Abstract
Female SNF1 hybrid mice spontaneously develop an immune complex–mediated glomerulonephritis as early as 24 weeks of age, whereas the disease onset in males is much slower. Further, a rise in concentration of glomerulus-specific autoantibodies via autoreactive B cells is critical to progression of the disease in this strain. Environmental factors contributing to the onset or degree of such autoimmunity are of interest yet poorly understood. In the present study, time-pregnant SWR × NZB dams (10/treatment) were gavaged on gestational 12 with 40 or 80 mg/kg 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), and the SNF1 offspring were evaluated at 24 weeks of age. Bone marrow B220lowCD24−AA4.1+ committed B lineage progenitors were increased in female offspring by TCDD, however, committed progenitors and pro-B cells were decreased in males. Splenic marginal zone B cells (CD21hiCD24low−int) were decreased and follicular B cells (CD21intCD24low) were increased across sex by prenatal TCDD, whereas transitional-2 B cells (CD21intCD24hi) and (CD23low−int CD1low−int) were decreased in males only. Antibodies to double-stranded DNA were significantly increased across sex by TCDD. Anti-IgG and anti-C3 immune complex renal deposition was visibly worsened in females, and present in TCDD-treated males. These data suggest that developmental exposure to TCDD permanently and differentially alters humoral immune function by sex, and exacerbates a type III hypersensitivity lupus-like autoimmune disease in genetically predisposed mice.
Keywords: TCDD, prenatal exposure, autoimmunity, lupus, SNF1 mouse
The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on the immune system are primarily mediated through the aromatic hydrocarbon (AhR) (Hogaboam et al., 2008; Hundeiker et al., 1999). During development, constant or cyclic AhR activation can result in permanent immune changes that persist into adulthood. These effects are greatly influenced by the affinity of the AhR (high vs. low) relative to the dose of receptor ligand encountered. For example, C57BL/6 mice express a high affinity AhR and are highly sensitive to TCDD. SNF1 mice, however, express a low affinity AhR and require an approximately 8-fold increase in TCDD dose to equal the C57BL/6 response (Mustafa et al., 2008).
Prenatal exposure of rodents to a growing list of immunotoxicants has been shown to result in persistent immune function deficits in the offspring (Dietert and Piepenbrink et al., 2006; Holladay and Smialowicz, 2000). Exposure of pregnant mice to TCDD caused, in offspring, depressed T-lymphocyte proliferation (Vos et al., 1974), graft-versus-host reactivity and skin graft rejection times (Vos et al., 1974), cytotoxic T-lymphocyte activity (Holladay et al., 1991), and delayed-type hypersensitivity (DTH) responses (Blaylock et al., 1992; Faith and Moore, 1977; Gehrs and Smialowicz et al., 1997; Gehrs et al., 1997). Dosing of mid-gestation pregnant rats with as little as 0.1 mg/kg TCDD similarly depressed T-lymphocyte activity, as measured by the DTH response, an effect still present in late adulthood (Gehrs and Smialowicz, 1999).
Besides causing immune suppression, several reports suggest that TCDD may increase the risk of autoimmunity. Silverstone et al. (1998) exposed autoimmune-predisposed SNF1 in mid-gestation to TCDD, and reported an accelerated postnatal onset of glomerulonephritis in the normally resistant male SNF1 offspring. The incidence of immune-mediated nephritis is low in autoimmune NZB mice, but when this strain is crossed with normal SWR mice, almost 100% of the female (SWR × NZB)F1 hybrids (SNF1) develop fatal glomerulonephritis by about 8 months of age. The progression of autoimmune disease in these lupus-like SNF1 mice, as in the human version of the disease, is critically dependent on accelerated antibody production. This autoimmune nephritis is then characterized by IgG deposition in kidney glomeruli (Mohan et al., 1993).
Recent work in our laboratory (Mustafa et al., 2008) showed that prenatal TCDD exposure increased late-life autoantibody production in nonautoimmune C57BL/6 mice. Based on this observation, we hypothesized that prenatal exposure to TCDD in autoimmune-prone SNF1 mice may affect B-cell development, maturation, and function in ways that correspond to acceleration or exacerbation of autoimmune lupus. Detecting such changes is a necessary first step toward determining how TCDD and other environmental agents may act as potential risk factors in the development of autoimmune diseases.
METHODS AND MATERIALS
Mice and TCDD exposure.
SWR × NZB (SNF1) mice were obtained by breeding male NZB mice with female SWR/J mice, both from Jackson Laboratories (Bar Harbor, ME). Mice (4–5 weeks of age) were acclimated to the animal care facility for at least 2 weeks prior to breeding. Briefly, 60 SWR females were bred to 30 NZB males overnight in cages containing one NZB male per two SWR females. Plug positive mice, evaluated the next morning, were designated gestation day (GD) 0. Pregnant SWR mice were orally gavaged on GD 12 with 0, 40, or 80 μg/kg TCDD dissolved in corn oil (n = 10 pregnant mice per treatment). This day of dosing was selected to include early establishment of B lymphopoiesis (Holladay and Smialowicz, 2000). The SNF1 offspring were weaned at 20–21 days, separated by treatment, allowed to mature to 24 weeks of age, and evaluated for changes in immune status. At 24 weeks, the untreated females are in the early stages of lupus nephritis, while males are free of clinical signs (Eastcott et al., 1983). All animals were fed a commercial pelleted diet, provided water ad libitum, and housed under controlled conditions of temperature (22°C), humidity (40–60%), and lighting (12:12-h light:dark cycle). Animal maintenance, care, and use were approved prior to initiation of experiments and at all times were in accordance with Institutional Animal Care and Use Committee guidelines at Virginia Tech.
Body weights and tissue collection.
Mice were euthanized at 24 weeks of age by cervical dislocation and weighed. Bone marrow and spleens were immediately collected posteuthanasia under aseptic conditions, using dissection scissors and curved forceps. The spleen was weighed and then all tissues were placed individually into prelabeled sterile Petri dishes (Corning, Corning, NY), containing 8 ml of RPMI-1640 culture medium (Mediatech, Herndon, VA). Dishes were placed on ice until tissue dissociation.
Cell dissociation and enrichment.
Spleens were gently dissociated over a stainless steel sieve screen (Sigma, St Louis, MO) using curved forceps. Cells were pipetted through the sieve screen following dissociation to remove debris, and then washed in RPMI-1640 for 10 min, 240 × g, and 23°C. The supernatant was discarded and cells were resuspended in 1 ml of incomplete RPMI-1640. To each tube, 2 ml of 0.83% ammonium chloride lysis buffer (ACK, pH 7.29) was then added, to lyse red blood cells, and tubes were incubated for 5 min at 23°C. After lysis incubation, spleen cells were resuspended in 5 ml of incomplete RPMI-1640 and washed twice (7 min, 290 × g and 7°C). The splenic leukocyte-rich cells were then resuspended in 5 ml of complete RPMI-1640 media containing 10% heat-inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA), 2mM l-glutamine (ICN, Costa Mesa, CA), 50 IU/ml penicillin (ICN), and 50 mg/ml of streptomycin (ICN), and maintained at 7–10°C. For bone marrow isolation, femurs were removed and the bone marrow cavities flushed with 2% FBS-phosphate-buffered saline (PBS) (2 ml), washed once (7 min, 290 × g and 7°C), resuspended in 1 ml of incomplete RPMI-1640 media and stored at 4°C.
Cell enumeration.
Cells were enumerated and size analyzed using a Beckman Multisizer 3 Cell Counter (Beckman Coulter, Fullerton, CA) according to the manufacturer's protocol. Briefly, a 10-μl aliquot of enriched cell suspension was transferred to a plastic counting chamber containing 10 ml of PBS (Mediatech). The plastic chamber was capped, mixed by repeated gentle inversion, and counted. The cells were enumerated and adjusted to 5.0 × 106 cells/ml in complete media.
Flow cytometric evaluation of cell-surface markers.
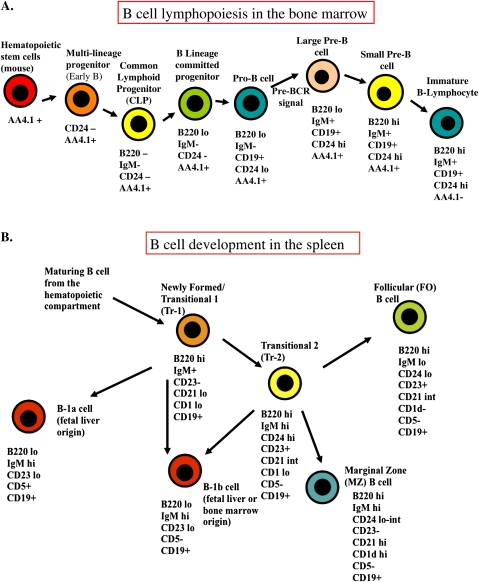
Cell suspensions (5 × 105/100 μl) from the spleen and bone marrow were dispensed into individual wells of a 96-well round-bottom tissue culture plate (Corning). Monoclonal antibodies (mAbs) with phycoerythrin (PE) fluorescent labels were used according to manufacturer's (BD Pharmingen, San Diego, CA) recommendation at a concentration of 0.2 μg/μl; mAbs with fluorescein isothiocyanate (FITC) fluorescent labels were similarly used at the recommended concentration of 0.5 μg/μl. Cells were stained as previously described (Mustafa et al., 2008). Briefly, lymphocyte aliquots (5 × 105 cells/100 μl) from spleen and bone marrow were incubated with the following primary mAbs: PE-anti CD45/B220, PE-CD5, Pe-Cy5-anti CD93 (AA4.1,C1qRp), FITC-anti CD19, FITC-anti IGM (ebioscience, San Diego, CA), FITC-anti CD45/B220, PE-anti CD24 (also know as heat stable antigen, BD Pharmingen), FITC-anti CD1, PE-anti CD23 (BD Pharmingen). For double or triple staining protocols, mAbs with different fluorescent labels were simultaneously added to the sample. For bone marrow analysis, aliquots of 5 × 105 cells were preblocked with anti-FcγIII/IIR (clone 2.4G2, Rat IgG2b). Following staining, cells were washed and evaluated on a Coulter Epics XL flow cytometer (Beckman Coulter). From each sample, 10,000 events were collected and analyzed using the FlowJo software (Tree Star, San Carlos, CA). Dead cells, clumps, and debris were excluded electronically by gating on forward scatter versus side scatter. Figure 1 shows the different B-cell subsets within the bone marrow and the spleen, including antibody markers used in this study to characterize these cell phenotypes.
FIG. 1.
Computer-generated diagram showing the key cell phenotypes highlighting B-cell lymphopoiesis in the bone marrow (A) and B-cell maturation in the spleen (B). The cell-surface markers identified under the specific cell types were the ones evaluated in this study.
ELISA for autoantibodies to double-stranded DNA, single-stranded DNA and cardiolipin.
To detect the presence of double-stranded DNA (dsDNA) antibody, 96-well medium-binding microtiter plates (Corning) were coated with heat-denatured calf thymus DNA (100 μg/ml; Sigma). For single stranded DNA (ssDNA) antibody titers, DNA from calf thymus was used (10 μg/ml; Sigma), and for autoantibodies to cardiolipin a solution from bovine heart was used (10 μg/ml; Sigma). All precoated plates were incubated at 4°C overnight. The plates were washed thrice with 300 μl of PBS/0.05% Tween 20 (Mediatech), blocked with 1% BSA (Sigma) for 2 h at 23°C, washed, and then incubated with diluted serum samples to be tested (1/100). For all three enzyme-linked immunosorbent assays (ELISAs), serial dilutions of each mouse serum were performed to optimize optical density (OD) readings. Serum diluted 1/100 was determined to be the optimal dilution to attain a wide spectrum in the OD among the samples. After 3 h at 23°C, the serum-coated plates were washed thrice with 300 μl of PBS-0.05% Tween 20. To each well, 0.2 ml of diluted alkaline phosphatase-conjugated anti-IgG antibody (1/3000) (Sigma) were added and the plates were incubated for 60 min at 23°C. The plates were then washed thrice with 300 μl of PBS-0.05% Tween 20. To each well, 0.2 ml of prepared substrate for alkaline phosphatase-conjugated secondary antibody (Sigma Fast p-nitrophenyl phosphate tablets) were added and allowed to develop for 45 min at 23°C before adding 50 μl of 3M NaOH stop solution (Sigma). The absorbance (A 405) of the initial dilution was measured. OD readings represent the average from sera from each mouse performed in duplicate.
Histology of the kidneys and spleen.
Kidneys were collected at the time of euthanasia and immediately fixed in 10% formalin (Fisher Scientific, Pittsburgh, PA) for 48 h. The spleen was collected previously from one male and one female offspring per litter, for cell counts and phenotyping. For histopathology, the spleen was also collected from littermates of the above mice (n = 4 mice per treatment) and immediately fixed in 10% formalin. After 48 h in formalin, the tissues were removed, routinely processed and embedded in a paraffin block. Following embedding, a 5-μm section was cut from each tissue block, and stained with hematoxylin and eosin (H&E, Richard-Allen Scientific, Kalamazoo, MI) using standard histologic methods. The prepared slides were then evaluated, with a light microscope, in a blinded manner by a veterinary pathologist (coauthor P.S.). For each kidney, 100 consecutive renal cortex glomeruli were evaluated. Each glomerulus was scored for the presence of fibrinoid necrosis or crescents and extent of lymphocytic infiltration. In the spleens, all fields were examined. The spleens were evaluated for changes within splenic follicles including the presence or absence of germinal centers, periarteriolar cuffs including cellular density, evidence of cell death and overall architecture.
Immunohistochemistry of the kidneys: C3 and IgG deposition.
Frozen kidneys were cut into 5-μm sections and stained with FITC conjugated antibodies. Briefly, tissue sections were thawed at room temperature and dried for 30 min. Slides were fixed in acetone for 10 min and then washed with PBS thrice for 3 min per wash. Goat anti-mouse IgG diluted 1:100 (MP Biomedicals, Santa Ana, CA) or goat anti-mouse C3 diluted 1:100 (MP Biomedicals) were incubated with tissues sections in a humid chamber for 60 min at 23°C. The sections were then rinsed thrice for 5 min per wash with PBS. The slides were mounted using Vectashield mounting media (Vector Labs, Burlingame, CA) and then examined using an Olympus BX-60 fluorescence microscope (Center Valley, PA). The severity of glomerulonephritis and immune complex deposition was scored using a range from 0 to 3+, where 0 corresponded to a nonautoimmune healthy mouse and 3+ to the maximal alteration observed in the study. All slides were scored in a blinded manner independently by two experienced investigators (coauthors C.R. and R.G.). Scores were averaged for the final tissue score.
Lymphocyte proliferation assay.
Splenocytes were plated into each well (5 × 105 cells/100 μl per well) of a 96-well round-bottom tissue culture plate (Corning Cell Wells, Corning). Cells were exposed to the B-cell mitogen, lipopolysaccharide (LPS, 50 μg/ml, Sigma). Nonstimulated cells were cultured with 100 μl of complete media alone. Triplicate wells were used and the total incubation volume was 200 μl per well. Following 48 h of incubation, 20 μl of alamarBlue dye (Serotec, Raleigh, NC) (10% of incubation volume) was added to each well of the culture plates (Ahmed et al., 1994). At 24- and 48-h postaddition, degree of absorbance was determined under dual wavelength (570 and 600 nm) using a Molecular Devices plate reader (Menlo Park, CA).
Statistical analysis.
Normal probability plots were generated to verify that data followed a normal distribution. Subsequently, data were summarized as arithmetical mean ± SEM. ANOVA was used with Dunnett's t-test to establish significant differences between treatment groups and control. After the ANOVA, residual plots were inspected to assess model adequacy (i.e., errors followed a normal distribution with a constant variance). All analyses were performed using JMP 7.0 (SAS Institute, Inc., Cary, NC). The pregnant dam was maintained as the statistical unit in all cases such that each offspring analyzed represented a separate dam. Group size was five SNF1 offspring per sex for all experiments (n = 5) except for the spleen and thymus histology (n = 4). Statistical significance was set a p ≤ 0.05.
RESULTS
Body and Organ Weights, and Organ Cellularity
Body weights of the 24-week-old adult SNF1 offspring were decreased in the males by prenatal exposure to 40 and 80 μg/kg TCDD and 80 μg/kg TCDD in the females. There were no significant differences in splenic weight or the spleen/body weight ratio across treatment groups. In contrast, splenic cellularity tended to increase by treatment reaching significance in the 80 μg/kg TCDD males (Table 1).
TABLE 1.
| 0 μg/kg (mean ± SEM) | 40 μg/kg (mean ± SEM) | 80 μg/kg (mean ± SEM) | |
| Body weight (g) | |||
| Female | 26.9 ± 0.9 | 25.9 ± 0.3 | 24.1 ± 0.6* |
| Male | 42.0 ± 0.8 | 36.1 ± 1.3* | 33.7 ± 1.1* |
| Splenic weight (g) | |||
| Female | 0.19 ± 0.05 | 0.22 ± 0.04 | 0.17 ± 0.33 |
| Male | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.01 |
| Splenic cellularity (×106) | |||
| Female | 106.3 ± 13.4 | 151.8 ± 22.3 | 144.5 ± 22.7 |
| Male | 64.4 ± 5.8 | 65.2 ± 4.8 | 88.8 ± 10.3* |
| Spleen body weight ratio | |||
| Female | 0.007 ± 0.001 | 0.008 ± 0.002 | 0.007 ± 0.00 |
| Male | 0.003 ± 0.00 | 0.003 ± 0.00 | 0.003 ± 0.00 |
Note. n = 5 mice per treatment per gender, *p ≤ 0.05, Dunnett's test, values in bold are significantly different from control.
B Lymphoid Progenitors in Bone Marrow
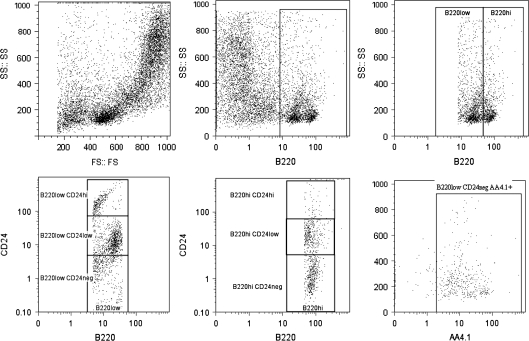
At 24 weeks of age, the 80 μg/kg TCDD females showed a significant decrease in total B220 cells (B220+). The percentage of these B220+ cells that were B220hi, representing both small pre-B cells that phenotypically immediately precede immature B lymphocytes as well as the immature B cells, was significantly decreased, whereas the percentage that were B220low, representing B lineage committed progenitors, pro-B cells, and large pre-B cells, was significantly increased in these females. The percentage of B220loCD24− cells that expressed AA4.1 (B220lowCD24−AA4.1+), representing B lineage committed progenitors, was significantly increased in the females. Prenatal TCDD-treated males did not show changed bone marrow total B220+ expression or B220low expression. However, the percentage of B220hi cells that were CD24low was significantly decreased, and the percentage of B220lo CD24low cells, representing B lineage committed progenitors and pro-B cells, was significantly decreased in 80 μg/kg TCDD males. Interestingly, the percentage of B220lowCD24− cells was significantly increased in both 40 μg/kg and 80 μg/kg TCDD males. The percentage of B220loCD24−AA4.1+ cells was not changed in males by TCDD (Table 2). Gating of the above-described B-cell subset distributions was as previously performed by us and others (Hardy and Hayakawa, 2001; Mustafa et al., 2008; Thurmond and Gasiewicz, 2000) (Fig. 2).
TABLE 2.
| Bone marrow | 0 μg/kg (mean ± SEM) | 40 μg/kg (mean ± SEM) | 80 μg/kg (mean ± SEM) |
| Total B220 | |||
| Female (%) | 21.5 ± 0.9 | 18.5 ± 1.6 | 16.6 ± 2.2* |
| Male | 18.6 ± 0.5 | 19.6 ± 1.1 | 19.8 ± 1.0 |
| B220low (%) | |||
| Female | 67.0 ± 1.7 | 67.7 ± 3.7 | 81.3 ± 2.1* |
| Male | 73.0 ± 3.2 | 67.1 ± 1.5 | 68.4 ± 2.2 |
| B220lowCD24+ | |||
| Female | 75.3 ± 1.3 | 79.1 ± 0.7 | 72.1 ± 3.1 |
| Male | 79.5 ± 0.4 | 75.2 ± 1.1* | 73.9 ± 1.1* |
| B220lowCD24lhi % of total B220lowCD24pos | |||
| Female | 8.5 ± 1.0 | 10.8 ± 1.1 | 6.7 ± 3.1 |
| Male | 12.9 ± 1.1 | 12.7 ± 1.2 | 15.3 ± 2.1 |
| B220lowCD24low % of total B220lowCD24pos | |||
| Female | 66.7 ± 1.4 | 68.3 ± 0.7 | 65.4 ± 3.8 |
| Male | 66.6 ± 1.1 | 62.5 ± 1.1 | 58.6 ± 1.3* |
| B220low CD24− | |||
| Female | 26.5 ± 1.1 | 21.0 ± 0.7* | 27.9 ± 2.0* |
| Male | 20.5 ± 0.4 | 24.8 ± 1.1* | 26.1 ± 1.3* |
| B220lowCD24−AA4.1+ % of total B220lowCD24− | |||
| Female | 64.6 ± 2.6 | 63.1 ± 3.9 | 74.5 ± 3.0* |
| Male | 61.9 ± 1.9 | 68.6 ± 3.0 | 68.3 ± 1.8 |
| B220hi | |||
| Female | 32.5 ± 2.0 | 32.4 ± 3.7 | 18.7 ± 3.0* |
| Male | 27.0 ± 3.2 | 32.9 ± 1.5 | 31.7 ± 2.2 |
| B220hiCD24+ | |||
| Female | 52.6 ± 6.8 | 61.6 ± 4.2 | 39.7 ± 5.6 |
| Male | 46.1 ± 5.9 | 35.8 ± 2.5 | 29.0 ± 1.1* |
| B220hiCD24lhi % of total B220hiCD24+ | |||
| Female | 15.2 ± 4.6 | 14.4 ± 3.7 | 7.3 ± 1.4 |
| Male | 8.4 ± 1.2 | 5.8 ± 0.5 | 5.9 ± 0.8 |
| B220hiCD24low % of total B220hiCD24+ | |||
| Female | 37.4 ± 2.4 | 47.3 ± 3.7 | 32.4 ± 4.5 |
| Male | 37.7 ± 5.6 | 30.0 ± 2.3 | 23.1 ± 1.1* |
| B220hiCD24− | |||
| Female | 47.4 ± 6.8 | 38.4 ± 4.2 | 60.3 ± 5.6 |
| Male | 53.9 ± 5.9 | 64.2 ± 2.3 | 71.0 ± 1.5* |
Note. n = 5 mice per treatment per gender, *p ≤ 0.05, Dunnett's test, values in bold are significantly different from control.
FIG. 2.
Representative dot plots showing the gating technique employed to differentiate select B-cell subsets associated with lymphopoiesis in the bone marrow. Bone marrow cells were adjusted based on size (FS) and granularity (SS) to elucidate the total cell population (A). Filters were employed to gate out dead cells and debris. Using the FloJo software (Tree Star), cells were then gated on for their ability to express CD45RB220 (B). The cell populations were then sub divided based on low fluorescence to CD45RB220 (B220 low) and high fluorescence to CD45RB220 (B220 high) (C). These two subpopulations of CD45RB220 cells were then evaluated based on their ability to express CD24 (D and E). Bone marrow cells with a CD45RB220lowCD24− phenotype were costained with AA4.1 (CD93, C1qRp) to identify the earliest B lineage cells (F).
Phenotype of Spleen Cells, and Migration of Mature B Cells in the Spleen
The relative percentage of splenic leukocytes that expressed B220 did not differ across treatment or sex compared with controls. However, the relative percentage and absolute number of CD19+CD5+ B cells (representing B-1a cells, a B-cell subset of fetal origin only, rather than bone marrow) were significantly increased in 40 μg/kg TCDD females, whilst the absolute number of cells expressing the CD19+CD5+ phenotype was increased in the 40 μg/kg TCDD females and 80 μg/kg TCDD males (Table 3). Although the relative percentage of splenic B cells was unchanged, there was a shift in the immature and mature B-cell compartments with reduced numbers of B220+CD24hi transitional B cells and increased numbers of B220+CD24low−int mature B cells (superscripts “low”, “int” and “hi” refer to low, intermediate and high fluorescence, respectively). Additionally, there was a significant difference within B-cell subsets expressing CD21/CD24. The 80 μg/kg females displayed decreased percentages of B marginal zone (MZ) cells (CD21intCD24low−int), and increased relative percentages of follicular (FO) B cells (CD21intCD24low). In TCDD males, the CD21intCD24low−int MZ cells and B transitional-2 cells (Tr-2) (CD21intCD24hi) were significantly decreased, whereas the percentage of CD21intCD24low−int FO cells significantly increased. Furthermore, B transitional-2 cells (CD23low−int CD1low−int) were significantly decreased in males, whereas other B-cell subsets expressing CD1/CD23 were not different compared with the control groups. Gating of the above-described B-cell subset distributions was as previously performed (Grimaldi et al., 2001; Pillai et al., 2005) (Table 4).
TABLE 3.
| Spleen | 0 μg/kg (mean ± SEM) | 40 μg/kg (mean ± SEM) | 80 μg/kg (mean ± SEM) |
| B220+ (%) | |||
| Female | 24.6 ± 2.4 | 23.4 ± 3.0 | 28.1 ± 3.2 |
| Male | 28.7 ± 2.9 | 30.4 ± 3.3 | 31.5 ± 1.6 |
| CD19+CD5+ (%) | |||
| Female | 5.4 ± 0.8 | 11.0 ± 1.4* | 9.3 ± 2.0 |
| Male | 3.4 ± 0.5 | 3.1 ± 0.6 | 4.5 ± 0.6 |
| Absolute number B220+ (×103) | |||
| Female | 2609.6 ± 383.1 | 3835.7 ± 1066.0 | 4144.4 ± 972.9 |
| Male | 1901.3 ± 320.0 | 2038.8 ± 372.1 | 2783.7 ± 333.1 |
| Absolute number CD19+CD5+ (×103) | |||
| Female | 599.9 ± 158.8 | 1719.9 ± 368.4* | 1229.1 ± 220.9 |
| Male | 215.1 ± 32.9 | 198.0 ± 34.7 | 414.7 ± 86.8* |
Note. n = 5 mice per treatment per gender, *p ≤ 0.05, Dunnett's test, values in bold are significantly different from control.
TABLE 4.
| Spleen % of total B220 | 0 μg/kg (mean ± SEM) | 40 μg/kg (mean ± SEM) | 80 μg/kg (mean ± SEM) |
| CD24hi (%) | |||
| Female | 10.9 ± 0.8 | 11.6 ± 0.5 | 7.9 ± 1.0* |
| Male | 8.8 ± 0.6 | 8.2 ± 0.7 | 6.1 ± 0.5* |
| CD24low−int (%) | |||
| Female | 88.5 ± 0.8 | 88.0 ± 0.5 | 91.8 ± 2.1* |
| Male | 85.6 ± 0.9 | 86.3 ± 0.9 | 88.4 ± 0.6* |
| CD21hiCD24low−int (%) | |||
| Female | 15.4 ± 2.9 | 12.8 ± 0.8 | 6.4 ± 1.0* |
| Male | 16.9 ± 1.1 | 11.6 ± 0.9 | 10.8 ± 1.3* |
| CD21intCD24hi (%) | |||
| Female | 7.4 ± 0.7 | 7.9 ± 0.5 | 7.7 ± 0.6 |
| Male | 8.4 ± 0.6 | 6.0 ± 0.6* | 4.3 ± 0.5* |
| CD21intCD24low (%) | |||
| Female | 65.2 ± 3.4 | 67.8 ± 1.3 | 73.4 ± 1.6* |
| Male | 64.3 ± 1.7 | 72.1 ± 1.6* | 75.3 ± 1.7* |
| CD21−/lowCD24hi (%) | |||
| Female | 12.9 ± 0.5 | 12.7 ± 0.5 | 13.2 ± 0.7 |
| Male | 10.9 ± 0.5 | 10.7 ± 0.7 | 9.9 ± 0.3 |
| CD23int−hiCD1− (%) | |||
| Female | 56.0 ± 2.8 | 56.9 ± 4.1 | 60.4 ± 1.7 |
| Male | 59.7 ± 4.2 | 55.7 ± 1.1 | 62.7 ± 2.6 |
| CD23low−intCD1low−int (%) | |||
| Female | 9.8 ± 1.0 | 10.1 ± 0.9 | 11.3 ± 0.5 |
| Male | 6.1 ± 0.4 | 5.5 ± 0.5 | 3.0 ± 0.4* |
| CD23−CD1low (%) | |||
| Female | 14.7 ± 1.6 | 15.9 ± 2.5 | 14.8 ± 1.6 |
| Male | 21.5 ± 4.2 | 26.7 ± 0.9 | 22.4 ± 2.0 |
| CD23−/lowCD1hi (%) | |||
| Female | 12.3 ± 0.5 | 11.2 ± 0.8 | 10.9 ± 0.8 |
| Male | 12.8 ± 0.6 | 12.3 ± 0.5 | 12.1 ± 0.7 |
Note. n = 5 mice per treatment per gender, *p ≤ 0.05, Dunnett's test, values in bold are significantly different from control.
Antibody Titers to ssDNA, dsDNA, and Cardiolipin
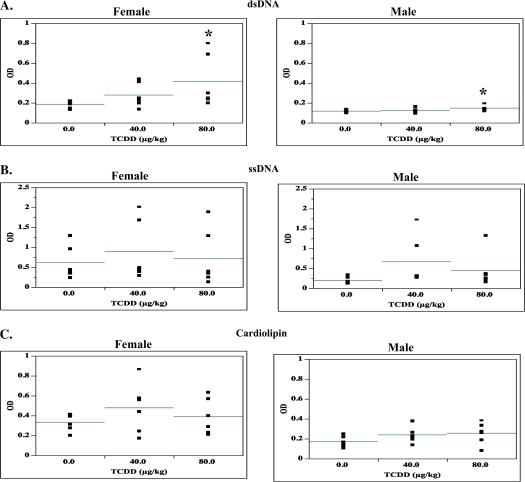
Based on the altered B-cell subset expression in the TCDD-treated spleens, the functionality of the B cells for autoreactivity was evaluated by measuring autoantibody titer to dsDNA, sDNA, and cardiolipin via ELISA assays. Anti-dsDNA antibodies were increased in both male and female offspring by 80 μg/kg TCDD. Anti-ssDNA and anti-cardiolipin titers showed numeric increasing trends in both the male and female offspring (Fig. 3).
FIG. 3.
Sera from 24-week-old SNF1 mice that were prenatally exposed to 0, 40, or 80 μg/kg TCDD were analyzed for the presence of autoantibodies to dsDNA (A), ssDNA (B), and cardiolipin (C). The data are arranged by sex and are based on five mice per treatment per sex (*p ≤ 0.05, Dunnett's test).
Kidney Pathology
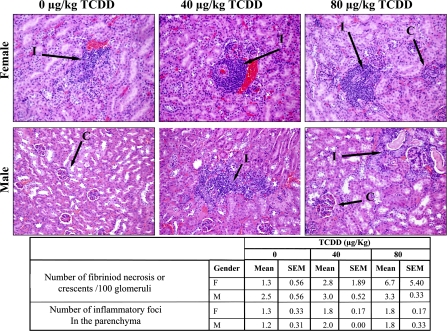
Immune complex deposition was evaluated in the kidneys to assess disease progression. Immune complex deposition in the kidney is a common signalment in lupus patients, and is used to follow disease progression in SNF1 mice. Glomeruli that manifested with fibriniod necrosis (N) or crescents (C) showed an increasing but nonsignificant trend by treatment and sex. The mean number of mononuclear inflammatory cell (I) foci also numerically increased, nonsignificantly, by treatment and sex (Fig. 4).
FIG. 4.
The kidneys from 24-week-old SNF1 mice that were prenatally exposed to 0, 40, or 80 μg/kg TCDD were collected, fixed, sectioned, and stained with H&E. Images are representative of renal sections by treatment and sex. All sections were scored for number of fibrinoid necrosis cells or crescents/100 glomeruli and number of inflammatory foci in the parenchyma. The data are based on five mice per treatment per sex (*p ≤ 0.05, Dunnett's test).
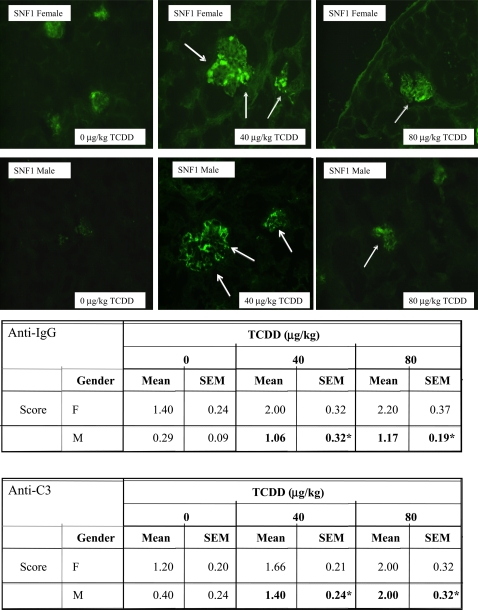
Deposition of Anti-IgG and Anti-C3 Immune Complexes in the Kidney
Based on the histopathologic suggestion of potential increased kidney pathology by TCDD, using light microscopy and H&E staining, immunofluorescent staining to examine IgG and C3 involvement in immune complex deposition was performed. Kidney sections from 24-week-old SNF1 offspring showed a TCDD dose-dependent increasing trend in deposition of immune complexes, for both anti-IgG and anti-C3, which was significant in the males (Fig. 5).
FIG. 5.
The kidneys from 24-week-old SNF1 mice that were prenatally exposed to 0, 40, or 80 μg/kg TCDD were collected, fixed, section, and stained with FITC-labeled anti-IgG and anti-C3. The above are representative images of kidneys stained with FITC-anti-IgG based on treatment and sex. The data are based on five mice per treatment per sex (*p ≤ 0.05, Dunnett's test).
Splenic Pathology
Following detection of changes in cytology and phenotypic analysis of the B cells in mice prenatally exposed to TCDD, histopathologic analysis of the spleen was also performed. Splenic sections showed large active follicles in the females for all treatment groups. In the male spleens, 75% of 40 μg/kg TCDD mice displayed small germinal centers, whereas in the 80 μg/kg TCDD mice the germinal centers were indistinct to absent and the follicles were large and coalescing (Fig. 6).
FIG. 6.
The spleens from 24-week-old SNF1 mice that were prenatally exposed to 0, 40, or 80 μg/kg TCDD were collected, fixed, sectioned, and stained with H&E. Images are representative of splenic sections presented by treatment and sex. Spleens from male mice prenatally exposed to 40 μg/kg TCDD frequently displayed follicles with small germinal centers (arrows). In the 80 μg/kg TCDD male mice, the germinal centers were few and discrete with coalescing of follicles and a loss of follicular architecture (arrows).
Mitogen-Stimulated Splenic B Lymphocyte Proliferation
Mitogen stimulation of enriched, cultured splenic lymphocytes was employed to assess the influence of prenatal TCDD on B lymphocyte functionality in the adult mouse. No significant effects on stimulation responses were seen using LPS in splenocytes, at 48 or 72 h (data not shown).
DISCUSSION
Autoantibodies produced by B cells tend to display strong polyreactivity to DNA and glomerular substrate, and are frequently localized as immune deposits in lupus kidneys (Xie et al., 2003). Presently, there is a paucity of data concerning the effects of developmental TCDD exposure on B maturation and function (Kantor et al., 1997; Sato et al., 2004) and no studies exist evaluating the effects of prenatal TCDD on the B cells of an autoimmune-prone strain. In this study, B-cell lymphopoiesis, B-cell colonization and phenotype, autoantibody production, renal immune complex deposition, and renal histopathology of SNF1 mice that received prenatal TCDD were examined. The 24-week-age group was selected because SNF1 females are in the early stages of autoimmune disease, whereas the males do not begin to show signs until 48–52 weeks (Eastcott et al., 1983).
Disruption in normal B-cell function can either initiate or promote autoimmunity, in which B cells may act via nonconventional mechanisms, for example, in addition to autoantibody production, including altered antigen presentation or cytokine production (Fujimoto and Sato, 2007). In female SNF1 mice dosed with 80 μg/kg TCDD, bone marrow cells expressing the pan B-cell marker (B220+) were decreased, whereas B220low B-cell progenitors and B220lowCD24−AA4.1+ early B-cell progenitors were increased. Male B-cell lymphopoiesis was also affected but not as dramatically, showing decreased large pre-B cells (B220loCD24hi) and small pre-B and/or IgM+ B cells (B220hi). These results clearly imply that prenatal TCDD permanently dysregulates B-cell lymphopoiesis, similar to thymic T-cell maturation (Faith and Moore, 1977). Potential relationships of these progenitor-compartment effects to impaired mature B-cell function remain undetermined but will be investigated in future studies.
In the secondary lymphoid compartment, a TCDD dose-related trend toward increased splenic B-cell numbers was observed in both sexes suggesting altered B-cell phenotypes. Total numbers of CD5+ B cells increased significantly in both sexes after TCDD, which is noteworthy. B-1a cells are distinguished from conventional B cells (B-2) by their fetal developmental origin, their surface marker expression of CD5, their FO location verses MZ and their function (Duan and Morel, 2006). These are long-lived, noncirculating B cells that display reduced B-cell receptor diversity and affinity compared with B-2 cells (Kantor et al., 1997). They also produce circulating low affinity and polyreactive IgM “natural antibodies” that function as a first line of defense against bacterial pathogens (O'Garra et al., 1992). The polyreactivity of these antibodies also mediates the recognition of autoantigens, which serves in the clearance of apoptosis products (Carroll and Prodeus, 1998). This unique autoreactivity of B-1a cells may play a role in autoimmune pathogenesis, as may production of high levels of IL-10 and enhanced antigen presentation capacities by these cells (O'Garra et al., 1992). Further, high levels of B-1a cells have been detected in patients with systemic lupus erythematosus (SLE), Sjogren's syndrome and rheumatoid arthritis, and numerous associations between expansion of this cell compartment and systemic autoimmunity have been found in murine models {Karpuzoglu-Sahin et al., 2001; Pillai et al., 2004). Thus, it is possible that B-1a cells may activate autoreactive T cells and produce autoantibodies against specific target organs, contributing to immune complex–mediated pathology (Sato et al., 2004). Histologic exam of the spleen showed large, active follicles across all treatments and across sex. However, germinal center size and number decreased by dose in the TCDD-treated males suggesting that TCDD may disrupt germinal center formation in the males, which warrant further investigation.
Production of anti-nuclear autoantibodies in human and mouse SLE involves interactions between select populations of autoimmune Th cells and B cells. These pathogenic antibodies tend to undergo high somatic mutation (O'Keefe et al., 1996) and are the products of T-dependent FO B-cell activity. In particular, autoantibody-inducing Th cell clones isolated from SNF1 mice with lupus nephritis rapidly induce immune complex glomerulonephritis when transferred into young pre-autoimmune mice (Mohan et al., 1993; Naiki et al., 1992; Rajagopalan et al., 1990; Shivakumar et al., 1989). In the absence of these Th cells, the autoantibody-producing B cells were not sustained and underwent apoptosis (Kalled et al., 1998). Further, in murine SLE models, B cells are needed to prime pathogenic T cells, for instance B-cell deficient MRL/lpr mice do not show expansion of activated T cells (Leitges et al., 1996). However, MZ B cells are more efficient than FO B cells at priming naïve T cells and are over-represented in lupus-prone strains, suggesting that MZ B cells help trigger the T-cell dependent disease (Cariappa et al., 2001). Thus, whereas MZ B cells may be important in initiating autoimmune disease, Th cells and FO B cells appear to be important in sustaining and promoting such disorders. In the present study, prenatal TCDD also caused decreased expression of transitional 2 (Tr2) B cells in spleens of the present mice. The significance of this finding is not yet known, however, at least two developmentally and functionally distinct types of Tr2 cells have been identified (Cariappa and Pillai, 2002; Pillai et al., 2004). Our collective B cell results suggest that these cells might play an important role in the early induction of SLE-like symptoms in male mice and exacerbation of SLE symptoms in the females after prenatal TCDD.
SNF1 female mice spontaneously produce moderate levels of autoreactive IgG antibodies by 24 weeks of age, whereas at the same age males produce low levels of autoreactive IgG. Serum analysis of prenatal TCDD-exposed SNF1 mice showed that both sexes had significantly increased anti-dsDNA levels, with the basal and TCDD-induced levels being higher in the females. There was a numeric increase in both the anti-ssDNA and anti-cardiolipin levels of the TCDD-exposed females, however, the control female levels were also elevated due to the natural progression of disease in SNF1 females at 24 weeks. Collectively, these results suggest that perinatal TCDD exposure enhanced autoantibody production in the 24-week-old SNF1 females, and induced early autoantibody production in the SNF1 males. Analysis of the kidneys from these animals supported this finding, in which immune complex IgG and C3 deposition was significantly increased in the TCDD-exposed males. In the TCDD-exposed females, a dose-dependent numeric increase in immune complex formation was noted but not significant due to measureable immune complexes in the controls correlating with the disease at this age. Females and males both also showed dose-related trends in autoimmune-related kidney pathology, in the form of fibrinoid necrosis, crescents, and inflammatory cells.
To summarize, both male and female SNF1 mice showed persistent changes in humoral immunity as a consequence of GD 12 exposure to TCDD. Among these were numerous sex-specific effects, suggesting a possible interaction with endogenous hormones. TCDD-exposed mice displayed a clear enhanced autoimmune profile, including increased splenic CD5+ and FO B cells, increased autoantibody production, and increased autoimmune kidney lesions. These collective data suggest that developmental exposure to TCDD permanently alters the postnatal immune system in autoimmune-prone SNF1 mice, in a manner beyond the well-established profile of immune suppression, to include increased risk of autoimmune responses. Therefore, autoimmune-prone individuals who are gestationally exposed to TCDD may be at increased risk for either accelerated or exacerbated autoimmune disease.
FUNDING
National Institute of Health (grant number NIH-R21-PAR-03-121).
Acknowledgments
We would like to thank Ms Melissa R. Makris, Flow Cytometry Lab Supervisor, for her assistance with the flow cytometry data analysis and Flowjo software and Mr Richard Kerr for his help with cell counting. The authors also express their gratitude to Dr S. Ansar Ahmed for his critical review of the manuscript, to Dr Keith Harris, Department of Pathology, University of Georgia for review of pathology interpretation and reporting in the manuscript, and to Dr Stephen Were, College of Veterinary Medicine, Virginia Tech for statistical support.
References
- Ahmed SA, Gogal RM, Walsh J. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to 3H-thymidine incorporation assay. J. Immunol. Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Blaylock BL, Holladay SD, Comment CE, Heindel JJ, Luster MI. Exposure to tetrachlorodibenzo-p-dioxin (TCDD) alters fetal thymocyte maturation. Toxicol. Appl. Pharmacol. 1992;112:207–213. doi: 10.1016/0041-008x(92)90189-y. [DOI] [PubMed] [Google Scholar]
- Cariappa A, Pillai S. Antigen-dependent B-cell development. Curr. Opin. Immunol. 2002;14:241–249. doi: 10.1016/s0952-7915(02)00328-x. [DOI] [PubMed] [Google Scholar]
- Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- Carroll MC, Prodeus AP. Linkages of innate and adaptive immunity. Curr. Opin. Immunol. 1998;10:36–40. doi: 10.1016/s0952-7915(98)80028-9. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Piepenbrink MS. Perinatal immunotoxicity: Why adult exposure assessment fails to predict risk. Environ. Health Perspect. 2006;114:477–483. doi: 10.1289/ehp.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun. Rev. 2006;5:403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Eastcott JW, Schwartz RS, Datta SK. Genetic analysis of the inheritance of B cell hyperactivity in relation to the development of autoantibodies and glomerulonephritis in NZB × SWR crosses. J. Immunol. 1983;131:2232–2239. [PubMed] [Google Scholar]
- Faith RE, Moore JA. Impairment of thymus-dependent immune functions by exposure of the developing immune system to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) J. Toxicol. Environ. Health. 1977;3:451–464. doi: 10.1080/15287397709529578. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Sato S. B cell signaling and autoimmune diseases: CD19/CD22 loop as a B cell signaling device to regulate the balance of autoimmunity. J. Dermatol. Sci. 2007;46:1–9. doi: 10.1016/j.jdermsci.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Gehrs BC, Riddle MM, Williams WC, Smialowicz RJ. Alterations in the developing immune system of the F344 rat after perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin: II. Effects on the pup and the adult. Toxicology. 1997;122:229–240. doi: 10.1016/s0300-483x(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Gehrs BC, Smialowicz RJ. Alterations in the developing immune system of the F344 rat after perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin I. [correction of II]. Effects on the fetus and the neonate. Toxicology. 1997;122:219–228. doi: 10.1016/s0300-483x(97)00098-x. [DOI] [PubMed] [Google Scholar]
- Gehrs BC, Smialowicz RJ. Persistent suppression of delayed-type hypersensitivity in adult F344 rats after perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicology. 1999;134:79–88. doi: 10.1016/s0300-483x(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Grimaldi CM, Michael DJ, Diamond B. Cutting edge: Expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J. Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K. Cell development pathways. Annu. Rev. Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- Hogaboam JP, Moore AJ, Lawrence BP. The aryl hydrocarbon receptor affects distinct tissue compartments during ontogeny of the immune system. Toxicol. Sci. 2008;102:160–170. doi: 10.1093/toxsci/kfm283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay SD, Lindstrom P, Blaylock BL, Comment CE, Germolec DR, Heindell JJ, Luster MI. Perinatal thymocyte antigen expression and postnatal immune development altered by gestational exposure to tetrachlorodibenzo-p-dioxin (TCDD) Teratology. 1991;44:385–393. doi: 10.1002/tera.1420440405. [DOI] [PubMed] [Google Scholar]
- Holladay SD, Smialowicz RJ. Development of the murine and human immune system: Differential effects of immunotoxicants depend on time of exposure. Environ. Health Perspect. 2000;108(Suppl. 3):463–473. doi: 10.1289/ehp.00108s3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundeiker C, Pineau T, Cassar G, Betensky RA, Gleichmann E, Esser C. Thymocyte development in Ah-receptor-deficient mice is refractory to TCDD-inducible changes. Int. J. Immunopharmacol. 1999;21:841–859. doi: 10.1016/s0192-0561(99)00053-3. [DOI] [PubMed] [Google Scholar]
- Kalled SL, Cutler AH, Datta SK, Thomas DW. Anti-CD40 ligand antibody treatment of SNF1 mice with established nephritis: Preservation of kidney function. J. Immunol. 1998;160:2158–2165. [PubMed] [Google Scholar]
- Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J. Immunol. 1997;158:1175–1186. [PubMed] [Google Scholar]
- Karpuzoglu-Sahin E, Zhi-Jun Y, Lengi A, Sriranganathan N, Ansar Ahmed S. Effects of long-term estrogen treatment on IFN-gamma, IL-2 and IL-4 gene expression and protein synthesis in spleen and thymus of normal C57BL/6 mice. Cytokine. 2001;14:208–217. doi: 10.1006/cyto.2001.0876. [DOI] [PubMed] [Google Scholar]
- Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Immunodeficiency in protein kinase cbeta-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: A major immunogen for pathogenic autoantibody-inducing T cells of lupus. J. Exp. Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A, Holladay SD, Goff M, Witonsky SG, Kerr R, Reilly CM, Sponenberg DP, Gogal RM., Jr An enhanced postnatal autoimmune profile in 24 week-old C57BL/6 mice developmentally exposed to TCDD. Toxicol. Appl. Pharmacol. 2008;232:51–59. doi: 10.1016/j.taap.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki M, Chiang BL, Cawley D, Ansari A, Rozzo SJ, Kotzin BL, Zlotnik A, Gershwin ME. Generation and characterization of cloned T helper cell lines for anti-DNA responses in NZB.H-2bm12 mice. J. Immunol. 1992;149:4109–4115. [PubMed] [Google Scholar]
- O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur. J. Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- O'Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- Pillai S, Cariappa A, Moran ST. Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunol. Rev. 2004;197:206–218. doi: 10.1111/j.0105-2896.2003.097.x. [DOI] [PubMed] [Google Scholar]
- Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu. Rev. Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Zordan T, Tsokos GC, Datta SK. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: Isolation of CD4-8-T helper cell lines that express the gamma delta T-cell antigen receptor. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7020–7024. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Ishikawa S, Akadegawa K, Ito T, Yurino H, Kitabatake M, Yoneyama H, Matsushima K. Aberrant B1 cell migration into the thymus results in activation of CD4 T cells through its potent antigen-presenting activity in the development of murine lupus. Eur. J. Immunol. 2004;34:3346–3358. doi: 10.1002/eji.200425373. [DOI] [PubMed] [Google Scholar]
- Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J. Immunol. 1989;143:103–112. [PubMed] [Google Scholar]
- Silverstone A, Gavalchin J, Silvin C, Staples J, Ames I, Shanley P. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on a murine model of a lupus-like nephritis. Chemosphere. 1998;18:11–23. [Google Scholar]
- Thurmond TS, Gasiewicz TA. A single dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin produces a time- and dose-dependent alteration in the murine bone marrow B-lymphocyte maturation profile. Toxicol. Sci. 2000;58:88–95. doi: 10.1093/toxsci/58.1.88. [DOI] [PubMed] [Google Scholar]
- Vos JG, Moore JA, Zinkl JG. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57B1/6 mice. Toxicol. Appl. Pharmacol. 1974;29:229–241. doi: 10.1016/0041-008x(74)90060-x. [DOI] [PubMed] [Google Scholar]
- Xie C, Liang Z, Chang S, Mohan C. Use of a novel elution regimen reveals the dominance of polyreactive antinuclear autoantibodies in lupus kidneys. Arthritis Rheum. 2003;48:2343–2352. doi: 10.1002/art.11092. [DOI] [PubMed] [Google Scholar]