Abstract
Microbial community structures in deep-sea hydrothermal vents fields are constrained by available energy yields provided by inorganic redox reactions, which are in turn controlled by chemical composition of hydrothermal fluids. In the past two decades, geochemical and microbiological studies have been conducted in deep-sea hydrothermal vents at three geographically different areas of the Southern Mariana Trough (SMT). A variety of geochemical data of hydrothermal fluids and an unparalleled microbiological dataset of various samples (i.e., sulfide structures of active vents, iron-rich mats, borehole fluids, and ambient seawater) are available for comparative analyses. Here, we summarize the geochemical and microbiological characteristics in the SMT and assess the relationship between the microbial community structures and the fluid geochemistry in the SMT by thermodynamic modeling. In the high temperature vent fluids, aerobic sulfide-oxidation has the potential to yield large amounts of bioavailable energy in the vent fluids, which is consistent with the detection of species related to sulfide-oxidizing bacteria (such as Thiomicrospira in the Gammaproteobacteria and Sulfurimonas in the Epsilonproteobacteria). Conversely, the bioavailable energy yield from aerobic iron-oxidation reactions in the low-temperature fluids collected from man-made boreholes and several natural vents were comparable to or higher than those from sulfide-oxidation. This is also consistent with the detection of species related to iron-oxidizing bacteria (Mariprofundus in the Zetaproteobacteria) in such low-temperature samples. The results of combination of microbiological, geochemical, and thermodynamic analyses in the SMT provide novel insights into the presence and significance of iron-based microbial ecosystems in deep-sea hydrothermal fields.
Keywords: deep-sea hydrothermal vent field, shallow sub-seafloor microbial ecosystem, chemolithoautotrophs, iron-oxidizing bacteria, thermodynamic modeling
Introduction
Microbial ecosystems require energy for maintenance and prosperity. On land and in the sea surface, solar power is the main energy source. In the “photosynthetic ecosystem,” photoautotrophs (e.g., plants and cyanobacteria) are the primary producers that fix inorganic carbon and transform it into organic carbon using solar energy. The resulting organic carbon supports the growth of various organisms as carbon and energy sources. In contrast, there are microbial ecosystems that are sustained by chemical energy derived from inorganic redox reactions between electron donors (such as H2, H2S, Fe2+, and CH4) and acceptors (such as O2, Fe3+, and CO2). Chemolithoautotrophs fix inorganic carbon using the chemical energy, sustaining “Chemosynthetic ecosystems” as primary producers. It is known that chemosynthetic ecosystems are widely distributed on land (e.g., acid mines, hot springs, and deep subsurface) and in oceans (e.g., cold seeps, hydrothermal vents, and potentially enormous sub-seafloor aquifers). Recently, much attention has been paid to deep-sea hydrothermal vents and sub-seafloor aquifers that are potential habitats harboring extensive chemosynthetic ecosystems sustained by a variety of chemolithoautotrophs (reviewed in Orcutt et al., 2011). Although the precise picture of the potentially enormous sub-seafloor biosphere is still not well understood, the elucidation of the distribution, function, activity, and productivity of the deep-sea chemosynthetic ecosystems is important for better understanding not only of the extent and limit of the biosphere on Earth but also the global cycling of elements related to biological activities.
Deep-sea hydrothermal vent fields were initially found in the late 1970s. To date, over 200 deep-sea hydrothermal fields have been found in various areas, mainly on mid-ocean ridges, arc volcanoes, back-arc basins, and hot-spot volcanoes (http://www.interridge.org/irvents/). The hydrothermal fluids contain a variety of electron donors that serve as energy sources for the life thriving there. The chemical disequilibria, which occur in deep-sea hydrothermal environments by rapid mixing of reduced hydrothermal fluids with oxygenated cold seawater, could provide energy sources for the growth of the chemolithoautotrophs (McCollom and Shock, 1997; Takai and Nakamura, 2010; Amend et al., 2011). In fact, the presence of hydrogen-, sulfide-, and methane-oxidizers was confirmed soon after the discovery of the vent field (Jannasch and Mottl, 1985). Subsequent studies have revealed the ecology of the chemolithoautotrophs inhabiting the deep-sea hydrothermal vent fields, which include mesophiles to hyperthermophiles belonging to the Epsilonproteobacteria, Gammaproteobacteria, and Archaea (Nakagawa and Takai, 2008).
In contrast to hydrogen-, sulfide-, and methane-oxidizers, our knowledge of iron-oxidizers, especially mesophilic and neutrophilic bacteria, in deep-sea hydrothermal fields has been quite limited and was reported only recently. The first neutrophilic, mesophilic, and iron-oxidizing chemolithoautotrophic marine bacterium, Mariprofundus ferrooxydans, which belongs to the Zetaproteobacteria, was isolated from iron-rich mats in the hydrothermal fields of the Loihi Seamount (Emerson et al., 2007), and the genome sequence of M. ferrooxydans was recently reported (Singer et al., 2011). This isolate produces unique helical-stalks consisting of organic compounds and iron oxides (Chan et al., 2011). It is thus reasonable to consider that the iron-oxidizers play a significant role in the generation of massive iron-rich mats on the seafloor (Emerson and Moyer, 2002). Diverse 16S rRNA gene sequences affiliated in the Zetaproteobacteria have been recovered from iron-rich mats from various marine hydrothermal fields (McAllister et al., 2011). In some cases, members of the Zetaproteobacteria dominate the communities in the iron-rich mats (Kato et al., 2009a) and in the sub-seafloor warm fluids (Kato et al., 2009b), although the physiology of these phylotypes in the Zetaproteobacteria is still unclear because few isolates have been reported. Considering the domination of the Zetaproteobacteria and the abundance of the unique stalk structures in the seafloor massive iron oxide mats, it is very likely that the Zetaproteobacteria contain many iron-oxidizing chemolithoautotrophic species, sustaining the iron-based microbial ecosystem present in the iron-rich mats.
In the deep-sea hydrothermal vent fields, sulfide deposits (like mounds and chimneys) are usually present and sulfide- and hydrogen-oxidizers have been detected in these deposits and venting fluids. The detection of sulfide- and hydrogen-oxidizers in these habitats is consistent with the high potential of bioavailable energy from sulfide- and hydrogen-oxidation reactions in chemical conditions of the habitats (Takai and Nakamura, 2010; Amend et al., 2011). In contrast, iron-rich mats are not always observed in deep-sea hydrothermal vent fields, and the Zetaproteobacterial phylotypes (putative iron-oxidizers) were relatively abundant, compared to the known sulfide- and hydrogen-oxidizers, only in the iron-rich mats and crustal fluids (Kato et al., 2009a,b). A comprehensive and comparative analysis is needed to answer the following question: what is the critical factor(s) for the appearance of iron-based microbial ecosystems. This information is important in order to understand global carbon and iron cycling because iron-based microbial ecosystems are potentially present in enormous sub-seafloor aquifers, which would be the largest chemosynthetic ecosystem on Earth (Bach and Edwards, 2003; Edwards et al., 2003).
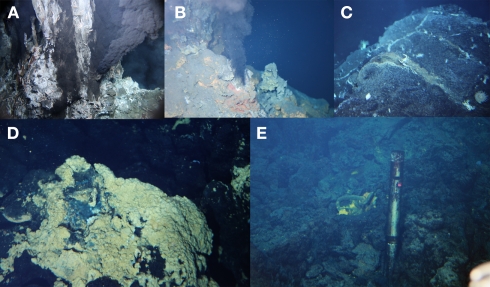
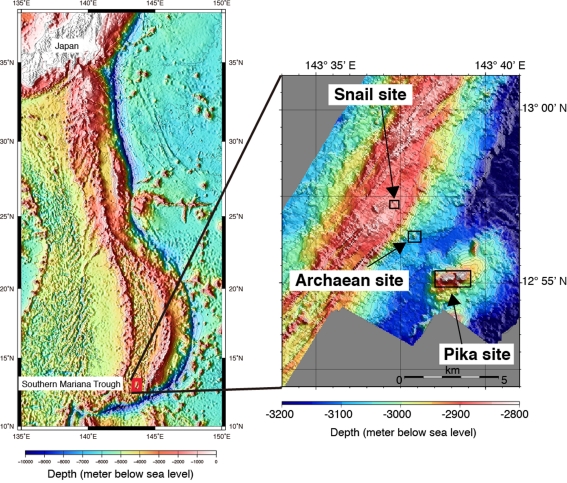
The Southern Mariana Trough (SMT; Figure A1A in Appendix) is one of the most suitable fields in which assess the factors leading to the appearance of iron-based microbial ecosystems. In the SMT, there are variable temperature habitats, from black and clear smoker sulfide chimneys (up to ∼340°C) to iron-rich mats with shimmering (∼110°C; Figure 1), in an area of 5 km × 5 km (Figure A1B in Appendix). Furthermore, there are several boreholes that were drilled using a shallow-seafloor drilling instrument, Benthic Multi-coring System (BMS; Marumo et al., 2008). Analysis of the collected fluids from the boreholes can provide valuable information about the geochemical conditions and microbiological activities of the sub-seafloor aquifers. Davis and Moyer (2008) first reported the presence of the Zetaproteobacteria in iron-rich mats in the SMT. Sequentially, comprehensive microbiological analyses of the various samples from the SMT hydrothermal fields have been done (Kato et al., 2009a,b, 2010). Here we summarize the variation of microbial communities in the SMT as shown by culture-independent molecular microbiological analyses, and discuss how these community structures were constructed. In addition, the bioavailable energy yields based on thermodynamic calculations were applied to discuss the geochemical factors leading to the appearance of the iron-based microbial ecosystem.
Figure 1.
On-site observation of the seafloor in the SMT. Photos were taken by Shinkai 6500 (JAMSTEC, Japan) in 2010. (A) A black smoker chimney at the Archaean site, (B) a black smoker chimney at the Pika site, (C) diffuse flows from crack of pillow lavas at the Snail site, (D) iron-rich mats at the Snail site, and (E) a casing pipe inserted into a borehole at the Snail site.
Geological Settings and Fluid Geochemistry
The SMT is a spreading back-arc basin that is located at the southern extension of the Izu–Bonin arc, western Pacific (Figure A1A in Appendix; Fryer, 1995; Ishibashi and Urabe, 1995). Hydrothermal activity hosted by basaltic rocks (Kakegawa et al., 2008) was found on the back-arc spreading ridge (Snail site) and on the off-ridge seamounts (Pika and Archaean sites) in the SMT (Figure A1B in Appendix; Wheat et al., 2003; Ishibashi et al., 2004, 2006). In the Pika and Archaean sites, sulfide chimneys venting high temperature fluids (up to >300°C) were found (Figures 1A,B). Low-temperature diffusing and shimmering fluids (<150°C) were also observed in both sites. In the Snail site, diffuse flows or shimmering with relatively low-temperature hydrothermal fluids (<120°C) from the fractures of pillow lavas (basalts; Figure 1C) and iron-rich mats (containing Fe, Si, and Mn; Figure 1D) were also observed (Wheat et al., 2003; Kato et al., 2009a), although chimney-like sulfide structures and associated high temperature hydrothermal venting have not been found.
Boreholes were created on the seafloor at the Snail and Pika sites using BMS (Marumo et al., 2008). These boreholes were cased with titanium pipes (Figure 1E). During fluid sampling from the boreholes, the casing pipes minimize the contamination from the seafloor materials into the crustal fluids.
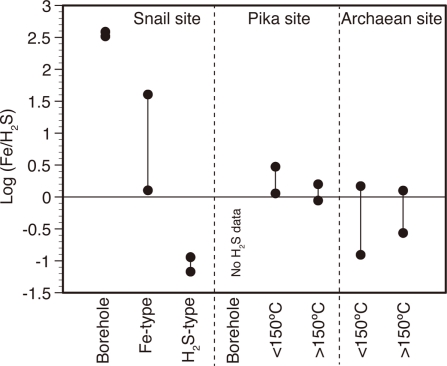
Preliminarily analyses of the geochemical composition of hydrothermal fluids collected in the SMT hydrothermal fields in 2003, 2004, and 2005 have been reported (Wheat et al., 2003; Ishibashi et al., 2004, 2006). Furthermore, we collected hydrothermal fluids from natural vents and boreholes in the SMT in 2010 and analyzed the fluid geochemistry as previously described (Takai et al., 2008; Toki et al., 2008). The geochemistry of the fluids is summarized in Table A1 in Appendix. Concentrations of O2, CO2, CH4, and H2 in deep seawater were referred from the previous publication (McCollom, 2007). It should be noted that a significant difference in the ratio of Fe to H2S was observed among the vent samples collected from the Snail site (Figure 2). These samples were divided into Fe-type (high Fe/H2S) and H2S-type (low Fe/H2S) and their geochemical data are shown in separate columns in Table A1 in Appendix.
Figure 2.
The ratio of Fe to H2S in fluid samples.
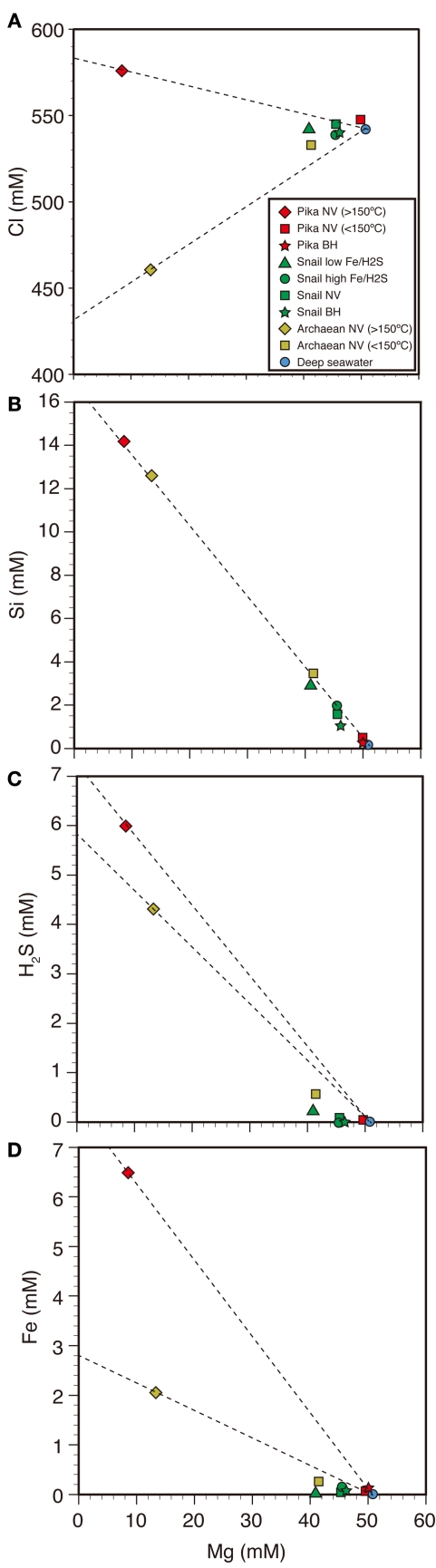
The concentrations of Cl, Si, H2S, and Fe are plotted against its Mg concentration (Figure 3). These so-called magnesium diagrams are conventionally used to estimate the hydrothermal end-member composition of a high temperature fluid from the deep-sea hydrothermal system (Von Damm et al., 1985). The collected fluid sample is often conceived to be simple mixture between the hydrothermal end-member (completely depleted in Mg) and seawater (Mg concentration is rather high as 50 mM). For conservative chemical species in mixing between the hydrothermal end-member and seawater, plots of the collected samples should be aligned along a simple linear trend.
Figure 3.
Mg diagrams against Cl, Si, H2S, and Fe for fluid samples. (A) Cl–Mg, (B) Si–Mg, (C) H2S–Mg, and (D) Fe–Mg diagrams are shown. NV and BH indicate natural vents and borehole fluids, respectively.
Chloride is predominant anion of hydrothermal fluid and seawater, which is a representative for such conservative species. In Cl–Mg diagram (Figure 3A), plots of the collected samples from the Pika and Archaean sites show clearly different trends. Substantial deviation of Cl concentration of hydrothermal fluid from that of seawater is attributed to sub-seafloor phase separation (e.g., Takai et al., 2008). Based on the data of high temperature (>150°C) fluids, Cl-enriched signature of the Pika fluid can be classified as vapor-lost (i.e., the gases such as H2, H2S, and CH4 are poor) hydrothermal fluid. In contrast, Cl-depleted signature of the Archaean fluid can be classified as vapor-rich (i.e., the gases are enriched) hydrothermal fluid. In the Archaean site, the collected sample of low-temperature (<150°C) shimmering was enriched in H2 and CH4 (Table A1 in Appendix), which is in accordance with occurrence of vapor-rich hydrothermal fluid in this site.
Si concentration of hydrothermal fluid is known as controlled by fluid-mineral equilibrium at the fluid reservoir (Von Damm et al., 1991). The Si–Mg diagram (Figure 3B) suggests Si concentrations of the hydrothermal fluid end-members for three sites are commonly as high as 16 mM, which corresponds to equilibrium at high temperature condition above 300°C. This result is reasonable for the Pika and Archaean fluids, since such high temperature fluid vents have been observed in these sites. Similar to these two sites, a high temperature fluid reservoir would exist below the seafloor in the Snail Site. In fact, the first report for the Snail site mentioned that fluid temperature was as high as 250°C (Wheat et al., 2003).
Significantly high H2S and Fe concentrations were observed for the samples collected from high temperature fluid vents in both Pika and Archaean sites (Table A1 in Appendix). Based on analogy to the high temperature fluids found at hydrothermal fields in Lau Basin, low pH signature of the fluid could be responsible for enrichment in these species (Takai et al., 2008). In contrast, H2S and Fe concentrations of the low-temperature fluid samples are noticeably lower than the mixing line between high temperature fluids and seawater (Figures 3C,D). It is likely that iron-sulfide mineral precipitation occurred during sub-seafloor mixing, causing the decrease of H2S and Fe concentrations in the low-temperature fluids. For the Snail site, it is difficult to estimate original H2S and Fe concentrations in the fluid reservoir, since no high temperature fluid venting was observed. However, it is reasonable to expect similar range to other two sites, since common Si concentration among three sites suggest existence of a high temperature fluid reservoir. If it is the case, the observed Fe and H2S concentrations of the samples collected from low-temperature shimmering can be interpreted as affected by mineral precipitation during sub-seafloor mixing between the high temperature fluid and seawater.
Bacterial Community Structures
The microbial community structures in various habitats (e.g., sulfide structures of active vents, iron-rich mats, borehole fluids, and ambient seawater; Figure 1) in the SMT have been investigated using culture-independent molecular microbiological methods (PCR clone library construction and DNA sequencing, fluorescence in situ hybridization (FISH) and quantitative real-time PCR; Kato et al., 2009a,b, 2010). The bacterial community structures in the habitats have been determined by 16S rRNA gene clone library analysis as described in our previous reports (Kato et al., 2009a,b, 2010). The microbiological data used in the present paper are originated from the previous reports. The relatively abundant taxonomic groups in the libraries for each habitat are summarized in Table 1. It should be noted that the abundance of the phylotypes in the libraries does not indicate their real abundance in the communities but only the relative abundance due to the inherent biases of PCR-based analyses (Wintzingerode et al., 1997).
Table 1.
Abundant taxonomic groups in the bacterial clone libraries for each habitat.
| Habitat | Site | Temp range of venting fluids (°C) | Relative abundant group (potential chemolithoautotrophs) | Potential electron donor* | Growth temperature* |
|---|---|---|---|---|---|
| Active sulfide structure | Snail, Pika, Archaean | 19–341 | Aquificae (e.g., Persephonella), Epsilonproteobacteria (e.g., Hydrogenimonas and Sulfurimonas) | Hydrogen, sulfide | Moderate–Hyperthermophiles |
| Deep seawater | Snail, Pika, Archaean | 2–3 | Gammaproteobacteria (SUP05 group) | Sulfide | Psychrophiles (?) |
| Borehole fluid | Snail, Pika | 6–40 | Zetaproteobacteria (Mariprofundus), Epsilonproteobacteria (e.g., Sulfurimonas), Gammaproteobacteria (Thiomicrospira) | Iron, sulfide | Mesophiles |
| Iron-rich mat | Snail | 33–116 | Zetaproteobacteria (Mariprofundus), Gammaproteobacteria (e.g., Methylomonas and Methylobacter) | Iron, methane | Mesophiles |
*Based on the physiological characteristics inferred from the phylogeny of the phylotypes.
In the sulfide structures of active vents, phylotypes belonging to the Aquificae or Epsilonproteobacteria were abundant in the libraries. The detection frequencies in the libraries were up to 44% of the total clone numbers. Members of the Aquificae included thermophilic hydrogen-oxidizers, such as Persephonella hydrogeniphila. Members of the Epsilonproteobacteria have a variety of metabolic and physiological characteristics: hydrogen- and sulfide-oxidation, microaerobic and anaerobic, and mesophilic to thermophilic. However, no iron-utilizing bacteria have been reported in the two taxonomic groups. The microbial community structures of these sulfide structures of active vents in the SMT were similar to those of each habitat in other deep-sea hydrothermal areas including other back-arc basins, arc volcanoes, and mid-ocean ridges (Takai et al., 2006).
The abundant phylotypes in the libraries from the seawater samples were different from those of the sulfide structures of active vents. The phylotypes related to SUP05 group and SAR11 cluster dominated in the libraries from the seawater samples. The SUP05 group belonging to the Gammaproteobacteria predominated in the hydrothermal plume of the Suiyo Seamount (Sunamura et al., 2004) and may include sulfide-oxidizing chemolithoautotrophs as suggested by metagenomic analysis (Walsh et al., 2009). The bottom seawater samples collected in the SMT are likely to be mixed with hydrothermal plumes.
The microbial community structures of the iron-rich mats and borehole fluids were distinguished from those in the chimneys and seawater. The phylotypes belonging to the Zetaproteobacteria were abundant (up to 50% of the total clone numbers) in the libraries from the iron-rich mats and borehole fluids. Quantitative PCR or FISH analysis indicated that the Zetaproteobacteria phylotypes accounted for up to 32% of the total cell numbers in the communities of these samples. In addition, the phylotypes related to sulfide-oxidizers, such as Thiomicrospira in the Gammaproteobacteria and Sulfurimonas in the Epsilonproteobacteria, were also detected in the libraries from the borehole fluids. However, such phylotypes were not detected in the iron-rich mats. In contrast, the phylotypes related to Methylomonas and Methylophaga (including methano/methylotrophs) in the Gammaproteobacteria were relatively abundant in the libraries from the iron-rich mats. Davis and Moyer (2008) have also provided 16S rRNA gene data from iron-rich mats in Snail site. They also detected the phylotypes related to Zetaproteobacteria and Gammaproteobacterial methano/methylotrophs, which is consistent with our results.
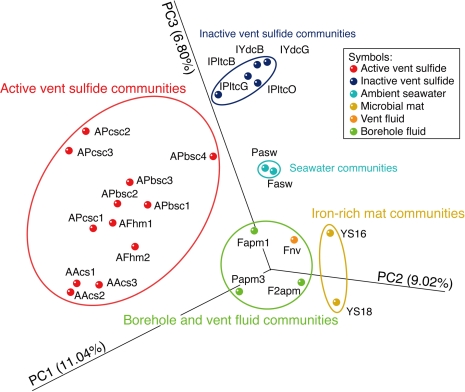
Based on the microbiological data that we reported previously (Kato et al., 2009a,b, 2010), the bacterial community structures in each habitat (i.e., active sulfide structures, iron-rich mats, borehole fluids and ambient seawater) in the SMT were compared by principal coordination analysis (PCoA) using Fast UniFrac (Hamady et al., 2009). Although Davis and Moyer (2008) have also provided 16S rRNA gene data from iron-rich mats in Snail site, we did not include their data in the comparative analysis to minimize methodological biases accompanied by different experimental procedures (e.g., DNA extraction and PCR). The distribution pattern of the communities for each sample corresponded well to their habitat types (Figure 4). The differences in the habitat types resulted in the differences in the abundant phylotypes in the libraries (Table 1). The physiology of the phylotypes cannot be determined from their phylogeny, but only inferred from the closest cultured species (Table 1). If we assume that the inferred metabolic functions of these phylotypes are correct, the observed differences in the microbial community structures among the habitats can be attributed to the presence/absence of each chemolithoautotroph (i.e., hydrogen-, sulfide-, methane-, or iron-oxidizer). In principle, the distribution pattern in PCoA is greatly influenced by the presence/absence of phylotypes that are phylogenetically distant from one another.
Figure 4.
Comparison of the bacterial community structures in the SMT habitats by principal coordination analysis. The used microbiological data are originated from our previous reports (Kato et al., 2009a,b, 2010). The principal coordination analysis was performed using Fast UniFrac (Hamady et al., 2009). Each axis (PC1-3) indicates the first, second, and third principal coordinates. The percentages in the axis labels represent the percentages of variation explained by the principal coordinates. AFhm1 and 2, sulfide mound venting 107°C fluid at the Snail site; AAcs1-3, clear smoker chimney venting 117°C fluid at the Archaean site; APbsc1-4, black smoker chimney venting 270°C fluid at the Pika site; APcsc1-4, clear smoker chimney venting 19°C fluid at the Pika site; IPltcB, G, and O, inactive chimney at Pika site; IYdcB, and G, inactive chimney at the Y site; Pasw, and Fasw, ambient seawater at the Pika and Snail sites, respectively; Fapm1 and F2apm, borehole fluids at the Snail site collected in 2004 and 2005, respectively; Papm3, borehole fluids at the Pika site; Fnv, natural vent fluid at the Snail site; YS16 and YS18, iron-rich mats at the Kaiko and Snail sites, respectively. The data from the Y and Kaiko sites are not mentioned in the present paper (please, see Kato et al., 2009a, 2010 for details).
Archaeal Community Structures
In addition to the bacterial communities, diverse archaeal communities were also detected in the habitats in the hydrothermal fields of the SMT (Kato et al., 2009a,b, 2010). The abundant archaeal taxonomic groups were different among the habitats (Table 2) like the bacterial communities. A large number of the recovered phylotypes were affiliated with uncultured clone groups, such as the miscellaneous crenarchaeotic group (MCG), terrestrial hot spring crenarchaeota (THSC), pSL12-related group, and Marine Benthic Group E (MBGE). Although the physiologies of these uncultured phylotypes is still unclear, some of them have been inferred from metagenomic and functional gene analyses, and the geochemical characteristics of the environments where they were detected: for example, the MCG includes anaerobic chemoorganotrophs (Teske and Sørensen, 2008), the pSL12-related group includes ammonia-oxidizers (Mincer et al., 2007), and MBGE includes iron-oxidizers (Takai and Nakamura, 2010), respectively.
Table 2.
Abundant taxonomic groups in the archaeal clone libraries for each habitat.
| Habitat | Site | Temp range of venting fluids (°C) | Relative abundant group (potential chemolithoautotrophs) | Potential electron donor* | Growth temperature* |
|---|---|---|---|---|---|
| Active sulfide structure | Snail, Pika, Archaean | 19–341 | Archaeoglobi, Thermoprotei | Hydrogen | (Hyper)thermophiles |
| Deep seawater | Snail, Pika, Archaean | 2–3 | MGI | Ammonia | Psychrophiles |
| Borehole fluid | Snail, Pika | 6–40 | MBGE, MGI, pSL12-related | Iron (?), ammonia | Mesophiles (?) |
| Iron-rich mat | Snail | 33–116 | MBGE, MGI, pSL12-related | Iron (?), ammonia | Mesophiles (?) |
*Based on the physiological characteristics inferred from the phylogeny of the phylotypes.
In the sulfide structures of active vents, phylotypes related to Archaeoglobi, Thermoprotei, and THSC were abundant in the libraries. The total detection frequencies of the three groups in the libraries were over 40%. Members of the Archaeoglobi and Thermoprotei include (hyper)thermophilic hydrogen-oxidizing anaerobes. No isolates of THSC have been reported. Only Marine Group I (MGI) phylotypes were detected in the libraries from the seawater samples. MGI members are common archaeal inhabitants in oceans (Fuhrman et al., 1992) and the group includes an ammonia-oxidizing chemolithoautotrophic isolate (Könneke et al., 2005). Like the bacterial community structures, the archaeal community structures of these sulfide structures and seawater samples were totally similar to those of each habitat in other deep-sea hydrothermal areas including other back-arc basins, arc volcanoes, and mid-ocean ridges (Takai et al., 2006).
All archaeal phylotypes recovered from the iron-rich mats and borehole fluids were classified in uncultured clone groups, such the MBGE, MCG, and pSL12-related group. These uncultured groups have been recovered from other deep-sea environments. For example, the MBGE phylotypes were first reported from deep-sea sediments (Vetriani et al., 1999) and were detected in iron-rich habitats (Suzuki et al., 2004; Takai, 2008). The MCG phylotypes are one of the widely distributed groups of Archaea in deep-sea environments (Teske and Sørensen, 2008). Interestingly, a MCG phylotype (NCBI accession number, AB213054) was closely related to those detected in crustal fluids from the IODP borehole 1026B (AY181048; Cowen et al., 2003) and from the inserting pipes at the Baby Bare seamount (AY704375; Huber et al., 2006). This MCG phylotype is potentially an indigenous archaeal member in sub-seafloor crustal aquifers.
Archaeal communities have often been ignored in microbiological analysis of iron-rich mats. However, the archaeal abundance in the communities of the SMT is not so small (over 10%) and should not be ignored (Davis and Moyer, 2008; Kato et al., 2009a). It is possible that archaeal communities play significant role in elemental cycling of Fe, N, and C in iron-rich deep-sea environments, and in the maintenance of the microbial ecosystems. Further cultivation efforts are needed to determine the physiology of these uncultured archaeal groups and to understand their significance in microbial ecosystems and elemental cycling in deep-sea hydrothermal systems.
Geochemical Constraints Shaping Microbial Community Structures
It has been proposed that fluid geochemistry constrains microbial community structures in deep-sea hydrothermal fields (McCollom and Shock, 1997). The available chemical energy for chemolithotrophs can be calculated thermodynamically for each metabolic reaction based on the geochemistry of hydrothermal fluids. To assess the relationship between microbial community structures and geochemical composition of hydrothermal fluids in the SMT, we calculated the bioavailable energy yields of metabolic reactions (aerobic hydrogen-, methane-, sulfide-, ammonia- or iron-oxidation, methanogenesis, sulfate-, iron- or nitrate-reducing hydrogen-oxidation, and anaerobic methane-oxidation) for each type of hydrothermal fluid, and compared them to the inferred metabolic ability for each taxonomic group from their phylogeny (Tables 1 and 2).
The procedure for the thermodynamic calculation was previously described in detail (Takai and Nakamura, 2010). In brief, the amounts of chemical energy potentially available for chemolithotrophic metabolisms in hydrothermal fluid were determined by calculating the Gibbs free energy of each of the metabolic reactions. The overall Gibbs free energy of reaction can be calculated using the equation:
| (1) |
where ΔGr is the Gibbs free energy of reaction, ΔGr° is the standard state Gibbs free energy of reaction, R is the universal gas constant, T is the temperature in Kelvin, and Q is the activity quotient of the compounds involved in the reaction. The Q term takes into account the contribution of the fluid composition to the Gibbs energy of each reaction. Chemical compositions of the hydrothermal fluids used for the calculation are (i) the measured data of the low-temperature fluids sampled and (ii) the calculated values for low-temperature fluids by mixing between end-member hydrothermal fluids and seawater. The mixing calculations were performed with the aid of the computer program EQ3/6, Version 8.0 (Wolery and Jarek, 2003). The thermodynamic database for the EQ3/6 operations and the values of the standard Gibbs energy for the chemolithotrophic metabolic reactions (ΔGr°) were generated using the SUPCRT92 code (Johnson et al., 1992) with a customized database.
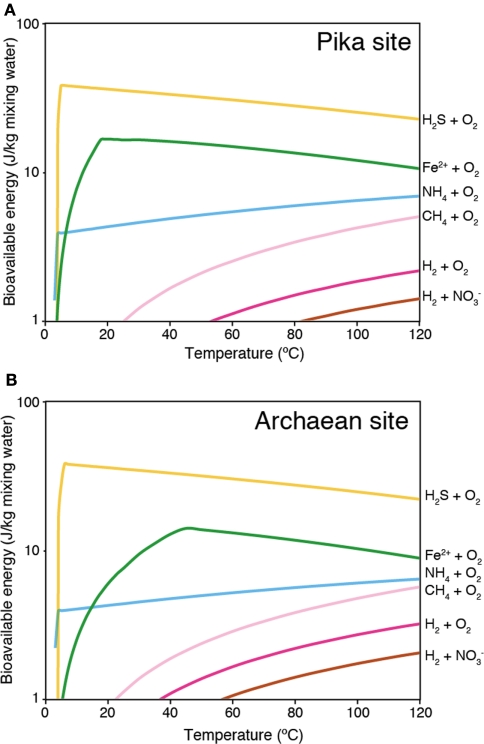
For reproducing the conditions inside of sulfide structures of active vents, the bioavailable energy yields for the temperature range between 3 and 125°C were calculated (Figure A2 in Appendix) using the geochemical data of high temperature hydrothermal fluids venting at the Pika and Archaean sites. We could not calculate the bioavailable energy yields for the Snail site because of lack of data for high temperature vent fluids in that site. In the calculation, simple mixing between hydrothermal fluid and seawater without any redox and mineral precipitation reactions was assumed (Takai and Nakamura, 2010). The results of the geochemical model calculations show that aerobic sulfide-oxidation yields the highest bioavailable energy under all temperature conditions (Figure A2 in Appendix). This suggests that sulfide-oxidizers using O2 and as electron acceptors can dominate in sulfide structures of active vents at both the Pika and Archaean sites. Indeed, phylotypes related to the Epsilonproteobacteria including aerobic sulfide-oxidizing chemolithoautotrophs (e.g., Sulfurimonas) were dominant in the libraries from the active chimneys from the Pika and Archaean sites (Table 1). This implies that the high-potentials of the energy availability from sulfide-oxidation reactions affect the relative abundance of these sulfide-oxidizing chemolithoautotrophs in bacterial communities in sulfide structures of active vents. The geochemical modeling results also suggest that iron-oxidation reactions produce the second largest amount of bioavailable energy. In the active chimney samples, however, none of the known iron-oxidizing chemolithoautotrophs was detected. This may suggest that the uncultured phylotypes detected in the sulfide structures contain unknown iron-oxidizers.
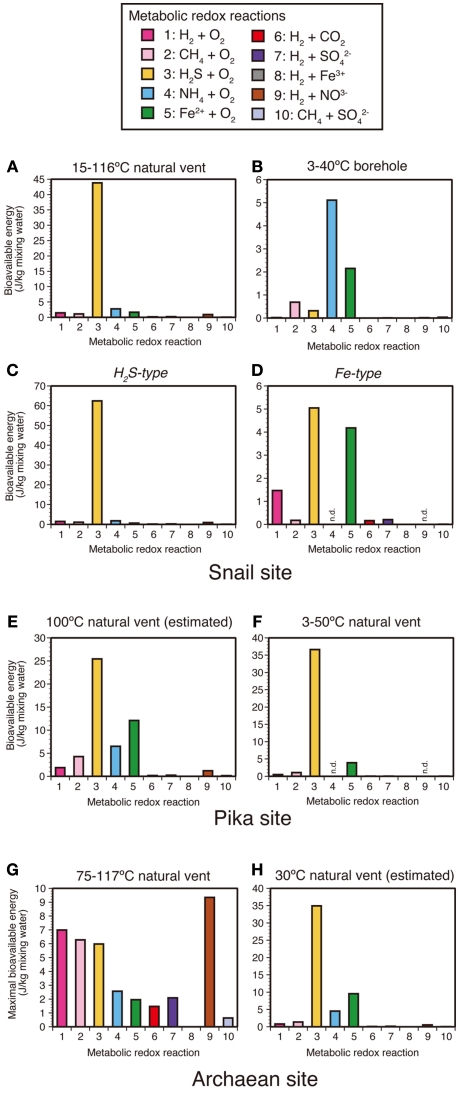
To assess the geochemical conditions of the relatively low-temperature (<150°C) fluids for chemolithoautotrophic microorganisms at the Pika, Archaean and Snail sites, the bioavailable energy yields were also calculated using the observed chemical data of the low-temperature shimmering fluids from the vent sites. The calculated bioavailable energy yields show that sulfide-oxidation reactions can produce higher bioavailable energy than other metabolic reactions (Figures 5A,E,F,H), except the Archaean site where hydrogen- and methane-oxidizing reactions yield higher bioavailable energy than sulfide-oxidation reactions (Figure 5G). The results are generally consistent with those estimated for vent fluids from active sulfide structures (Figure A2 in Appendix). Regarding the 75–115°C vent fluids of the Archaean site (Figure 5G), the bioavailable energy yields from aerobic and anaerobic hydrogen-oxidation reactions are slightly higher than those values estimated for active sulfide structures (Figure A2 in Appendix) due to relatively high H2 concentrations of the low-temperature shimmering fluids (comparable to high temperature fluids). Although the hydrogen concentration of the Archaean vent fluids would be variable and fortuitously fluctuating by phase separation and subsequent sub-seafloor mixing, the geochemical modeling result is generally consistent with microbiological observations that putative (hyper)thermophilic hydrogen-oxidizing chemolithoautotrophs belonging to both domains Bacteria (e.g., Hydrogenimonas) and Archaea (e.g., Archaeoglobi) were detected in the Archaean site.
Figure 5.
Bioavailable energy yields for metabolic redox reactions for each sample type. For the Snail site, the energy yields result from (A) 15–116°C natural vent, (B) 3–40°C borehole, (C) H2S-type, and (D) Fe-type fluids. For the Pika site, the energy yields result from (E) 100°C natural vent and (F) 3–50°C natural vent fluids. For the Archaean site, the energy yields result from (G) 75–117°C natural vent and (H) 30°C natural vent fluids. The bioavailable energy yields for (E) 100°C natural vent in the Pika site and for (H) 30°C natural vent in the Archaean site were calculated using the geochemical data estimated from a simple mixing model of hydrothermal fluids and seawater.
It should be noted that the bioavailable energy yields for iron-oxidation in the borehole fluid and high Fe/H2S vent fluids (i.e., “Fe-type fluids”) in the Snail site may be comparable to or higher than those for sulfide-oxidation (Figures 5B,D). The result is quite different from those estimated by a simple mixing model of high temperature hydrothermal fluids and seawater (Figure A2 in Appendix) and those calculated using the observed chemical data of the other low-temperature shimmering fluids (Figures 5A,C,E–H). This leads us to propose that there are habitats of iron-oxidizers where bioavailable energy yields from iron-oxidation reactions may be preferable for chemolithoautotrophs compared to those from sulfide-oxidation reactions. Indeed, the phylotypes related to the Zetaproteobacteria including an iron-oxidizing chemolithoautotroph (M. ferrooxydans) were abundant in the iron-rich mats accompanied by Fe-type vent fluids and the borehole fluids in the SMT (Table 1; Kato et al., 2009a,b). This microbiological result is consistent with the geochemical–thermodynamic result showing the presence of habitats with relatively high bioavailable energy yields for iron-oxidation in the Snail site. The same putative iron-oxidizers were also detected in borehole fluid from the Pika site. Although H2S and H2 concentration data for the Pika borehole fluid are not available and thus bioavailable energy yields for hydrogen- and sulfide-oxidation reactions cannot be calculated, Fe-rich, and H2S-depleted fluids similar to that observed in the Snail site are expected for the Pika borehole. Massive iron-rich mats harboring the Zetaproteobacteria were found in other hydrothermal fields, such as on arc volcanoes (Forget et al., 2010), hot-spot volcanoes (Rassa et al., 2009), and mid-ocean ridges (Davis et al., 2009). Furthermore, the phylotypes related to the Zetaproteobacteria were detected in the basaltic oceanic rocks (Santelli et al., 2008). Bach and Edwards (2003) have suggested that oxidation of reduced iron contained in the basaltic oceanic crust can provide energy for chemosynthetic ecosystems, which is corresponding to ∼2 × 1011 g cellular carbon/year as estimated by thermodynamic calculation. More detailed geochemical–microbiological characterization will provide insight into the relationship between the bioavailable energy yields by iron-oxidation and the abundance and distribution of the Zetaproteobacteria on and below the seafloor.
As shown above, assuming that the vent fluids were simply generated by mixing of end-member hydrothermal fluids with seawater, sulfide-oxidation yields the high energy for chemolithotrophs at all temperature ranges in the SMT hydrothermal fields (Figure A2 in Appendix). However, in fact, our thermodynamic calculations using the actual geochemical data indicate the presence of habitats in the Snail site where relatively high energy yields are available from iron-oxidation rather than sulfide- and hydrogen-oxidation. This discrepancy between the observation and theoretical expectation may result in part from incomplete modeling of the geochemical composition of the venting fluids as mentioned previously (Amend et al., 2011); for example, residence time, mineral depositions, and/or microbial metabolisms can influence the geochemistry of actual vent fluids, especially for low-temperature shimmering fluids that are likely to migrate in the crust at a slow rate. In the Snail site, many diffuse flows from cracks of the seafloor basalts were observed (Figure 1C), while no black smoker chimneys were found. In addition, pyrites (FeS2) were found in the fractures and vesicles of the sub-seafloor basalts collected by drilling (Kato et al., 2009b). These facts suggest that upwelling hydrothermal fluids are gradually mixed and cooled in the shallow sub-seafloor environments pervaded with the penetrating seawater and then iron and sulfide in the fluids are precipitated as pyrite. Depending on the end-member concentrations of Fe and H2S, the sub-seafloor mixing, and subsequent pyrite precipitation may result in the presence of high Fe/H2S low-temperature fluids. However, so far we have not determined the end-member concentrations of iron and sulfide because of lack of high temperature vent fluids in the Snail site. Further investigations are needed to assess the model of the generation of the Fe-type fluids.
It is remarkable that ammonia-oxidation yields the highest bioavailable energy for the borehole fluid in the Snail site followed by iron-oxidation (Figure 5B), although the bioavailable energy yields for the borehole fluid in the Pika site could not be calculated due to incomplete geochemical data (Table A1 in Appendix). Archaea accounted for 27–58% of the total cell number in the borehole fluids (Kato et al., 2009b), and putative ammonia-oxidizers, such as MGI and pSL12-related group, were detected in the borehole fluids (Table 2). This may be comparable to or higher than the result that Zetaproteobacteria accounted for 6–32% in the borehole fluids (Kato et al., 2009b) and is consistent with the thermodynamic calculation results: considerable numbers of putative ammonia-oxidizers as well as putative iron-oxidizers are expected to be present in the borehole fluids.
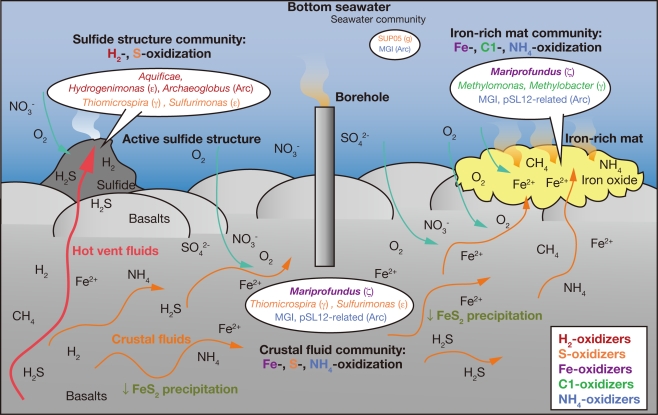
Model of the Appearance of the Microbial Community Structures in the SMT
Considering the inferred metabolic functions of the abundant phylotypes in each library from the samples as described above (Tables 1 and 2), it seems that the microbial community structures observed in the SMT hydrothermal fields are constrained by the energy availability of the fluids. We propose a model for the appearance of the microbial community structures in the SMT based on the results of microbiological, geochemical, and thermodynamic analyses (Figure 6). Although this model is mainly based on the results from the Snail site, our results from the Pika and Archaean sites can partially fit this model (e.g., the detection of putative sulfide-oxidizers in the active sulfide structures in the Pika and Archaean sites and that of putative iron- and ammonia-oxidizers in the borehole fluids in Pika site).
Figure 6.
A model for the appearance of the microbial community structures in each habitat in the SMT. This model is based on the microbiological and geochemical characteristics in the Snail site.
Upwelling high temperature hydrothermal fluids discharged from vents rapidly react with seawater, and lead to the precipitation of metal sulfides, which results in the formation of the chimney- or mound-like structures on the seafloor. Sequentially, hydrothermal fluids are mixed with seawater within the wall of the sulfide structures, which leads to the occurrence of steep chemical disequilibria. In such environments, hydrogen, and sulfide-oxidation reactions yield high bioavailable energy, and accordingly hydrogen-oxidizers and sulfide-oxidizers may be relatively abundant in the microbial communities.
In contrast, a portion of the upwelling hydrothermal fluids can permeate into porous basaltic pillow lava. The hydrothermal fluids may be gradually mixed and cooled in the shallow sub-seafloor environments pervaded with the penetrating seawater and then iron and sulfide in the fluids may be precipitated as pyrite. This sub-seafloor process would generate “Fe-type fluids.” In the Fe-type fluids, iron-oxidation yields high bioavailable energy, and accordingly, iron-oxidizers can dominate in the microbial communities as observed in the borehole fluids and iron-rich mats.
Conclusion and Perspective
In this paper, we summarized the microbial community structures in various habitats in the SMT. These findings highlight the uniqueness of the microbial communities in each habitat. On the whole, the results of thermodynamic calculations using the actual geochemical data are consistent with the presence/absence of each chemolithoautotroph in the microbial communities. The high bioavailable energy yield of iron-oxidation in the borehole and several vent fluids may be related to the appearance of microbial communities with abundant Zetaproteobacteria (putative iron-oxidizing chemolithoautotrophs), i.e., the iron-based ecosystem, in such environments. Based on the microbiological–geochemical–thermodynamic results, a model for the appearance of the microbial community structures in the SMT is proposed. It should be noted that the molecular microbiological methods we used mainly targeted DNA extracted from environmental samples and have some shortcomings (Wintzingerode et al., 1997). Firstly, we cannot determine whether the detected phylotypes are active in the environments or not. RNA-based analysis (such as reverse transcript PCR and FISH) can identify active members thriving in environments. Secondly, the physiologies of the phylotypes cannot be directly determined from the phylogeny. We believe that the diverse phylotypes in the Zetaproteobacteria include iron-oxidizing chemolithoautotrophs, although this must be verified by culture-dependent analyses. As shown here, the thermodynamic modeling by simple mixing of hydrothermal fluids with seawater is a powerful tool for predicting the microbial community structures in active chimneys where the mixing of hydrothermal fluids with seawater occurs immediately (Takai and Nakamura, 2010; Amend et al., 2011). Further careful calculations accompanied by other factors such as mineral precipitation will provide more helpful information for understanding the mechanisms shaping the microbial community structures in global deep-sea hydrothermal systems.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all of the crew, the operation team and scientists joining the YK03-05, TN167A, YK05-09, YK10-10, and YK10-13 cruises for their cooperation. This research was funded by the Ministry of Education, Culture, Science, and Technology (MEXT), Japan, through a special coordination fund (Project TAIGA: Trans-crustal Advection and In situ biogeochemical processes of Global sub-seafloor Aquifer) and partly by the RIKEN Special Postdoctoral Researchers Program.
Appendix
Table A1.
Summary of the geochemical characteristics of fluid samples collected in the SMT.
| Sampling site | Snail (2861 m) |
Pika (2773 m) |
Archaean (2986 m) |
Deep seawater | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample type | Borehole | Natural vent |
Fe–H2S available* |
Borehole | Natural vent |
Natural vent |
||||
| H2S-type | Fe-type | <150°C | >150°C | <150°C | >150°C | |||||
| T ave. | 25 | 55 | 54 | 41 | 10 | 28 | 265 | 101 | 251 | 3 |
| (Range; °C) | (3–40) | (15–116) | (15–81) | (18–59) | (3–50) | (230–330) | (75–117) | (153–343) | ||
| pH | 6.69 | 6.65 | 5.85 | 6.38 | 7.57 | 6.59 | 3.33 | 5.61 | 3.60 | 7.73 |
| Alk. (meq) | 2.22 | 2.09 | 2.16 | 1.94 | 2.37 | 2.28 | −0.95 | 0.95 | −0.63 | 2.48 |
| Mg (mM) | 46.2 | 45.6 | 40.9 | 45.5 | 50.0 | 49.8 | 8.5 | 41.3 | 13.3 | 50.8 |
| Ca (mM) | 10.7 | 11.2 | 12.9 | 11.1 | 9.7 | 10.2 | 34.4 | 10.7 | 16.0 | 9.6 |
| Sr (μM) | 85.0 | 89.7 | 82.4 | 91.6 | 83.5 | 87.5 | 123 | 100 | 102 | 84.8 |
| Na (mM) | 460 | 455 | 450 | 463 | 469 | 460 | 457 | 424 | 370 | 457 |
| K (mM) | 10.8 | 11.4 | 13.0 | 11.7 | 9.7 | 9.7 | 24.9 | 13.5 | 25.6 | 9.6 |
| Li (mM) | 0.046 | 0.076 | 0.118 | 0.081 | 0.028 | 0.044 | 0.530 | 0.158 | 0.391 | 0.033 |
| B (μM) | 388 | 401 | 353 | 481 | n.d. | 362 | 695 | 524 | 984 | 375 |
| C1 (mM) | 540 | 545 | 542 | 539 | n.d. | 548 | 576 | 533 | 461 | 542 |
| Br (mM) | 0.85 | 0.85 | 0.83 | 0.85 | 0.84 | 0.84 | 0.93 | n.d. | 0.77 | 0.86 |
| SO4 (mM) | 25.3 | 25.0 | 22.9 | 23.9 | 25.7 | 26.9 | 3.5 | 23.0 | 7.6 | 27.4 |
| Si (mM) | 1.05 | 1.60 | 2.98 | 1.97 | 0.21 | 0.49 | 14.2 | 3.47 | 12.6 | 0.17 |
| NH4 (μM) | 14.3 | 7.9 | 5.2 | 7.3 | n.d. | 3.5 | 36.3 | 8.4 | 31.1 | 11.0 |
| NO3 (μM) | 4.4 | 13.2 | 2.8 | 0.0 | n.d. | 25.1 | 6.8 | 20.7 | 8.3 | 33.8 |
| H2S (μM) | 0.41 | 57.1 | 229 | 6.6 | n.d. | 34.6 | 6001 | 572 | 4313 | 0.04 |
| Mn (mM) | 0.15 | 0.23 | 0.32 | 0.20 | 0.12 | 0.02 | 0.99 | 0.23 | 0.89 | 0.0018 |
| Fe (μM) | 62.4 | 57.0 | 19.5 | 119 | 115 | 82.8 | 6494 | 244 | 2056 | 8.9 |
| Ba (μM) | 1.67 | 22.1 | n.d. | 33.4 | n.d. | 4.60 | 46.2 | 44.7 | 40.2 | 1.66 |
| O2 (μM) | 286 | 164 | n.d. (164) | n.d. | 98 | 18 | 16 | 18 | 100** | |
| N2 (μM) | 1630 | 1771 | n.d. | n.d. | n.d. | n.d. | 1099 | 1358 | 967 | n.d. |
| CO2 (mM) | 7.20 | 7.76 | n.d. (7.76) | 3.11 | n.d. | 8.42 | 36.2 | 16.8 | 16.6 | 2.3** |
| CH4 (μM) | 0.86 | 1.37 | n.d. (1.37) | 0.22 | n.d. | 1.46 | 14.9 | 20.9 | 15.8 | 0.0003** |
| H2 (μM) | 0.10 | 6.62 | n.d. (6.62) | n.d. | 0.69 | 23.8 | 67.8 | 33.2 | 0.0004** | |
Average values for each sample type at each sampling site, i.e., boreholes, low-temperature (<150°C) natural vents and high temperature (>150°C) natural vents are shown. These data were obtained from the samples collected in 2003, 2004, 2005, and 2010. *The chemical data for the samples of the Snail site with iron and sulfide concentrations, which are shown in italic, were divided into H2S-type (high H2S/Fe) and Fe-type (high Fe/H2S). For thermodynamic calculation, the average values in parentheses were used. **Concentrations of O2, CO2, CH4, and H2 in deep seawater were obtained previously (McCollom, 2007).
Figure A1.
(A) Topographic map of the Southern Mariana Trough with (B) an enlarged view of the hydrothermal areas.
Figure A2.
Thermodynamic model calculation of bioavailable energy. Simple mixing between hydrothermal fluid and seawater without any redox and mineral precipitation reactions are assumed in the calculation. The results of (A) Pika and (B) Archaean sites are shown.
References
- Amend J. P., McCollom T. M., Hentscher M., Bach W. (2011). Catabolic and anabolic energy for chemolithoautotrophs in deep-sea hydrothermal systems hosted in different rock types. Geochim. Cosmochim. Acta 75, 5736–5748 10.1016/j.gca.2011.07.041 [DOI] [Google Scholar]
- Bach W., Edwards K. J. (2003). Iron and sulfide oxidation within the basaltic ocean crust: implications for chemolithoautotrophic microbial biomass production. Geochim. Cosmochim. Acta 67, 3871–3887 10.1016/S0016-7037(03)00304-1 [DOI] [Google Scholar]
- Chan C. S., Fakra S. C., Emerson D., Fleming E. J., Edwards K. J. (2011). Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation. ISME J. 5, 717–727 10.1038/ismej.2010.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen J., Giovannoni S., Kenig F., Johnson H., Butterfield D., Rappé M., Hutnak M., Lam P. (2003). Fluids from aging ocean crust that support microbial life. Science 299, 120–123 10.1126/science.1075653 [DOI] [PubMed] [Google Scholar]
- Davis R., Moyer C. (2008). Extreme spatial and temporal variability of hydrothermal microbial mat communities along the Mariana island arc and Southern Mariana back-arc system. J. Geophys. Res. 113, B08S15. 10.1029/2008JA013116 [DOI] [Google Scholar]
- Davis R. E., Stakes D. S., Wheat C. G., Moyer C. L. (2009). Bacterial variability within an iron-silica-manganese-rich hydrothermal mound located off-axis at the Cleft Segment, Juan de Fuca Ridge. Geomicrobiol. J. 26, 570–580 10.1080/01490450902889080 [DOI] [Google Scholar]
- Edwards K. J., Bach W., Rogers D. R. (2003). Geomicrobiology of the ocean crust: a role for chemoautotrophic Fe-bacteria. Biol. Bull. 204, 180–185 10.2307/1543555 [DOI] [PubMed] [Google Scholar]
- Emerson D., Moyer C. L. (2002). Neutrophilic Fe-oxidizing bacteria are abundant at the Loihi seamount hydrothermal vents and play a major role in Fe oxide deposition. Appl. Environ. Microbiol. 68, 3085–3093 10.1128/AEM.68.6.3085-3093.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D., Rentz J. A., Lilburn T. G., Davis R. E., Aldrich H., Chan C., Moyer C. L. (2007). A novel lineage of proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS ONE 2, e667. 10.1371/journal.pone.0000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget N. L., Murdock S. A., Juniper S. K. (2010). Bacterial diversity in Fe-rich hydrothermal sediments at two South Tonga Arc submarine volcanoes. Geobiology 8, 417–432 10.1111/j.1472-4669.2010.00247.x [DOI] [PubMed] [Google Scholar]
- Fryer P. (1995). “Geology of the Mariana Trough,” in Backarc Basins: Tectonics and Magmatism, ed. Taylor B. (New York: Plenum Press; ), 237–279 [Google Scholar]
- Fuhrman M., McCallum K., Davis A. A. (1992). Novel major archaebacterial group from marine plankton. Nature 356, 148–149 10.1038/356148a0 [DOI] [PubMed] [Google Scholar]
- Hamady M., Lozupone C., Knight R. (2009). Fast Unifrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and phylochip data. ISME J. 4, 17–27 10.1038/ismej.2009.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. A., Johnson H. P., Butterfield D. A., Baross J. A. (2006). Microbial life in ridge flank crustal fluids. Environ. Microbiol. 8, 88–99 10.1111/j.1462-2920.2005.00872.x [DOI] [PubMed] [Google Scholar]
- Ishibashi J., Suzuki R., Yamanaka T., Toki T., Kimura H., Noguchi T., Urabe T. (2006). Seafloor hydrothermal activity at off-axial seamounts of backarc spreading in Southern Mariana Trough. Geochim. Cosmochim. Acta 70, A279. 10.1016/j.gca.2006.06.566 [DOI] [Google Scholar]
- Ishibashi J., Urabe T. (1995). “Hydrothermal activity related to arc-backarc magmatism in the western Pacific,” in Backarc Basins: Tectonics and Magmatism, ed. Taylor B. (New York: Plenum Press; ), 451–495 [Google Scholar]
- Ishibashi J., Yamanaka T., Kimura H., Hirota A., Toki T., Tsunogai U., Gamo T., Utsumi M., Roe K., Miyabe S. (2004). “Geochemistry of hydrothermal fluids in South Mariana backarc spreading center,” in American Geophysical Union, Fall Meeting 2004, San Francisco, abstr. #V44A-05. [Google Scholar]
- Jannasch H. W., Mottl M. J. (1985). Geomicrobiology of deep-sea hydrothermal vents. Science 229, 717–725 10.1126/science.229.4715.717 [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Oelkers E. H., Helgeson H. C. (1992). SUPCRT92: a software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C. Comput. Geosci. 18, 899–947 10.1016/0098-3004(92)90029-Q [DOI] [Google Scholar]
- Kakegawa T., Utsumi M., Marumo K. (2008). Geochemistry of sulfide chimneys and basement pillow lavas at the Southern Mariana Trough (12.55ºN-12.58ºN). Resour. Geol. 58, 249–266 10.1111/j.1751-3928.2008.00060.x [DOI] [Google Scholar]
- Kato S., Kobayashi C., Kakegawa T., Yamagishi A. (2009a). Microbial communities in iron-silica-rich microbial mats at deep-sea hydrothermal fields of the Southern Mariana Trough. Environ. Microbiol. 11, 2094–2111 10.1111/j.1462-2920.2009.01930.x [DOI] [PubMed] [Google Scholar]
- Kato S., Yanagawa K., Sunamura M., Takano Y., Ishibashi J., Kakegawa T., Utsumi M., Yamanaka T., Toki T., Noguchi T., Kobayashi K., Moroi A., Kimura H., Kawarabayasi Y., Marumo K., Urabe T., Yamagishi A. (2009b). Abundance of Zetaproteobacteria within crustal fluids in back-arc hydrothermal fields of the Southern Mariana Trough. Environ. Microbiol. 11, 3210–3222 10.1111/j.1462-2920.2009.01930.x [DOI] [PubMed] [Google Scholar]
- Kato S., Takano Y., Kakegawa T., Oba H., Inoue K., Kobayashi C., Utsumi M., Marumo K., Kobayashi K., Ito Y., Ishibashi J., Yamagishi A. (2010). Biogeography and biodiversity in sulfide structures of active and inactive vents at deep-sea hydrothermal fields of the Southern Mariana Trough. Appl. Environ. Microbiol. 76, 2968–2979 10.1128/AEM.00478-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könneke M., Bernhard A. E., De La Torre J. R., Walker C. B., Waterbury J. B., Stahl D. A. (2005). Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437, 543–546 10.1038/nature03911 [DOI] [PubMed] [Google Scholar]
- Marumo K., Urabe T., Goto A., Takano Y., Nakaseama M. (2008). Mineralogy and isotope geochemistry of active submarine hydrothermal field at Suiyo Seamount, Izu–Bonin Arc, west Pacific Ocean. Resour. Geol. 58, 220–248 10.1111/j.1751-3928.2008.00059.x [DOI] [Google Scholar]
- McAllister S. M., Davis R. E., McBeth J. M., Tebo B. M., Emerson D., Moyer C. L. (2011). Biodiversity and emerging biogeography of the neutrophilic iron-oxidizing Zetaproteobacteria. Appl. Environ. Microbiol. 77, 5445–5457 10.1128/AEM.00533-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollom T. M. (2007). Geochemical constraints on sources of metabolic energy for chemolithoautotrophy in ultramafic-hosted deep-sea hydrothermal systems. Astrobiology 7, 933–950 10.1089/ast.2006.0119 [DOI] [PubMed] [Google Scholar]
- McCollom T. M., Shock E. L. (1997). Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim. Cosmochim. Acta 61, 4375–4391 10.1016/S0016-7037(97)00241-X [DOI] [PubMed] [Google Scholar]
- Mincer T. J., Church M. J., Taylor L. T., Preston C., Karl D. M., Delong E. F. (2007). Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ. Microbiol. 9, 1162–1175 10.1111/j.1462-2920.2007.01239.x [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Takai K. (2008). Deep-sea vent chemoautotrophs: diversity, biochemistry and ecological significance. FEMS Microbiol. Ecol. 65, 1–14 10.1111/j.1574-6941.2008.00502.x [DOI] [PubMed] [Google Scholar]
- Orcutt B. N., Sylvan J. B., Knab N. J., Edwards K. J. (2011). Microbial ecology of the dark ocean above, at, and below the seafloor. Microbiol. Mol. Biol. Rev. 75, 361–422 10.1128/MMBR.00039-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassa A. C., McAllister S. M., Safran S. A., Moyer C. L. (2009). Zeta-proteobacteria dominate the colonization and formation of microbial mats in low-temperature hydrothermal vents at Loihi Seamount, Hawaii. Geomicrobiol. J. 26, 623–638 10.1080/01490450903263350 [DOI] [Google Scholar]
- Santelli C. M., Orcutt B. N., Banning E., Bach W., Moyer C. L., Sogin M. L., Staudigel H., Edwards K. J. (2008). Abundance and diversity of microbial life in ocean crust. Nature 453, 653–656 10.1038/nature06899 [DOI] [PubMed] [Google Scholar]
- Singer E., Emerson D., Webb E. A., Barco R. A., Kuenen J. G., Nelson W. C., Chan C. S., Comolli L. R., Ferriera S., Johnson J., Heidelberg J. F., Edwards K. J. (2011). Mariprofundus ferrooxydans PV-1 the first genome of a marine Fe(II) oxidizing zetaproteobacterium. PLoS ONE 6, e25386. 10.1371/journal.pone.0025386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunamura M., Higashi Y., Miyako C., Ishibashi J., Maruyama A. (2004). Two bacteria phylotypes are predominant in the Suiyo Seamount hydrothermal plume. Appl. Environ. Microbiol. 70, 1190–1198 10.1128/AEM.70.2.1190-1198.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Inagaki F., Takai K., Nealson K. H., Horikoshi K. (2004). Microbial diversity in inactive chimney structures from deep-sea hydrothermal systems. Microb. Ecol. 47, 186–196 10.1007/s00248-003-1014-y [DOI] [PubMed] [Google Scholar]
- Takai K. (2008). Variability in the microbial communities and hydrothermal fluid chemistry at the newly discovered mariner hydrothermal field, Southern Lau Basin. J. Geophys. Res. 113 [Google Scholar]
- Takai K., Nakagawa S., Reysenbach A. L., Hoek J. (2006). Microbial ecology of mid-ocean ridges and back-arc basins. Geophys. Monogr. 166, 185–213 10.1029/166GM10 [DOI] [Google Scholar]
- Takai K., Nakamura K. (2010). “Compositional, physiological and metabolic variability in microbial communities associated with geochemically diverse, deep-sea hydrothermal vent fluids,” in Geomicrobiology: Molecular and Environmental Perspective, eds Barton L. L., Mandl M., Loy A. (Dordrecht: Springer; ), 251–283 [Google Scholar]
- Takai K., Nunoura T., Ishibashi J.-I., Lupton J., Suzuki R., Hamasaki H., Ueno Y., Kawagucci S., Gamo T., Suzuki Y., Hirayama H., Horikoshi K. (2008). Variability in the microbial communities and hydrothermal fluid chemistry at the newly discovered mariner hydrothermal field, Southern Lau Basin. J. Geophys. Res. 113, G02031. 10.1029/2007JG000636 [DOI] [Google Scholar]
- Teske A., Sørensen K. B. (2008). Uncultured archaea in deep marine subsurface sediments: have we caught them all? ISME J. 2, 3–18 10.1038/ismej.2007.90 [DOI] [PubMed] [Google Scholar]
- Toki T., Tsunogai U., Ishibashi J., Utsumi M., Gamo T. (2008). Methane enrichment in low-temperature hydrothermal fluids from the Suiyo Seamount in the Izu-Bonin Arc of the western Pacific Ocean. J. Geophys. Res. 113, B08S13. 10.1029/2007JB005476 [DOI] [Google Scholar]
- Vetriani C., Jannasch H. W., Macgregor B. J., Stahl D. A., Reysenbach A.-L. (1999). Population structure and phylogenetic characterization of marine benthic archaea in deep-sea sediments. Appl. Environ. Microbiol. 65, 4375–4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Damm K. L., Bischoff J. L., Rosenbauer R. J. (1991). Quartz solubility in hydrothermal seawater; an experimental study and equation describing quartz solubility for up to 0.5 m NaCl solutions. Am. J. Sci. 291, 977–1007 10.2475/ajs.291.10.977 [DOI] [Google Scholar]
- Von Damm K. L., Edmond J. M., Grant B., Measures C. I., Walden B., Weiss R. F. (1985). Chemistry of submarine hydrothermal solutions at 21ºN, East Pacific Rise. Geochim. Cosmochim. Acta 49, 2197–2220 10.1016/0016-7037(85)90222-4 [DOI] [Google Scholar]
- Walsh D. A., Zaikova E., Howes C. G., Song Y. C., Wright J. J., Tringe S. G., Tortell P. D., Hallam S. J. (2009). Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326, 578–582 10.1126/science.1174010 [DOI] [PubMed] [Google Scholar]
- Wheat C. G., Fryer P., Hulme S., Becker N., Curtis A., Moyer C. (2003). “Hydrothermal venting in the southern most portion of the mariana backarc spreading center at 12.57 degrees N,” in American Geophysical Union, Fall Meeting 2004, San Francisco, abstr. T32A-0920. [Google Scholar]
- Wintzingerode F., Gobel U. B., Stackebrandt E. (1997). Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 21, 213–229 10.1111/j.1574-6976.1997.tb00351.x [DOI] [PubMed] [Google Scholar]
- Wolery T. W., Jarek R. L. (2003). Software User’s Manual. EQ3/6, Version 8.0. U.S. Department of Energy Report 10813-UM-8.0-00, Albuquerque, NM: Sandia National Laboratories, 376 [Google Scholar]