Abstract
In the present study, we observed that the adenocarcinoma component in the mucosa was continuous with neuroendocrine carcinoma (NEC) in the deeper layers; this suggests the normal course of NEC carcinogenesis at the histological level. A 72-year-old man was admitted to our hospital with a chief complaint of tarry stools. Endoscopic examination of the upper gastrointestinal tract revealed a 2-cm tumor, with a deep central depression, surrounded by a smooth elevated area, in the middle of the stomach body. A biopsy showed that the tumor was a moderately differentiated gastric adenocarcinoma. The patient underwent total gastrectomy and standard lymph node dissection. The resected tumor was a 3.5 × 2.5 cm type 2 lesion. It comprised two elements at the histological level: (i) a moderately differentiated adenocarcinoma in the superficial portion of the mucous membrane layer, and (ii) NEC-like cells with dark, round nuclei and scant cytoplasm, presenting a solid and trabecular pattern, in the submucosal and muscularis propria layers. Immunohistochemical findings showed that the NEC-like cells were diffusely positive for chromogranin A, synaptophysin, neural cell adhesion molecule, and neuron-specific enolase, but were negative for carcinoembryonic antigen. The Ki-67 labeling index was 95%. The final pathological diagnosis was gastric NEC with an adenocarcinoma component and a high cellular proliferative potential.
Key Words: Neuroendocrine carcinoma, Endocrine cell carcinoma, Gastric cancer, Adenocarcinomatous differentiation
Introduction
Neuroendocrine carcinoma (NEC) rarely occurs in the gastrointestinal tract and accounts for approximately 1% of cancers in the esophagus, 0.2% in the colon, and 0.1–0.4% in the stomach [1, 2]. NEC has been variously termed ‘endocrine cell carcinoma’, ‘small cell carcinoma’, ‘atypical carcinoid’, and ‘oat-cell carcinoma’ [3]. Gastric NEC is characterized by endocrine cell differentiation and classified as either a pure-type tumor or a composite-type tumor containing an admixture of adenocarcinoma and/or differentiated squamous type cells [4]. Gastric NEC arises predominantly from endocrine precursor cell clones that develop in the preceding adenocarcinoma component. These clones transform into NEC and the NEC develops rapidly in the submucosal and deeper layers [5]. Therefore, it is difficult to make a definitive diagnosis of NEC by endoscopic biopsy. Immunohistochemical staining for several neuroendocrine markers – chromogranin A, synaptophysin, etc. – is helpful in making a definite diagnosis of NEC. The prognosis of gastric NEC is extremely poor, and lymph node and liver metastases are observed frequently. However, there is no standardized chemotherapy. In the present study, we observed that the adenocarcinoma component in the mucosa was continuous with the NEC in the deeper layers; this suggests the normal course of NEC carcinogenesis at the histological level.
Case Report
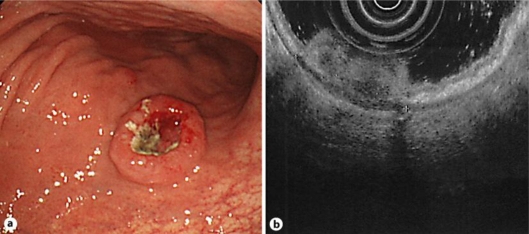
The patient was a 72-year-old man who was born healthy. He was admitted to our hospital with a chief complaint of tarry stools. Physical examination revealed anemia in the palpebral conjunctiva, but did not show a mass in the abdomen or enlargement of any superficial lymph nodes. The hemoglobin level was 9.8 g/dl and the albumin level was 3.2 g/dl, but the other hematological tests, including those for the serum levels of tumor markers such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9, yielded normal results. Endoscopic examination of the upper gastrointestinal tract revealed a 2-cm tumor, with a deep central depression, surrounded by a smooth elevated area, in the middle of the stomach body. A biopsy showed that the tumor was a moderately differentiated gastric adenocarcinoma (fig. 1a). Ultrasound endoscopic examination showed that the tumor invaded the subserosa (fig. 1b). Radiography of the upper gastrointestinal tract showed the presence of a mass with central ulceration and a clear margin, surrounded by a smooth elevated area, in the posterior wall of the stomach body. Computed tomography (CT) showed two lymph nodes each measuring 10 mm in diameter on the lesser curve of the stomach, suggestive of metastasis, but we neither observed a thickened gastric wall, which could be due to the tumor, nor distant metastasis. Total gastrectomy, standard lymph node dissection and Roux-en-Y reconstruction with a jejunal pouch were performed without any complication.
Fig. 1.
a Endoscopic examination of the upper gastrointestinal tract revealed a 2-cm tumor, with a deep central depression, surrounded by a smooth elevated area, in the middle of the stomach body. b Ultrasound endoscopic examination showed that the tumor invaded the subserosa.
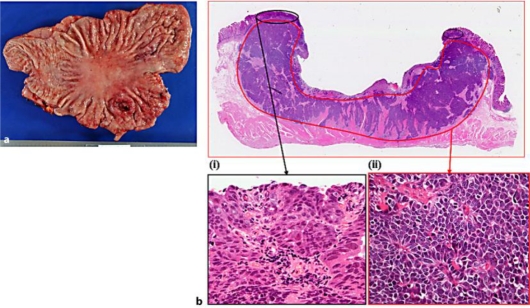
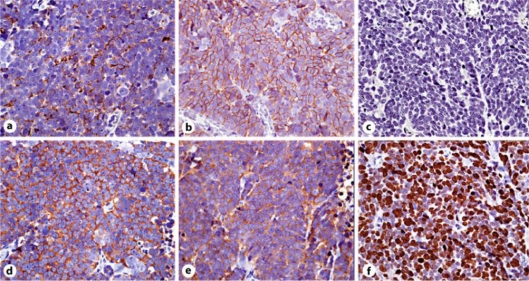
Gross pathological examination showed that the tumor was a 3.5 × 2.5 cm type 2 lesion in the middle of the posterior wall of the stomach body (fig. 2a). Microscopic examination of the resected specimen revealed a large ulcerated tumor invading the muscularis propria, with vascular and lymphatic invasions. The NEC had also metastasized to the regional lymph nodes. Hematoxylin-eosin staining showed that the tumor comprised two elements at the histological level: (i) a moderately differentiated adenocarcinoma in the superficial portion of the mucous membrane layer, and (ii) NEC-like cells with dark, round nuclei and scant cytoplasm, presenting a solid and trabecular pattern, in the submucosal and muscularis propria layers (fig. 2b). Immunohistochemical findings showed that the NEC-like cells were diffusely positive for chromogranin A, synaptophysin, neural cell adhesion molecule (NCAM), and neuron-specific enolase (NSE), but were negative for CEA. The Ki-67 labeling index was 95% (fig. 3). The final pathological diagnosis was gastric NEC with an adenocarcinoma component and a high cellular proliferative potential.
Fig. 2.
a Gross pathological examination showed that the tumor was a 3.5 × 2.5 cm type 2 lesion in the middle of the posterior wall of the stomach body. b At microscopic examination the tumor comprised two elements at the histological level (hematoxylin-eosin staining, ×2): (i) A moderately differentiated adenocarcinoma in the superficial portion of the mucous membrane layer (×40). (ii) NEC-like cells with dark, round nuclei and scant cytoplasm, presenting a solid and trabecular pattern, in the submucosal and muscularis propria layers (×40).
Fig. 3.
Immunohistochemical findings (×40). The NEC-like cells were diffusely positive for chromogranin A (a), synaptophysin (b), NCAM (d) and NSE (e), but were negative for CEA (c). The Ki-67 labeling index (f) was 95%.
Adjuvant chemotherapy consisting of S-1 (tegafur/gimeracil/oteracil potassium) at a dose of 120 mg was administered orally. 6 months after the operation, the patient underwent a CT, the results of which did not show recurrence or metastasis.
Discussion
Most digestive neuroendocrine tumors (NETs) are referred to as carcinoids. Recently, a new classification system for digestive NETs has been formulated and is presented in the 2010 revision of the WHO classification of digestive tumors [6]. It recognizes five main categories: NET G1, NET G2, small cell-type NEC, large cell-type NEC, and mixed adenoneuroendocrine carcinoma. This classification is based on cellular atypia, nuclear abnormalities, the number of mitotic figures, and the Ki-67 labeling index by which the tumor's proliferative activity can be determined. Our patient was diagnosed with gastric small cell-type NEC on the basis of the cells’ high proliferative potential and scant cytoplasm. Moreover, cytoplasmic argyrophilia and presence of structural neurosecretory granules support this diagnosis; however, previous studies have reported that several tumors did not have these features [4, 7]. Therefore, an immunohistochemically positive staining for several neuroendocrine markers – chromogranin A, synaptophysin, synaptic vesicle protein, Leu 7 (CD57), NSE, protein gene product 9.5 (PGP 9.5), NCAM (CD56), and cytokeratin – is helpful in making a definite diagnosis of NEC, in contrast to negative staining for CEA [4, 8]. The patient in our study, too, was positive for some of these neuroendocrine markers.
NEC in the gastrointestinal tract occasionally shows multidirectional differentiation, with adenocarcinoma and/or squamous cell proliferation [4, 9, 10]. Nishikura et al. [5] demonstrated that 70.6% of gastric NEC cases included an adenocarcinoma component in the mucosa and/or submucosa. Furthermore, gastric NEC arises predominantly from endocrine precursor cell clones that develop in the preceding adenocarcinoma component. These clones transform into NECs during rapid clonal expansion, and the NEC develops rapidly in the submucosal and deeper layers [5]. Similar to this observation, our patient had an adenocarcinoma component that was restricted to the mucosal surface, whereas the NEC was found in the deeper layers. Moreover, the two components were continuous; this finding suggested a NEC carcinogenesis at the histological level.
Gastric NEC invades lymphatic and vascular lumens and frequently metastasizes to the lymph nodes and liver, even during the early stages, because of its aggressive biological behavior [4]. Therefore, intensive chemotherapy, with or without surgery, is recommended for gastric NEC at any stage [10]. Previously, cisplatin and etoposide (PE) and cyclophosphamide, doxorubicin, and etoposide (CDE) combinations have been employed for the chemotherapeutic treatment of NEC [11, 12]; however, the chemotherapy has not yet been standardized. Recently, Okita et al. [13] reported that cisplatin plus irinotecan regimens elicited a good response in 12 cases of metastatic or recurrent gastric NEC (the response rate was 75%, the median progression-free survival time was 212 days, and the median survival time was 679 days). The neoadjuvant or adjuvant first-line chemotherapy regimens used for the treatment of gastric adenocarcinoma (S-1 and S-1/cisplatin) have been effective for treating gastric NEC [14, 15]. However, no conclusions can be drawn regarding the most effective regimen because of the small number of patients.
Conclusion
Gastric NEC can coexist with an adenocarcinoma component at the mucosal surface. Therefore, it is difficult to make a definitive diagnosis of NEC preoperatively by endoscopic biopsy. The tumor in our patient was resected curatively without distant metastasis, and this may explain the disease-free survival of the patient for 8 months after the operation. However, we think that it is necessary to follow up this patient carefully and administer adjuvant chemotherapy, keeping in mind the poor prognosis and high cellular proliferative potential of gastric NEC.
References
- 1.Nobin A, Ahren B, Ahlman H. Endocrine tumors in the gastrointestinal tract. Nord Med. 1988;103:12–14. [PubMed] [Google Scholar]
- 2.Jass JR, Sobin LH, Watanabe H. The World Health Organization's histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer. 1990;66:2162–2167. doi: 10.1002/1097-0142(19901115)66:10<2162::aid-cncr2820661020>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Chiba N, Suwa T, Hori M, Sakuma M, Kitajima M. Advanced gastric endocrine cell carcinoma with distant lymph node metastasis: a case report and clinicopathological characteristics of the disease. Gastric Cancer. 2004;7:122–127. doi: 10.1007/s10120-004-0279-2. [DOI] [PubMed] [Google Scholar]
- 4.Matsui K, Kitagawa M, Miwa A, Kuroda Y, Tsuji M. Small cell carcinoma of the stomach: a clinicopathologic study of 17 cases. Am J Gastroenterol. 1991;86:1167–1175. [PubMed] [Google Scholar]
- 5.Nishikura K, Watanabe H, Iwafuchi M, Fujiwara T, Kojima K, Ajioka Y. Carcinogenesis of gastric endocrine cell carcinoma: analysis of histopathology and p53 gene alteration. Gastric Cancer. 2003;6:203–209. doi: 10.1007/s10120-003-0249-0. [DOI] [PubMed] [Google Scholar]
- 6.Scoazec JY, Couvelard A. The new WHO classification of digestive neuroendocrine tumors. Ann Pathol. 2011;31:88–92. doi: 10.1016/j.annpat.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Matsuyama M, Suzuki H. Differentiation of immature mucous cells into parietal, argyrophil, and chief cells in stomach grafts. Science. 1970;169:385–387. doi: 10.1126/science.169.3943.385. [DOI] [PubMed] [Google Scholar]
- 8.Sakaki M, Sano T, Hirokawa M, Takahashi M, Kiyoku H. Immunohistochemical study of endocrine cells in ductal adenocarcinoma of the pancreas. Virchows Arch. 2002;441:249–255. doi: 10.1007/s00428-002-0652-7. [DOI] [PubMed] [Google Scholar]
- 9.Mori M, Matsukuma A, Adachi Y, et al. Small cell carcinoma of the esophagus. Cancer. 1989;63:564–573. doi: 10.1002/1097-0142(19890201)63:3<564::aid-cncr2820630328>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda T, Ohnishi Y, Nishimaki T, Ohtani H, Tachikawa S. Early gastric cancer of the small cell type. Am J Gastroenterol. 1988;83:1176–1179. [PubMed] [Google Scholar]
- 11.Van der Gaast A, Verwey J, Prins E, Splinter TA. Chemotherapy as treatment of choice in extrapulmonary undifferentiated small cell carcinomas. Cancer. 1990;65:422–424. doi: 10.1002/1097-0142(19900201)65:3<422::aid-cncr2820650308>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Moertel CG, Kvols LK, O'Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Okita NT, Kato K, Takahari D, et al. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric Cancer. 2011;14:161–165. doi: 10.1007/s10120-011-0025-5. [DOI] [PubMed] [Google Scholar]
- 14.Hanada N, Inoue K, Osako T, Kuriwaki K, Shimajiri S, Mochinaga M. A case of partial response in liver metastatic lesion from gastric endocrine cell carcinoma treated with TS-1. Gan To Kagaku Ryoho. 2006;33:1143–1146. [PubMed] [Google Scholar]
- 15.Tsushima T, Tsuji Y, Abe S, et al. A case of metastatic gastric endocrine cell carcinoma which could be curably resected after chemotherapy with S-1/CDDP. Gan To Kagaku Ryoho. 2008;35:817–820. [PubMed] [Google Scholar]