Abstract
BACKGROUND
Clinical inertia, provider failure to appropriately intensify treatment, is a major contributor to uncontrolled blood pressure (BP). Some clinical inertia may result from physician uncertainty over the patient’s usual BP, adherence, or value of continuing efforts to control BP through lifestyle changes.
OBJECTIVE
To test the hypothesis that providing physicians with uncertainty reduction tools, including 24-h ambulatory BP monitoring, electronic bottle cap monitoring, and lifestyle assessment and counseling, will lead to improved BP control.
DESIGN
Cluster randomized trial with five intervention clinics (IC) and five usual care clinics (UCC).
SETTING
Six public and 4 private primary care clinics.
PARTICIPANTS
A total of 665 patients (63 percent African American) with uncontrolled hypertension (BP ≥140 mmHg/90 mmHg or ≥130/80 mmHg if diabetic).
INTERVENTIONS
An order form for uncertainty reduction tools was placed in the IC participants’ charts before each visit and results fed back to the provider.
OUTCOME MEASURES
Percent with controlled BP at last visit. Secondary outcome was BP changes from baseline.
RESULTS
Median follow-up time was 24 months. IC physicians intensified treatment in 81% of IC patients compared to 67% in UCC (p < 0.001); 35.0% of IC patients and 31.9% of UCC patients achieved control at the last recorded visit (p > 0.05). Multi-level mixed effects longitudinal regression modeling of SBP and DBP indicated a significant, non-linear slope difference favoring IC (p time × group interaction = 0.048 for SBP and p = 0.001 for DBP). The model-predicted difference attributable to intervention was −2.8 mmHg for both SBP and DBP by month 24, and −6.5 mmHg for both SBP and DBP by month 36.
CONCLUSIONS
The uncertainty reduction intervention did not achieve the pre-specified dichotomous outcome, but led to lower measured BP in IC patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-011-1888-1) contains supplementary material, which is available to authorized users.
KEY WORDS: hypertension control, cluster randomized trial, physician uncertainty
INTRODUCTION
Hypertension is a major risk factor for cardiovascular morbidity and mortality. While the most recent findings from national health surveys indicate a marked improvement in hypertension control over the past decade, 31% of persons treated with drugs for hypertension are still above the treatment goal of 140/90 mmHg recommended by the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure for individuals without serious cardiovascular co-morbities1,2.
Both individual and patient-level factors have been proposed to explain the residual lack of blood pressure (BP) control in treated patients. There is limited evidence that individual behaviors, such as appointment-keeping or medication adherence, are the most significant contributors to poor BP control. National health survey data show that the great majority of the uncontrolled are insured and have frequent health care visits3, and objective data indicate that most patients with uncontrolled BP are actually compliant with medications4. Berlowitz et al.5 and Rose et al.6 have documented that poorly controlled BP in treated patients is largely attributable to physicians’ failure to intensify treatment when confronted with BP measurements that are only modestly above the recommended control thresholds.
The term “clinical inertia”7 has been coined to refer to physicians’ failure to intensify treatment according to clear clinical practice guidelines. In hypertension management, clinical inertia was operationalized by O’Conner as an office visit in which no therapeutic action was taken to lower the BP of a patient with uncontrolled hypertension8. While instances of clinical inertia are generally viewed as undesirable, growing evidence suggests that it might be rooted in a legitimate uncertainty over the need for treatment intensification. Recent studies suggest that the major sources of uncertainty in hypertension management center on whether the BP recorded at the visit is representative of the patient’s usual BP, and whether the patient is actually adherent to the currently prescribed regimen9,10. In past surveys, physicians openly disagreed with the recommended target BP11, but this was not given as a reason not to titrate in the more recent evaluations cited above.
African Americans (AAs) have both a disproportionately higher prevalence and higher burden of complications from hypertension, compared to non-Hispanic whites (NHW) in the US12. Although awareness and treatment of hypertension and self-reported lifestyle actions to control hypertension are now greater in AAs than NHW, the proportion of AAs controlled on treatment still remains significantly lower than NHWs1,12–14. There is no evidence that clinical inertia is significantly different when treating AAs than hypertensives from other racial/ethnic groups15,16. However, because of their increased risk for complications of poorly controlled hypertension, it is important that AAs be well represented in evaluations of new treatment approaches.
We designed a clinic-level intervention to reduce physician uncertainty about the patient’s actual typical BP, actual adherence to medications, and likelihood of achieving BP control through lifestyle changes. We hypothesized that reducing physician uncertainty would counteract clinical inertia and lead to improved blood pressure control. In this paper, we report on the primary BP control outcomes of this intervention carried out in ten clinics with substantial numbers of AA patients.
METHODS
Design Overview
The details of this cluster randomized trial have been reported previously17. Ten primary care clinics belonging to two different health care systems served as the units of intervention. Five clinics (ICs) were randomized to implement the uncertainty reduction intervention, and the remaining clinics served as usual care clinics (UCCs). Six of the clinics belonged to a public health care delivery system, and four of the clinics were part of a large, multi-specialty group practice. All participating physicians were general internists or family physicians. Randomization of clinics to intervention or control condition was stratified by system. Before randomization, the physicians in all ten clinics received a baseline knowledge survey and a comprehensive 2-h educational program regarding the JNC 7 treatment guidelines, effective patient-physician communication, and special considerations for treating AAs.
In the ICs, study research staff placed an updated graph of recent BP measurements in the patient chart before each visit, along with a referral form to order: (1) 24-h ambulatory BP monitoring (ABPM), (2) electronic bottle cap assessment of medication adherence, followed by medication adherence counseling in non-adherent patients, and (3) lifestyle assessment and counseling followed by 24-h ABPM approximately 3 months after completion of the counseling protocol. The study staff carried out these procedures and fed back the results to the ordering physician. The physicians could order any combination of the tools at any visit. The tools were not provided in the five clinics assigned to UCC.
We included the assessment of dietary and physical activity habits18,19, followed by a telephone-based behavioral counseling program developed for a previous intervention20, because lifestyle changes, including weight loss and sodium restriction, have long been considered important elements of a hypertension control regimen2,21. In focus groups conducted to develop lifestyle counseling messages in a previous study, we found that participants assigned considerable value to controlling BP through non-pharmacological means20; thus, we considered it likely that some instances of clinical inertia occur when patients request more time to implement intended lifestyle changes.
Participant Inclusion Criteria
Details of the inclusion and exclusion criteria have been reported previously17. Research assistants identified potential study participants by screening the medical records of patients who presented for a routine primary care appointment. Patients had to have at least two clinic visits in the previous 12 months, with BP on the most recent two consecutive visits of ≥140 mmHg systolic or ≥ 90 mmHg diastolic, or if diabetic, ≥ 130 systolic or 80 mmHg diastolic. Patients with cognitive impairment, renal insufficiency, or a serious concomitant illness such as cancer, recent MI, or unstable angina, were excluded. Informed consent was obtained from both the patient and provider.
Outcome Measures
The defined primary endpoint was the proportion of patients with clinic BP <140/90 mmHg (<130/80 if the patient had diabetes) at the last visit. Actual change in measured clinic systolic and diastolic BP from baseline was the secondary endpoint. The planned duration of follow-up was 2 to 3 years, depending on randomization date. At each visit we collected data on the number and class of anti-hypertensive drugs prescribed and whether treatment was intensified. Intensification was defined as an increase in the dose of an existing drug or the addition of a drug.
Sample Size
We powered the study for a difference in proportion controlled of 30% in the control clinics vs. 50% in the intervention clinics using sample size adjustments to account for the cluster randomized design22. The effect size assumed a temporal improvement in BP control in the control clinics. The required sample size to detect this effect size with alpha = 0.05 (two-sided) and power of 0.90, an intra-class correlation of 0.008 (to account for the cluster design) was 160 per group. We increased this number to 335 per group (670 total) to allow for an expected 20% attrition over 2 years of follow-up and to allow for 40% of the sample to consist of non-African Americans while providing sufficient power to examine the effects in African Americans as a subgroup of interest.
Statistical Analysis
We used an intention-to-treat analysis in which all patients enrolled, and who did not request to be withdrawn from the study, were included in the analysis. We first examined the raw unadjusted changes in proportion of patients controlled and changes in SBP and DBP using standard two-group comparisons (chi-square or independent samples t-test). However, to adjust for clustering, an unbalanced number of observations within patients and clinics, and a relatively long planned follow-up time, our principal, analytic approach was to use multi-level, mixed effects, longitudinal linear and logistic regression models to assess the intervention effect23,24. In this approach, the test for differences in the slope of change in outcomes between the intervention and control groups, expressed as a time by outcome interaction term, accounts for any difference in baseline values. Because patients could be seen by different providers at the clinic at different times, it was not possible to also account for individual provider effects. We conducted additional linear and logistic regression analyses to explore the relationship between intervention uptake and outcomes.
The endpoint for each patient was defined as the clinic BP recorded on their last visit during the study follow-up period. In the mixed effects regression models, we expressed follow-up time as number of months from the baseline visit. The fixed effects in the models were patient’s baseline characteristics including age, sex, race, education, and presence or absence of diabetes. In the model specification for random effects, patients were nested within clinic and BP measurements within patients. We fitted models with a random intercept for clinic effects and a random intercept and slope for patient-level effects. The structure of the covariance matrix for within-patient BP change was selected after testing a number of possible alternatives, including autoregressive and spatial. Based on the AIC fit statistic and the correlation of slope of BP change with time, we fit the models with an unstructured covariance for the patient-specific intercepts and slopes. To account for possible non-linear BP change, we tested models that included a quadratic term for follow-up months. We selected the final models with a quadratic trend based on improvement in fit statistics. Analyses were performed using STATA version 10 and SAS 9.2.
RESULTS
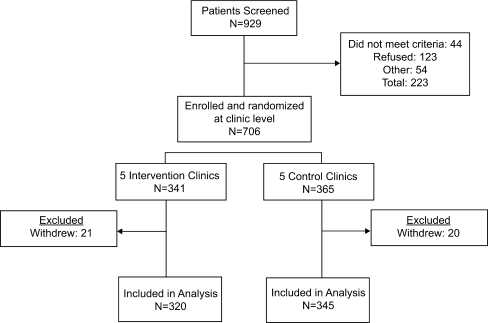
Figure 1 reflects the number of patients screened, enrolled, and included in the analysis. Recruitment occurred between January 2006 and March 2007. By chance, the clinic with the smallest total population was assigned to the intervention condition and had fewer uncontrolled hypertensives than expected. As a result, there were slightly more patients in the UCCs than the ICs.
Figure 1.
Consort diagram. Patients were included in the analysis until death or censoring at their last recorded visit. Five deaths occurred in intervention patients (1 at 9 months and 4 after >12 months of follow-up), and 8 death occurred in the control group (all after >12 months of follow-up).
Table 1 describes the characteristics of the patients in the ICs compared to the UCCs. The two groups were balanced with regard to age, race/ethnicity, education, BMI, and prevalence of type 2 diabetes. However, fewer patients in the ICs were male (25.9% compared to 37.1%) and employed (44.8% compared to 53.0%), and more patients in the ICs were smokers (34.5% compared to 25.4%). Baseline clinic SBP and DBP were higher in the IC patients.
Table 1.
Patient Characteristics at Baseline
| ICs | UCCs | Total* | p | |
|---|---|---|---|---|
| (n = 320) | (n = 345) | (n = 665) | ||
| Age (years, mean ± SD) | 55.03 ± 10.3 | 55.2 ± 10.6 | 55.2 ± 10.5 | 0.890 |
| Sex (% male) | 83 (25.9) | 128 (37.1) | 211 (31.7) | 0.002 |
| Public clinic patients | 221 (69.1) | 207 (60.0) | 428 (64.4) | 0.015 |
| Race/ethnicity | N/A | N/A | N/A | 0.600 |
| Black/African American | 204 (63.9) | 216 (64.0) | 420 (63.3) | N/A |
| Hispanic | 85 (26.6) | 87 (25.2) | 172 (25.9) | N/A |
| Non-Hispanic White | 27 (8.5) | 34 (9.9) | 61 (9.2) | N/A |
| Other | 4 (1.3) | 8 (2.3) | 12 (1.8) | N/A |
| Employment status | N/A | N/A | N/A | 0.003 |
| Employed | 143 (44.8) | 183 (53.0) | 326 (49.1) | N/A |
| Not working | 115 (36.1) | 83 (24.1) | 198 (29.8) | N/A |
| Retired | 61 (19.1) | 79 (22.9) | 140 (21.1) | N/A |
| Education | N/A | N/A | N/A | 0.348 |
| Less than high school | 105 (33.4) | 105 (30.5) | 210 (31.9) | N/A |
| High school or GED | 80 (25.5) | 105 (30.5) | 185 (28.1) | N/A |
| Some college and above | 129 (41.1) | 134 (39.0) | 263 (40.0) | N/A |
| Body mass index (mean ± SD) | 34.57 ± 7.74 | 34.23 ± 7.81 | 34.39 ± 7.77 | 0.566 |
| Diabetes (%) | 150 (46.9) | 181 (52.5) | 331 (49.8) | 0.150 |
| Current smoker (%)* | 108 (34.5) | 87 (25.4) | 195 (29.7) | 0.011 |
| Systolic blood pressure (mmHg) | 150.22 ± 20.34 | 145.41 ± 18.93 | 147.73 ± 19.75 | 0.002 |
| Diastolic blood pressure (mmHg) | 85.83 ± 3.50 | 83.23 ± 13.11 | 84.48 ± 13.35 | 0.012 |
| Number of antihypertensives in regimen | 2.02 ± 1.26 | 1.91 ± 1.38 | 1.96 ± 1.32 | 0.318 |
*Some variables have a small number of missing values
Table 2 presents a set of process measures that reflect the average amount of follow-up time accrued by intervention and control group patients, the number of hypertensive treatment intensifications ordered by physicians in both groups, and the number of uncertainty reduction interventions ordered by physicians in the intervention group clinics over the course of the study. Eighty percent of patients had four or more visits, and the difference in number of clinic visits and follow-up time was not significantly different in the intervention and control groups. Table 2 also reports the use of uncertainty reduction tools by the physicians in the intervention arm. Overall, 40% of patients were referred for one or more of the uncertainty reduction interventions. Of those referred for ABPM, 24% were found to be controlled out of office. Of those referred for electronic bottle cap monitoring, 71% were compliant. The majority of patients referred for lifestyle assessment needed counseling on reduction of dietary sodium, increase in fruit and vegetable consumption, and increased physical activity.
Table 2.
Follow-up Time, Treatment Intensification and Intervention Exposure by Number of Clinic Follow-up Visits
| Number of visits to clinic | Number (%) of patients | Follow-up time (months)* | Number of treatment intensifications (dose increase or addition of a drug)† | Number (%) of patients referred for an uncertainty reduction intervention (intervention group only)‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC | UCC | IC | UCC | IC | UCC | ABPM§ | MEMS || | Lifestyle¶ | Any Referral | |
| 1 | 14 (4) | 17 (5) | 0 | 0 | 0.5 ± 0.52 | 0.3 ± 0.5 | 1 (7) | 0 | 0 | 1 |
| 2–3 | 36 (11) | 59 (17) | 11.8 ± 9.3 | 12.9 ± 10.3 | 0.9 ± 0.77 | 0.6 ± 0.6 | 6 (17) | 5 (14) | 8 (22) | 12 (27) |
| 4–5 | 60 (19) | 65 (19) | 17.9 ± 8.7 | 16.9 ± 7.7 | 1.3 ± 1.0 | 0.9 ± 0.8 | 10 (17) | 8 (13) | 14 (23) | 19 (31) |
| 6–9 | 117 (37) | 142 (41) | 22.7 ± 5.1 | 23.5 ± 5.2 | 1.7 ± 1.2 | 1.3 ± 1.3 | 29 (25) | 31 (25) | 32 (27) | 57 (49) |
| ≥10 | 84 (26) | 62 (18) | 24.5 ± 4.2 | 24.9 ± 4.5 | 2.8 ± 2.1 | 2.2 ±1.7 | 26 (31) | 26 (31) | 31 (37) | 42 (50) |
| Column total or overall mean | 320 | 345 | 19.8 ± 8.7 | 19.5 ± 9.1 | 1.8 ± 1.5 | 1.3 ± 1.5 | 72 (23) | 70 (22) | 85 (27) | 131 (40) |
*p = 09 for overall mean follow-up months
†p < 0.001 for overall mean difference
‡Denominator for percents = 320
§25% controlled
||71% adherent (≥80% of prescribed doses taken)
¶45 (53%) had high fat diet pattern; 76(89%) had low fruit/vegetable intake; 60 (71%) had sedentary lifestyle; 18 (21%) smoked
We report the raw, unadjusted changes in overall BP control, and in clinic SBP and DBP in IC patients compared to UCC patients in Table 3. As dictated by the eligibility criteria, all patients were uncontrolled at baseline. The proportion defined as controlled at their last observed clinic visit was 35.0% in the intervention group and 31.9% in the control group, a difference that was not statistically significant. However, the reduction in clinic SPB and DBP was significantly greater in intervention clinic patients than control clinic patients.
Table 3.
Unadjusted Changes in Blood Pressure Control in Intervention and Control Group Patients
| IC | UCC | p | |
|---|---|---|---|
| Defined control (percent) | 35.0 | 31.9 | 0.395 |
| Change in SBP (mean ± SD) | −10.02 ±24.67 | −4.60 ± 22.87 | 0.003 |
| Change in DBP (mean ± SD) | −5.34 ± 14.33 | −0.49 ± 13.62 | <0.001 |
*p values are for the comparison between change values for intervention and control clinic patients. A simple independent samples chi-square test was used to test the difference in proportion controlled at last visit, and the independent samples t-test was used to test mean change in SBP and DBP
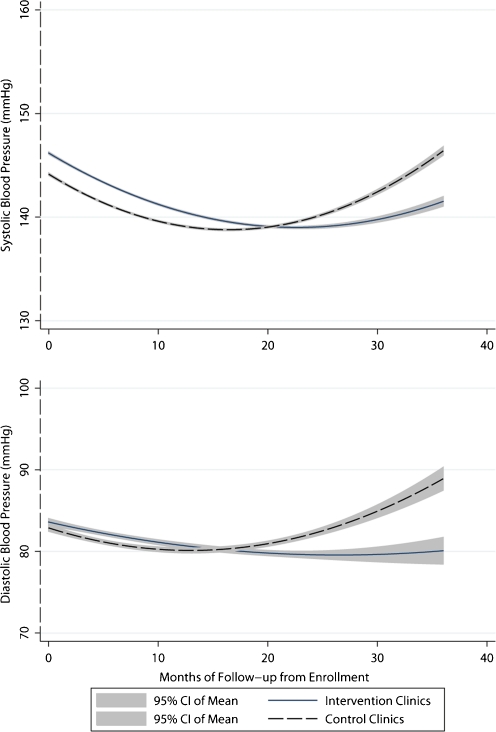
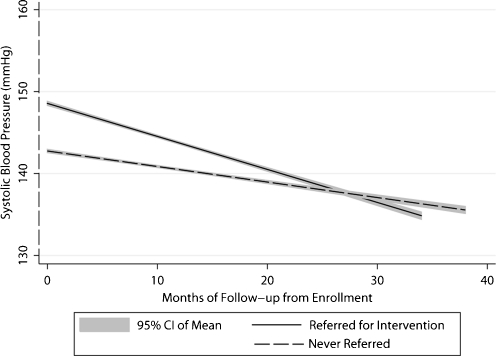
In mixed-effects logistic regression modeling, the dichotomous outcome remained non-significant. The modeling of SBP and DBP as continuous variables indicated that the slope of both SBP and DBP was best described with a quadratic trend over time, which favored lower BPs in ICs compared to UCCs. The graphs in Figure 2 indicate that during the early months of the intervention, SBP and DBP declined in both intervention and control clinics. After approximately 18 months, both SBP and DBP began to rise in UCCs but not ICs. By 36 months, the model-predicted SBP and DBP were 6.5 mmHg lower in ICs than UCCs. (The equivalence of this difference is coincidental, with results from the coefficient for group by time squared interaction being the same for SBP and DBP). Additional details on modeling results and predicted differences are provided in the appendix material (accessible online). Since providers in ICs chose to order one or more intervention tools for only 40% of enrolled patients, we examined the effects of exposure to the intervention within ICs. As shown in Figure 3, patients referred for monitoring or lifestyle assessment and counseling had higher baseline BPs that improved significantly compared to patients not referred. Because of the large proportion of patients who received more than one uncertainty reduction procedure simultaneously, we cannot assess the impact of each tool individually.
Figure 2.
Systolic and diastolic blood pressure trend in intervention compared to control clinics. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) trends in intervention and control clinics predicted from the mixed effects models. The SBP prediction equation was: 141.48 + 070 (age) - 1.738 (sex) + 2.540 (black) - 0.658 (follow-up month) + 2.291(group assignment) + 0.020 (follow-up month squared) - 0.005 (follow-up month squared * group assignment). The DBP prediction equation was: 110.03 - 0.393 (age) - 3.786 (sex) + 3.215 (black) - 2.577 (diabetes) - 0.256 (follow-up month) + 0.1.026 (group assignment) + 0.010 (follow-up month squared) - 0.005 (follow-up month squared * group assignment). Education, smoking, and health system were not significant predictors of SBP or DBP change, and were omitted from final models to preserve degrees of freedom. Diabetes was not associated with SBP change. The p-values for (months squared × group) interaction term were 0.048 and 0.001 for the SBP and DPB models, respectively. The model-predicted difference in SBP in the intervention group compared to the control group is -0.749 mmHg at 12 months, -3.00 mmHg at 24 months, and −6.48 mmHg at 36 months. The model predicted difference in DBP in the intervention group compared to the control group is -0.720 mmHg at 12 months, -2.88 mmHg at 24 months, and -6.48 mmHg at 36 months.
Figure 3.
Blood pressure trend in patients referred for any uncertainty reduction intervention compared to patients never referred (intervention clinics only). SBP trend in patients referred for any uncertainty reduction intervention compared to patients never referred in the intervention clinics. The mixed effects linear regression coefficient associated with referral was 5.70 ± 1.70 (p < 0.001), and the coefficient for the interaction of referral by follow-up month was -0.21 ± 0.11 (p = 0.048).
To determine whether the BP reduction in ICs could be attributed to treatment intensification, we constructed longitudinal logistic regression models to calculate the odds ratio (OR) for adding a drug or increasing a drug dosage in ICs compared to UCCs. Adjusting for SBP at the encounter, the OR for treatment intensification in ICs vs. UCCs was 1.29 (95% CI = 1.10–1.51, p = 0.001), and adjusting for DBP at the encounter, the OR was 1.35 (95% CI = 1.14–1.50, p ≤ 0.001). In mixed models associating longitudinal changes in SBP and DBP with the number of treatment intensifications, a greater number of treatment intensifications was significantly associated with lower SBP over time (beta for the number of intensifications by follow-up months interaction = -0.078, p ≤ 0.001). The interaction term in the DBP model did not reach statistical significance (beta = -0.02, p = 0.085). The absolute probability of a treatment intensification was strongly related to BP at the visit; in ICs, treatment intensification occurred in 24% of encounters where SBP was in the 140–149 mmHg range, 34% where SBP was in the 150–159 mmHg range, and 41% of encounters when SBP ≥ 160 mmHg. Presence of diabetes was not associated with the probability of treatment intensification in the ICs (OR = 1.01, 95% CI = 0.81, 1.25 for the SBP model, and OR = 1.24, 95% CI 0.99, 1.56 for the DBP model). AAs had significantly higher SBP and DBP than non-AAs throughout the follow-up period, and our longitudinal modeling did not show a significantly different rate of BP change in AAs. Adverse events, including ER visits and hospitalizations for cardiovascular and non-cardiovascular causes, were similar in the IC and UCC groups.
DISCUSSION
This clinic-level intervention did not achieve the pre-specified difference in proportion of initially uncontrolled hypertensives who reached the threshold of <140/90 mmHg (130/80 mmHg if diabetic). However, it was clear that patients in intervention clinics had a significantly different trajectory of measured BP change over time that supported an effect of the uncertainty reduction tools in BP management. Process measures, including evidence of more aggressive drug titration, and a significant decline in BP in patients within the ICs who were referred for monitoring and counseling, supported the hypothesis that reducing uncertainty about the need for treatment intensification would help to overcome clinical inertia and thereby lead to better BP control. In addition, the finding that 25% of patients referred for ABPM had adequate BP control and 30% of monitored patients were non-adherent to medications suggests that clinician uncertainty over the need to intensify treatment is often warranted.
Despite the significantly lower BP achieved in ICs compared to UCCs over time, hypertension control as a categorical construct did not improve. Several factors may have limited the categorical effect size. To maximize acceptance by providers and generalizability, our intervention left the decisions regarding which patients to refer for monitoring and/or counseling, and which management actions to take after receiving the results to the individual providers. Our baseline educational program stressed the importance of treating BP to less than 140/90 mmHg (130/80 mmHg), and in the knowledge and attitudes survey that preceded the educational session, over 90 percent of providers reported that they sought these targets in their practices. However, the trend line in Figure 3 and evidence that treatment intensifications were relatively unlikely when SBP was less than 150 mmHg indicate clearly that providers’ real threshold for an action to lower SBP was closer to 150 mmHg. Since the mean baseline BP was higher in ICs than UCCs, the BP lowering achieved with uncertainty reduction tools did not translate to the expected difference in categorically defined control. Finally, physicians did not act aggressively to reach the lower recommended treatment targets in the large number of patient with diabetes who qualified for the study with SBP ≥130–139 mmHg or DBP ≥80 mmHg.
The clinicians in our system are not unique in failing to adhere stringently to the recommended treatment goals. A recent study in North Carolina also found that physicians are unlikely to take treatment actions when SBP is just above the 140 mmHg line25. The discussion about the appropriate goals for hypertension management may become more complex with recent publication of trials that do not clearly support targets less than 140 mmHg, even in high-risk patients26–28. While uncertainty reduction tools can help clinicians make a treatment decision, they cannot be expected to change the actual treatment target.
Our intervention design had both strengths and limitations. We did not attempt to alter routine care delivery; thus, patients had variable numbers of visits at variable intervals. Both clinicians and patients may have had multiple concerns and were not obligated to address BP control issues at all visits. Many successful hypertension interventions rely on an additional provider, such as a nurse or pharmacist, who focuses exclusively on BP control29. In our study, providers were allowed to make independent judgments about the value of ordering uncertainty reduction procedures in a particular patient and the actions that should be taken after receiving the results. An alternative approach that has shown substantial BP reductions requires providers to follow a rigid drug titration protocol to achieve guideline mandated targets27,30. Although our reliance on providers’ individual decision-making may have attenuated the effect size, our results are applicable to the large number of practice settings that do not have a nurse or pharmacist to augment hypertension management. Many aspects of our intervention could easily be incorporated into current office practice models, and all components could be implemented in a team-based medical home model.
It is encouraging that the magnitude of the intervention effect was similar in public vs. private clinics, and in AA patients compared to non-AAs. Nevertheless, the finding that BP levels in AAs remained higher than in non-AAs exposed to the same treatment conditions indicates that achieving satisfactory BP control in AAs remains a challenge.
Baseline BP and group assignment were not related to the number of follow-up visits or time. Thus, our results are unlikely to be biased by differential losses to follow-up. However, the longitudinal modeling results make it clear that patients with a longer follow-up period were more likely to benefit from the intervention than those with shorter follow-ups.
In summary, we demonstrated that the introduction of a set of tools to reduce uncertainty over usual BP and medication adherence led to significantly lower SBP and DBP in five intervention clinics compared to clinics where the tools were not available. Within the ICs, BP was reduced sharply in patients who were referred for monitoring or lifestyle assessment and counseling. However, the expectation that a clinic level intervention in which providers determined when to employ the uncertainty reduction tools would lead to a 20% difference in proportion controlled was not met. The study supports our hypothesis that reducing uncertainty about the reason patients’ BP is poorly controlled may be an effective strategy to improved BP control, but additional research on the conditions under which these tools can be optimally effective is needed.
Electronic Supplementary Material
(DOC 45 kb)
Acknowledgements
The study was funded by the National Heart Lungs and Blood Institute (1 RO1 HL078589). The sponsor did not participate in the design, analysis, or interpretation of study results.
Conflicts of Interest
Dr. Victor Simms was on the speaker panels of Novartis and Forest pharmaceuticals. These relationships were terminated at the beginning of 2011.
REFERENCES
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345(7):479–486. doi: 10.1056/NEJMoa010273. [DOI] [PubMed] [Google Scholar]
- 4.Wetzels GE, Nelemans P, Schouten JS, Prins MH. Facts and fiction of poor compliance as a cause of inadequate blood pressure control: a systematic review. J Hypertens. 2004;22(10):1849–1855. doi: 10.1097/00004872-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Berlowitz D, Ash A, Hickey R, Friedman R, Glickman M, Kader B, Moskowitz M. Inadequate Management of Blood Pressure in a Hypertensive Population. N Engl J Med. 1998;339:1957–1963. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 6.Rose AJ, Berlowitz DR, Orner MB, Kressin NR. Understanding uncontrolled hypertension: is it the patient or the provider? J Clin Hypertens (Greenwich) 2007;9(12):937–943. doi: 10.1111/j.1524-6175.2007.07332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677–2678. doi: 10.1001/archinte.163.22.2677. [DOI] [PubMed] [Google Scholar]
- 9.Rose AJ, Shimada SL, Rothendler JA, et al. The accuracy of clinician perceptions of "usual" blood pressure control. J Gen Intern Med. 2008;23(2):180–183. doi: 10.1007/s11606-007-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr EA, Zikmund-Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148(10):717–727. doi: 10.7326/0003-4819-148-10-200805200-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hyman DJ, Pavlik VN. Self-reported hypertension treatment practices among primary care physicians: blood pressure thresholds, drug choices, and the role of guidelines and evidence-based medicine. Arch Intern Med. 2000;160(15):2281–2286. doi: 10.1001/archinte.160.15.2281. [DOI] [PubMed] [Google Scholar]
- 12.Hurley LP, Dickinson LM, Estacio RO, Steiner JF, Havranek EP. Prediction of cardiovascular death in racial/ethnic minorities using Framingham risk factors. Circ Cardiovasc Qual Outcomes. 2010;3(2):181–187. doi: 10.1161/CIRCOUTCOMES.108.831073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings DM, Doherty L, Howard G, et al. Blood pressure control in diabetes: temporal progress yet persistent racial disparities: national results from the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study. Diabetes Care. 2010;33(4):798–803. doi: 10.2337/dc09-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 15.Umscheid CA, Gross R, Weiner MG, Hollenbeak CS, Tang SS, Turner BJ. Racial disparities in hypertension control, but not treatment intensification. Am J Hypertens. 2010;23(1):54–61. doi: 10.1038/ajh.2009.201. [DOI] [PubMed] [Google Scholar]
- 16.Manze M, Rose AJ, Orner MB, Berlowitz DR, Kressin NR. Understanding racial disparities in treatment intensification for hypertension management. J Gen Intern Med. 2010;25(8):819–825. doi: 10.1007/s11606-010-1342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlik V, Greisinger A, Pool J, Haidet P, Hyman D. Does reducing physician uncertainty improve hypertension control: Rationale and Methods. Circ Cardiovasc Qual Outcomes. 2009;2:257–263. doi: 10.1161/CIRCOUTCOMES.109.849984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18(4):284–288. doi: 10.1016/S0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 19.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 20.Hyman DJ, Pavlik VN, Taylor WC, Goodrick GK, Moye L. Simultaneous vs sequential counseling for multiple behavior change. Arch Intern Med. 2007;167(11):1152–1158. doi: 10.1001/archinte.167.11.1152. [DOI] [PubMed] [Google Scholar]
- 21.Appel LJ, Giles TD, Black HR, et al. ASH position paper: dietary approaches to lower blood pressure. J Am Soc Hypertens. 2010;4(2):79–89. doi: 10.1016/j.jash.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Donner A, Klar N. Design and Analysis of Cluster Randomization Trials in Health Research. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 23.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons, Inc.; 2004. [Google Scholar]
- 24.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. College Station, TX: Stata Press; 2008. [Google Scholar]
- 25.Viera AJ, Schmid D, Bostrom S, Yow A, Lawrence W, DuBard CA. Level of blood pressure above goal and clinical inertia in a Medicaid population. J Am Soc Hypertens. 2010;4(5):244–254. doi: 10.1016/j.jash.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appel LJ, Wright JT, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Group JS. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS) Hypertens Res. 2008;31(12):2115–2127. doi: 10.1291/hypres.31.2115. [DOI] [PubMed] [Google Scholar]
- 29.Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169(19):1748–1755. doi: 10.1001/archinternmed.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naik AD, Rodriguez E, Rao R, Teinert D, Abraham NS, Kalavar J. Quality improvement initiative for rapid induction of hypertension control in primary care. Circ Cardiovasc Qual Outcomes. 2010;3(5):558–564. doi: 10.1161/CIRCOUTCOMES.109.913137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 45 kb)