Abstract
Solitary neurofibromal colonic polyps are a rare entity, particularly outside the setting of neurofibromatosis type 1. The clinical significance of such lesions has not yet been established. Though typically benign tumors, neurofibromas have been reported to undergo malignant transformation, with an increased risk of malignancy when associated with neurofibromatosis. In this case report, we present the rare case of a man found to have an isolated colonic neurofibroma without any personal/family history or clinical features of neurofibromatosis. A 59-year-old man with a history of dyslipidemia and degenerative joint disease presented for a routine screening colonoscopy. The colonoscopy revealed no abnormalities except a 3 mm transverse colon polyp and another 4 mm polyp in the descending colon. Biopsy results showed the descending colonic polyp to be a tubular adenoma; however, multiple levels of the 3 mm transverse colon polyp revealed interlacing bundles of spindle cells extending into the lamina propria with comma-shaped nuclei consistent with findings seen in neurofibroma. Isolated colonic neurofibromas are rare and understudied. While they are usually benign, they may undergo malignant transformation, especially when associated with neurofibromatosis. Thus, patients presenting with isolated neurofibromas should be followed for development of neurofibromatosis and malignancies.
Key Words: Neurofibroma, Neurofibromatosis, Polyp
Introduction
Neurofibromas typically are benign nerve sheath tumors of the peripheral nervous system, arising from Schwann cells, classically associated with neurofibromatosis type 1 (NF1, von Recklinghausen's disease). Two types of neurofibromas are known to develop in NF1: superficial dermal or cutaneous neurofibromas, composed of a single peripheral nerve, and deep plexiform neurofibromas, which involve multiple nerve fascicles. Dermal neurofibromas typically arise in the second decade of life, often coinciding with the appearance of puberty. Plexiform neurofibromas, on the other hand, are typically congenital lesions and tend to grow mainly in the first decade of life. Unlike dermal neurofibromas, plexiform neurofibromas on rare occasions have been known to undergo malignant transformation and to develop into malignant peripheral nerve sheath tumors, which are aggressive, almost uniformly fatal cancers [1, 2]. About 10% of patients with NF1 are seen to develop these tumors, either from a plexiform neurofibroma or without such precursors [3].
NF1 is a common neurocutaneous disorder with an incidence of approximately 1 in 3,000 individuals [4]. It is an autosomal dominant disorder with few cases of sporadic inheritance, caused by any of a number of mutations of the Nf1 gene, mapped on chromosome 17q11.2. Nf1 is a tumor suppressor gene and produces the protein neurofibromin, which in turn is responsible for downregulating Ras, another cell protein that is thought to promote cell growth and proliferation [2, 5]. Increased risk of tumorigenesis is only seen in the homozygous state [6]. Nf1 has a high penetrance, and so most patients with the mutation have some clinical features. Current genetic testing is able to identify 95% of Nf1 mutations [7]. However, genetic testing is usually unnecessary, since clinical features can establish the diagnosis in about 95% of cases.
According to the NIH, two of the following diagnostic criteria should be present to make the diagnosis of NF1: =6 café au lait macules, =2 neurofibromas of any type or 1 plexiform neurofibroma, freckling in the axillary or inguinal regions, optic glioma, =2 iris hamartomas (Lisch nodules), bony lesions (pseudoarthrosis), or a first-degree relative with NF1. This condition has variable clinical expressions with manifestations involving many systems, such as the skin, eyes, bones, nervous system and gastrointestinal tract. The disease is equally prevalent in all ethnic groups.
Gastrointestinal involvement has been documented in 25% of patients with NF1, mostly involving the stomach and small intestine [8, 9]. The involvement consists of (1) ganglioneuromas: hyperplasia and hypertrophy of the nerve plexuses and ganglionic cells in the mucosa, and/or (2) neurofibromas: hyperplasia of neuronal cells in the submucosa, muscularis propria or even from the serosa. Sometimes it is also associated with gastrointestinal stromal tumors with different degrees of neuronal and smooth muscle differentiation [8, 10].
Case Report
A 59-year-old black man with a history of controlled dyslipidemia, hypertension, depression, benign prostatic hyperplasia, erosive gastritis, chronic pain syndrome and degenerative joint disease presented for a routine screening colonoscopy. His medical history was also significant for bilateral hydroceles and one previous hospitalization 6 years before for community-acquired pneumonia. His medications included aspirin 81 mg daily, finasteride 5 mg daily, risperidone 6 mg daily, trazedone 100 mg daily, fluoxetine 40 mg daily, hydrochlorothiazide 25 mg daily, omeprazole 20 mg daily, and tramadol 50 mg three times per day. He did not have any significant history of abdominal pain or bloating, diarrhea or constipation, or any melena or hematochezia. The patient denied any recent episodes of fevers, chills, nausea, or vomiting. He reported a stable appetite and weight. He had no history of alcohol, smoking, or drug abuse. His family history did not include any neurofibromatosis or any gastrointestinal malignancies. Physical examination of the head, neck, lungs, heart, skin and other systems did not reveal any significant findings. Ophthalmological examination revealed no Lisch nodules. Laboratory examinations, including a complete blood count, metabolic panel, hepatic panel and coagulation tests were all within normal limits.
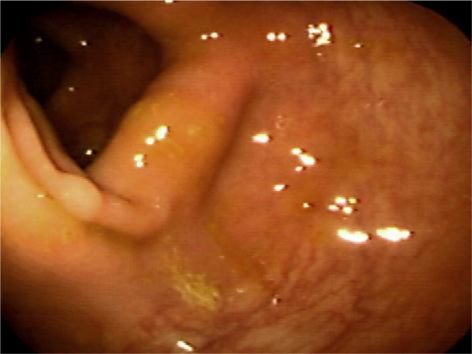
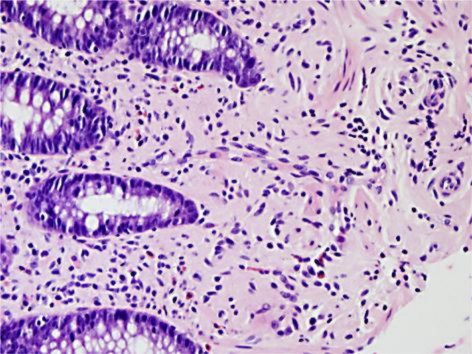
On colonoscopic examination, a 3 mm polyp was found in the transverse colon (fig. 1) and another 4 mm polyp in the descending colon; both were biopsied. The remainder of the colonoscopy did not reveal any additional abnormal macroscopic pathology. Histological examination of the descending colonic polyp revealed findings consistent with a tubular adenoma; however, multiple levels of the 3 mm transverse colon polyp revealed interlacing bundles of spindle cells extending into the lamina propria with comma-shaped nuclei consistent with findings seen in neurofibroma (fig. 2). Subsequent immunohistochemical stains were performed on the specimen, which disclosed that S100 was expressed in the majority of the cell nuclei, also compatible with neurofibroma. CD117 staining was performed twice but had to be voided because of tissue loss.
Fig. 1.
Photograph of a 3 mm polyp of the transverse colon, found on biopsy to be a neurofibroma.
Fig. 2.
Photomicrograph of a transverse colon polyp. A high-power field showed interlacing bundles of spindle cells, with comma-shaped nuclei, extending into the lamina propria, consistent with findings of a neurofibroma. Staining was positive for nuclear S100.
Discussion
In this case, the patient had no personal or familial history of neurofibromatosis, nor did he have any of the physical manifestations that define NF1. He presented for routine screening colonoscopy that detected two polyps, with subsequent histology revealing a neurofibroma, confirmed by S100 staining. This is the fourth reported case of isolated colonic neurofibroma in a patient without NF1, and only the second one found in an asymptomatic patient undergoing routine colorectal cancer screening[11,12,13]. Unfortunately, this patient was lost to follow-up and no repeat colonoscopies were performed. It was discovered later that he died of complications related to an aggressive lymphoma.
The clinical significance of isolated, intestinal neurofibromas has not been well established. No consensus has yet been reached in the medical community as to whether such lesions represent different phenotypic manifestations of neurofibromatosis or separate and distinct entities entirely [14]. Though typically benign lesions, they have been reported to undergo malignant transformation, particularly when associated with NF1 and in larger, plexiform lesions [15]. Though uncommon, there are reports in the literature of neurofibromas and nerve sheath tumors presenting as lymphomas [16, 17].
Gastrointestinal neurofibromas are usually asymptomatic, but may present with bleeding, anemia, or signs or symptoms of obstruction. Indications for evaluation of gastrointestinal complications in the setting of NF1 include unexplained anemia, weight loss, gastrointestinal bleeding, abdominal pain, emesis, chronic diarrhea or signs of malabsorption [10, 18]. Occasionally, isolated intestinal neurofibromas may be the initial sign of NF1 in patients without any other clinical manifestations of the disease. As discussed previously, NF1 is a tumor predisposition syndrome and thus requires some degree of surveillance, consisting mainly of regular, comprehensive physical examinations aimed at the early detection and treatment of complications as they occur [19]. This case highlights the need for close clinical follow-up of patients found to have isolated neurofibromas to exclude NF1, as isolated neurofibromas are rare entities and have an increased risk of malignant transformation when associated with NF1 [20].
References
- 1.Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62:1573–1577. [PubMed] [Google Scholar]
- 2.Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the gap. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 3.Rubin JB, Gutmann DH. Neurofibromatosis type 1 – a model for nervous system tumour formation? Nat Rev Cancer. 2005;5:557–564. doi: 10.1038/nrc1653. [DOI] [PubMed] [Google Scholar]
- 4.Goldman L, Ausiello DA. Cecil Textbook of Medicine. ed 22. Philadelphia: WB Saunders; 2004. ch 459. [Google Scholar]
- 5.Gutmann DH, Collins FS. The neurofibromatosis type 1 gene and its protein product, neurofibromin. Neuron. 1993;10:335–343. doi: 10.1016/0896-6273(93)90324-k. [DOI] [PubMed] [Google Scholar]
- 6.Knudson AG. Two genetic hits (more or less) to cancer. Nat Rev Cancer. 2001;1:157–162. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- 7.Messiaen LM, Callens T, Mortier G, Beysen D, Vandenbroucke I, Van Roy N, Speleman F, Paepe AD. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat. 2000;15:541–555. doi: 10.1002/1098-1004(200006)15:6<541::AID-HUMU6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Bononi M, De Cesare A, Stella MC, Fiori E, Galati G, Atella F, Angelini M, Cimitan A, Lemos A, Cangemi V. Isolated intestinal neurofibromatosis of colon. Single case report and review of the literature. Dig Liver Dis. 2000;32:737–742. doi: 10.1016/s1590-8658(00)80340-0. [DOI] [PubMed] [Google Scholar]
- 9.Kim HR, Kim YJ. Neurofibromatosis of the colon and rectum combined with other manifestations of von Recklinghausen's disease: case report. Dis Colon Rectum. 1998;41:1187–1192. doi: 10.1007/BF02239443. [DOI] [PubMed] [Google Scholar]
- 10.Fuller CE, Williams GT. Gastrointestinal manifestations of type 1 neurofibromatosis. Histopathology. 1991;19:1–11. doi: 10.1111/j.1365-2559.1991.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 11.Panteris V, Vassilakaki T, Vaitsis N, Elemenoglou I, Mylonakou I, Karamanolis DG. Solitary colonic neurofibroma in a patient with transient segmental colitis: case report. World J Gastroenterol. 2005;11:5573–5576. doi: 10.3748/wjg.v11.i35.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abramson LP, Orkin BA, Schwartz AM. Isolated colonic neurofibroma manifested by massive lower gastrointestinal bleeding and intussusception. South Med J. 1997;90:952–954. doi: 10.1097/00007611-199709000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Weisen A, Davidoff S, Sideridis K, Greenberg R, Bank S, Falkowski O. Neurofibroma in the colon. J Clin Gastroenterol. 2006;40:85–86. doi: 10.1097/01.mcg.0000190777.42656.19. [DOI] [PubMed] [Google Scholar]
- 14.Carter JE, Laurini JA. Isolated intestinal neurofibromatous proliferations in the absence of associated systemic syndromes. World J Gastroenterol. 2008;14:6569–6571. doi: 10.3748/wjg.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddle ND, Gorden L, Rojiani MV, Hakam A, Rojiani AM. CD44 and p53 immunoexpression patterns in NF1 neoplasms – indicators of malignancy and infiltration. Int J Clin Exp Pathol. 2010;3:515–521. [PMC free article] [PubMed] [Google Scholar]
- 16.Kapoor R, Resvesz T, Powell M. Solitary cervical lymphoma presenting as a neurofibroma. Br J Neurosurg. 1992;6:583–586. doi: 10.3109/02688699209002376. [DOI] [PubMed] [Google Scholar]
- 17.Radi MJ, Foucar E, Palmer CH, Gooding RA. Malignant lymphoma arising in a large congenital neurofibroma of the head and neck. Report of a case. Cancer. 1988;61:1667–1673. doi: 10.1002/1097-0142(19880415)61:8<1667::aid-cncr2820610826>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Rudolph CD, Hyman PE, Altschuler SM, Christensen J, Colletti RB, Cucchiara S, Di Lorenzo C, Flores AF, Hillemeier AC, McCallum RW, Vanderhoof JA. Diagnosis and treatment of chronic intestinal pseudo-obstruction in children: report of consensus workshop. J Pediatr Gastroenterol Nutr. 1997;24:102–112. doi: 10.1097/00005176-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Hersh JH, American Academy of Pediatrics Committee on Genetics Health supervision for children with neurofibromatosis. Pediatrics. 2008;121:633–642. doi: 10.1542/peds.2007-3364. [DOI] [PubMed] [Google Scholar]
- 20.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]