Abstract
Pancreatic carcinoma is an extremely high-grade malignant tumor with fast development and high mortality. The incidence of pancreatic carcinoma continues to increase. Peripancreatic invasion and metastasis are the main characteristics and important prognostic factors in pancreatic carcinoma, especially invasion into the nervous system; pancreatic nerve innervation includes the intrapancreatic and extrapancreatic nerves. A strong grasp of pancreatic nerve innervation may contribute to our understanding of pancreatic pain modalities and the metastatic routes for pancreatic carcinomas. Computed tomography (CT) and magnetic resonance imaging (MRI) are helpful techniques for depicting the anatomy of extrapancreatic nerve innervation. The purpose of the present work is to show and describe the anatomy of the extrapancreatic neural plexus and to elucidate its characteristics using CT and MRI, drawing on our own previous work and the research findings of others.
Keywords: Computed tomography, Magnetic resonance imaging, Extrapancreatic neural plexus
INTRODUCTION
Pancreatic carcinoma is a type of tumor associated with high mortality, and even in the early stages, peripancreatic invasion and metastasis are observed[1,2]. The pattern of cancer invasion via neural routes (perineural invasion) has been studied extensively, and 53% to 100% of results have reported neural invasion in pancreatic carcinoma. The perineural invasion and the degree of perineural invasion, especially of the extrapancreatic neural plexus, can provide some useful information for planning surgery, and it has also been shown to be an important prognostic factor for pancreatic carcinoma by several studies[3-16]. The innervation of the pancreas is a very important factor in the progression of disease, primarily of the biliary tract and pancreas and in surgical procedures[3,6,11,16]. It is critical for the anatomy of the extrapancreatic innervation of the pancreas to be made clear. Several studies have offered descriptions of the innervation of the pancreas[3,17,18].
The major celiac ganglia are distributed around the pancreas, and its nerve fiber branches not only mediated effective internal and external secretion in the pancreas but are also related to abdominal algesia. Neurotropic growth is one of the important biological features of pancreatic carcinoma[19,20]. Extrapancreatic neural plexus invasion was not only the prognostic indicator of pancreatic carcinoma but also related to the retroperitoneum recurrence after operation[21]. For the relief of intractable epigastric and back pain caused by advanced pancreatic carcinoma or other advanced epigastric tumors, computed tomography (CT)-guided celiac plexus block was widely launched in clinics[22-25]. Therefore, to understand the celiac ganglia that are invaded by epigastric malignancy, improving the likelihood of success for celiac plexus block, reducing complications, and correctly identifying celiac ganglia are essential.
The development of modern imaging technology has permitted imaging of the extrapancreatic neural plexus[12,26,27].
ANATOMY OF EXTRAPANCREATIC NERVE PLEXUS
Innervation of the pancreas by the sympathetic division of the autonomic nervous system occurs via the splanchnic nerves, and innervation by the parasympathetic division occurs via the vagus nerve[28]. Both types of nerves are generally accompanied by blood vessels. Both nerve divisions contribute efferent (motor) fibers to the walls of the blood vessels, the pancreatic ducts, and the pancreatic acini and visceral afferent (pain) fibers. For vagal afferent innervation, the major portion descends in the gastroduodenal branch toward the duodenum, pancreas, and pylorus[29].
The abundance of nerve fibers and their encasement of the neural plexus of pancreas, which consists of the intrapancreas nerve interlaced with the extrapancreatic, and the peripancreatic and retroperitoneal distribution of many netlike nerve fibers are the main reasons that pancreatic carcinoma easily causes nerve invasion and metastasis. The extrapancreatic neural plexus has six parts[30,31], including the following.
The neural plexus of the pancreatic head
Concerning the innervation of the pancreas, Yoshioka et al[32] and Yi et al[3] explained that the plexus pancreaticus capitalis (PLX) could be divided into two parts, PLX-I and -II. The main routes for both of the nerves from the celiac plexus and the ganglia to the pancreas include two parts, one being the direct route from the celiac ganglia to the posterior surface of the head of the pancreas (PLX-I) and the other route extending from the bilateral celiac ganglion to the left margin of the uncinate process, via the plexus, around the superior mesenteric artery (SMA) (PLX-II)[1] (Figures 1 and 2). Yi et al[3] also reported that the plexus from the celiac plexus to the pancreas head was divided into the anterior hepatic plexus and the posterior hepatic plexus; the former ran along the common hepatic artery, and the latter ran below and behind the portal venous system. The PLX-I represents approximately 20% of the fibers derived from the posterior hepatic plexus. The PLX-II is the portion most often invaded by pancreatic carcinoma, and it is reported to be involved in 74%-90% of cases of pancreatic head carcinoma[4,33].
Figure 1.
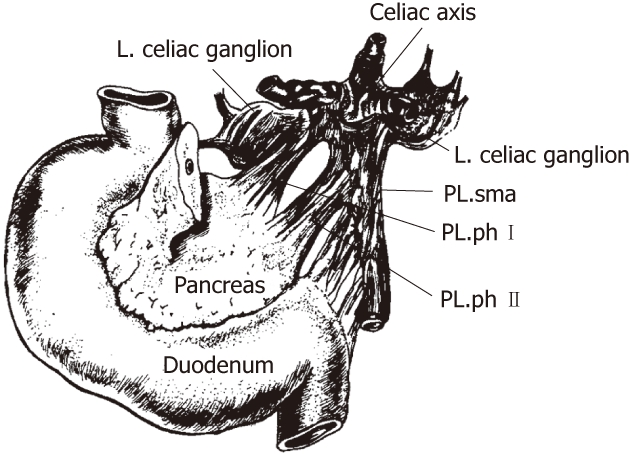
The anatomy of the extrapancreatic plexus[4]. PL: Plexus; sma: Superior mesenteric artery; ph: Pancreatic head.
Figure 2.
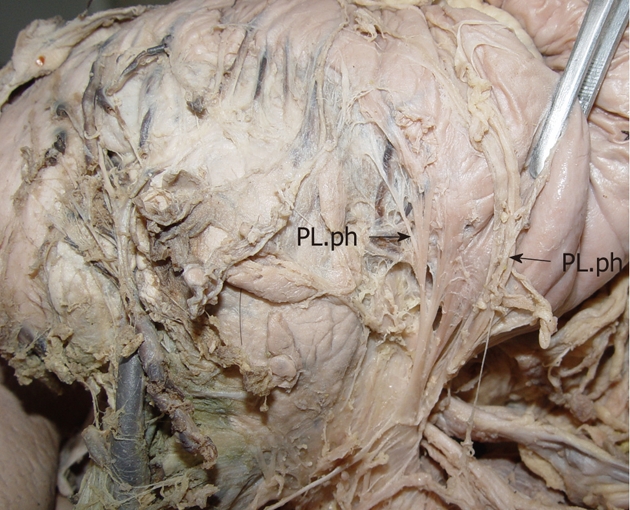
The anatomy of the pancreatic head plexus in a cadaver (arrows). The plexus extends from the celiac ganglia to the posterior surface of the head of the pancreas, the uncinate process. PL.ph: The plexus of the pancreatic head.
The celiac plexus
The celiac plexus is the center of the viscus and composed of celiac ganglia, several large and small nerves that terminate with the celiac ganglia, several nerve fibers that originate from the ganglia, and the abdominal branch of the posterior vagal trunk. The celiac plexus is located in front of the superior segment of the abdominal aorta, surrounding the celiac trunk and the root of the SMA[34,35] (Figure 3). At dissection, most of the celiac ganglia were between thoracic 12 and lumbar 1 (T12 and L1), and these ganglia were found in the upper part of the retroperitoneum, in front of the diaphragmatic crura, medial to the adrenal glands, and near the aorta between the origin of the celiac artery and the SMA[26] (Figures 4 and 5).
Figure 3.
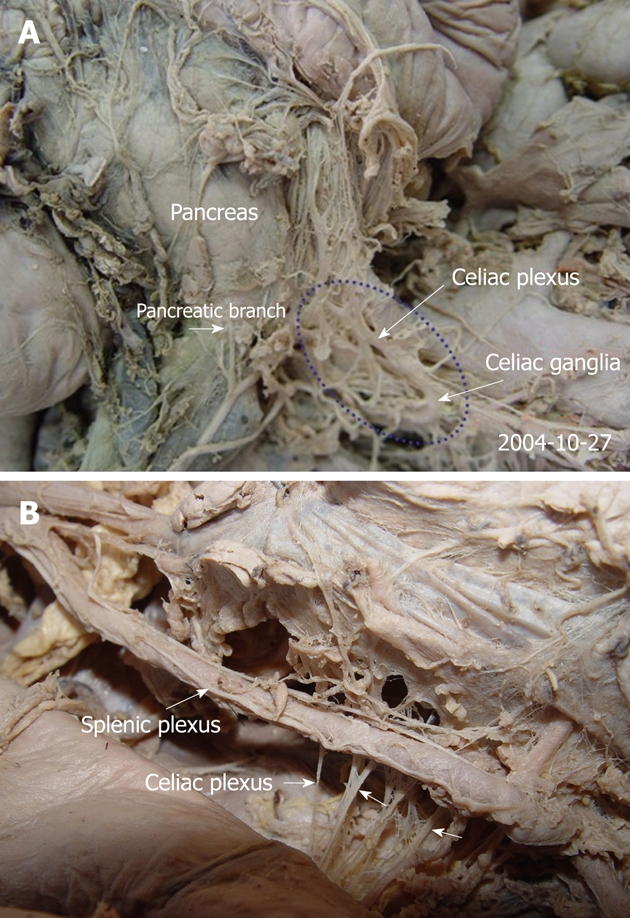
The anatomy of the celiac plexus in cadavers (A, B). The pancreas was moved upward. The pictures demonstrate that the celiac plexus is the center of the viscus and composed of the celiac ganglia and several large and small nerves that terminate with the celiac ganglia (arrows).
Figure 4.
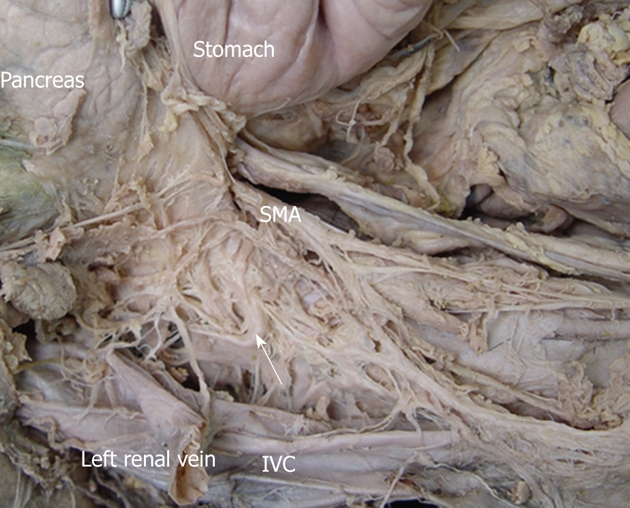
The anatomy of the celiac ganglia in a cadaver. The celiac ganglion (white arrows) was close to the aorta at a level intermediate to the origin of the celiac artery and superior to the mesenteric artery. It was located in the space bound by the inferior vena cava (IVC), the head of pancreas and the superior mesenteric artery (SMA). The pancreas was moved upward. The IVC was cut and moved laterally.
Figure 5.
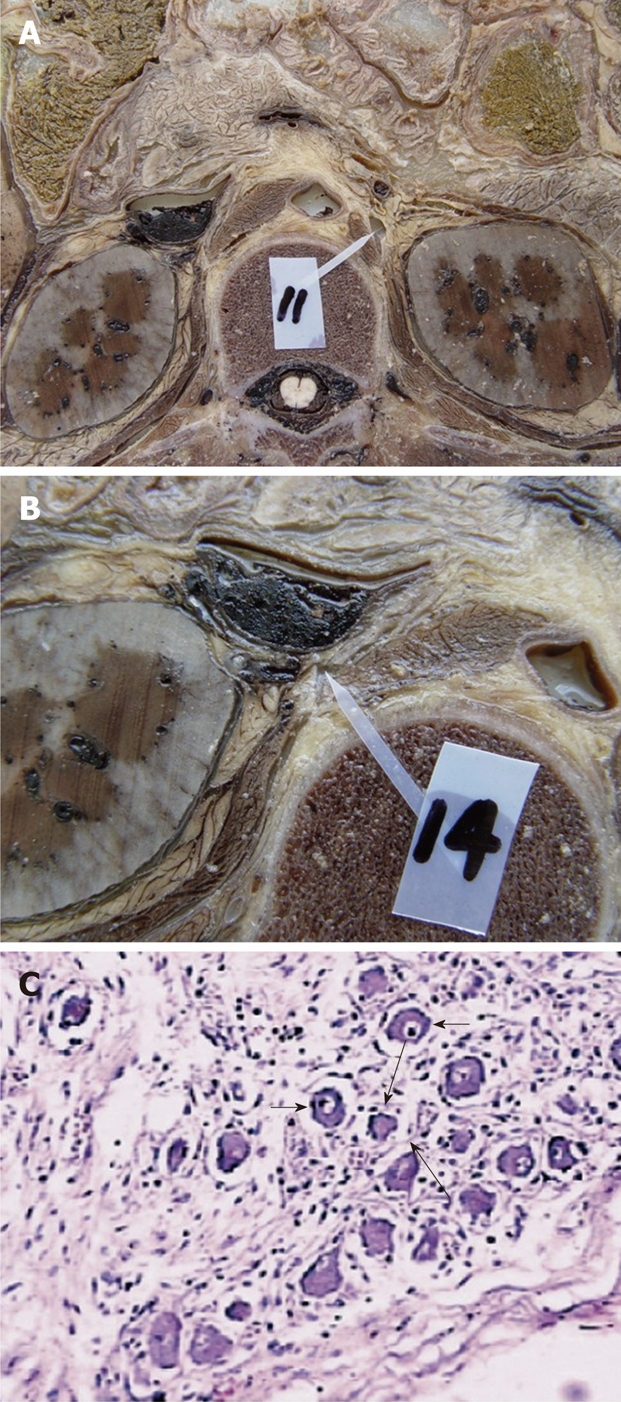
The dissection of the celiac ganglia in a cadaver at the L1 level. A: The left celiac ganglia; B: The right celiac ganglia; C: The histologic specimen stained with hematoxylin-eosin staining (× 100). With light microscopy, the celiac ganglion shows scattered ganglion cells (short arrows) and sparse nerve fibers (long arrows) among these ganglion cells.
The plexus around the superior mesenteric artery
There are many pancreatic branches from the SMA to the right edge of incisure of the pancreas, the center and to the right of the region behind the head of pancreas, which bypass the dorsal mesentery (Figure 6).
Figure 6.
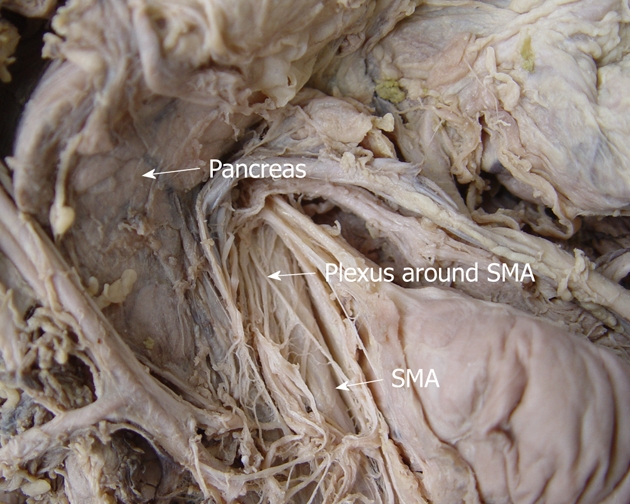
The anatomy of the plexus around the superior mesenteric artery in a cadaver. This route extends from the superior mesenteric artery (SMA) to the right edge of incisure of the pancreas.
The hepatic plexus
This route and its branches go from the liver to the back and superior borders of the pancreatic head along the common hepatic artery and proper hepatic artery (Figure 7).
Figure 7.
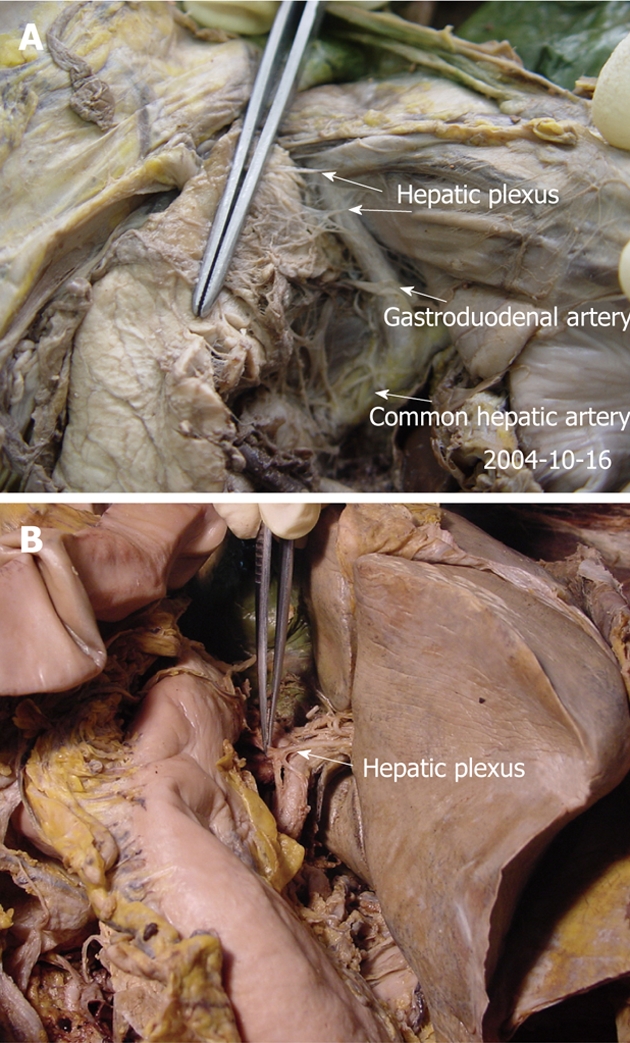
The anatomy of the hepatic plexus in cadavers (A, B). This route goes from the liver to the back and superior border of the pancreatic head, along the common hepatic and gastroduodenal artery (arrows).
The aortic plexus
This route and its branches travel around the aorta and extend to the head and uncinate process of the pancreas (Figure 8A).
Figure 8.
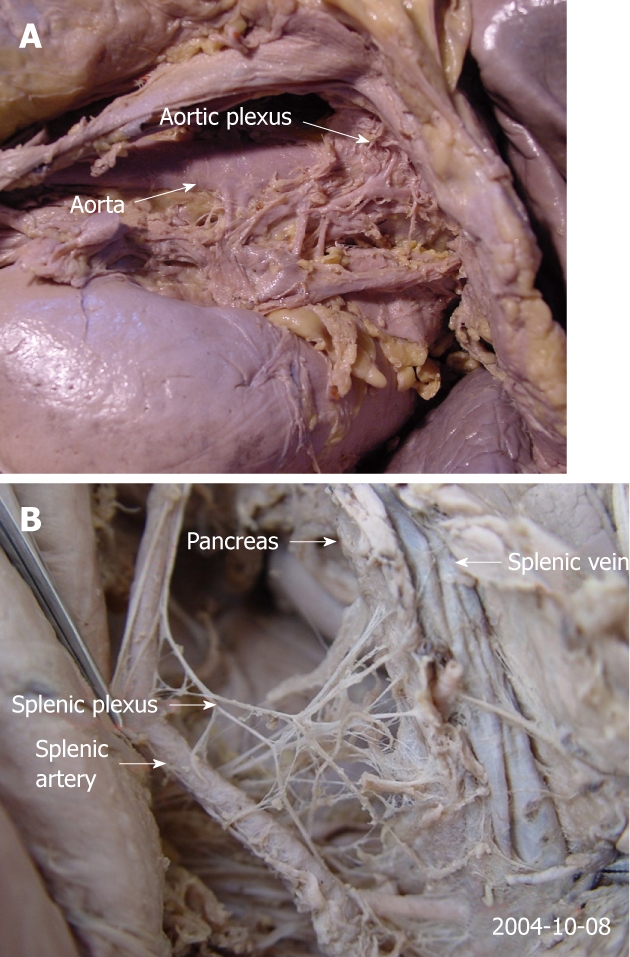
The anatomy of the aortic plexus in a cadaver. A: This route and its branches surround the aorta and extend to the head and uncinate process of the pancreas; B: The route travels along the splenic artery, and the main route and its many branches reach the tail of the pancreas.
The splenic plexus
This route travels along the splenic artery, and the main route and its branches reach the tail of the pancreas (Figure 8B).
CT FINDINGS OF EXTRAPANCREATIC NERVE PLEXUS
Multi-detector row CT allows thinner images (1.0 or 0.5 mm) to be reconstructed, enabling clear identification of the details of the pancreatic and peripancreatic anatomies[36].
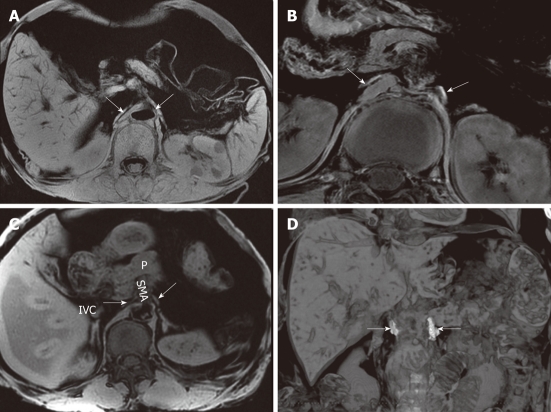
The neural plexus of the pancreatic head can be demonstrated clearly with CT imaging (Figures 9 and 10), showing a characteristic strand-like structure (Figure 10). Dal Pozzo et al[37] performed CT at the level of the celiac trunk and the SMA to identify the celiac ganglia. The celiac ganglia appeared as small lines, oval or laminar structures lower in density than the diaphragm; some were the same density as the diaphragm. In cadavers, the celiac ganglia on CT thus indicated corresponded exactly-by position, morphology, and dimensions-to the anatomic structures previously described in vivo (Figures 11 and 12). Rathmell et al[38] report the anatomy of the celiac plexus block using CT. We performed CT scanning on six cadavers and found that all of the left celiac ganglia were satisfactorily observed, whereas five out of six of the right celiac ganglia were satisfactorily shown. All of the celiac ganglia in the 6 cadavers were located between T12- L1, and their morphology was primarily laminar. The appearance of the celiac ganglia was high density (Figure 11). In addition, we also observed celiac ganglion in normal adults using CT. We found that both sides of the ganglia were of moderate density, the same as the liver and spleen or slightly lower than the diaphragma crura, in 650 cases (Figure 12).
Figure 9.
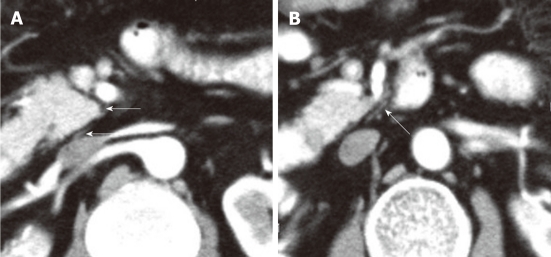
A contrast-enhanced computed tomography shows the plexus (PLX-II) (A, B). The PLX-II extends to the left margin of the uncinate process via the plexus surrounding the superior mesenteric artery (arrows).
Figure 10.
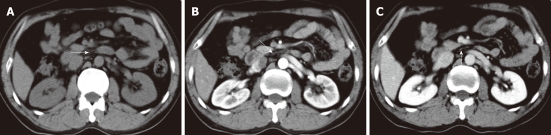
Non-enhanced computed tomography and contrast-enhanced computed tomography images show the neural plexus of the head of the pancreas (A-C). The plexus was a strand-like structure located in the area bound by the superior mesenteric artery, the inferior vena cava and the abdominal aorta (arrows).
Figure 11.
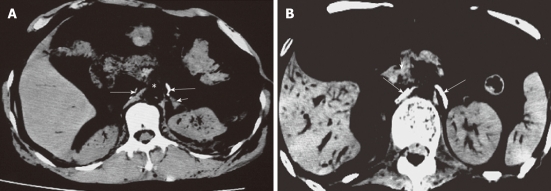
Computed tomography images of celiac ganglion in cadavers (labeled with contras media). The left was crescent-shaped and in front of the left adrenal gland, and the right was line-shaped and adjacent to the right crura of diaphragm (long arrows). Short arrows indicate left adrenal gland (A) and superior mesenteric artery (B).
Figure 12.
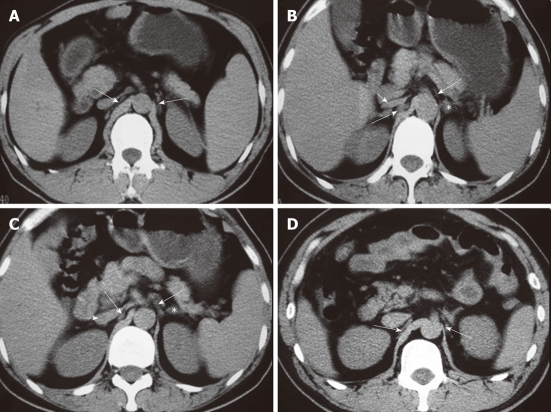
Computed tomography images show the celiac ganglia at different levels. A: A celiac ganglion (long arrows) at the root of the superior mesenteric artery; its density is the same as the liver and spleen or slightly less than the diaphragma crura; B: The celiac ganglia (long arrows) at the level of the pancreatic head and neck. The left celiac ganglia were lamina-shaped and located lengthwise in the space in the front of the left adrenal gland (*); the right celiac ganglia were a thick, line-shaped structure dorsal to the inferior vena cava (IVC) and lateral to the right diaphragma crura; C: A celiac ganglion (long arrows) at the level of the root of the celiac trunk. It appeared lamina- and nodule-shaped; D: The celiac ganglia (long arrows) at the level of the uncinate process, with well-defined margins. The left ganglia were lamina-shaped and located lengthwise in the space to the front and the left of the adrenal gland (*); the right ganglia were thick and line-shaped, located dorsal to the IVC and lateral to the right diaphragma crura. Short arrows indicate IVC (B, C).
MR FINDINGS OF EXTRAPANCREATIC NERVE PLEXUS
We studied the magnetic resonance imaging (MRI) findings of the celiac ganglia in cadavers and found that MRI can show the celiac ganglia accurately when the ganglia are large and labeled with gadolinium. These findings in cadavers can be a reference for identifying the celiac ganglia in vivo[26] (Figure 13).
Figure 13.
The celiac ganglia on an magnetic resonance imaging. A: A gradient-refocused-echo T1-weighted image shows that both the right and left celiac ganglia (arrows), labeled with Gd-DTPA, have a higher signal intensity than that of the viscus, such as the liver and spleen; B: 3D T1-weighted image shows that both the right and left celiac ganglia labeled with Gd-DTPA (arrows) have a higher signal intensity than the kidneys; C: A gradient-refocused-echo, T1-weighted, out-of-phase image shows the right and left celiac ganglia (labeled with arrows). The right celiac ganglion was located in the space formed by the inferior vena cava (IVC), right adrenal gland, right diaphragmatic crura, head of pancreas, and superior mesenteric artery (SMA). The left ganglion was located in the open space formed by the left adrenal gland, left diaphragmatic crura, and SMA. The celiac ganglia are labeled with gadolinium; D: Coronal imaging on T1-weighted images shows the celiac ganglia labeled with Gd-DTPA (arrows). P: Pancreas.
On MRI in cadavers, all of the right and left ganglia were identified and found to be hyperintense relative to the liver and spleen (Figure 13). Seventy-five percent (75%) of celiac ganglia were located at the level between the celiac artery and the SMA, in front of the diaphragmatic crura and close and medial to the aorta. Twenty-five percent (25%) of celiac ganglia were at the level of the SMA. The celiac ganglion appeared lamina-shaped (85.38%), nodule-shaped (10%) and sickle-shaped (4.62%) (Figure 13). Both celiac ganglia were depicted at the same level on 83.33% of MRI images. Almost all celiac ganglia could be seen at the level of the pancreas. At the level of the head and body of the pancreas, the right (41.67%) and left (58.33%) celiac ganglia could be seen; at the level of the head of the pancreas, the right (25%) and left (16.67%) celiac ganglia could be seen; at the level of the body and tail of the pancreas, the right (33.33%) and the left (25%) celiac ganglia could be seen[26]. In healthy adults, the celiac ganglia characteristics were identical, in terms of position, morphology, and dimensions, to the anatomic structures previously described in cadavers. Most of the celiac ganglia were lamina- or line-shaped (Figures 13 and 14).
Figure 14.
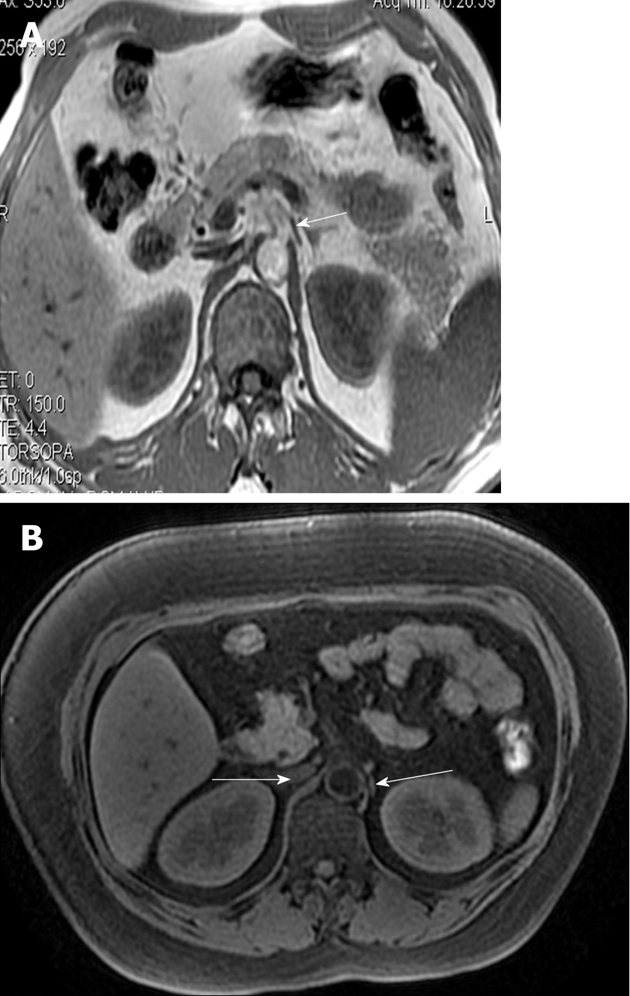
The magnetic resonance imagings of normal adults clearly show the celiac ganglia (A, B). Both celiac ganglia were seen at the level between the celiac artery and the superior mesenteric artery (arrows).
CONCLUSION
The anatomy of extrapancreatic neural plexus should be emphasized. The plexus includes six main parts, and the neural plexus of the pancreatic head is very important because it is easily invaded. These modern imaging techniques (CT and MRI) satisfactorily demonstrated the anatomy of the extrapancreatic nerve, which corresponded to the anatomy of the cadaver. The clear recognition and understanding of the innervation of the pancreas is necessary for surgical treatment of patients who have suffered nerve invasion by pancreatic carcinoma.
Footnotes
Supported by National Nature Science Foundation of China, No. 30370436
Peer reviewers: Aytekin Oto, MD, Associate Professor of Radiology, Chief of Abdominal Imaging and Body MRI, Department of Radiology, University of Chicago, 5841 S Maryland Ave, MC 2026 Chicago, IL 60637, United States; Weiguang Yao, PhD, Department of Medical Physics, Regional Cancer Program, Sudbury Regional Hospital, 41 Ramsey Lake Road, Sudbury, Ontario P3E 5J1, Canada; Dr. Charikleia Triantopoulou, Konstantopouleion Hospital, 3-5, Agias Olgas street, Athens 14233, Greece
S- Editor Cheng JX L- Editor A E- Editor Zheng XM
References
- 1.Tian H, Mori H, Matsumoto S, Yamada Y, Kiyosue H, Ohta M, Kitano S. Extrapancreatic neural plexus invasion by carcinomas of the pancreatic head region: evaluation using thin-section helical CT. Radiat Med. 2007;25:141–147. doi: 10.1007/s11604-006-0115-1. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Ishikura H, Kato H, Tanabe T, Yoshiki T. Intra-pancreatic, extra-tumoral perineural invasion (nex). An indicator for the presence of retroperitoneal neural plexus invasion by pancreas carcinoma. Acta Pathol Jpn. 1992;42:99–103. [PubMed] [Google Scholar]
- 3.Yi SQ, Miwa K, Ohta T, Kayahara M, Kitagawa H, Tanaka A, Shimokawa T, Akita K, Tanaka S. Innervation of the pancreas from the perspective of perineural invasion of pancreatic cancer. Pancreas. 2003;27:225–229. doi: 10.1097/00006676-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Kayahara M, Nagakawa T, Konishi I, Ueno K, Ohta T, Miyazaki I. Clinicopathological study of pancreatic carcinoma with particular reference to the invasion of the extrapancreatic neural plexus. Int J Pancreatol. 1991;10:105–111. doi: 10.1007/BF02924113. [DOI] [PubMed] [Google Scholar]
- 5.Kayahara M, Nagakawa T, Tsukioka Y, Ohta T, Ueno K, Miyazaki I. Neural invasion and nodal involvement in distal bile duct cancer. Hepatogastroenterology. 1994;41:190–194. [PubMed] [Google Scholar]
- 6.Kayahara M, Nagakawa T, Ueno K, Ohta T, Tsukioka Y, Miyazaki I. Surgical strategy for carcinoma of the pancreas head area based on clinicopathologic analysis of nodal involvement and plexus invasion. Surgery. 1995;117:616–623. doi: 10.1016/s0039-6060(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 7.Kayahara M, Nagakawa T, Futagami F, Kitagawa H, Ohta T, Miyazaki I. Lymphatic flow and neural plexus invasion associated with carcinoma of the body and tail of the pancreas. Cancer. 1996;78:2485–2491. doi: 10.1002/(sici)1097-0142(19961215)78:12<2485::aid-cncr6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Nagakawa T, Kayahara M, Ohta T, Ueno K, Konishi I, Miyazaki I. Patterns of neural and plexus invasion of human pancreatic cancer and experimental cancer. Int J Pancreatol. 1991;10:113–119. doi: 10.1007/BF02924114. [DOI] [PubMed] [Google Scholar]
- 9.Nagakawa T, Mori K, Nakano T, Kadoya M, Kobayashi H, Akiyama T, Kayahara M, Ohta T, Ueno K, Higashino Y. Perineural invasion of carcinoma of the pancreas and biliary tract. Br J Surg. 1993;80:619–621. doi: 10.1002/bjs.1800800526. [DOI] [PubMed] [Google Scholar]
- 10.Nakao A, Harada A, Nonami T, Kaneko T, Nomoto S, Koyama H, Kanazumi N, Nakashima N, Takagi H. Lymph node metastasis in carcinoma of the body and tail of the pancreas. Br J Surg. 1997;84:1090–1092. doi: 10.1046/j.1365-2168.1997.02754.x. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T, Tsunoda T, Suzuki T, Monden M, Saitoh Y, et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16–22. doi: 10.1007/BF02482964. [DOI] [PubMed] [Google Scholar]
- 12.Ochotorena IJ, Kiyosue H, Hori Y, Yokoyama S, Yoshida T, Mori H. The local spread of lower bile duct cancer: evaluation by thin-section helical CT. Eur Radiol. 2000;10:1106–1113. doi: 10.1007/s003309900055. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko T, Inoue S, Sugimoto H, Takeda S, Harada A, Nakao A. Intraoperative diagnosis of pancreatic cancer extension using IVUS. Hepatogastroenterology. 2001;48:944–948. [PubMed] [Google Scholar]
- 14.Nano M, Lanfranco G, Ferronato M, Dal Corso H, Solej M. [Contribution to the study of innervation of the pancreas with a view to its relevance to neoplasm surgery] Chir Ital. 2001;53:587–594. [PubMed] [Google Scholar]
- 15.Hirai I, Kimura W, Ozawa K, Kudo S, Suto K, Kuzu H, Fuse A. Perineural invasion in pancreatic cancer. Pancreas. 2002;24:15–25. doi: 10.1097/00006676-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Kimura W, Watanabe T. [Anatomy of the pancreatic nerve plexuses and significance of their dissection] Nihon Geka Gakkai Zasshi. 2011;112:170–176. [PubMed] [Google Scholar]
- 17.Mitchell GAG. Anatomy of the autonomic nervous system. 1st ed. Edinburgh: Livingstone; 1953. pp. 147–200. [Google Scholar]
- 18.Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MWJ. Gray’s Anatomy. 38th ed. Edinburgh: Churchill Livingstone; 1995. pp. 1292–1312. [Google Scholar]
- 19.Makino I, Kitagawa H, Ohta T, Nakagawara H, Tajima H, Ohnishi I, Takamura H, Tani T, Kayahara M. Nerve plexus invasion in pancreatic cancer: spread patterns on histopathologic and embryological analyses. Pancreas. 2008;37:358–365. doi: 10.1097/mpa.0b013e31818166e6. [DOI] [PubMed] [Google Scholar]
- 20.Mitsunaga S, Hasebe T, Kinoshita T, Konishi M, Takahashi S, Gotohda N, Nakagohri T, Ochiai A. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol. 2007;31:1636–1644. doi: 10.1097/PAS.0b013e318065bfe6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang XM, Mitchell DG, Byun JH, Verma SK, Bergin D, Witkiewicz A. MR imaging for predicting the recurrence of pancreatic carcinoma after surgical resection. Eur J Radiol. 2010;73:572–578. doi: 10.1016/j.ejrad.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Yang IY, Oraee S, Viejo C, Stern H. Computed tomography celiac trunk topography relating to celiac plexus block. Reg Anesth Pain Med. 2011;36:21–25. doi: 10.1097/AAP.0b013e318203067f. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Eroles X, Mayoral V, Montero A, Serra J, Porta J. Celiac plexus block: a new technique using the left lateral approach. Clin J Pain. 2007;23:635–637. doi: 10.1097/AJP.0b013e31812e6aa8. [DOI] [PubMed] [Google Scholar]
- 24.Zhang CL, Zhang TJ, Guo YN, Yang LQ, He MW, Shi JZ, Ni JX. Effect of neurolytic celiac plexus block guided by computerized tomography on pancreatic cancer pain. Dig Dis Sci. 2008;53:856–860. doi: 10.1007/s10620-007-9905-2. [DOI] [PubMed] [Google Scholar]
- 25.Konan AV, Rajhi H, Mnif N, Hamza R. [Treating pain related to inoperable pancreatic cancer in tropical areas: the advantage of CT-guided celiac plexus block and splanchnic nerves neurolysis] Sante. 2005;15:105–107. [PubMed] [Google Scholar]
- 26.Zhang XM, Zhao QH, Zeng NL, Cai CP, Xie XG, Li CJ, Liu J, Zhou JY. The celiac ganglia: anatomic study using MRI in cadavers. AJR Am J Roentgenol. 2006;186:1520–1523. doi: 10.2214/AJR.04.1765. [DOI] [PubMed] [Google Scholar]
- 27.Mochizuki K, Gabata T, Kozaka K, Hattori Y, Zen Y, Kitagawa H, Kayahara M, Ohta T, Matsui O. MDCT findings of extrapancreatic nerve plexus invasion by pancreas head carcinoma: correlation with en bloc pathological specimens and diagnostic accuracy. Eur Radiol. 2010;20:1757–1767. doi: 10.1007/s00330-010-1727-5. [DOI] [PubMed] [Google Scholar]
- 28.Teff KL. Visceral nerves: vagal and sympathetic innervation. JPEN J Parenter Enteral Nutr. 2008;32:569–571. doi: 10.1177/0148607108321705. [DOI] [PubMed] [Google Scholar]
- 29.Berthoud HR. Anatomy and function of sensory hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:827–835. doi: 10.1002/ar.a.20088. [DOI] [PubMed] [Google Scholar]
- 30.Japan Pancreas Society. The general rules of clinical and pathological management for carcinoma of the pancreas. 3rd ed. Tokyo: Kanehara Pubcomp; 1986. pp. 35–37. [Google Scholar]
- 31.Kuroda A, Nagai H. Surgical anatomy of the pancreas. In: Howard JM, Idezuki Y, Ihse I, Prinz RA, editors. Surgical diseases of the pancreas. 3rd ed. Baltimore: Williams and Wilkins; 1998. pp. 11–21. [Google Scholar]
- 32.Yoshioka H, Wakabayashi T. Therapeutic neurotomy on head of pancreas for relief of pain due to chronic pancreatitis; a new technical procedure and its results. AMA Arch Surg. 1958;76:546–554. doi: 10.1001/archsurg.1958.01280220066013. [DOI] [PubMed] [Google Scholar]
- 33.Kaneko T, Nakao A, Inoue S, Nomoto S, Nagasaka T, Nakashima N, Harada A, Nonami T, Takagi H. Extrapancreatic nerve plexus invasion by carcinoma of the head of the pancreas. Diagnosis with intraportal endovascular ultrasonography. Int J Pancreatol. 1996;19:1–7. doi: 10.1007/BF02788369. [DOI] [PubMed] [Google Scholar]
- 34.Jin G, Sugiyama M, Tuo H, Oki A, Abe N, Mori T, Masaki T, Fujioka Y, Atomi Y. Distribution of lymphatic vessels in the neural plexuses surrounding the superior mesenteric artery. Pancreas. 2006;32:62–66. doi: 10.1097/01.mpa.0000194607.16982.d7. [DOI] [PubMed] [Google Scholar]
- 35.Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1:469–476. [PubMed] [Google Scholar]
- 36.Prokesch RW, Schima W, Chow LC, Jeffrey RB. Multidetector CT of pancreatic adenocarcinoma: diagnostic advances and therapeutic relevance. Eur Radiol. 2003;13:2147–2154. doi: 10.1007/s00330-003-1926-4. [DOI] [PubMed] [Google Scholar]
- 37.Dal Pozzo G, Bozza A, Fargnoli R, Brizzi E. CT identification of coeliac ganglia. Eur J Radiol. 1985;5:24–26. [PubMed] [Google Scholar]
- 38.Rathmell JP, Gallant JM, Brown DL. Computed tomography and the anatomy of celiac plexus block. Reg Anesth Pain Med. 2000;25:411–416. doi: 10.1053/rapm.2000.7618. [DOI] [PubMed] [Google Scholar]