Abstract
Malaria infections normally consist of more than one clonally-replicating lineage. Within-host interactions between sensitive and resistant parasites can have profound effects on the evolution of drug resistance. Here, using the Plasmodium chabaudi mouse malaria model, we ask whether the costs and benefits of resistance are affected by the number of co-infecting strains competing with a resistant clone. We found strong competitive suppression of resistant parasites in untreated infections and marked competitive release following treatment. The magnitude of competitive suppression depended on competitor identity. However, there was no overall effect of the diversity of susceptible parasites on the extent of competitive suppression or release. If these findings generalize, then transmission intensity will impact on resistance evolution because of its effect on the frequency of mixed infections, not because of its effect on the distribution of clones per host. This would greatly simplify the computational problems of adequately capturing within-host ecology in models of drug resistance evolution in malaria.
Keywords: Plasmodium chabaudi, malaria, drug resistance, within-host dynamics, competition, competitive release, multiplicity of infection, complexity of infection, selection coefficient
Introduction
The evolution of drug-resistant malaria parasites is a major public health challenge. Resistance against most widely-used antimalarial drugs has spread globally (Mita et al., 2009, Plowe, 2009) and there are recent reports of the first stirrings of resistance against even the most recently deployed drugs (Dondorp et al., 2009, Dondorp et al., 2010). Clearly, there is an urgent need for new front-line drugs (Roll Back Malaria, 2008, Alonso et al., 2011, Baum et al., 2011); an equally urgent need is for evidence-based resistance management strategies for the drugs we already have (Read & Huijben, 2009, Read et al., 2011, zur Wiesch et al., 2011). A prerequisite for that is a better understanding of the selective forces involved in drug resistance evolution (Hastings, 1997, Hastings & D'Alessandro, 2000, White & Pongtavornpinyo, 2003, Stepniewska & White, 2008).
A feature of malaria natural history which critically impacts drug resistance evolution is the genetic diversity of infections. Multi-genotype malaria infections are common in people (e.g. Arnot, 1998, Babiker et al., 1999, Smith et al., 1999, Bruce et al., 2000, Jafari et al., 2004, A-Elbasit et al., 2007, Nwakanma et al., 2008, Vafa et al., 2008, Baruah et al., 2009, Soulama et al., 2009). These arise from infections initiated by multiply-infected mosquitoes or from infections from multiple mosquitoes (Walliker et al., 1987, Kobbe et al., 2006, Nwakanma et al., 2008). The number of parasite strains observed within a person, termed the ‘multiplicity of infection’, MoI (e.g. Schoepflin et al., 2009, Hastings et al., 2010), or the ‘complexity of infection’, CoI (e.g. Paul et al., 1995, Sutton et al., 2009), range from one to more than five, and sometimes well in excess of ten (e.g. Babiker et al., 1999, Konate et al., 1999, Beck et al., 2001, Magesa et al., 2002, Sutherland et al., 2002, Schoepflin et al., 2009, Juliano et al., 2010). Broadly speaking, the average number of clones per person in a population is a function of transmission intensity (e.g. Arnot, 1998), with high levels of multigenotype infections common in populations receiving many hundreds of infective bites per year (Trape & Rogier, 1996, Beier et al., 1999, Smith et al., 2005, Gemperli et al., 2006). Globally, most malaria parasite clones share their host with at least one other clone.
This fact has a critical impact on drug resistance evolution because crowding occurs within hosts, whereby the density of a replicating parasite lineage can be suppressed when co-infecting parasite strains are present. Direct experimental evidence of competition between Plasmodium falciparum genotypes in the same host, particularly when drug-resistant parasites are involved, cannot be ethically obtained from human infections. However, correlational field data are consistent with suppression of parasite genotypes when other genotypes are present (e.g. Daubersies et al., 1996, Mercereau-Puijalon, 1996, Smith et al., 1999, Bruce et al., 2000, Hastings, 2003, Talisuna et al., 2006, Bousema et al., 2008, Farnert, 2008, Harrington et al., 2009, Baliraine et al., 2010). Additionally, there is considerable direct experimental evidence for crowding in rodent malaria models (e.g. Jarra & Brown, 1985, Anderson & May, 1991, Taylor et al., 1997, de Roode et al., 2004a, de Roode et al., 2004b, de Roode et al., 2005b, Bell et al., 2006, Wargo et al., 2007, Huijben et al., 2010, Read et al., 2011). This competition is likely resource based (e.g. over red blood cells) and/or immune-mediated (e.g. Hellriegel, 1992, Read & Taylor, 2001, Haydon et al., 2003, Råberg et al., 2006, Antia et al., 2008, Mideo et al., 2008, Hastings, 2011b).
Competition has a profound impact on drug resistance because it greatly magnifies both the costs of resistance in untreated hosts and the benefits of resistance in treated hosts (Hastings, 1997, Mackinnon & Hastings, 1998, Hastings & D'Alessandro, 2000). Resistant parasites are likely to be competitively suppressed in untreated hostsif there is a performance cost associated with resistance. In contrast, drug treatment of mixed infections of resistant and sensitive parasites can greatly enhance the relative fitness of the resistant parasites, not only as a result of their survival advantage, but also because of the removal of their susceptible competitors. If resistant parasites can expand to fill this newly vacated niche-space, their absolute fitness will be substantially increased. Such drug-induced competitive release has been demonstrated in rodent malaria infections following both prophylactic (de Roode et al., 2004a) and therapeutic (Wargo et al., 2007, Huijben et al., 2010) drug treatment.
To date, all experimental work on the ecology of resistant parasites in infections with sensitive parasites has involved a resistant clone competing with a single susceptible genotype (i.e. MoI = 2). However, as Hastings and D’Alessandro (2000) first pointed out, the extent of competitive suppression, and hence competitive release, could vary with MoI. For instance, in the simplest case where limited ‘infection-space’ is equally divided among co-infecting clones and all else is equal then, relative to the single clone case, the density of a resistant clone in an untreated infection will be halved by co-infection if MoI=2, but reduced to 20% when MoI=5, and 10% when MoI=10. The flip side of that is that drug treatment could double the transmission rate of resistant parasites when MoI is 2, and increase it many many-fold more when MoI is substantially higher. The cost and benefits of resistance will be even higher if competition is asymmetric, where the resistant clone takes up even less ‘infection space’ than expected were infection space simply divided equally among clones (Read & Huijben, 2009). Alternatively, the effect of increasing MoI on resistant parasites might go the other way. For example, it may be that genetically diverse infections are less readily controlled by host immunity, and/or that clones occupy different niches, so that competition does not intensify with increasing MoI. If so, it could be that all that matters for the evolution of drug resistance is whether any competitor clones are present at all, not how many competing lineages are present.
The aim of this study was to determine whether the intensity of competitive suppression of resistant parasites – and hence the extent of competitive release – is affected by the multiplicity of infection. Using a P. chabaudi mouse model, the performance of up to four genetically distinct clonal lineages in mixed infections were quantified through time. We found that the extent of competitive suppression and competitive release of resistant parasites was independent of the number of co-infecting susceptible clones.
Material and methods
Parasites and hosts
Four genetically distinct P. chabaudi clones were used: drug-resistant clone AS8p(pyr-1A) (hereafter referred to as clone R), and drug-sensitive clones AJ8p, AT2p and CB2p (hereafter referred to as clones AJ, AT and CB respectively). All clones were originally isolated from thicket rats and subsequently cloned (Beale et al., 1978). Clone R was made resistant in the laboratory by exposure to a high dose of pyrimethamine in a single passage (Walliker et al., 1975). Ten week old female C57Bl/6 laboratory mice (Charles River Laboratories) were used as hosts. All mice received 0.05% PABA-supplemented drinking water to enhance parasite growth (Jacobs, 1964). The mice were fed on Laboratory Rodent Diet 5001 (LabDiet, PMI Nutrition International) and were kept on a 12:12 L:D cycle.
Experimental design, infections and drug treatment
Mice were infected with either a single-clone infection of clone R, or a mixed infection with clone R and one, two or all three of the other clones. All possible combinations of co-infecting clones were included and half of the mice were drug treated (Table 1). Each treatment group consisted of 5 mice, except for the untreated infections with three and four clones, which consisted of respectively 7 and 9 mice, since we anticipated higher mortality in these groups.
Table 1.
Experimental set-up. The number mice in each treatment group were inoculated with clone R and none, one, two or all of the clones AJ, AT and CB. The number in brackets specifies the number of mice that survived the infection and that were included in the analysis.
| Treatment | No drugs | Drug treated |
|---|---|---|
| R | 5 (5) | 5 (5*) |
| R+AJ | 5 (5) | 5 (5**) |
| R+AT | 5 (5) | 5 (5) |
| R+CB | 5 (2) | 5 (5***) |
| R+AJ+AT | 7 (6) | 5 (5) |
| R+AJ+CB | 7 (3) | 5 (5) |
| R+AT+CB | 7 (5) | 5 (5*) |
| R+AJ+AT+CB | 9 (5) | 5 (5) |
| Total | 50(36) | 40(40) |
Asterisks indicate mice that had a significant lag in parasite growth but were included in the analysis.
Infections were initiated with an intraperitoneal injection of 106 parasites of each clone. Multi-clone infections were established by mixing the different parasite clones in the inoculum, so that only a single inoculation was necessary. In multi-clone infections, each clone was initiated with 106 parasites, so that the four-clone infection received a fourfold higher parasite dose than the single-clone R infection. We did this because analysing competition requires comparison of the performance of an individual clone in the presence and absence of competition, starting from the same initial parasite dose. Up to a fourfold higher dose has little effect on overall parasite dynamics or host health (Timms et al., 2001).
Chemotherapy was initiated on day 6 post-infection (PI), when parasite-induced weight loss and anaemia became pronounced, and subsequently repeated on days 7 to 9. Drug treatment consisted of 8 mg pyrimethamine/kg mouse weight dissolved in dimethyl sulfoxide (DMSO) administered by intraperitoneal injection of 50 µl. This treatment regime was chosen because in previous work it cleared all susceptible parasites (de Roode et al., 2004a). Untreated controls were given the same volume of DMSO only.
Monitoring of infections
Mice were monitored daily from day 3 to day 21 PI and three times a week up to day 35 PI. During sampling, mouse weight (to the nearest 0.1 gram) and red blood cell density using flow-cytometry (Beckman Coulter) were measured, and a thin blood smear was taken. Additionally, 5µl of blood was taken to estimate total parasite density and a further 10µl to estimate gametocyte density, both by using quantitative PCR, as follows.
DNA was extracted from the 5µl blood sample using the ABI Prism® 6100 Nucleic Acid PrepStation according to manufacturer’s instructions. RNA was extracted from the 10µl sample, using the ‘RNA 8 Blood-DNA’ method on the ABI Prism® 6100 Nucleic Acid PrepStation. RNA was converted to single stranded cDNA immediately following extraction, using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Wargo et al., 2006). Both DNA and cDNA were stored at - 80°C until quantification.
To measure total parasite density (asexual parasites and gametocytes combined) of each clone, quantitative PCR was performed on extracted DNA using clone-specific assays. Clone R and AJ were measured using the R-specific and AJ-specific PcCG1 assay respectively, as described in Drew and Reece (2007). Clone AT was quantified using the msp-1 AT assay and clone CB was quantified using the msp-1 CB assay, both described by Bell et al. (2006). PCR conditions were as described by Bell et al. (2009). Additionally, gametocyte density of clone R was quantified using cDNA on the R-specific PcCG1 assay (Drew & Reece, 2007). Parasite densities for clone R were determined for days 3–35 PI, for non-focal clones AJ, AT and CB parasite densities were only followed during the initial phase of infection (up to day 23). Due to accidental loss of some samples, gametocyte data was only available from day 3 to day 17.
Selection coefficient
To estimate the temporal pattern of the strength and direction of within-host selection, the coefficient of selection on the resistant clone was estimated. The selection coefficient is the difference in the per-capita growth rate of the resistant clone and all susceptible clones combined. This strength of selection can be calculated from the frequency of the competitors as described by Huijben et al. (2010), with all susceptible clones pooled to function as one group of competitors. Using this method, we studied the effect of multiple competing genotypes on the selection for resistance in the presence and absence of chemotherapy. The analysis was performed as described in Huijben et al. (2010). Net selection was calculated for each mouse and each bootstrap replicate by integrating the selection coefficient curve from day 3 to day 15 PI, which was the maximum time period that we had selection coefficients for most mice.
Statistical analysis
Analysis was done using analysis of variance in R 2.9.0 (R Development Core Team, 2009). For analysis of competition in untreated infections, the geometric mean parasite density was calculated over days 3–35 PI for clone R and over days 3–23 PI for clones AJ, AT and CB. For analysis of competitive release in drug-treated infections, geometric mean parasite density of clone R post-treatment was calculated (day 7–35 PI). The total parasite density of all parasite clones combined in the infection was estimated by summing the parasite densities of each infecting clone in mixed infections up to day 23. As a measure of transmission potential, the predicted infectiousness was calculated for clone R after the start of treatment (day 7 PI) until day 17 using gametocyte densities in the density-infectivity function q1 as described by Huijben et al. (2010) which relates gametocyte concentration to prevalence of infected mosquitoes. The resulting dynamics of mosquito infection risk were integrated over time to give predicted proportion of mosquitoes infected over the course of the infection, assuming constant biting rate per day and no change in gametocyte infectivity over time. As a measure of virulence, mean weight and mean red blood cell density during the infection were calculated. Analysis of variance was used, with the following explanatory variables: drug treatment (drug-treated/untreated) and number of co-infecting clones (0/1/2/3) or co-infecting clones (AJ/AT/CB/AJ-AT/AJ-CB/AT-CB/AJ-AT-CB). The validity of all models was checked by inspection of residual distributions. Full models were used (with non-significant interactions removed to obtain minimal models), or comparisons for untreated or drug-treated infections only were performed. For some comparisons, only mixed infections were used. Details of the specific dataset used are given for each reported test-statistic.
Seven mice apparently received a significant lower inoculum of parasites than intended, seen as a lag in growth of the parasites of a day or more (Timms et al. 2001). These late mice were coincidently all in the drug treated groups (see Table 1 and Figure S2), however, since this lag was observed prior to the initiation of drug treatment and all inoculations were randomized by cage, we do not suspect this to be a treatment effect. Since the different clones were mixed into a single inoculum, mice that received a lower dose of parasites received an overall lower dose of all parasite-clones, but the parasite ratio was maintained. Parasite dynamics in individual mice are given in figures S1 and S2 in the Supporting Information. To account for any effect of the lower inocula, the R parasite density at the first sampling day (day 3) was initially included in the models as a covariate. This factor did not have a significant effect in any of the tests and was therefore eliminated from the models. The exclusion of these late mice in the analysis did not alter any of the conclusions presented below.
A total of 14 mice died or were euthanized during the course of the infection, all of them in untreated groups (Table 1). All deaths occurred between days 8 and 13 post-infection, with a peak on day 10. These 14 mice were excluded from the analyses.
Results
Asexual parasite dynamics
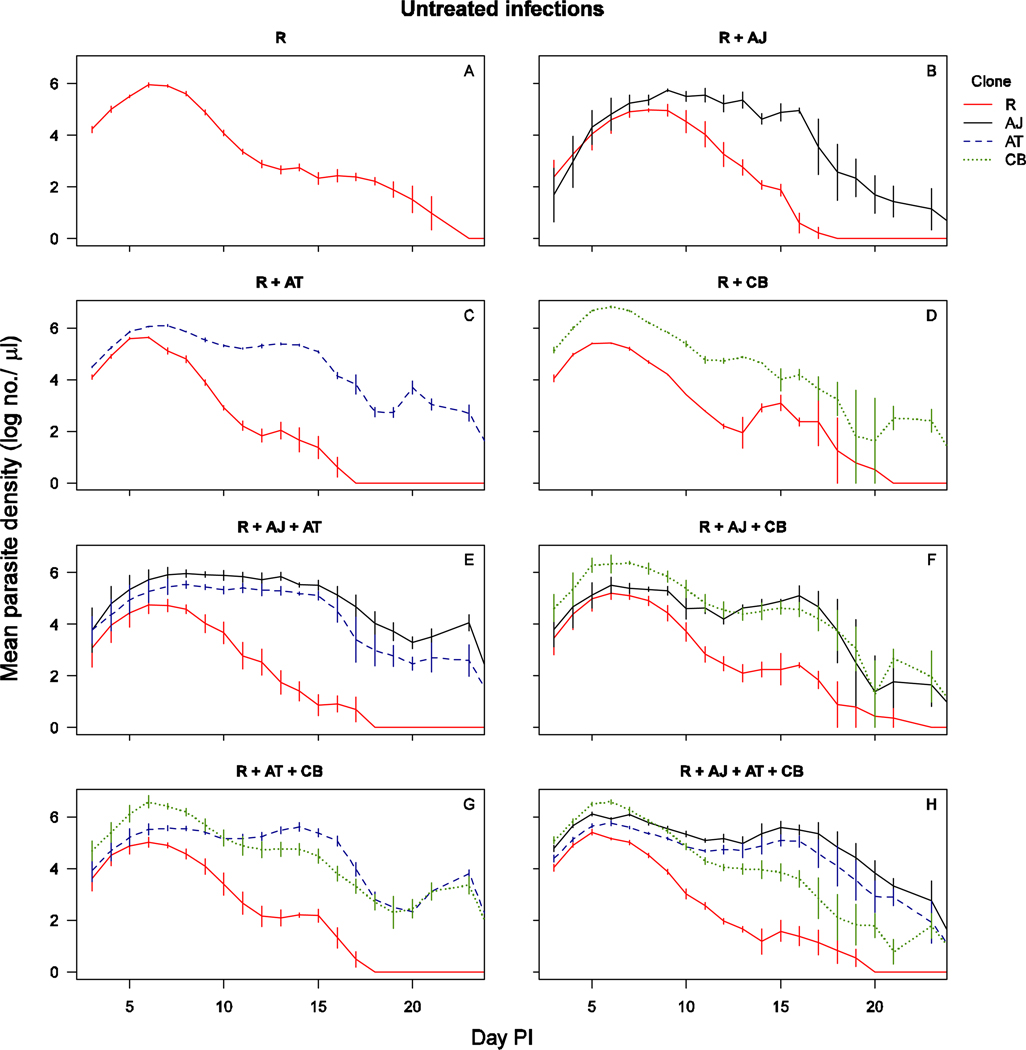
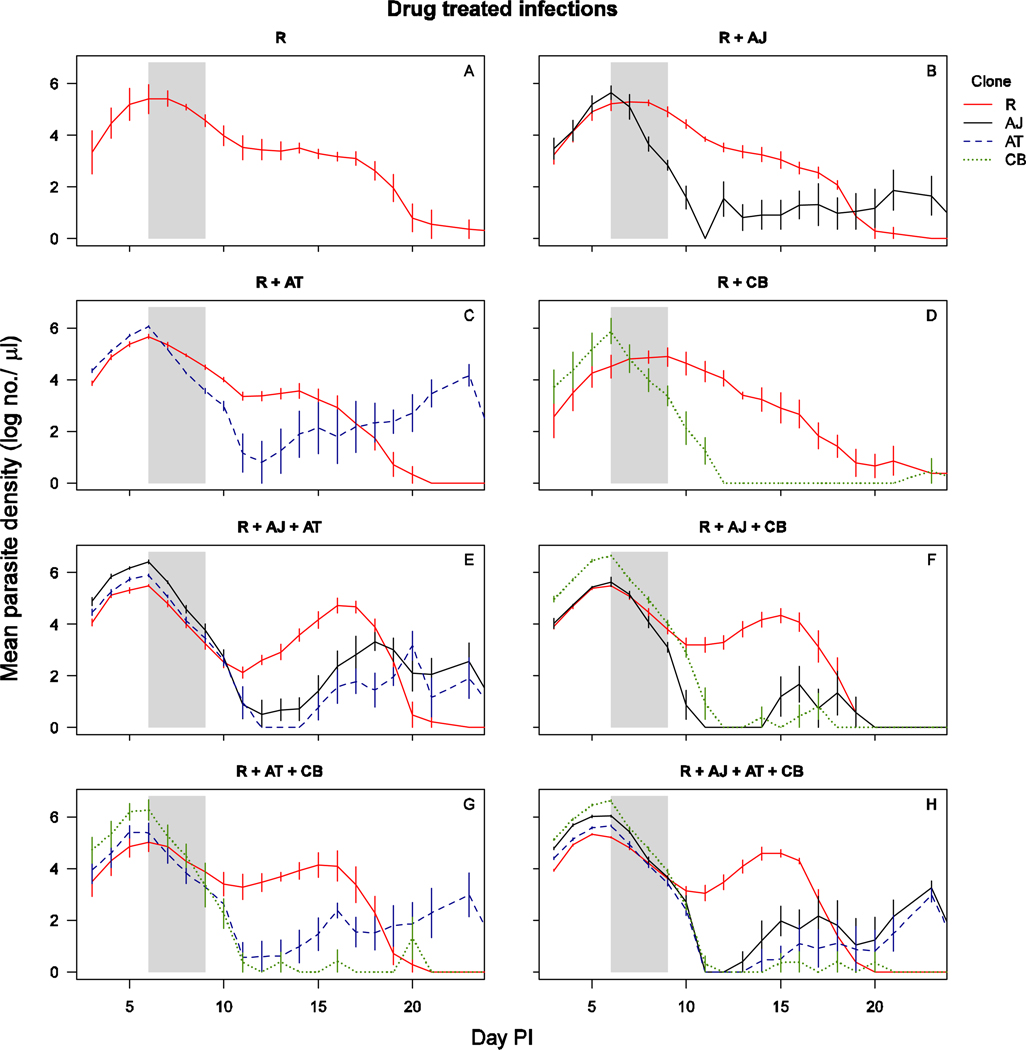
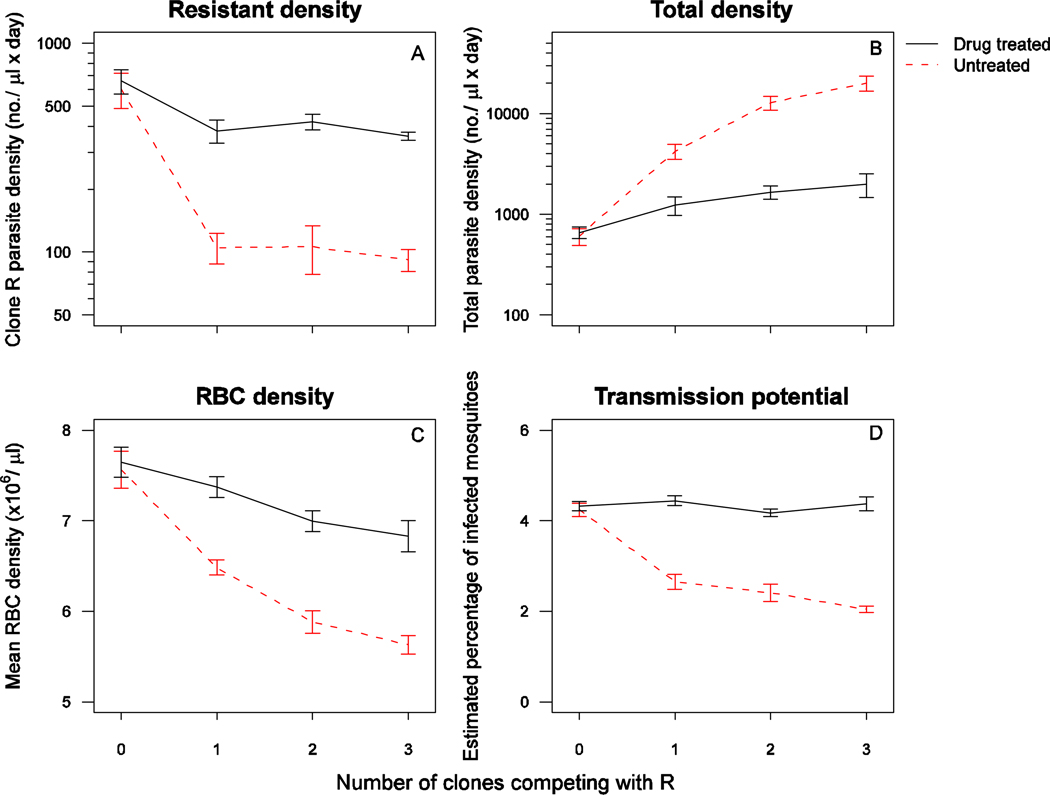
In untreated infections, all parasite clones reached peak parasite density around day 6, after which susceptible clones AJ, AT and CB persisted at higher parasite densities than resistant clone R (Figure 1, Figure S1). When the infections were drug-treated, the densities of each susceptible parasite clone dropped rapidly to around or below detection threshold by day 12 post-infection, at which time the density of clone R increased substantially with distinct second peaks when in competition with two or more susceptible clones (Figure 2, Figure S2). In the absence of drug treatment, the resistant parasites were competitively suppressed by all three clones (all untreated infections, presence of competitors: F1,34=61, p<0.001). The number of parasites produced by clone R during the entire infection was similar between co-infections with a different number of competing clones (Figure 3A – untreated mixed infections, number co-infecting clones: F2,28=0.1, p=0.89). Similarly, resistant parasite densities in drug-treated infections were comparable across the different number of co-infecting clones (drug-treated infections, number of co-infecting clones: F2,32=0.23, p=0.79). Consequently, competitive release did not increase with increasing number of competing clones (Figure 3A; all mixed infections, drugs×no. of co-infecting clones: F2,60=0.05, p=0.95) However, of note is that the dynamics of clone R varied slightly with different numbers of competing genotypes; resistant parasites in more diverse co-infections appeared to be somewhat more suppressed prior to treatment (Figure 2). Resistant parasites that were competing with two or three susceptible clones relapsed to higher densities after treatment than resistant parasites competing with only one susceptible clone (Figure 2 – drug treated mixed infections, no. of co-infecting clones: F2,32=7.8, p=0.002).
Figure 1.
Parasite dynamics of untreated infections of clone(s): R alone (a), R with AJ (b), R with AT (c), R with CB (d), R with AJ and AT (e), R with AJ and CB (f), R with AT and CB (g), R with AJ, AT and CB (h). The total parasite densities for clone R are shown in solid red line, for clone AJ in solid black line, for clone AT in dashed blue line and for clone CB in dotted green line. Data are means (± standard error) of up to 6 mice (table 1). The mixed infection of R with CB consisted of only two surviving mice.
Figure 2.
Parasite dynamics of drug treated infections of clone(s): R alone (a), R with AJ (b), R with AT (c), R with CB (d), R with AJ and AT (e), R with AJ and CB (f), R with AT and CB (g), R with AJ, AT and CB (h). The total parasite densities for clone R are shown in solid red line, for clone AJ in solid black line, for clone AT in dashed blue line and for clone CB in dotted green line. Data are means (± standard error) of 5 mice (table 1). Drug treatment was given on days 6–9 post-infection, as indicated by the shaded area.
Figure 3.
Geometric mean daily parasite density of clone R (A), geometric mean daily total parasite density of all clones combined (B), mean daily red blood cell density (C) and estimated percentage of infected mosquitoes of clone R (D), in drug treated (solid black line) and untreated (dashed red 27 line) infections of either clone R alone (0 competing clones) or in a co-infection with 1, 2 or 3 other clones. Data are means (± standard error) for the entire infection period (panel A and C, day 3–35 PI), for the period with recorded susceptible parasite densities (panel B, day 323) or for the post-treatment period with gametocyte data (panel D, day 7–17). Note the y-axis varies between the left panel plots.
All three susceptible clones were strong competitors, but, in pair-wise competition with the resistant clone, the strength of competitive suppression in untreated infections was less strong with a co-infection with clone CB than with AJ or AT (Figure S3A, untreated 2-clone infections, clone: F2,9=6.4, p=0.019). Similarly, the combination AJ + CB exerted less competitive suppression than the other two-competitor combinations (Figure S3B, untreated 3-clone infections F2,11=7.4, p=0.009). The genotypes of the co-infecting clones did not affect the extent of competitive release (all 2-clone infections, co-infecting clone × drugs: F2,21=1.4, p=0.30; all 3-clone infections: F2,23=2.9, p=0.07). Each of the susceptible clones had similar or higher parasite densities when sharing the host with one or two additional susceptible parasite clones compared to only clone R (untreated infections, presence of other susceptible competitors: AJ – F2,16=9.2, p=0.002; AT – F2,18=1.29, p=0.30; CB – F2,12=3.2, p=0.08), so that the total parasite density increased with an increasing number of co-infecting clones (Figure 3B, all untreated infections, number of infecting clones: F3,32=41, p<0.001). The impact of drug treatment varied with the number of co-infecting clones (all infections, F3,68=6.5, p<0.001). Drug-treated infections had a lower total parasite density than untreated infections (drugs: F1,68=117, p<0.001), but the total parasite densities in drug-treated infections were not significantly different for double, triple or quadruple infected mice (drug treated mixed infections, no. of co-infecting clones: F2,32=2.3, p=0.12).
Consistent with the effects of MoI on total parasite densities, mice with more clones in the infection were more anaemic during the infection (Figure 3C, all infections, drugs×no. of co-infecting clones: F3,68=4.3, p=0.007; no. of co-infecting clones: F3,68=27, p<0.001). This result could not be explained by the inclusion of the presence of any of the three susceptible clones as a co-variate, nor did the different susceptible genotypes in different combinations cause different levels of anaemia (untreated 2-clone infections, co-infecting clones,: F2,9=1.56, p=0.26; untreated 3-clone infections: F2,11=0.31, p=0.74). Thus with increasing MoI, infections had a higher parasite density, and resource (red blood cell) exploitation was greater.
Transmission potential
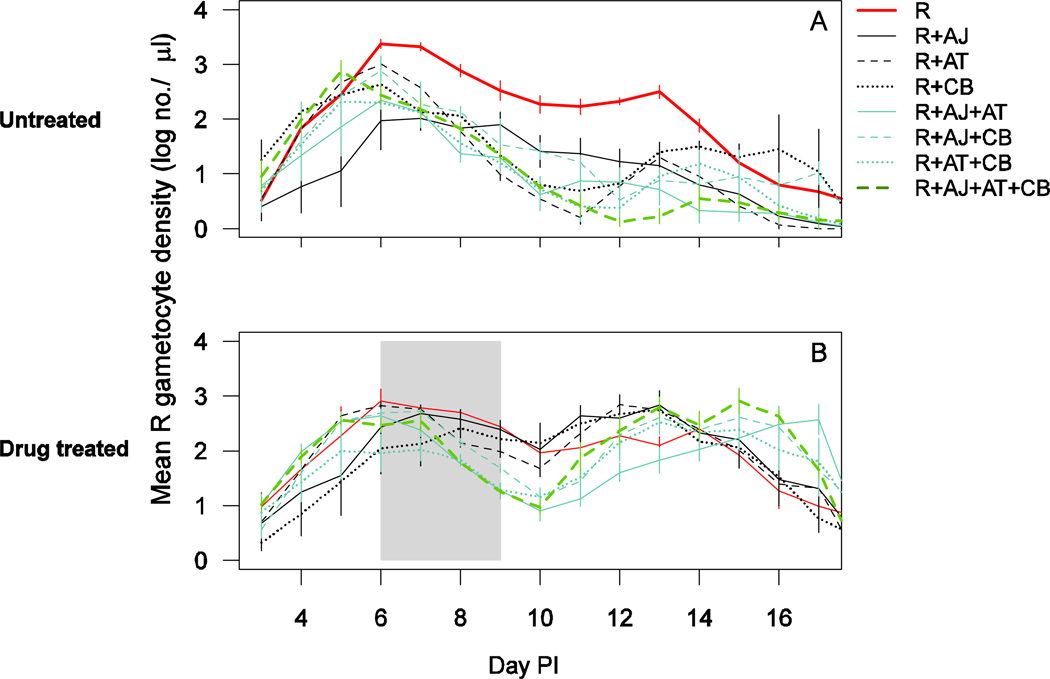
The gametocytes of clone R peaked around day 6 in untreated infections, after which densities dropped and peaked again around day 13 (Figure 4A). Clone R appeared to produce higher gametocyte densities throughout the infection in the absence of competition (Figure 4A). When drug treatment was given, resistant parasites in all mixed infections had a more pronounced second peak in gametocyte production (Figure 4B). The statistical analyses of the transmission potential based on these gametocyte densities are as follows.
Figure 4.
Gametocyte dynamics of clone R (day 3–17) in untreated (a) and drug treated (b) infections of clone R alone (solid thick red line), R with AJ (solid black line), R with AT (dashed black line), R with CB (dotted black line), R with AJ and AT (solid blue line), R with AJ and CB (dashed blue line), R with AT and CB (dotted blue line) and R with AJ, AT and CB (thick dashed green line). Drug treatment was given on days 6–9 post-infection. Data are means (± standard error) of up to six mice (table 1). The mixed infection of R with CB consisted of only two surviving mice.
The transmission potential of the resistant parasites was reduced when co-infecting strains were present (untreated infections, presence of competitors: F1,34=37, p<0.001), but the extent of the suppression was unrelated to the number of competing strains (Figure 3D, untreated mixed infections, no. of co-infecting clones: F2,28=1.7, p=0.20). Drug treatment doubled the transmission potential of the resistant clones (Figure 3D, all infections, drugs: F1,68=214, p<0.001). The extent of this competitive release was not affected by the number of co-infecting clones (all mixed infections, no. of co-infecting clones × drugs: F2,60=1.3, p=0.29). The transmission potential of clone R was independent of the particular genotype(s) of co-infecting parasites (p>0.05 in all cases).
Selection coefficient
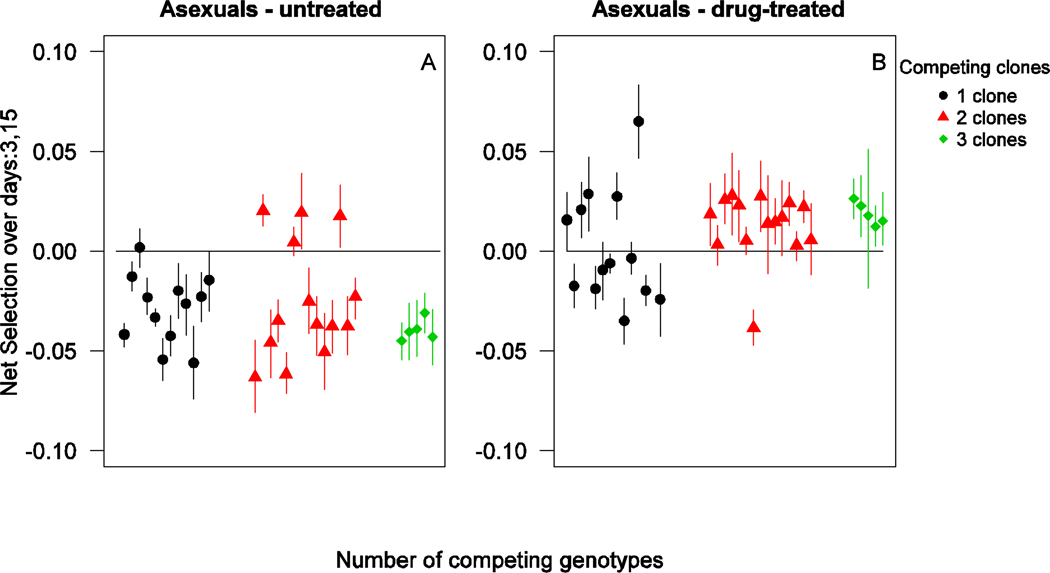
In untreated infections, drug resistant parasites experienced strong negative selection during the acute phase of the infection. In drug treated infections, resistant parasites experienced strong positive selection from the time of treatment until several days after termination of treatment (up to day 15 PI; Figure S5). The net selection on the resistant parasite over the time period of day 3 to day 15 PI was negative in most mixed infections, and the strength of the negative selection was independent of the number of competing genotypes (Figure 5A – untreated mixed infections, no. of co-infecting clones: F2,28=0.74, p=0.49). Drug treatment led to strong positive selection during the same time frame (all mixed infections, drugs: F1,60=31, p<0.001), with competitive release not leading to increased selection at higher complexity of infection (Figure 5B – all mixed infections, drugs×no. of co-infecting clones: F2,60=0.43, p=0.65).
Figure 5.
Net selection on clone R competing with one (black circles), two (red triangles) and three (green squares) susceptible genotypes over days 3–15 in untreated (left panel) and drug-treated (right panel) infections. Each datapoint represents a mean value (± 95% confidence interval) of bootstrap replicates for an individual mouse.
Discussion
Multi-clone infections can exacerbate both the cost of drug resistance (in the absence of treatment) and benefit of resistance (in treated hosts) (Hastings, 1997, Mackinnon & Hastings, 1998, Hastings & D'Alessandro, 2000, Mackinnon, 2005, Read & Huijben, 2009, Read et al., 2011). Here, we found strong competitive suppression of the resistant parasites in the absence of treatment and competitive release following drug-treatment (Figures 1,2,3A), as have others in two-clone infections (de Roode et al., 2004a, Wargo et al., 2007, Read & Huijben, 2009, Huijben et al., 2010). However, we found that the number of co-infecting susceptible genotypes did not affect the extent of competitive suppression, or the extent of competitive release. Resistant parasites in multi-clonal environments were not worse off than resistant parasites sharing their host with only one susceptible genotype: a single competitor strain had the same effect as up to three. However, temporal dynamics did differ slightly, with resistant parasites in more complex infections being more suppressed initially but also released to higher densities following drug treatment. This pattern suggests that there is a transient fitness advantage for resistant parasites competing with more than one susceptible genotype in drug-treated infections, which is likely due to a relatively greater relaxation of competitive suppression after treatment in these more complex infections. This did not have a detectable effect on overall transmission potential (Figure 3D).
Competition between co-infecting malaria parasites is thought to arise through two mechanisms. The first is competition for resources, with red blood cell densities considered most likely limiting resource (Yap & Stevenson, 1994, Hetzel & Anderson, 1996, Haydon et al., 2003, Antia et al., 2008, Mideo et al., 2008, Miller et al., 2010), though others such as glucose may also play a role (de Roode et al., 2003). The other possibility is immune-mediated competition, whereby immune responses produced against one parasite genotype are cross-reactive against other genotypes (Mota et al., 2001, Read & Taylor, 2001, Råberg et al., 2006, Barclay et al., 2008). The experiments we report here were not designed to test for the impact of competition on anything other than a single focal clone (R), but we observed that infections with an increasing number of parasite clones experienced a higher degree of anaemia during the acute infection. Additionally, a higher total parasite density was observed in these higher genotype diversity infections. This suggests that the carrying capacity of the infection increased with increasing genotypic diversity, leading to, or indeed caused by, enhanced resource exploitation. This observation is different from observations in the field, where a stable carrying capacity has been found across a range of asymptomatic children (Bruce et al., 2000). We note, however, that these infections were in asymptomatic patients, opposed to naïve hosts used in our experiments. The carrying capacity in these semi-immune children is likely predominantly driven by immune factors, whereas the carrying capacity in naïve hosts is expected to be also greatly controlled by resource availability. The generality of our findings across hosts of different immune status warrants further exploration.
Enhanced exploitation in this system can conceivably be explained when different genotypes occupy slightly different niches, allowing for a wider range of suitable red blood cells to be invaded and hence leading to a greater total parasite density (e.g. Antia et al., 2008). Such niche partitioning leading to enhanced host exploitation has been much discussed in the classical ecological literature and demonstrated empirically in a variety of ecological communities (reviewed in Hooper et al., 2005, Finke & Snyder, 2010). It is tempting to conclude that the parasite clones in our experiment have subtly different niches, so that infections with higher MoI have a higher parasite density and induce more anaemia because clonal diversity allows some niche expansion. This would provide an explanation why competitive suppression of resistant parasites did not increase with MoI: through niche partitioning, a wider range of resources were available for the parasite population as a whole, and hence less competition for each one of them. Presumably there is a limit to such enhanced exploitation of the host; with the combination of four clones in this experiment, we may not have reached that limit. We are in the process of directly testing the presence of enhanced host exploitation in more diverse infections with experimental designs borrowed from community ecology where people are interested in the relationship between biodiversity and ecosystem productivity (Finke & Snyder, 2008). The alternative hypothesis is that the immune system is overwhelmed by the more diverse infections, leading to less effective control of the parasite population as a whole. More diverse P. chabaudi infections take longer to clear (e.g. Taylor et al., 1998, de Roode et al., 2003). A new statistical approach has recently been developed to disentangle the strength of the immune system from dynamics driven by resource depletion (Metcalf et al., in press). We hope to use this approach in the near future to understand the relative roles of the immune system and resource depletion in these complicated mixed-genotype infections.
Our results suggest that resistant parasites experience no additional cost to being in an infection with more than one susceptible parasite strain and no additional advantages from competitive release following drug treatment. If this is true in nature, it would greatly simplify the structural requirements of epidemiological-evolutionary models of drug resistance in malaria. Minimally adequate models of drug resistance evolution need to take into account the costs and benefits of resistance; in malaria, these are so clearly influenced by co-infection that the effect of co-infection must be incorporated in order to assess the impact of force of infection and medical interventions on evolutionary trajectories (Hastings, 2006, Read & Huijben, 2009, Antao & Hastings, 2011, Hastings, 2011a, Read et al., 2011). In the worst case scenario, this would require keeping track of the distribution of clones per host, rather like a classic epidemiological macroparasite model (Anderson & May, 1991). Our results suggest that this can be simplified to a single dichotomy: the infections in a population that contain two or more clones versus the fraction of infections consisting of only a single clone.
However, an important question is whether experiments in a rodent model can lead to generalizable conclusions. Clearly, mice are not men (as discussed extensively in Råberg et al., 2006, Wargo et al., 2007), and we necessarily can only deal with a limited number of clones, in a single host genotype, of uniform immunological status, during acute stage infections. It remains unclear how real world heterogeneities in these sorts of variables and others would affect our conclusions. For instance, studies in areas of seasonal malaria have shown that resistant parasites can persist during the long dry season in mixed infections with susceptible parasites (Abdel-Muhsin et al., 2004, Ord et al., 2007, Babiker, 2009), which likely contain a variety of susceptible genotypes, and are not competitively excluded. Such infections are chronic and asymptomatic, whereas in our experiment we predominantly studied the acute phase of the infection. We did so because acute phase infections are those associated with severe symptoms and so most likely to be treated. But competitive interactions could differ in the acute and chronic phases. Competition in chronic infections is likely more immune-mediated, since red blood cell densities are recovered and thus less of a limiting factor. In P. chabaudi infections, the dynamics of co-infecting clones during the chronic phase of the infection are more or less independent from each other (Bell et al., 2006). The effects of competition on the selective advantages of drug resistance in such chronic infections become important where mass drug administration is being attempted (Antao, 2011), or in intermittent preventive therapy in pregnancy (Harrington et al., 2009). They will also be important where chronic infections maintain resistant parasites in seasonal environments.
More complex within-host ecological contexts will also affect the competitive outcome. One important complexity which we did not study here is the situation where multi-genotype infections arise from multiple infectious bites days or weeks apart. A turn-over of different parasite clones has frequently been observed in the field (Daubersies et al., 1996, Bruce et al., 2000, Farnert, 2008, Baliraine et al., 2010). The dynamics following such super-infections may result in different competitive outcomes, whereby competition may be more intense for genotypes that infect later on in the infection (de Roode et al., 2005a). The competitive outcome will be importantly dependent on the role of the immune system in mediating competition. Furthermore, Huijben et al (submitted) show that competitive suppression and competitive release are pronounced when resistant parasites are at low frequency in the infection; resistant parasites may be more disposed to pronounced competitive suppression with increasing multiplicity of infection when they are at low abundance. These results additionally show that the competitive outcomes in multi-genotype infections could vary depending on the ecological context.
We have elsewhere argued that the within-host dynamics of resistant and susceptible parasites may be utilized as a resistance management tool by adjusting drug treatment regimes such that competitive suppression of drug-resistant parasites is maintained (Wargo et al., 2007, Read & Huijben, 2009, Huijben et al., 2010, Read et al., 2011). This suggestion has proved highly controversial (Antao, 2011, Goncalves & Paul, 2011, Hastings, 2011b). Assessing this possibility, and the consequences of other management options such as mass drug administration, requires a thorough understanding of the within-host dynamics of resistant and susceptible parasites. The results presented here suggest that clone multiplicity does not influence within-host dynamics for resistant parasites. The generalizability of these findings clearly needs further investigation, but at least for now, there is no reason to incorporate at least this potential complexity into models predicting the spread of resistance and informing rational resistance management strategies.
Supplementary Material
Acknowledgements
We thank R Hallett, S Reece, W Snyder, members of the Read group and members of the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health, for stimulating discussion. Sarah Knowles, as well as an anonymous reviewer, provided stimulating comments that greatly helped to improve the manuscript. The empirical work reported here was supported by Award Number R01GM089932 from the National Institute of General Medical Sciences, and Penn State. Work under awards R01AI089819 and U19AI089676 from the National Institute of Allergy And Infectious Diseases contributed to conceptual development. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, the National Institute of Allergy and Infectious Diseases, or the National Institutes of Health.
References
- A-Elbasit IE, ElGhazali G, A-Elgadir TME, Hamad AA, Babiker HA, Elbashir MI, Giha HA. Allelic polymorphism of MSP2 gene in severe P. falciparum malaria in an area of low and seasonal transmission. Parasitol Res. 2007;102:29–34. doi: 10.1007/s00436-007-0716-3. [DOI] [PubMed] [Google Scholar]
- Abdel-Muhsin AMA, Mackinnon MJ, Ali E, Nassir EKA, Suleiman S, Ahmed S, Walliker D, Babiker HA. Evolution of drug-resistance genes in Plasmodium falciparum in an area of seasonal malaria transmission in Eastern Sudan. J Infect Dis. 2004;189:1239–1244. doi: 10.1086/382509. [DOI] [PubMed] [Google Scholar]
- Alonso PL, Djimde A, Kremsner P, Magill A, Milman J, Najera J, Plowe CV, Rabinovich R, Wells T, Yeung S, ma ICGD. A research agenda for malaria eradication: drugs. PLoS Med. 2011;8:e1000402. doi: 10.1371/journal.pmed.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, May RM. Infectious diseases of humans. Oxford, U.K.: Oxford University Press; 1991. [Google Scholar]
- Antao T. Evolutionary parasitology applied to control and elimination policies. Trends Parasitol. 2011;27:233–234. doi: 10.1016/j.pt.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Antao T, Hastings IM. Environmental, pharmacological and genetic influences on the spread of drug-resistant malaria. Proc R Soc Lond B Biol Sci. 2011;278:1705–1712. doi: 10.1098/rspb.2010.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antia R, Yates A, de Roode JC. The dynamics of acute malaria infections. I. Effect of the parasite's red blood cell preference. Proc R Soc Lond B Biol Sci. 2008;275:1449–1458. doi: 10.1098/rspb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnot DE. Clone multiplicity of Plasmodium falciparum infections in individuals exposed to variable levels of disease transmission. Trans R Soc Trop Med Hyg. 1998;92:580–585. doi: 10.1016/s0035-9203(98)90773-8. [DOI] [PubMed] [Google Scholar]
- Babiker HA. Seasonal fluctuation of drug-resistant malaria parasites: a sign of fitness cost. Trends Parasitol. 2009;25:351–352. doi: 10.1016/j.pt.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Babiker HA, Ranford-Cartwright LC, Walliker D. 3. Genetic structure and dynamics of Plasmodium falciparum infections in the Kilombero region of Tanzania. Trans R Soc Trop Med Hyg. 1999;93:S11–S14. doi: 10.1016/s0035-9203(99)90321-8. [DOI] [PubMed] [Google Scholar]
- Baliraine FN, Afrane YA, Amenya DA, Bonizzoni M, Vardo-Zalik AM, Menge DM, Githeko AK, Yan GY. A cohort study of Plasmodium falciparum infection dynamics in Western Kenya Highlands. BMC Infect Dis. 2010;10:283. doi: 10.1186/1471-2334-10-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay VC, Raberg L, Chan BHK, Brown S, Gray D, Read AF. CD4(+)T cells do not mediate within-host competition between genetically diverse malaria parasites. Proc R Soc Lond B Biol Sci. 2008;275:1171–1179. doi: 10.1098/rspb.2007.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruah S, Lourembam SD, Sawian CE, Baruah I, Goswami D. Temporal and spatial variation in MSP1 clonal composition of Plasmodium falciparum in districts of Assam, Northeast India. Infect Genet Evol. 2009;9:853–859. doi: 10.1016/j.meegid.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Baum J, Bousema T, Dinglasan R, McGovern V. A research agenda for malaria eradication: basic science and enabling technologies. PLoS Med. 2011;8:e1000399. doi: 10.1371/journal.pmed.1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale GJ, Carter R, Walliker D. Genetics. In: Killick-Kendrick R, Peters W, editors. Rodent Malaria. London: Academic Press; 1978. pp. 213–245. [Google Scholar]
- Beck S, Mockenhaupt FP, Bienzle U, Eggelte TA, Thompson WNA, Stark K. Multiplicity of Plasmodium falciparum infection in pregnancy. Am J Trop Med Hyg. 2001;65:631–636. doi: 10.4269/ajtmh.2001.65.631. [DOI] [PubMed] [Google Scholar]
- Beier JC, Killeen GF, Githure JI. Short report: Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- Bell AS, Blanford S, Jenkins N, Thomas MB, Read AF. Real-time quantitative PCR for analysis of candidate fungal biopesticides against malaria: technique validation and first applications. J Invertebr Pathol. 2009;100:160–168. doi: 10.1016/j.jip.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AS, De Roode JC, Sim D, Read AF. Within-host competition in genetically diverse malaria infections: Parasite virulence and competitive success. Evolution. 2006;60:1358–1371. [PubMed] [Google Scholar]
- Bousema JT, Drakeley CJ, Mens PF, Arens T, Houben R, Omar SA, Gouagna LC, Schallig H, Sauerwein RW. Increased Plasmodium falciparum gametocyte production in mixed infections with P. malariae. Am J Trop Med Hyg. 2008;78:442–448. [PubMed] [Google Scholar]
- Bruce MC, Donnelly CA, Alpers MP, Galinski MR, Barnwell JW, Walliker D, Day KP. Cross-species interactions between malaria parasites in humans. Science. 2000;287:845–848. doi: 10.1126/science.287.5454.845. [DOI] [PubMed] [Google Scholar]
- Daubersies P, SallenaveSales S, Magne S, Trape JF, Contamin H, Fandeur T, Rogier C, Mercereau-Puijalon O, Druilhe P. Rapid turnover of Plasmodium falciparum populations in asymptomatic individuals living in a high transmission area. Am J Trop Med Hyg. 1996;54:18–26. doi: 10.4269/ajtmh.1996.54.18. [DOI] [PubMed] [Google Scholar]
- de Roode JC, Culleton R, Bell AS, Read AF. Competitive release of drug resistance following drug treatment of mixed Plasmodium chabaudi infections. Malar J. 2004a;3:33. doi: 10.1186/1475-2875-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roode JC, Culleton R, Cheesman SJ, Carter R, Read AF. Host heterogeneity is a determinant of competitive exclusion or coexistence in genetically diverse malaria infections. Proc R Soc Lond B Biol Sci. 2004b;271:1073–1080. doi: 10.1098/rspb.2004.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roode JC, Helinski MEH, Anwar MA, Read AF. Dynamics of multiple infection and within-host competition in genetically diverse malaria infections. Am Nat. 2005a;166:531–542. doi: 10.1086/491659. [DOI] [PubMed] [Google Scholar]
- de Roode JC, Pansini R, Cheesman SJ, Helinski MEH, Huijben S, Wargo AR, Bell AS, Chan BHK, Walliker D, Read AF. Virulence and competitive ability in genetically diverse malaria infections. Proc Natl Acad Sci USA. 2005b;102:7624–7628. doi: 10.1073/pnas.0500078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roode JC, Read AF, Chan BHK, Mackinnon MJ. Rodent malaria parasites suffer from the presence of conspecific clones in three-clone Plasmodium chabaudi infections. Parasitology. 2003;127:411–418. doi: 10.1017/s0031182003004001. [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NPJ, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Yeung S, White L, Nguon C, Day NPJ, Socheat D, von Seidlein L. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- Drew DR, Reece SE. Development of reverse-transcription PCR techniques to analyse the density and sex ratio of gametocytes in genetically diverse Plasmodium chabaudi infections. Mol Biochem Parasitol. 2007;156:199–209. doi: 10.1016/j.molbiopara.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnert A. Plasmodium falciparum population dynamics: only snapshots in time? Trends Parasitol. 2008;24:340–344. doi: 10.1016/j.pt.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Finke DL, Snyder WE. Niche partitioning increases resource exploitation by diverse communities. Science. 2008;321:1488–1490. doi: 10.1126/science.1160854. [DOI] [PubMed] [Google Scholar]
- Finke DL, Snyder WE. Conserving the benefits of predator biodiversity. Biol Conserv. 2010;143:2260–2269. [Google Scholar]
- Gemperli A, Sogoba N, Fondjo E, Mabaso M, Bagayoko M, Briet OJT, Anderegg D, Liebe J, Smith T, Vounatsou P. Mapping malaria transmission in West and Central Africa. Trop Med Int Health. 2006;11:1032–1046. doi: 10.1111/j.1365-3156.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Goncalves BPA, Paul REL. Sub-clearance treatment to slow malaria drug resistance? Trends Parasitol. 2011;27:50–51. doi: 10.1016/j.pt.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Harrington WE, Mutabingwa TK, Muehlenbachs A, Sorensen B, Bolla MC, Fried M, Duffy PE. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc Natl Acad Sci USA. 2009;106:9027–9032. doi: 10.1073/pnas.0901415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings IM. A model for the origins and spread of drug-resistant malaria. Parasitology. 1997;115:133–141. doi: 10.1017/s0031182097001261. [DOI] [PubMed] [Google Scholar]
- Hastings IM. Malaria control and the evolution of drug resistance: an intriguing link. Trends Parasitol. 2003;19:70–73. doi: 10.1016/s1471-4922(02)00017-x. [DOI] [PubMed] [Google Scholar]
- Hastings IM. Complex dynamics and stability of resistance to antimalarial drugs. Parasitology. 2006;132:615–624. doi: 10.1017/S0031182005009790. [DOI] [PubMed] [Google Scholar]
- Hastings IM. How artemisinin-containing combination therapies slow the spread of antimalarial drug resistance. Trends Parasitol. 2011a;27:67–72. doi: 10.1016/j.pt.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Hastings IM. Why we should effectively treat malaria. Trends Parasitol. 2011b;27:51–52. doi: 10.1016/j.pt.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Hastings IM, D'Alessandro U. Modelling a predictable disaster: The rise and spread of drug-resistant malaria. Parasitol Today. 2000;16:340–347. doi: 10.1016/s0169-4758(00)01707-5. [DOI] [PubMed] [Google Scholar]
- Hastings IM, Nsanzabana C, Smith TA. A comparison of methods to detect and quantify the markers of antimalarial drug resistance. Am J Trop Med Hyg. 2010;83:489–495. doi: 10.4269/ajtmh.2010.10-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon DT, Matthews L, Timms R, Colegrave N. Top-down or bottom-up regulation of intra-host blood-stage malaria: do malaria parasites most resemble the dynamics of prey or predator? Proc R Soc Lond B Biol Sci. 2003;270:289–298. doi: 10.1098/rspb.2002.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellriegel B. Modeling the immune-response to malaria with ecological concepts: short-term behavior against long-term equilibrium. Proc R Soc Lond B Biol Sci. 1992;250:249–256. doi: 10.1098/rspb.1992.0156. [DOI] [PubMed] [Google Scholar]
- Hetzel C, Anderson RM. The within-host cellular dynamics of bloodstage malaria: theoretical and experimental studies. Parasitology. 1996;113:25–38. doi: 10.1017/s0031182000066245. [DOI] [PubMed] [Google Scholar]
- Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setala H, Symstad AJ, Vandermeer J, Wardle DA. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- Huijben S, Chan BHK, Nelson WA, Read AF. The fitness of drug-resistant malaria parasites in a rodent model: the effect of rarity in an infection. doi: 10.1111/j.1420-9101.2011.02369.x. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijben S, Nelson WA, Wargo AR, Sim DG, Read AF. Chemotherapy, within-host ecology and the fitness of drug-resistant malaria parasites. Evolution. 2010;64:2952–2968. doi: 10.1111/j.1558-5646.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RL. Role of p-aminobenzoic acid in Plasmodium berghei infection in the mouse. Exp Parasitol. 1964;15:213. doi: 10.1016/0014-4894(64)90017-7. [DOI] [PubMed] [Google Scholar]
- Jafari S, Le Bras J, Bouchaud O, Durand R. Plasmodium falciparum clonal population dynamics during malaria treatment. J Infect Dis. 2004;189:195–203. doi: 10.1086/380910. [DOI] [PubMed] [Google Scholar]
- Jarra W, Brown KN. Protective immunity to malaria: studies with cloned lines of Plasmodium chabaudi and P. berghei in CBA/Ca mice. I. The effectiveness and inter-and intra-species specificity of immunity induced by infection. Parasite Immunol. 1985;7:595–606. doi: 10.1111/j.1365-3024.1985.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Juliano JJ, Porter K, Mwapasa V, Sem R, Rogers WO, Ariey F, Wongsrichanalai C, Read A, Meshnick SR. Exposing malaria in-host diversity and estimating population diversity by capture-recapture using massively parallel pyrosequencing. Proc Natl Acad Sci USA. 2010;107:20138–20143. doi: 10.1073/pnas.1007068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobbe R, Neuhoff R, Marks F, Adjei S, Langefeld I, von Reden C, Adjei O, Meyer CG, May J. Seasonal variation and high multiplicity of first Plasmodium falciparum infections in children from a holoendemic area in Ghana, West Africa. Trop Med Int Health. 2006;11:613–619. doi: 10.1111/j.1365-3156.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Konate L, Zwetyenga J, Rogier C, Bischoff E, Fontenille D, Tall A, Spiegel A, Trape JF, Mercereau-Puijalon O. The epidemiology of multiple Plasmodium falciparum infections -5. Variation of Plasmodium falciparum msp1 block 2 and msp2 allele prevalence and of infection complexity in two neighbouring Senegalese villages with different transmission conditions. Trans R Soc Trop Med Hyg. 1999;93:S21–S28. doi: 10.1016/s0035-9203(99)90323-1. [DOI] [PubMed] [Google Scholar]
- Mackinnon MJ. Drug resistance models for malaria. Acta Trop. 2005;94:207–217. doi: 10.1016/j.actatropica.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Mackinnon MJ, Hastings IM. The evolution of multiple drug resistance in malaria parasites. Trans R Soc Trop Med Hyg. 1998;92:188–195. doi: 10.1016/s0035-9203(98)90745-3. [DOI] [PubMed] [Google Scholar]
- Magesa SM, Mdira KY, Babiker HA, Alifrangis M, Farnert A, Simonsen PE, Bygbjerg IC, Walliker D, Jakobsen PH. Diversity of Plasmodium falciparum clones infecting children living in a holoendemic area in north-eastern Tanzania. Acta Trop. 2002;84:83–92. doi: 10.1016/s0001-706x(02)00179-1. [DOI] [PubMed] [Google Scholar]
- Mercereau-Puijalon O. Revisiting host-parasite interactions: Molecular analysis of parasites collected during longitudinal and cross-sectional surveys in humans. Parasite Immunol. 1996;18:173–180. doi: 10.1046/j.1365-3024.1996.d01-79.x. [DOI] [PubMed] [Google Scholar]
- Metcalf CJE, Graham AL, Huijben S, Barclay VC, Long GH, Grenfell BT, Read AF, Bjømstad ON. Partitioning and quantifying regulatory mechanisms acting on within host malaria using the effective propagation number. Science. doi: 10.1126/science.1204588. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mideo N, Barclay VC, Chan BHK, Savill NJ, Read AF, Day T. Understanding and predicting strain-specific patterns of pathogenesis in the rodent malaria Plasmodium chabaudi. Am Nat. 2008;172:E214–E238. doi: 10.1086/591684. [DOI] [PubMed] [Google Scholar]
- Miller MR, Råberg L, Read AF, Savill NJ. Quantitative analysis of immune response and erythropoiesis during rodent malarial infection. PLoS Comput Biol. 2010;6:e1000946. doi: 10.1371/journal.pcbi.1000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita T, Tanabe K, Kita K. Spread and evolution of Plasmodium falciparum drug resistance. Parasitol Int. 2009;58:201–209. doi: 10.1016/j.parint.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Mota MM, Brown KN, Do Rosario WE, Holder AA, Jarra W. Antibody recognition of rodent malaria parasite antigens exposed at the infected erythrocyte surface: Specificity of immunity generated in hyperimmune mice. Infect Immun. 2001;69:2535–2541. doi: 10.1128/IAI.69.4.2535-2541.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwakanma D, Kheir A, Sowa M, Dunyo S, Jawara M, Pinder M, Milligan P, Walliker D, Babiker HA. High gametocyte complexity and mosquito infectivity of Plasmodium falciparum in the Gambia. Int J Parasitol. 2008;38:219–227. doi: 10.1016/j.ijpara.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ord R, Alexander N, Dunyo S, Hallett R, Jawara M, Targett G, Drakeley CJ, Sutherland CJ. Seasonal carriage of pfcrt and pfmdr1 alleles in Gambian Plasmodium falciparum imply reduced fitness of chloroquine-resistant parasites. J Infect Dis. 2007;196:1613–1619. doi: 10.1086/522154. [DOI] [PubMed] [Google Scholar]
- Paul REL, Packer MJ, Walmsley M, Lagog M, Ranford-Cartwright LC, Paru R, Day KP. Mating patterns in malaria parasite populations of Papua New Guinea. Science. 1995;269:1709–1711. doi: 10.1126/science.7569897. [DOI] [PubMed] [Google Scholar]
- Plowe CV. The evolution of drug-resistant malaria. Trans R Soc Trop Med Hyg. 2009;103:S11–S14. doi: 10.1016/j.trstmh.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. pp. [Google Scholar]
- Råberg L, de Roode JC, Bell AS, Stamou P, Gray D, Read AF. The role of immune-mediated apparent competition in genetically diverse malaria infections. Am Nat. 2006;168:41–53. doi: 10.1086/505160. [DOI] [PubMed] [Google Scholar]
- Read AF, Day T, Huijben S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1100299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read AF, Huijben S. Evolutionary biology and the avoidance of antimicrobial resistance. Evol Appl. 2009;2:40–51. doi: 10.1111/j.1752-4571.2008.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read AF, Taylor LH. The ecology of genetically diverse infections. Science. 2001;292:1099–1102. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- Roll Back Malaria. [accessed February 9, 2009];The global malaria action plan. 2008 http://www.rbm.who.int/gmap/gmap.pdf.
- Schoepflin S, Valsangiacomo F, Lin E, Kiniboro B, Mueller I, Felger I. Comparison of Plasmodium falciparum allelic frequency distribution in different endemic settings by high-resolution genotyping. Malar J. 2009;8:8. doi: 10.1186/1475-2875-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Felger I, Tanner M, Beck H-P. The epidemiology of multiple Plasmodium falciparum infections. 11. Premunition in Plasmodium falciparum infection: insights from the epidemiology of multiple infections. Trans R Soc Trop Med Hyg. 1999;93:S59–S64. doi: 10.1016/s0035-9203(99)90329-2. [DOI] [PubMed] [Google Scholar]
- Soulama I, Nebie I, Ouedraogo A, Gansane A, Diarra A, Tiono AB, Bougouma EC, Konate AT, Kabre GB, Taylor WRJ, Sirima SB. Plasmodium falciparum genotypes diversity in symptomatic malaria of children living in an urban and a rural setting in Burkina Faso. Malar J. 2009;8:8. doi: 10.1186/1475-2875-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska K, White NJ. Pharmacokinetic determinants of the window of selection for antimalarial drug resistance. Antimicrob Agents Chemother. 2008;52:1589–1596. doi: 10.1128/AAC.00903-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland CJ, Alloueche A, Curtis J, Drakeley CJ, Ord R, Duraisingh M, Greenwood BM, Pinder M, Warhurst D, Targett GAT. Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. Am J Trop Med Hyg. 2002;67:578–585. doi: 10.4269/ajtmh.2002.67.578. [DOI] [PubMed] [Google Scholar]
- Sutton PL, Neyra V, Hernandez JN, Branch OH. Plasmodium falciparum and Plasmodium vivax infections in the Peruvian Amazon: propagation of complex, multiple allele-type infections without super-infection. Am J Trop Med Hyg. 2009;81:950–960. doi: 10.4269/ajtmh.2009.09-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talisuna AO, Erhart A, Samarasinghe S, Van Overmeir C, Speybroeck N, D'Alessandro U. Malaria transmission intensity and the rate of spread of chloroquine resistant Plasmodium falciparum: Why have theoretical models generated conflicting results? Infect Genet Evol. 2006;6:241–248. doi: 10.1016/j.meegid.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Taylor LH, Mackinnon MJ, Read AF. Virulence of mixed-clone and single-clone infections of the rodent malaria Plasmodium chabaudi. Evolution. 1998;52:583–591. doi: 10.1111/j.1558-5646.1998.tb01656.x. [DOI] [PubMed] [Google Scholar]
- Taylor LH, Walliker D, Read AF. Mixed-genotype infections of malaria parasites: within-host dynamics and transmission success of competing clones. Proc R Soc Lond B Biol Sci. 1997;264:927–935. doi: 10.1098/rspb.1997.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms R, Colegrave N, Chan BHK, Read AF. The effect of parasite dose on disease severity in the rodent malaria Plasmodium chabaudi. Parasitology. 2001;123:1–11. doi: 10.1017/s0031182001008083. [DOI] [PubMed] [Google Scholar]
- Trape JF, Rogier C. Combating malaria morbidity and mortality by reducing transmission. Parasitol Today. 1996;12:236–240. doi: 10.1016/0169-4758(96)10015-6. [DOI] [PubMed] [Google Scholar]
- Vafa M, Troye-Blomberg M, Anchang J, Garcia A, Migot-Nabias F. Multiplicity of Plasmodium falciparum infection in asymptomatic children in Senegal: relation to transmission, age and erythrocyte variants. Malar J. 2008;7:9. doi: 10.1186/1475-2875-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walliker D, Carter R, Sanderson A. Genetic studies on Plasmodium chabaudi - recombination between enzyme markers. Parasitology. 1975;70:19–24. doi: 10.1017/s0031182000048824. [DOI] [PubMed] [Google Scholar]
- Walliker D, Quakyi IA, Wellems TE, McCutchan TF, Szarfman A, London WT, Corcoran LM, Burkot TR, Carter R. Genetic analysis of the human malaria parasites Plasmodium falciparum. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Wargo AR, Huijben S, de Roode JC, Shepherd J, Read AF. Competitive release and facilitation of drug-resistant parasites after therapeutic chemotherapy in a rodent malaria model. Proc Natl Acad Sci USA. 2007;104:19914–19919. doi: 10.1073/pnas.0707766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo AR, Randle N, Chan BHK, Thompson J, Read AF, Babiker HA. Plasmodium chabaudi: Reverse transcription PCR for the detection and quantification of transmission stage malaria parasites. Exp Parasitol. 2006;112:13–20. doi: 10.1016/j.exppara.2005.08.013. [DOI] [PubMed] [Google Scholar]
- White NJ, Pongtavornpinyo W. The de novo selection of drug-resistant malaria parasites. Proc R Soc Lond B Biol Sci. 2003;270:545–554. doi: 10.1098/rspb.2002.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap GS, Stevenson MM. Blood-transfusion alters the course and outcome of Plasmodium chabaudi AS infection in mice. Infect Immun. 1994;62:3761–3765. doi: 10.1128/iai.62.9.3761-3765.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Wiesch PA, Kouyos R, Engelstadter J, Regoes RR, Bonhoeffer S. Population biological principles of drug-resistance evolution in infectious diseases. Lancet Infect. Dis. 2011;11:236–247. doi: 10.1016/S1473-3099(10)70264-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.