table 2.
Patient Demographic Characteristics of PHR Users and Non-users
| Variable | Non-Users | Users | P-Value |
|---|---|---|---|
| Number of Patients | 6,710 | 4,036 | |
| Age, Years, mean (SD) | 62 (10) | 59 (10) | < 0.01 |
| Female,% | 50 | 46 | < 0.01 |
| Insurance Type,% | < 0.01 | ||
| Commercial | 51 | 69 | |
| Medicaid | 1 | < 1 | |
| Medicare | 46 | 29 | |
| Self-Pay | 2 | 2 | |
| Race/Ethnicity,% | < 0.01 | ||
| Caucasian | 66 | 84 | |
| African American | 28 | 11 | |
| Hispanic | <1 | <1 | |
| Other | 2 | 3 | |
| Unknown | 2 | 2 | |
| Household Income, USD in thousands, mean (SD) | 47.5 (16.1) | 53.0 (14.8) | < 0.01 |
| Less Than High School Graduation,% in ZCTA | 16 | 13 | < 0.01 |
ZCTA = zip code tabulation area
p-values for continuous variables: two-sample t test or Welch’s t test
p-values for categorical variables: Fisher’s exact test or Chi-square test
Baseline Diabetes Quality Measure Differences between PHR Users and Non-users
Personal health record users generally had slightly more favorable diabetes quality measure values than PHR non-users (Table 3). Users were more likely than non-users to have a documented HbA1c measurement within the study period (96% versus 94%) and to be documented non-smokers (91% versus 86%) [p-values all < 0.01]. Users also had significantly lower mean HbA1c values compared to non-users (7.0% versus 7.3%), lower mean systolic blood pressure (126 versus 129 mmHg), and lower mean LDL cholesterol (90 versus 93 mg/dL) [p-values all < 0.01]. In separate analyses among PHR users, diabetes quality measure results varied little between users with many (use >5% of days within year) and few PHR use days (data not shown).
Table 3.
Comparison of Unadjusted Diabetes Quality Care Measure Values Between PHR Users and Non-users
| Variable | Non-Users | Users | P-value |
|---|---|---|---|
| Number in Group | 6,710 | 4,036 | |
| Dilated Eye Exam, count (%) | 2,081 (31) | 1,319 (33) | 0.07 |
| Pneumococcal Vaccine, count (%) | 5,846 (87) | 3,565 (88) | 0.07 |
| ACEi/ARB or Microalbumin Test, count (%) | 6,197 (92) | 3,761 (93) | 0.12 |
| Foot exam, count (%) | 4,729 (70) | 2,898 (72) | 0.15 |
| Non-smoker, count (%) | 5,801 (86) | 3,686 (91) | <0.01 |
| HbA1c Measurement, count (%) | 6,313 (94) | 3,895 (96) | <0.01 |
| Hemoglobin A1c value,%, mean (SD) | 7.3 (1.5) | 7.0 (1.3) | <0.01 |
| Last SBP, mmHg, mean (SD) | 129 (16) | 126 (15) | <0.01 |
| Last DBP, mmHg, mean (SD) | 74 (10) | 75 (9.5) | 0.51 |
| Last LDL, mg/dL, mean (SD) | 93 (32) | 90 (30) | <0.01 |
| BMI, kg/m2, mean (SD) | 33.6 (7.3) | 34.0 (7.5) | <0.01 |
p-values for continuous variables: two-sample t test or Welch’s t test
p-values for categorical variables: Fisher’s exact test or Chi-square test
Associating PHR Use with Diabetes Quality Measure Values
After adjusting for patient demographic and provider characteristics, PHR users continued to demonstrate slightly more favorable diabetes quality measure values compared to PHR non-users for eight of eleven measures (Tables 4 and 5). Users were more likely to have a documented dilated eye exam (OR 1.11), have documented lifetime pneumococcal vaccination (OR 1.38), be prescribed ACEi/ARB medications or have a test for microalbuminuria performed (OR 1.26), be documented non-smokers (OR 1.74), and to have a documented HbA1c test performed within the study period (OR 2.06) [p-values all <0.05]. Further, PHR users had lower HbA1c values (by 0.29%), SBP values (by 1.13 mmHg), and DBP values (by 0.54 mmHg) than PHR non-users [p-values all < 0.01].
Table 4.
Multivariable Logistic Regression Models Estimating the Effects of both PHR Use and Increases in Use on Diabetes Quality Measures
| Variable | Comparison | Odds Ratio | 95% CI | P-value |
|---|---|---|---|---|
| Dilated Eye Exam | User versus non-user | 1.11 | 1.01, 1.21 | 0.03 |
| ∆ 10 use days | 1.03 | 0.99, 1.06 | 0.14 | |
| Pneumococcal Vaccine | User versus non-user | 1.38 | 1.21, 1.56 | <0.01 |
| ∆ 10 use days | 1.08 | 1.02, 1.16 | 0.01 | |
| ACEi/ARB or Microalbumin Test | User versus non-user | 1.26 | 1.07, 1.48 | <0.01 |
| ∆ 10 use days | 1.00 | 0.93, 1.06 | 0.89 | |
| Foot exam | User versus non-user | 1.07 | 0.97, 1.17 | 0.17 |
| ∆ 10 use days | 1.04 | 1.00, 1.08 | 0.08 | |
| Nonsmoker | User versus non-user | 1.74 | 1.51, 2.00 | <0.01 |
| ∆ 10 use days | 1.05 | 0.98, 1.12 | 0.18 | |
| HbA1c Test Performed | User versus non-user | 2.06 | 1.67, 2.53 | <0.01 |
| ∆ 10 use days | 1.28 | 1.09, 1.49 | <0.01 |
*∆ 10 use days explores the effect of a PHR increasing use days by 10, e.g. from 1 day to 11 days
**Adjusted for age, gender, race/ethnicity, income, insurance, and provider engagement
Table 5.
Multivariable Linear Regression Models Estimating the Effects of both PHR Use and Increases in Use on Diabetes Quality Measures
| Variable | Comparison | Estimate | 95% CI | P-value |
|---|---|---|---|---|
| HbA1c value,% | User versus non-user | −0.29 | −0.35, −0.23 | <0.01 |
| ∆ 10 use days | −0.02 | −0.04, 0.00 | 0.02 | |
| Last SBP, mmHg | User versus non-user | −1.13 | −1.78, −0.48 | <0.01 |
| ∆ 10 use days | −0.04 | −0.29, 0.21 | 0.75 | |
| Last DBP, mmHg | User versus non-user | −0.54 | −0.93, −0.14 | <0.01 |
| ∆ 10 use days | −0.12 | −0.27, 0.04 | 0.15 | |
| Last LDL, mg/dL | User versus non-user | −0.01 | −1.40, 1.38 | 0.99 |
| ∆ 10 use days | 0.34 | −0.24, 0.91 | 0.25 | |
| BMI, kg/m2 | User versus non-user | 0.03 | −0.28, 0.35 | 0.84 |
| ∆ 10 use days | −0.04 | −0.17, 0.09 | 0.55 |
*∆ 10 use days explores the effect of a PHR increasing use days by 10, e.g. from 1 day to 11 days
**Adjusted for age, gender, race/ethnicity, income, insurance, and provider engagement
Among PHR users, an incremental increase in PHR use days was associated with more favorable diabetes quality measure values for only three of eleven quality measures, including documented pneumococcal vaccination, HbA1c testing, and HbA1c value (Tables 4 and 5). An incremental increase in PHR use days by 10 (e.g. 15 days versus 5 days) was associated with slightly greater odds of documented HbA1c testing (OR 1.28, p < 0.01) and only a minimal decrease in HbA1c values (0.02%, p < 0.01).
In summary, most quality measures were better, on average, among the group who used the personal health record, but the differences were quite small and of marginal clinical significance. More intensive use of the PHR did not result in any clinically important differences compared to minimal use.
DISCUSSION
Our study provides new insight into the association between PHR adoption and level of use and diabetes quality measures. The PHR evaluated in this study contains a number of features that might assist in diabetes management, including electronic communication with providers, access to diabetes-specific preventive care reminders, and high-quality diabetes-related educational content. However, we found little evidence that use of the PHR substantially improved diabetes quality measures; in our large sample of patients, the improvements we noted were small and of marginal clinical relevance.
As demonstrated in previous studies,7,8 we found significant demographic differences in PHR users and non-users, including such factors as lower age and higher incomes. Given these differences, it was not surprising to find better unadjusted diabetes quality measure profiles in PHR users.
After adjusting for patient demographic characteristics and level of provider engagement with the PHR, we continued to observe better profiles in PHR users versus non-users for a variety of important diabetes quality measures, such as lower HbA1c and blood pressure values. However, amongst PHR users, we typically found minimal or no association between increasing PHR use days and better diabetes quality measure profiles in the regression models (a poor “exposure-response” relationship). This leads us to suspect that those individuals who take steps to register and login to a PHR program have a different baseline level of engagement with the management of their disease than those who do not. These individuals may be more proactive in seeking health care and more engaged in learning about their medical conditions through a wide variety of media in addition to PHRs, such as through health-related websites or books. They may also be more “health-conscious” regardless of PHR use. We found, for example, lower odds of smoking in PHR users compared to non-users, which we cannot reasonably explain through PHR use.
This type of study design has a number of strengths compared to previous studies. Rather than looking at use or non-use categorically, we gained a more nuanced understanding of potential PHR value by examining the quantity of use among PHR users with diabetes quality measures. The observational approach we employed allowed for sample sizes that are typically unattainable in health informatics randomized trials. Eligible patients also had no knowledge of the study that might have influenced engagement with the PHR.
Among study limitations, this is an association study in which we cannot infer causality between PHR adoption and use and quality of diabetes management. We were unable to capture care received outside of the Cleveland Clinic health system, which might have affected results. However, as patients were seen at least twice at the practice site of their primary care physician during the 12-month study period, we are confident that much or most of the diabetes related care in our patient sample was received at the Cleveland Clinic or documented within the EMR. Our patient population differed from the overall population of the United States. For example, we had few Hispanic patients. This could be important, as health literacy and cultural differences and language background might influence adoption and engagement with the PHR. Finally, some patients may have had surrogates accessing their PHR account, which we could not capture in our PHR data repository. This would have but unknown effects on quality measure profiles for such patients.
To be effective, PHRs need to engage patients on a regular basis and provide tailored, action-oriented advice to improve self-management. The amount of patient PHR use in this study provides evidence that PHRs should be substantially improved to incorporate functionality that is useful and engaging to make the PHR more of an integral, valued tool for diabetes self-management. For example, PHR users only logged into MyChart a median of 9 days, or less than once a month. Next-generation PHRs might engage patients by giving action-oriented advice to patients for self-care via cell phones or through other means9. The rapid consumer adoption of iPads, smartphones such as iPhones and Androids, and televisions with the internet built-in opens up possibilities for the future PHR. Such innovations should be validated through future research studies correlating not just use of these tools but also the levels of engagement of these tools with disease outcomes.
Conflict of Interests
None disclosed.
References
- 1.American Diabetes Association Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008;31(3):596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.Pagliari C, Detmer D, Singleton P. Potential of electronic personal health records. BMJ. 2007; 335(7615):330-3. [DOI] [PMC free article] [PubMed]
- 3.Tenforde M, Jain A, Hickner J. The value of personal health records for chronic disease management: what do we know? Fam Med. 2011;43(5):351–4. [PubMed] [Google Scholar]
- 4.Grant RW, Wald JS, Schnipper JL, et al. Practice-linked online personal health records for type 2 diabetes mellitus: A randomized controlled trial. Arch Intern Med. 2008;168(16):1776–82. doi: 10.1001/archinte.168.16.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holbrook A, Thabane L, Keshavjee K, et al. Individualized electronic decision support and reminders to improve diabetes care in the community: COMPETE II randomized trial. CMAJ. 2009;181(1–2):37–44. doi: 10.1503/cmaj.081272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ralston JD, Hirsch IB, Hoath J, Mullen M, Cheadle A, Goldberg HI. Web-based collaborative care for type 2 diabetes: A pilot randomized trial. Diabetes Care. 2009;32(2):234–9. doi: 10.2337/dc08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goel MS, Brown TL, Williams A, Hasnain-Wynia R, Thompson JA, Baker DW. Disparities in enrollment and use of an electronic patient portal. J Gen Intern Med. 2011 [DOI] [PMC free article] [PubMed]
- 8.Yamin CK, Emani S, Williams DH, et al. The digital divide in adoption and use of a personal health record. Arch Intern Med. 2011;171(6):568–74. doi: 10.1001/archinternmed.2011.34. [DOI] [PubMed] [Google Scholar]
- 9.Brennan PF, Downs S, Casper G. Project HealthDesign: Rethinking the power and potential of personal health records. J Biomed Inform. 2010;43(5):S3–S5. doi: 10.1016/j.jbi.2010.09.001. [DOI] [PubMed] [Google Scholar]