Abstract
A prodrug strategy was applied to guanidino-containing analogs to increase oral absorption via hPEPT1 and hVACVase. L-Valine, L-isoleucine and L-phenylalanine esters of [3-(hydroxymethyl)phenyl]guanidine (3-HPG) were synthesized and evaluated for transport and activation. In HeLa/hPEPT1 cells, Val-3-HPG and Ile-3-HPG exhibited high affinity to hPEPT1 (IC50: 0.65 and 0.63 mM, respectively), and all three L-amino acid esters showed higher uptake (2.6- to 9-fold) than the parent compound 3-HPG. Val-3-HPG and Ile-3-HPG demonstrated remarkable Caco-2 permeability enhancement, and Val-3-HPG exhibited comparable permeability to valacyclovir. In rat perfusion studies, Val-3-HPG and Ile-3-HPG permeabilities were significantly higher than 3-HPG, and exceeded/matched the high-permeability standard metoprolol, respectively. All the L-amino acid 3-HPG esters were effectively activated in HeLa and Caco-2 cell homogenates, and were found to be good substrates of hVACVase (kcat/Km in mM−1·s−1: Val-3-HPG, 3370; Ile-3-HPG, 1580; Phe-3-HPG, 1660). In conclusion, a prodrug strategy is effective at increasing the intestinal permeability of polar guanidino analogs via targeting hPEPT1 for transport and hVACVase for activation.
Keywords: prodrug approach, guanidino functionality, hPEPT1, hVACVase, intestinal absorption
Introduction
Prodrugs are bioreversible derivatives of drug molecules designed to overcome pharmaceutical, pharmacokinetic or pharmacodynamic barriers, such as low oral absorption, lack of site specificity, insufficient chemical stability, poor solubility, toxicity, etc.1 In order to improve oral absorption, a classic prodrug approach can be adopted to enhance drug lipophilicity and passive diffusion. In recent years, the understanding of membrane transporters has promoted a novel targeted prodrug approach, utilizing carrier-mediated transport to increase intestinal permeability.2 Once the goal is achieved, prodrugs must be converted to the active parent drug to exert therapeutic effect. This activation process is not necessary to be specific; however, a good understanding of the possible activating enzymes will help the rational design of successful prodrugs.
Human peptide transporter 1 (hPEPT1), the oligopeptide transporter expressed in the apical brush border membrane of the small intestine, mediates the transport of di- and tripeptides and a variety of peptidomimetics across the intestinal wall.3, 4 The broad substrate specificity of hPEPT1 makes it an attractive target for prodrug design. By conjugating to an amino acid promoiety, a low permeability drug can be recognized by hPEPT1 transporter and obtain significantly higher oral absorption. As examples, valacyclovir and valganciclovir, the valine ester prodrugs of poorly absorbed antiviral drugs acyclovir and ganciclovir, respectively, exhibit more than 50% oral bioavailability by hPEPT1-mediated membrane transport and extensive activation to their corresponding parent drugs.5–8 The rapid activation of valacyclovir and valganciclovir is due to the cooperation of chemical hydrolysis and multiple activating enzymes. However, some particular esterase(s), such as a specific amino acid ester prodrug-activating enzyme we identified recently, human valacyclovirase (hVACVase), may be primarily responsible for this activation process.9 Encouraged by the success of valacyclovir and valganciclovir, the amino acid ester prodrug strategy has also been applied to other poorly absorbed drugs, especially nucleosides,10 such as zidovudine,11 floxuridine,12–15 gemcitabine,16 levovirin17, and cytarabine18. Results suggested that the membrane permeability was increased greatly by targeting hPEPT1, and many of the prodrugs could be activated by hVACVase.19
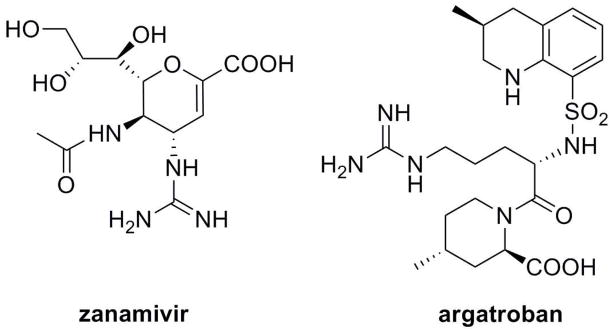
Frequently required for the high potency of drugs and drug candidates, the guanidino functionality is also an obstacle for oral absorption due to polarity and positive charge. Unless paracellular or active transport mechanism exists, such as the cases of metformin20, 21 and famotidine,22 guanidino-containing drugs are expected to have very limited oral absorption. For example, the influenza neuraminidase inhibitor zanamivir and the thrombin inhibitor argatroban (Scheme 1) are dosed by inhalation23 and intravenous administration24, respectively, due to low oral bioavailability. When oral route of administration is desired, guanidino group has to be replaced in many cases, although activity may also be compromised. In order to retain the potency as well as increase oral bioavailability, the classic prodrug strategy can be used to mask the charge and facilitate passive diffusion, following activation to the parent drug.25–28 However, this strategy has met with only limited success, partly due to the insufficient membrane transport and complicated activation. Moreover, if the drug is zwitterionic such as zanamivir and argatroban, all the charges need to be shielded, which further complicates both the synthesis and the in vivo activation. On the other hand, oral bioavailability can be increased by a targeted prodrug strategy, using mucosal transporters which may tolerate charges, such as hPEPT1, as prodrug carrier. It is well-known that guanidino group occurs in arginine-containing small peptides, which are natural substrates of hPEPT1 transporter. Although hPEPT1 appears to prefer neutral side chains instead of the charged ones, 3, 4, 29, 30 many arginine-containing small peptides still have at least medium affinity to hPEPT1 transporter and show evidence of transport.29–32 As for the amino acid ester prodrug strategy, the questions may arise are: (1) whether the guanidino functionality on the parent drug structure affects recognition and transport by hPEPT1; and (2) if the guanidino functionality plays a negative role, whether it can be compensated by a good amino acid promoity so the prodrug will be transported. If the guanidino group can be tolerated by hPEPT1, the amino acid ester prodrug strategy may be applied to guanidino-containing drugs, as long as an ester bond can be made through a hydroxyl group in the drug structure or a linker.
Scheme 1.
Structures of guanidino-containing drugs zanamivir and argatroban.
Another challenge for prodrug design is the activation process; the prodrug form should be retained prior to absorption, and be quickly metabolized to the parent compound following absorption. The classic prodrug strategy suffered from the unpredictable activation of the shielded guanidino functionality. The targeted prodrug strategy, however, circumvents this problem by leaving the guanidino group intact and at the same time, introducing a well-studied and relatively predictable amino acid ester bond. We have found that one of the primary amino acid ester prodrug-activating enzymes is hVACVase9, a serine hydrolase containing a catalytic triad S122-H255-D227. The very specific preference for amino acid ester substrates is attributed to the critical residue D123 forming electrostatic interaction with the a-amino group of substrate. hVACVase contains a large leaving group accommodating groove, which accommodates various leaving groups, including nucleoside analogs acyclovir, ganciclovir, floxuridine, gemcitabine, zidovudine and BDCRB, as well as simple alcohols such as methanol, ethanol and benzyl alcohol.9, 19, 33 In addition, the crystal structure of hVACVase revealed negative electrostatic potential in the groove, which may prefer a positively charged leaving group. Therefore, hVACVase may be a good target for guanidino-containing prodrugs, in order to control the activation process and secure the quick release of parent drug in vivo.
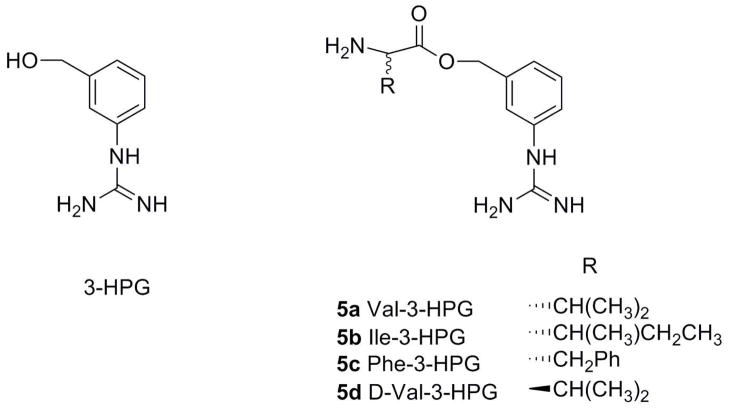
The purpose of this study was to apply a double-targeted (hPEPT1 and hVACVase) prodrug strategy for guanidino-containing molecules. We have designed and synthesized L-valine, L-isoleucine and L-phenylalanine esters of a model parent compound, [3-(hydroxymethyl)phenyl]guanidine (3-HPG, Scheme 2). These promoieties were chosen based on affinity to hPEPT1 transporter and stability profiles learned from our previous studies of amino acid nucleoside prodrugs. D-valine ester of 3-HPG was synthesized and used as a control to help determine the transport mechanism. The different model compounds were tested for hPEPT1-mediated uptake and transport across HeLa/hPEPT1 and Caco-2 cells, intestinal permeability in the rats, and activation by hVACVase. This set-up allowed us to evaluate the feasibility of the suggested strategy to improve oral absorption of low permeable guanidino-containing drugs.
Scheme 2.
Structures of 3-HPG and its L-valine, L-isoleucine, L-phenylalanine and D-valine esters.
Results
Synthesis of 3-HPG and its valine, isoleucine and phenylalanine esters
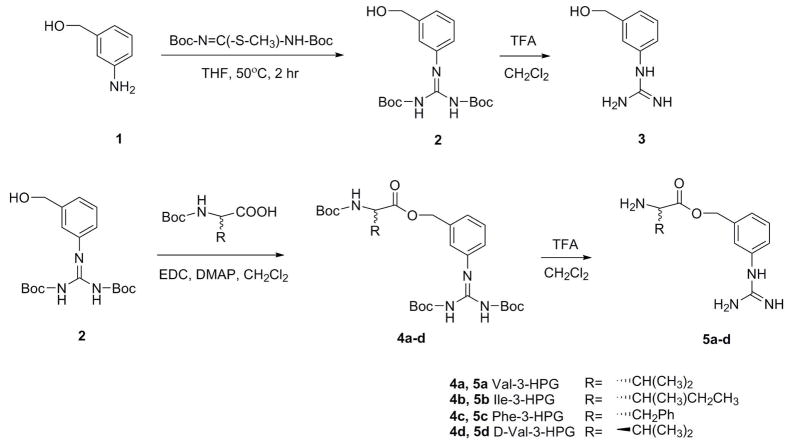
To synthesize 3-HPG, 3-amino benzyl alcohol was treated with 1,3-bis(tert-butoxycarbonyl)-2-methyl-2-thiopseudourea to convert the amino group to Boc-protected guanidino group. Followed by cleavage of the Boc group, 3-HPG was obtained as TFA salt (Scheme 3).
Scheme 3.
Synthesis of 3-HPG and its valine, isoleucine, phenylalanine and D-valine esters.
Amino acid esters were synthesized by coupling intermediate 2 and Boc-protected amino acids followed by deprotection (Scheme 3).
Hydrolysis in buffer and cell homogenates
Hydrolysis studies of 3-HPG and its L-amino acid esters were conducted in buffer and cell homogenates and the estimated half-lives (t1/2) obtained from linear regression of pseudo-first-order plots are shown in Table 1. The half-life of the reference amino acid ester prodrug, valacyclovir is also listed for comparison. In all buffers and cell homogenates, the parent compound 3-HPG exhibited good stability, which makes it a good model compound for subsequent studies. All the L-amino acid esters were also stable in pH 6 uptake buffer, and relatively stable in pH 7.4 phosphate buffer. In both HeLa and Caco-2 cell homogenates, the estimated half-lives of L-amino acid esters were much shorter than in pH 7.4 buffer, suggesting predominant contribution of enzymatic hydrolysis.
Table 1.
Estimated half-lives in pH 6 uptake buffer, pH 7.4 phosphate buffer, HeLa cell homogenates and Caco-2 cell homogenates (mean ± SEM; n=3).
| Half-lives (min)
|
||||
|---|---|---|---|---|
| pH 6 buffer | pH 7.4 buffer | HeLa cell homogenates | Caco-2 cell homogenates | |
| 3-HPG | stable* | stable* | stable* | stable* |
| Val-3-HPG (5a) | stable* | 493.6 ± 10.4 | 20.3 ± 0.7 | 4.8 ± 0.2 |
| Ile-3-HPG (5b) | stable* | 704.8 ± 9.7 | 137.2 ± 3.6 | 59.6 ± 2.3 |
| Phe-3-HPG (5c) | stable** | 261.8 ± 5.0 | ≪ 5 | ≪ 1 |
| valacyclovir | stable* | 829.4 ± 25.0 | 142.2 ± 1.2 | 31.3 ± 0.8 |
no area decrease detected in HPLC after 2 h
less than 5% decrease after 2 h
hVACVase-mediated hydrolysis
The estimated Michaelis-Menten kinetic parameters of 3-HPG esters as well as valacyclovir are listed in Table 2. All L-amino acid esters of 3-HPG showed high affinity to hVACVase, with Km values ranging from 9 to 207 μM. Interestingly, the kcat values followed the same order as Km values (Phe-3-HPG > Val-3-HPG > Ile-3-HPG), so the specificity constant, kcat/Km, didn’t differ as much as Km or kcat value itself. When compared with valacyclovir, Val-3-HPG exhibited higher, but not significantly different affinity. All the 3-HPG esters showed higher specificity constant than valacyclovir, clearly indicating that they are good substrates of hVACVase.
Table 2.
Michaelis-Menten kinetic parameters for hVACVase-mediated hydrolysis (mean ± SEM; n=3).
| Km (μM) | Vmax (nmol/min/μg) | kcat (s−1) | kcat/Km (mM−1·s−1) | |
|---|---|---|---|---|
| Val-3-HPG | 46 ± 5 | 321 ± 9 | 154 ± 4 | 3370 ± 460 |
| Ile-3-HPG | 9.0 ± 1.2 | 30 ± 1 | 14 ± 1 | 1580 ± 260 |
| Phe-3-HPG | 207 ± 16 | 718 ± 16 | 345 ± 8 | 1660 ± 160 |
| valacyclovir | 68 ± 4 | 120 ± 2 | 58 ± 1 | 850 ± 66 |
[3H]Gly-Sar uptake inhibition
The IC50 values of 3-HPG, its valine, isoleucine and phenylalanine esters to inhibit [3H]Gly-Sar uptake in HeLa/hPEPT1 cells are listed in Table 3. The amino acid benzyl esters were used for comparison to obtain the contribution of guanidino group. Valacyclovir, a known substrate of hPEPT1, was used as positive control.
Table 3.
[3H]Gly-Sar uptake inhibition in HeLa/hPEPT1 cells (mean ± SEM; valacyclovir, n=6; others n=4).
| IC50 (mM) | |
|---|---|
| 3-HPG | >3 |
| Val-3-HPG (5a) | 0.65 ± 0.04 |
| Val-OBz | 0.39 ± 0.04 |
| Ile-3-HPG (5b) | 0.63 ± 0.04 |
| Ile-OBz | 1.05 ± 0.13 |
| Phe-3-HPG (5c) | 2.33 ± 0.45 |
| Phe-OBz | > 3 |
| valacyclovir | 1.42 ± 0.18 |
The parent compound 3-HPG showed high IC50 value in [3H]Gly-Sar uptake inhibition study. Valine and isoleucine esters of 3-HPG, however, exhibited lower IC50 values than valacyclovir, indicating good affinity to the hPEPT1 transporter. Phe-3-HPG also showed some affinity to hPEPT1, although it was not as good as valacyclovir. Comparing with the corresponding benzyl esters, all the 3-HPG esters showed similar or higher affinity to hPEPT1.
Direct uptake
The results of direct uptake study in HeLa/hPEPT1 cells and normal HeLa cells are summarized in Table 4. The parent compound 3-HPG, its valine and isoleucine esters and valacyclovir were very stable in donor solution (> 95% remaining after 1 h). Phe-3-HPG was also relatively stable (> 80% remaining after 1h). Valacyclovir, a known substrate of hPEPT1 transporter, showed more than 25-fold higher uptake in HeLa/hPEPT1 cells compared to that in normal HeLa cells. The 3-HPG esters displayed 8- to more than 25-fold increased uptake in HeLa/hPEPT1 cells compared with in normal HeLa cells, whereas 3-HPG only showed little enhancement. In HeLa/hPEPT1 cells, the uptake of the amino acid esters was 2.6- to 9-fold higher than 3-HPG.
Table 4.
Direct uptake and stability in HeLa/hPEPT1 and normal HeLa cells (mean ± SEM; n = 3).
| HeLa/hPEPT1 cells | HeLa cells | ||||
|---|---|---|---|---|---|
| uptake (nmol/mg in 60 min) | stability* (%) | uptake (nmol/mg in 60 min) | stability* (%) | hPEPT1/control | |
| 3-HPG | 5.5 ± 0.6 | - | 3.8 ± 0.1 | - | 1.4 ± 0.2 |
| Val-3-HPG (5a) | 50.2 ± 1.6 | 96.9 ± 0.0 | < 2 | 98.0 ± 0.1 | > 25 |
| Ile-3-HPG (5b) | 27.7 ± 1.4 | 98.7 ± 0.0 | < 2 | 99.0 ± 0.1 | >13 |
| Phe-3-HPG (5c) | 14.8 ± 1.7 | 83.8 ± 0.3 | 1.7 ± 0.2 | 87.2 ± 0.9 | 8.8 ± 2.0 |
| valacyclovir | 5.5 ± 0.3 | 96.8 ± 0.1 | < 0.2 | 99.2 ± 0.0 | > 25 |
percentage of ester form in donor solution at 60 min
Caco-2 monolayer permeability studies
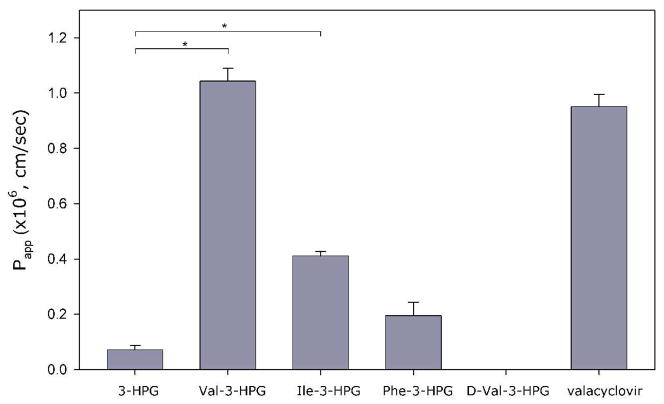
The permeability of 3-HPG and its amino acid esters across Caco-2 monolayers is shown in Figure 1. Val-3-HPG and Ile-3-HPG exhibited higher permeability than the parent compound 3-HPG and the permeability of Val-3-HPG was comparable to valacyclovir. Since both Val-3-HPG and Ile-3-HPG were stable in the donor solution (Table 5), and only the parent compound was detected in the receiver solution, most of the esters appeared to be metabolized in the cells prior penetration of the basolateral membrane. Phe-3-HPG didn’t show significantly different permeability than 3-HPG. However, due to its low stability in the donor solution (Table 5), the permeability may be underestimated. The stability issues, the lower affinity to hPEPT1 transporter and uptake in HeLa/hPEPT1 cells indicated that Phe-3-HPG was not a favorable candidate for in vivo studies.
Figure 1.
Apparent apical-to-basolateral permeability coefficient (Papp) across Caco-2 monolayers (mean ± SEM; n=3; *difference is statistically significant).
Table 5.
Stability in apical side during permeability study across Caco-2 monolayers (mean ± SEM; n = 3).
| Stability* (%) | |
|---|---|
| 3-HPG | - |
| Val-3-HPG (5a) | 86.5 ± 0.3 |
| Ile-3-HPG (5b) | 96.6 ± 0.6 |
| Phe-3-HPG (5c) | 3.9 ± 2.0 |
| D-Val-3-HPG (5d) | 98.8 ± 0.1 |
| valacyclovir | 94.7 ± 0.3 |
percentage of ester form in donor solution at 120 min
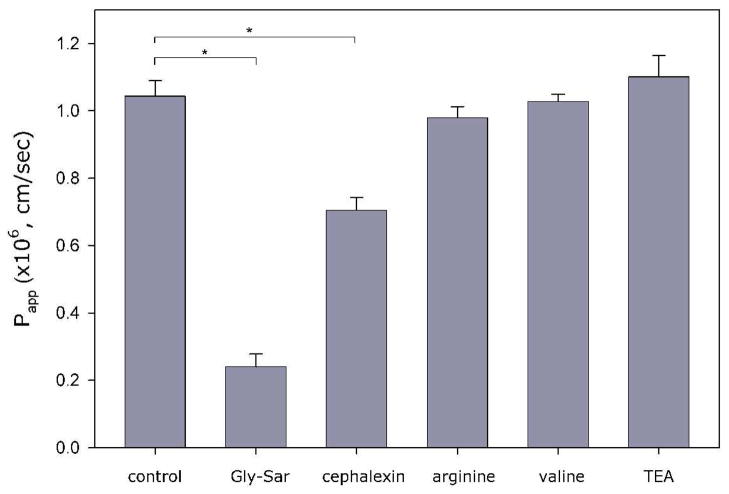
In contrast to Val-3-HPG, no transport of D-Val-3-HPG was detected. Since D-Val-3-HPG and Val-3-HPG are enantiomers, they should have similar permeability if the transport mechanism is predominantly passive diffusion. The much higher permeability of Val-3-HPG suggested that the transport was carrier mediated. In order to identify the transporter(s) involved in Val-3-HPG transport, we also investigated the effect of inhibitors on Val-3-HPG permeability, including hPEPT1 inhibitors Gly-Sar and cephalexin, amino acid transporter inhibitors L-valine and L-arginine, and organic cation transporter (OCT) inhibitor tetraethylammonium (TEA). As shown in Figure 2, only hPEPT1 inhibitors reduced Val-3-HPG permeability, suggesting hPEPT1 playing a dominant role in Val-3-HPG transport.
Figure 2.
Apparent apical-to-basolateral permeability coefficient (Papp) of Val-3-HPG without or with 5 mM inhibitors across Caco-2 monolayers. (mean ± SEM; n=3; * difference is statistically significant).
Rat perfusion studies
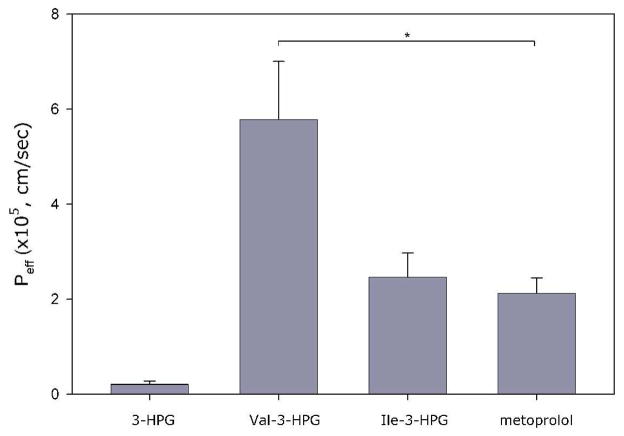
The effective permeability (Peff) of 3-HPG, Val-3-HPG and Ile-3-HPG in rat jejunum perfusion study is shown in Figure 3. Metoprolol was co-perfused with the test compounds as a reference standard for permeability in close proximity to the low/high permeability class boundary. It can be seen that Val-3-HPG and Ile-3-HPG exhibited higher or similar permeability compared to metoprolol, respectively, whereas the permeability of 3-HPG was very low. Hence, the amino acid ester prodrug approach significantly improved the rat jejunal permeability of 3-HPG, bringing it to high-permeability level.
Figure 3.
Rat jejunal membrane permeability (mean ± SEM; metoprolol, n=12; others, n=4; *difference is statistically significant).
Discussion and Conclusions
The positively charged guanidino functionality provides favorable interactions with important drug targets such as thrombin and influenza neuraminidase. However, the permanent positive charge at all relevant pHs can also cause low membrane permeability and poor oral bioavailability. To overcome this drug delivery barrier, we have utilized a targeted prodrug strategy, aiming to exploit hPEPT1 as the prodrug transport carrier and hVACVase as the predominant activating enzyme. This double-targeted prodrug approach may have the advantages of higher transport accompanied by more predictable activation, leading to favorable properties for oral delivery.
A primary concern of this research was whether guanidino functionality can be tolerated and transported by hPEPT1 transporter. In [3H]Gly-Sar uptake inhibition studies, both the valine and isoleucine esters of 3-HPG showed high affinity to hPEPT1, while the comparison between benzyl esters and 3-HPG esters didn’t show significant difference. Therefore, at least in the framework of this research, there was no evidence that the guanidino functionality hindered the binding to hPEPT1 transporter. The results in direct uptake, Caco-2 monolayer permeability and rat perfusion studies further support our hypothesis that the amino acid esters of 3-HPG can be transported by hPEPT1 for improved permeability. In normal HeLa cells, the uptake of the amino acid esters was even lower than the parent compound 3-HPG. However, in HeLa/hPEPT1 cells, the amino acid esters showed 2.6- to 9-fold higher uptake than 3-HPG, which can be attributed to hPEPT1-mediated transport. In the same fashion, both valine and isoleucine esters of 3-HPG exhibited considerably higher permeability across Caco-2 monolayers than the parent compound. The low permeability of D-Val-3-HPG and the studies with inhibitors clearly demonstrate that this enhancement can be attributed to hPEPT1. In rat perfusion studies, the effective permeability of Val-3-HPG and Ile-3-HPG exceeded or matched the high-permeability drug metoprolol, indicating high intestinal permeability. Taken together, these results demonstrate that it is feasible to target guanidino-containing compounds to hPEPT1 transporter for enhanced oral absorption. Up to now, the amino acid ester prodrug strategy has mainly been limited to nucleoside analogs. The positive results with guanidino functionality illustrated great promise to expand this strategy to other polar and charged low permeability drugs.
In addition to the guanidino functionality, this study provided more insights into the influence of amino acid promoieties on the hPEPT1-mediated transport. Amongst the three amino acid esters, valine ester exhibited the highest permeability in both cell culture and rat perfusion model, which is consistent with 5′-amino acid ester prodrugs of floxuridine,12 gemcitabine,16 levovirin17 and cytarabine.18 The phenylalanine ester exhibited the lowest affinity to hPEPT1, smallest uptake enhancement and no significant improvement in permeability studies. However, the poor stability in the donor solution of Caco-2 monolayer (Table 5) inevitably leads to underestimation of the permeability. This is also the case with 5′-phenylalanine ester of levovirin,17 where the low Caco-2 permeability accompanied low apical stability. Indeed, the 5′-phenylalanine ester prodrugs of floxuridine and gemcitabine exhibited enhanced uptake and permeability compared to corresponding parent drugs. Therefore, phenylalanine should not be excluded as a promising promoiety for hPEPT1 targeting. However, when phenylalanine ester prodrugs are designed, attention must be paid to make sure the prodrug is relatively stable in the brush border membrane to realize their full potential.
When utilizing the prodrug approach to increase oral absorption, the activation mechanism is often overlooked. As long as the parent drug can be regenerated, the activation will be considered successful, and the enzymes responsible for the prodrug activation may not capture much further attention. However, the activation process is actually the unique and one of the most critical steps for a prodrug to exert therapeutic effect. If the possible activating enzymes are identified, prodrugs can be designed to target these enzymes, which greatly increase the chances for the effective production of the parent drug. Although the amino acid ester prodrug strategy has been applied to many nucleoside analogs and improved the oral absorption by targeting hPEPT1, the activation was not well-studied and was considered nonspecific until the identification of the amino acid prodrog activating enzyme hVACVase9. hVACVase is at least one of the primary enzymes activating valacyclovir in vivo and is capable of activating many other amino acid ester prodrugs of nucleoside analogs.19 The large leaving group accommodating grove of hVACVase makes it an ideal target for prodrug design. The data presented in this paper demonstrate that a positively charged leaving group, 3-HPG, can be well tolerated by hVACVase, although whether the guanidino functionality helped the binding is still unclear. The high specificity constant (kcat/Km) of all the 3 L-amino acid esters of 3-HPG is consistent with their fast activation in Caco-2 cell homogenates as well as Caco-2 monolayer permeability studies. Therefore, hVACVase can be exploited as the target for activating amino acid ester prodrugs of guanidino-containing drugs. At the same time, the possibility that other enzymes can also contribute to the activation of amino acid ester prodrugs cannot be ruled out. We have estimated the hydrolysis rate of 3-HPG esters in Caco-2 cell homogenates with the kinetic data in recombinant hVACVase, based on the assumption that hVACVase was the predominant enzyme activating valacyclovir. After comparison with the hydrolysis data in Caco-2 homogenates, it turns out that hVACVase was also the primary enzyme responsible for Val-3-HPG and Ile-3-HPG hydrolysis, whereas the hydrolysis of Phe-3-HPG may involve other enzymes.
In conclusion, the amino acid esters of a guanidino-containing model compound exhibited high affinity to hPEPT1 transporter, leading to enhanced intestinal permeability accompanied by hVACVase-mediated activation to the parent compound. Hence, it may be feasible to apply the same strategy to other poorly absorbed guanidino analogs. This double-targeted approach makes the prodrug design more rational and less empirical, with the ability to mechanistically control the two most important aspects governing prodrug success.
Experimental Section
Materials
The tert-butyloxycarbonyl (Boc) protected amino acids, Boc-L-valine, Boc-L-isoleucine, Boc-L-phenylalanine and Boc-D-valine were obtained from Calbiochem-Novabiochem (San Diego, CA). Valacyclovir was a gift from GlaxoSmithKline, Inc. (Research Triangle Park, NC). High-performance liquid chromatography (HPLC) grade acetonitrile was obtained from Fisher Scientific (St. Louis, MO). L-Isoleucine benzyl ester 4-toluenesulfonate salt was obtained from Chem-Impex (Wood Dale, IL). 3-Amino benzyl alcohol, 1,3-bis(tert-butoxycarbonyl)-2-methyl-2-thiopseudourea, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC3HCl), 4-(dimethylamino)pyridine (DMAP), trifluoroacetic acid (TFA), L-valine benzyl ester hydrochloride, L-phenylalanine benzyl ester hydrochloride, metoprolol, phenol red and all other reagents and solvents were purchased from Sigma-Aldrich Co (St. Louis, MO). Cell culture reagents were obtained from Invitrogen (Carlsbad, CA), and cell culture supplies were obtained from Corning (Corning, NY) and Falcon (Lincoln Park, NJ). All chemicals were either analytical or HPLC grade.
Synthesis
NMR spectra were obtained on a Bruker AVANCE DRX500 NMR spectrometer. Electronspray ionization mass spectra were obtained on a Micromass LCT Time-of-Flight mass spectrometer. The purity of all synthesized test compounds was at least 95% as determined by HPLC.
[3-(hydroxymethyl)phenyl]guanidine (3-HPG, 3)
To a stirred solution of 3-amino benzyl alcohol (154 mg, 1.25 mmol) in 1 mL of dry tetrahydrofuran (THF), 1,3-bis(tert-butoxycarbonyl)-2-methyl-2-thiopseudourea (145 mg, 0.5 mmol) in 1.5 mL of THF was added dropwise, and then temperature was increased to 50°C. After 2 hours, the reaction was stopped and solvents were removed. The residue was dissolved in 30 mL of dichloromethane and washed twice with 10 mL of brine. The organic phase was dried over anhydrous MgSO4 and concentrated in vacuo. The product 2 was purified by column chromatography (CH2Cl2 : MeOH, 60 : 1) as colorless oil. Yield: 82.4%. 119.2 mg of 2 was dissolved in 4 mL of trifluoroacetic acid (TFA):CH2Cl2 (1:1) and stirred at room temperature for 5 hours. Then solvents were removed and the residue was dissolved in 0.1 % TFA, filtered and lyophilized. The raw product was further purified by semi-prep HPLC to give a colorless oil. Yield: 69.1%. 1H NMR (D2O) δ 7.40 (1H, dd, J = 7.8 Hz, 7.8 Hz, H-5), 7.29 (1H, d, J = 7.8 Hz, H-4 or H-6), 7.22 (1H, s, H-2), 7.17 (1H, d, J = 7.8 Hz, H-4 or H-6), 4.56 (2H, s, -CH2OH); ESI-MS: 166.0 (M+H)+.
L-Isoleucine [3-[(aminoiminomethyl)amino]phenyl]methyl ester (Ile-3-HPG, 5b)
33.1 mg of 2 (0.09 mmol), 35.6 mg of Boc-L-isoleucine (0.154 mmol) and 20.5 mg of 4-(dimethylamino)pyridine were dissolved in 1 mL of dry CH2Cl2 and stirred in ice bath. 30.5 mg of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC3HCl, 0.159 mmol) was slowly added to the above solution. The reaction mixture was stirred overnight at room temperature until reaction is complete. The solvents were removed and residue was dissolved in 30 mL of CH2Cl2 and washed with 10% (w/v) citric acid, saturated NaHCO3 and brine. The organic phase was dried over anhydrous MgSO4 and concentrated in vacuo. The mixture was then chromatographed on silica gel to obtain 4b as colorless oil. 4b was treated with 2.2 mL of TFA:CH2Cl2 (1:1.2) under argon for 2 hours. Solvents were removed and residue was dissolved in 0.1% TFA, filtered and lyophilized to give 5b as white solid. 5a, 5c and 5d were synthesized similarly. Yield of two steps: 70.9%. 1H NMR (methanol-d4) δ 7.53 (1H, dd, J=7.8 Hz, 7.8 Hz, H-5), 7.44 (1H, d, J=7.8 Hz, H-4 or H-6), 7.38 (1H, s, H-2), 7.33 (1H, d, J=7.8 Hz, H-4 or H-6), 5.35 (2H, m, - CH2-O-), 4.10 (1H, d, J=3.9 Hz, H-a), 2.02 (1H, m, H-β), 1.51 (1H, m, CH2- ), 1.35 (1H, m, CH2-γ), 0.99 (6H, m, CH3-γ, CH3-d); ESI-MS: 279.1 (M+H)+.
L-Valine [3-[(aminoiminomethyl)amino]phenyl]methyl ester (Val-3-HPG, 5a)
Yield of two steps: 39.5%. 1H NMR (methanol-d4) δ 7.53 (1H, dd, J=7.8 Hz, 7.8 Hz, H-5), 7.45 (1H, d, J=7.8 Hz, H-4 or H-6), 7.38 (1H, s, H-2), 7.33 (1H, d, J=7.8 Hz, H-4 or H-6), 5.36 (2H, m, -CH2-O-), 4.02 (1H, d, J=4.5 Hz, H-a), 2.32 (1H, m, H-β), 1.07 (6H, m, H- ); ESI-MS: 265.1 (M+H)+.
L-Phenylalnine [3-[(aminoiminomethyl)amino]phenyl]methyl ester (Phe-3-HPG, 5c)
Yield of two steps: 79.7%. 1H NMR (methanol-d4) δ 7.49 (1H, dd, J=7.9 Hz, 7.8 Hz, H-5), 7.20–7.38 (8H, m, aromatic protons), 5.27 (2H, s, - CH2-O-), 4.41 (1H, t, J=7.0 Hz, H-a), 3.25 (2H, m); ESI-MS: 313.1 (M+H)+.
D-Valine [3-[(aminoiminomethyl)amino]phenyl]methyl ester (D-Val-3-HPG, 5d)
Yield of two steps: 86.6%. 1H NMR (methanol-d4) δ 7.53 (1H, dd, J=7.8 Hz, 7.8 Hz, H-5), 7.44 (1H, d, J=7.8 Hz, H-4 or H-6), 7.38 (1H, s, H-2), 7.32 (1H, d, J=7.8 Hz, H-4 or H-6), 5.35 (2H, m, -CH2-O-), 4.02 (1H, d, J=4.5 Hz, H-a), 2.32 (1H, m, H-β), 1.06 (6H, m, H- ); ESI-MS: 265.2 (M+H)+.
Cell culture
HeLa cells (passage 17–31) and Caco-2 cells (passage 15–32) from American Type Culture Collection (Rockvile, MD) were routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 1% nonessential amino acids, 1 mmol/L sodium pyruvate and 1% L-glutamine. Cells were grown in an atmosphere of 5% CO2 and 90% relative humidity at 37°C.
Hydrolysis in HeLa and Caco-2 cell homogenates
HeLa cells or Caco-2 cells were washed with 0.15 M NaCl solution and then collected with 10 mM phosphate buffer (pH 7.4). The cell suspension was ultrasonicated in ice bath to extract enzymes. The suspension was spun at 10,000 rpm for 10 minutes at 4°C. Supernatant was placed in a new tube. The concentration of enzyme was determined as protein amount with the Bio-Rad DC assay (Hercules, CA) and adjusted to 500 μg/mL.
Hydrolysis in cell homogenates was determined at 37 °C. The hydrolysis reaction was initiated by adding 0.75 μl of test compound solution (200 mM in DMSO) to a reaction tube containing 749.25 μl cell homogenates. At various time points (0, 5, 10, 30, 60 and 120 min), 100 μl of the reaction mixture was removed and added to a quenching plate containing 100 μl of 10% TFA (in water) and stored in ice. Following the collection of all samples, quenching plate was filtered (2,000 rpm, 4 °C, 10 min). The filtrate was removed and assayed by HPLC.
The apparent first-order degradation rate constants were determined by plotting the natural logarithm of test compound remaining as a function of time. All the R2 values were higher than 0.98. The slope equals to negative rate constant (k). The degradation half-lives were then estimated by the equation:
Hydrolysis in pH 6.0 uptake buffer and pH 7.4 phosphate buffer
The hydrolysis in buffer was carried out the same way as above except each reaction well contains 749.25 μl of uptake buffer (pH 6.0, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaC12, 0.8 mM MgSO4, 5 mM D-glucose, and 25 mM MES) or 10 mM phosphate buffer (pH 7.4), and quenching plate contains 100 μl of 0.1% TFA (in water).
hVACVase-mediated hydrolysis
Recombinant hVACVase was overexpressed and purified from Escherichia coli as described previously.33 The purified hVACVase was concentrated and stored at −80 °C until it was used. The protein concentration was determined by Bio-Rad DC assay (Hercules, CA) with bovine serum albumin as a standard. The kinetic parameters of hVACVase-catalyzed hydrolysis were determined as follows. Kinetic measurements were carried out in 50 mM HEPES (pH 7.4) buffer at 37°C. After preincubation of the buffer for 5 min, recombinant hVACVase was added, and then the reaction was initiated by the addition of substrate. Aliquots were taken at different time points, and quenched by adding to same volume of 10% (v/v) trifluoroacetic acid. Initial velocities were calculated from the linear time course for the product formation. The kinetic parameters Km and Vmax were determined by fitting the initial velocity data to the Michaelis-Menten equation by the non-linear least-square regression analysis in GraphPad Prism software version 4.01. The kcat value was calculated from Vmax/[enzyme]0 based on the 28.83 kDa molecular mass of VACVase. Specific activity of valacyclovir was routinely monitored to normalize active protein concentration.
[3H]Gly-Sar uptake inhibition
The affinity to hPEPT1 transporter was evaluated by measuring its ability to inhibit the uptake of [3H]Gly-Sar, a standard hPEPT1 substrate, in HeLa/hPEPT1 cells. Prior to experiment, HeLa cells were grown in 12-well plates for 5 days, and infected with adenovirus containing hPEPT1 3 days before experiment.34 Cells were washed twice with uptake buffer (pH 6.0, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaC12, 0.8 mM MgSO4, 5 mM D-glucose, and 25 mM MES) and incubated with 10 μM Gly-Sar (9.9 μM Gly-Sar and 0.1 μM [3H]Gly-Sar) and various concentrations (0.05–5 mM) of test compounds in 0.3 mL uptake buffer for 30 min in an atmosphere of 5% CO2 and 90% relative humidity at 37°C. After 30 min, the donor solutions were aspirated and the cells were washed three times with ice-cold uptake buffer and solubilized with 0.1% SDS. Aliquots of the suspensions were used for scintillation counting (Beckman LS 6000SC). IC50 values were determined using nonlinear data fitting (GraphPad Prism software version 4.01).
Direct uptake
The carrier-mediated uptake of the test compounds was screened in HeLa/hPEPT1 cells and nomal HeLa cells. Prior to experiment, HeLa cells were grown in 12-well plates for 5 days, and infected with adenovirus containing hPEPT1 3 days before experiment (HeLa/hPEPT1 cells only).34 Cells were washed with uptake buffer (pH 6.0, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaC12, 0.8 mM MgSO4, 5 mM D-glucose, and 25 mM MES) and incubated with 1 mL/well of fresh uptake buffer in an atmosphere of 5% CO2 and 90% relative humidity at 37°C. After 15 min, uptake buffer was removed and 0.5 mL of freshly prepared test compound solution (1 mM) in uptake buffer was added to each well. The cell plate was incubated at 37°C for 60 min. Then, the cells were washed twice with ice-cold Dulbecco’s Phosphate Buffered Saline, and collected in 10 mM phosphate buffer (pH 7.4) with cell scrapers. The cell suspension was ultrasonicated in ice bath, and cell lysate was treated with ice-cold trifluoroacetic acid (final concentration of 7%), vortexed, and centrifuged for 10 min at 10,000 rpm. The supernatant was then filtered (0.22 μm) and analyzed by HPLC. Control experiments were performed in normal HeLa cells. The protein amount of each sample was determined with the Bio-Rad DC Protein Assay using bovine serum albumin as a standard.
Caco-2 monolayer permeability studies
Caco-2 cells were grown on 6-well collagen-coated transwell inserts (Corning, 3.0 μm pore size; area 4.67 cm2) and permeability studies were performed 22 to 23 days post-seeding. Transepithelial electrical resistance (TEER) was monitored before and after experiment. Each well was rinsed with 1.5 mL of MES buffer (pH 6.0, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaC12, 0.8 mM MgSO4, 5 mM D-glucose, and 25 mM MES) at apical side and 2.5 mL of HEPES buffer (pH 7.5, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaC12, 0.8 mM MgSO4, 5 mM D-glucose, and 25 mM HEPES) at basolateral side. 1.5 mL of MES buffer and 2.5 mL of HEPES buffer were added to the apical side and basolateral side of each well respectively and the plate was incubated in an atmosphere of 5% CO2 and 90% relative humidity at 37°C for 15 min. Experiments were initiated by replacing the apical buffer with 1.5 mL of 200 μM test compound solution in MES buffer and the basolateral buffer with 2.5 mL of fresh HEPES buffer and incubate the plate at 37 °C. Two hundred microliter aliquots of the basolateral receiver solution were withdrawn at predetermined intervals and replaced with fresh HEPES buffer. The concentrations were determined by HPLC.
The apparent permeability (Papp; cm/sec) across Caco-2 cell monolayers was calculated using the following equation:
Where C0 is the initial test compound concentration in the donor solution, A is the surface area of the exposed monolayer, and dQ/dt is the steady-state appearance rate of the test compound in the receiver solution.
Rat perfusion studies
Male albino Wistar rats (Charles River, IN) weighing 250–280 g were used for all perfusion studies. Prior to each experiment, the rats were fasted over night (12–18 h) with free access to water. Animals were randomly assigned to the different experimental groups.
The procedure for the in situ single-pass intestinal perfusion followed previously published reports.35, 36 Briefly, rats were anesthetized with an intra-muscular injection of 1 mL/kg of ketamine-xylazine solution (9%:1%, respectively) and placed on a heated surface maintained at 37°C (Harvard Apparatus Inc., Holliston, MA). The abdomen was opened by a midline incision of 3–4 cm. A jejunal segment of approximately 10 cm was carefully exposed and cannulated on two ends with flexible PVC tubing (2.29 mm i.d., inlet tube 40 cm, outlet tube 20 cm, Fisher Scientific Inc., Pittsburgh, PA). Care was taken to avoid disturbance of the circulatory system, and the exposed segment was kept moist with 37°C normal saline solution (Hospira, Lake Forest, IL). The isolated segment was rinsed with saline solution in order to clean out any residual debris.
The perfusion buffer (pH 6.5, 10 mM MES, 135 mM NaCl, 5 mM KCl, 0.1 mg/mL phenol red, 0.4 mg/mL metorpolol and 0.1 mM test compound) was incubated in a 37°C water bath. At the start of the study, perfusion buffer was pumped through the jejunal segment at a flow rate of 0.2 mL/min (Watson Marlow Pumps 323S, Watson-Marlow Bredel Inc, Wilmington, MA). Phenol red was added to the perfusion buffer as a nonabsorbable marker for measuring water flux. Metoprolol was co-perfused as a compound with known permeability that serves as a marker for the integrity of the experiment, and as a reference standard for permeability in close proximity to the low/high permeability class boundary.37 The perfusion buffer was first perfused for 1 h, in order to assure steady state conditions (as also assessed by the inlet over outlet concentration ratio of phenol red which approaches 1 at steady state). Following reaching steady state, samples were taken in 10 min intervals for 1 h (10, 20, 30, 40, 50, and 60 min). All samples including perfusion samples at different time points, original drug solution, and inlet solution taken at the exit of the syringe were immediately assayed by HPLC. Following the termination of the experiment, the length of the perfused intestinal segment was accurately measured.
The effective permeability (Peff) in the in situ rat perfusion experiments was calculated using the following equation:
Where Q is the perfusion flow rate (0.2 mL/min), R is radius of the intestine, L is the perfused intestinal lenth, Cin and Cout are the inlet and outlet solution concentrations, respectively. The latter was corrected by multiplying the outlet concentration with [phenol red]in/[phenol red]out in order to account for water flux.
HPLC analysis
The concentrations of test compounds were determined on a Waters HPLC system (Waters Inc., Milford, MA). The HPLC system consisted of two Waters pumps (Model 515), a Waters auto-sampler (WISP model 712) and a Waters UV detector (996 Photodiode Array Detector). The system was controlled by Waters Millennium 32 software (Version 3.0.1). Samples were resolved in an Agilent ZORBAX Eclipse XDB-C18 column (3.5μm, 4.6×150 mm) equipped with a guard column. The mobile phase consisted of 0.1% (v/v) TFA in milli-Q water (solvent A) and 0.1% (v/v) TFA in acetonitrile (solvent B) with the solvent B gradient changing from 2–30% at a rate of 2%/min during a 20 min run. The retention times for 3-HPG, Val-3-HPG, Ile-3-HPG, Phe-3-HPG, D-Val-3-HPG, acyclovir and valacyclovir were 6.7, 9.7, 11.2, 12.1, 9.7, 4.9 and 7.9 minutes, respectively. The detection wavelength was 235 nm for 3-HPG and its amino acid esters and 254 nm for acyclovir and valacyclovir.
Statistical Analysis
All the [3H]Gly-Sar uptake inhibition and animal experiments were n=4 unless stated otherwise, and all the hydrolysis, direct uptake and Caco-2 permeability experiments were performed in triplicate. The data are presented as mean ± SEM. To determine statistically significant differences among the experimental groups, the two-tailed nonparametric Mann-Whitney U test was used for two-group comparison and one-way analysis of variance followed by Dunnett’s test was performed for comparison of several groups against one control group. The difference was termed significant when p-value is smaller than 0.05.
Acknowledgments
The authors would like to thank Dr. Yasuhiro Tsume for cell culture trainings and invaluable suggestions. This research was supported by grant U01AI061457 awarded by the National Institutes of Health.
Abbreviations
- hPEPT1
human peptide transporter 1
- hVACVase
human valacyclovirase
- 3-HPG
[3-(hydroxymethyl)phenyl]guanidine
- Boc
tert-butyloxycarbonyl
- OCT
organic cation transporter
- TEA
tetraethylammonium
References
- 1.Ettmayer P, Amidon GL, Clement B, Testa B. Lessons learned from marketed and investigational prodrugs. J Med Chem. 2004;47 (10):2393–2404. doi: 10.1021/jm0303812. [DOI] [PubMed] [Google Scholar]
- 2.Han HK, Amidon GL. Targeted prodrug design to optimize drug delivery. AAPS PharmSci. 2000;2 (1):E6. doi: 10.1208/ps020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandsch M, Knutter I, Leibach FH. The intestinal H+/peptide symporter PEPT1: structure-affinity relationships. Eur J Pharm Sci. 2004;21(1):53–60. doi: 10.1016/s0928-0987(03)00142-8. [DOI] [PubMed] [Google Scholar]
- 4.Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447(5):610–618. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- 5.Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39(12):2759–2764. doi: 10.1128/aac.39.12.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balimane PV, Tamai I, Guo A, Nakanishi T, Kitada H, Leibach FH, Tsuji A, Sinko PJ. Direct evidence for peptide transporter (PepT1)-mediated uptake of a nonpeptide prodrug, valacyclovir. Biochem Biophys Res Commun. 1998;250(2):246–251. doi: 10.1006/bbrc.1998.9298. [DOI] [PubMed] [Google Scholar]
- 7.Jung D, Dorr A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J Clin Pharmacol. 1999;39(8):800–804. doi: 10.1177/00912709922008452. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara M, Huang W, Fei YJ, Leibach FH, Ganapathy V, Ganapathy ME. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J Pharm Sci. 2000;89(6):781–789. doi: 10.1002/(SICI)1520-6017(200006)89:6<781::AID-JPS10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Kim I, Chu XY, Kim S, Provoda CJ, Lee KD, Amidon GL. Identification of a human valacyclovirase: biphenyl hydrolase-like protein as valacyclovir hydrolase. J Biol Chem. 2003;278(28):25348–25356. doi: 10.1074/jbc.M302055200. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Maag H, Alfredson T. Prodrugs of nucleoside analogues for improved oral absorption and tissue targeting. J Pharm Sci. 2008;97(3):1109–1134. doi: 10.1002/jps.21047. [DOI] [PubMed] [Google Scholar]
- 11.Han H, de Vrueh RL, Rhie JK, Covitz KM, Smith PL, Lee CP, Oh DM, Sadee W, Amidon GL. 5′-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm Res. 1998;15(8):1154–1159. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
- 12.Landowski CP, Song X, Lorenzi PL, Hilfinger JM, Amidon GL. Floxuridine amino acid ester prodrugs: enhancing Caco-2 permeability and resistance to glycosidic bond metabolism. Pharm Res. 2005;22(9):1510–1518. doi: 10.1007/s11095-005-6156-9. [DOI] [PubMed] [Google Scholar]
- 13.Landowski CP, Vig BS, Song X, Amidon GL. Targeted delivery to PEPT1-overexpressing cells: acidic, basic, and secondary floxuridine amino acid ester prodrugs. Mol Cancer Ther. 2005;4(4):659–667. doi: 10.1158/1535-7163.MCT-04-0290. [DOI] [PubMed] [Google Scholar]
- 14.Tsume Y, Vig BS, Sun J, Landowski CP, Hilfinger JM, Ramachandran C, Amidon GL. Enhanced absorption and growth inhibition with amino acid monoester prodrugs of floxuridine by targeting hPEPT1 transporters. Molecules. 2008;13(7):1441–1454. doi: 10.3390/molecules13071441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsume Y, Hilfinger JM, Amidon GL. Enhanced cancer cell growth inhibition by dipeptide prodrugs of floxuridine: increased transporter affinity and metabolic stability. Mol Pharm. 2008;5(5):717–727. doi: 10.1021/mp800008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song X, Lorenzi PL, Landowski CP, Vig BS, Hilfinger JM, Amidon GL. Amino acid ester prodrugs of the anticancer agent gemcitabine: synthesis, bioconversion, metabolic bioevasion, and hPEPT1-mediated transport. Mol Pharm. 2005;2(2):157–167. doi: 10.1021/mp049888e. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Hong L, Mau CI, Chan R, Hendricks T, Dvorak C, Yee C, Harris J, Alfredson T. Transport of levovirin prodrugs in the human intestinal Caco-2 cell line. J Pharm Sci. 2006;95(6):1318–1325. doi: 10.1002/jps.20434. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Sun J, Shi S, Jing Y, Yin S, Chen Y, Li G, Xu Y, He Z. Synthesis, transport and pharmacokinetics of 5′-amino acid ester prodrugs of 1-beta-D-arabinofuranosylcytosine. Mol Pharm. 2009;6(1):315–325. doi: 10.1021/mp800200a. [DOI] [PubMed] [Google Scholar]
- 19.Kim I, Song X, Vig BS, Mittal S, Shin HC, Lorenzi PJ, Amidon GL. A novel nucleoside prodrug-activating enzyme: substrate specificity of biphenyl hydrolase-like protein. Mol Pharm. 2004;1(2):117–127. doi: 10.1021/mp0499757. [DOI] [PubMed] [Google Scholar]
- 20.Nicklin P, Keates AC, Page T, Bailey CJ. Transfer of metformin across monolayers of human intestinal Caco-2 cells and across rat intestine. Int J Pharm. 1996;128(1–2):155–162. [Google Scholar]
- 21.Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35(10):1956–1962. doi: 10.1124/dmd.107.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourdet DL, Pritchard JB, Thakker DR. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3) J Pharmacol Exp Ther. 2005;315(3):1288–1297. doi: 10.1124/jpet.105.091223. [DOI] [PubMed] [Google Scholar]
- 23.Cass LM, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin Pharmacokinet. 1999;36 (Suppl 1):1–11. doi: 10.2165/00003088-199936001-00001. [DOI] [PubMed] [Google Scholar]
- 24.Linkins LA, Weitz JI. Pharmacology and clinical potential of direct thrombin inhibitors. Curr Pharm Des. 2005;11(30):3877–3884. doi: 10.2174/138161205774580598. [DOI] [PubMed] [Google Scholar]
- 25.Saulnier MG, Frennesson DB, Deshpande MS, Hansel SB, Vyas DM. An efficient method for the synthesis of guanidino prodrugs. Bioorg Med Chem Lett. 1994;4(16):1985–1990. [Google Scholar]
- 26.Humphreys WG, Obermeier MT, Chong S, Kimball SD, Das J, Chen P, Moquin R, Han WC, Gedamke R, White RE, Morrison RA. Oxidative activation of acylguanidine prodrugs: intestinal presystemic activation in rats limits absorption and can be inhibited by co-administration of ketoconazole. Xenobiotica. 2003;33(1):93–106. doi: 10.1080/0049825021000012592. [DOI] [PubMed] [Google Scholar]
- 27.Arafa RK, Brun R, Wenzler T, Tanious FA, Wilson WD, Stephens CE, Boykin DW. Synthesis, DNA affinity, and antiprotozoal activity of fused ring dicationic compounds and their prodrugs. J Med Chem. 2005;48(17):5480–5488. doi: 10.1021/jm058190h. [DOI] [PubMed] [Google Scholar]
- 28.Arafa RK, Ismail MA, Munde M, Wilson WD, Wenzler T, Brun R, Boykin DW. Novel linear triaryl guanidines, N-substituted guanidines and potential prodrugs as antiprotozoal agents. Eur J Med Chem. 2008;43(12):2901–2908. doi: 10.1016/j.ejmech.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandsch M, Knutter I, Thunecke F, Hartrodt B, Born I, Borner V, Hirche F, Fischer G, Neubert K. Decisive structural determinants for the interaction of proline derivatives with the intestinal H+/peptide symporter. Eur J Biochem. 1999;266(2):502–508. doi: 10.1046/j.1432-1327.1999.00885.x. [DOI] [PubMed] [Google Scholar]
- 30.Vig BS, Stouch TR, Timoszyk JK, Quan Y, Wall DA, Smith RL, Faria TN. Human PEPT1 pharmacophore distinguishes between dipeptide transport and binding. J Med Chem. 2006;49(12):3636–3644. doi: 10.1021/jm0511029. [DOI] [PubMed] [Google Scholar]
- 31.Yang XD, Ma JY, Barger MW, Ma JK. Transport and utilization of arginine and arginine-containing peptides by rat alveolar macrophages. Pharm Res. 2002;19(6):825–831. doi: 10.1023/a:1016132200104. [DOI] [PubMed] [Google Scholar]
- 32.Biegel A, Gebauer S, Hartrodt B, Brandsch M, Neubert K, Thondorf I. Three-dimensional quantitative structure-activity relationship analyses of beta-lactam antibiotics and tripeptides as substrates of the mammalian H+/peptide cotransporter PEPT1. J Med Chem. 2005;48(13):4410–4419. doi: 10.1021/jm048982w. [DOI] [PubMed] [Google Scholar]
- 33.Lai L, Xu Z, Zhou J, Lee KD, Amidon GL. Molecular basis of prodrug activation by human valacyclovirase, an alpha-amino acid ester hydrolase. J Biol Chem. 2008;283(14):9318–9327. doi: 10.1074/jbc.M709530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu CP, Hilfinger JM, Walter E, Merkle HP, Roessler BJ, Amidon GL. Overexpression of human intestinal oligopeptide transporter in mammalian cells via adenoviral transduction. Pharm Res. 1998;15(9):1376–1381. doi: 10.1023/a:1011993303397. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Mitchell S, Kijek P, Tsume Y, Hilfinger J, Amidon GL. The suitability of an in situ perfusion model for permeability determinations: utility for BCS class I biowaiver requests. Mol Pharm. 2006;3(6):686–694. doi: 10.1021/mp060042f. [DOI] [PubMed] [Google Scholar]
- 36.Dahan A, Amidon GL. Small intestinal efflux mediated by MRP2 and BCRP shifts sulfasalazine intestinal permeability from high to low, enabling its colonic targeting. Am J Physiol Gastrointest Liver Physiol. 2009;297(2):G371–377. doi: 10.1152/ajpgi.00102.2009. [DOI] [PubMed] [Google Scholar]
- 37.Dahan A, West BT, Amidon GL. Segmental-dependent membrane permeability along the intestine following oral drug administration: Evaluation of a triple single-pass intestinal perfusion (TSPIP) approach in the rat. Eur J Pharm Sci. 2009;36(2–3):320–329. doi: 10.1016/j.ejps.2008.10.013. [DOI] [PubMed] [Google Scholar]