Abstract
Surgical management of cystic hygroma is very challenging since it has a thin wall consisting of endothelium which can easily be torn during its enucleation leading to recurrence. The other treatment options are intralesional injection of OK-432, triamcinolone followed by surgical excision, if necessary, injection of sclerosing agents, repeated aspiration, radiotherapy and finally spontaneous regression without any form of treatment. This is a case report of cystic hygroma of parotid region where regression occurred without any form of treatment.
Keywords: Cystic hygroma, spontaneous regression, cavernous lymphangioma
INTRODUCTION
Cystic hygroma, a cystic subtype of lymphangioma is a developmental tumor of lymphatic origin. It is considered to be a relatively rare lesion[1,2] and accounts for 6% of all benign lesions of infancy and childhood.[3] About 50% of the cystic hygromas reported are present since birth and most of the remaining 50% appear by the age of 2 years and are usually found in the head and neck region, but may also arise anywhere in the developing lymphatic system.[4–6] Exceptionally it can become so large so as to obstruct labor.
The treatment modalities of cystic hygroma are surgical excision, injection of sclerosing agents, repeated aspiration of the contents, incision and drainage, radiotherapy, etc.
This is a case report of a cystic hygroma, where complete regression occurred and depending upon the clinical course and history of the patient it was judicious to wait, without any form of a ive treatment.
CASE REPORT
A 5-year-old girl reported to the Department Of Oral and Maxillofacial Surgery, SDC, Lucknow, for evaluation of a swelling present over the right cheek region [Figure 1] since birth, which was slowly increasing in size. The swelling was painless. The patient had undergone surgery twice for this mass 1 month back before coming to the department. The histologic diagnosis of the tumor was unknown. There was no history of pain and the only concern of the patient was facial asymmetry.
Figure 1.
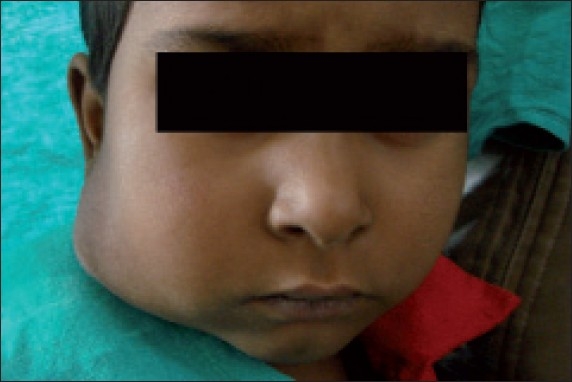
Frontal view at the time of presentation
On examination, a scar was present over the lower border of mandible and swelling was present over the region of parotid and masseter muscle of size approximately 5 × 5 cm on the right side [Figure 2]. The swelling was soft on palpation, with poorly defined margins, was nontender and when subjected to light test was brilliantly translucent. Intraorally, there was no significant finding. Routine blood examination was carried out and the findings were: hemoglobin (Hb) 9.1 g/dl, total leukocyte count (TLC) 11,600, differential leukocyte count as – polymorph nuclear cells 53%, lymphocytes 43%, monocytes 2% and eosinophils 2%. Fine needle aspiration cytology revealed that the mass contained clear yellow fluid in which no tumor cells were present. Ultrasonographic images of the swelling revealed that right masseter muscle was heterogeneous with loss of fibrillary pattern with a 6.5 × 6.2 × 2.4 cm (volume approximately 50 ml) ill-defined multicystic, nonvascular heterogeneous lesion that involved subcutaneous fat plane and was showing floating internal echoes. Right parotid gland pushed posteriorly but normal in size, shape and echogenecity. Interface of the lesion with underlying mandibular ramus was maintained [Figure 3]. On the basis of this finding, a diagnosis of myositis of right masseter or lymphangioma was made.
Figure 2.
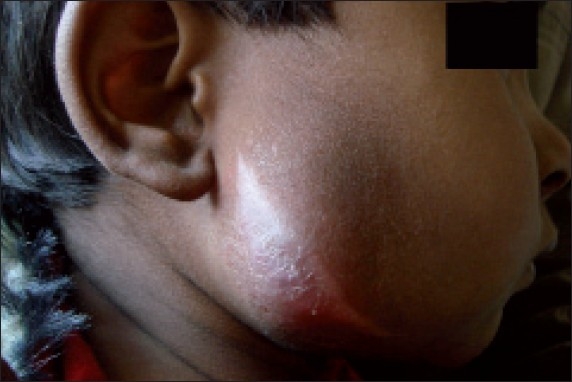
Profile view at the time of presentation
Figure 3.
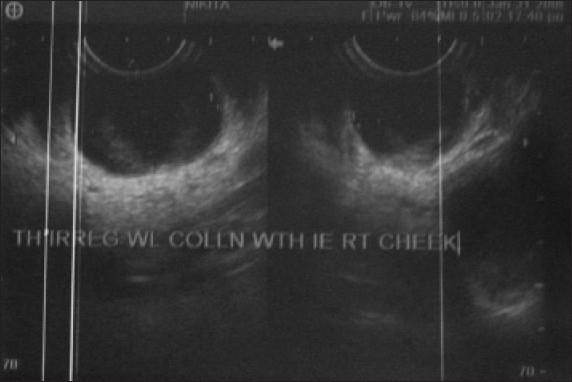
Ultra sonogram showing heterogeneous multiple cysts
Magnetic resonance and imaging (MRI) of the lesion showed multiple well-defined cystic lesions [homogenous and isointense to cerebrospinal fluid (CSF)] of varying sizes within the right masseter muscle, extending into the subcutaneous fat plane. The largest cyst had a size of 4.4 × 2.9 × 2.6 cm. No evidence of abnormal flow voids within or near the lesion was present. There was hyperintense T2 weighed image and hypointense T1 weighed image [Figures 4 and 5]. On the basis of MRI findings, a diagnosis of hemangioma or lymphangioma was made. On the basis of its brilliantly translucent character with a clear fluid, and ultrasound scanning (US) and MRI findings, a final diagnosis of cystic hygroma was made.
Figure 4.
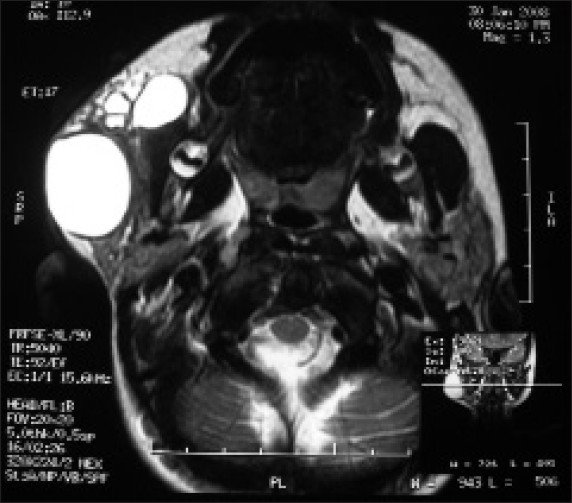
Axial view MRI showing multiple cysts with hyperintense T2 weighed images
Figure 5.
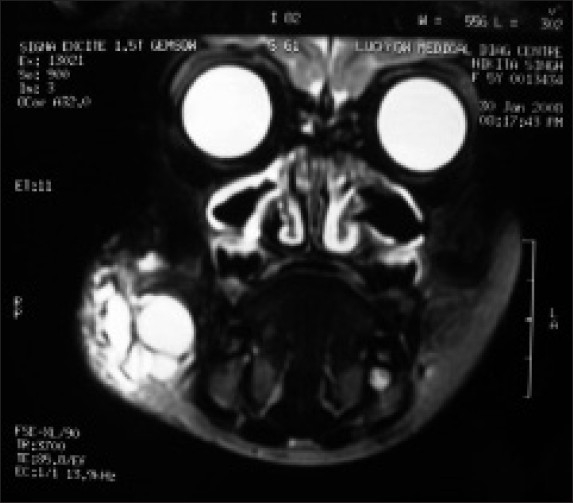
Coronal view MRI showing multiple cysts with hyperintense T2 weighed images
Surgical enucleation of the lesion was planned but since hemoglobin was low and the patient had undergone surgery 1 month back for the same problem, the patient was advised to take hematinics to increase hemoglobin and was recalled after a month for definitive management.
After a month's period, the patient presented with a minimal swelling, hence it was prudent to wait for a longer duration. The patient was closely observed for a period of 2 years during which the swelling gradually decreased and the patient did not present with any signs of recurrence [Figures 6 and 7].
Figure 6.
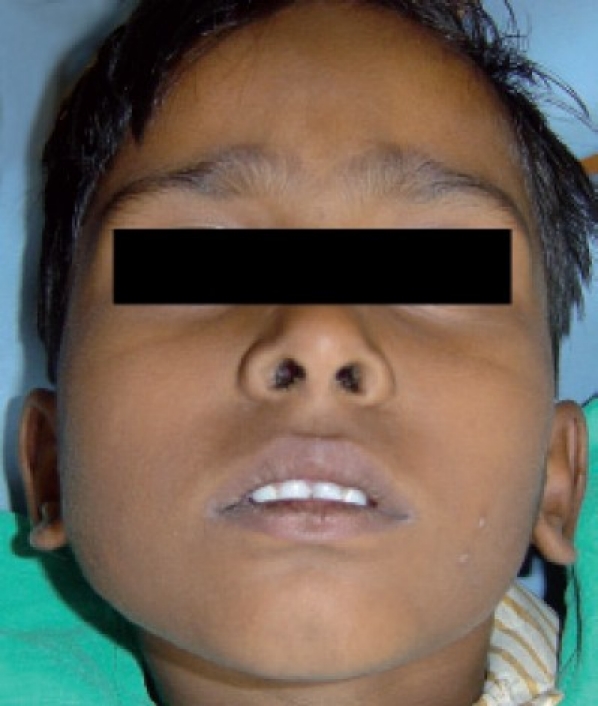
Frontal view after two years of follow up
Figure 7.
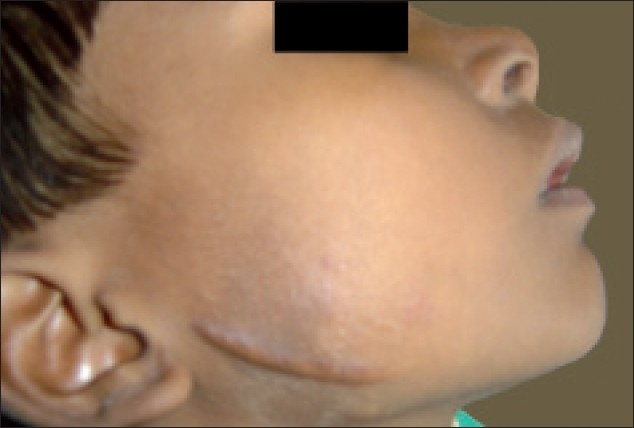
Profile view after two years of follow up
DISCUSSION
Cystic hygroma (syn: cavernous lymphangioma, lymphatic cysts, chylous cysts) is a congenital abnormalities of the lymphatic vessels. The primitive lymph sacs develop in mesoblast at about the sixth week of embryonic life, the principal pair being situated in the neck between the jugular and subclavian veins. It probably arises either from sequestration of primitive embryonic lymphatic tissue (jugular lymph sac) or from a congenital blocking of the regional lymph drainage.[6,7] The swelling consists of an aggregation of cysts like a mass of soap bubbles. The larger cysts are present near the surface, while the smaller ones lie deeper and tend to infiltrate muscle planes.[6] Histologically, it is characterized by a thin walled structure (single layer endothelium) containing clear watery fluid (lymph) showing no microscopic features apart from this. It may be either unilocular or multilocular and contains either serous or chylous fluid; sometimes thin septations within the mass can be seen.[8] This benign, painless, soft and fluctuant (partially compressible) malformation affects both sexes equally. It visibly increases in size when the patient (child) coughs or cries, but the characteristic which distinguishes it from all other cervical swellings is that it is brilliantly translucent.[6] It neither becomes malignant nor has a familial tendency.[9] Although these lesions are benign, they often involve extensive invasion of tissue planes by tiny daughter cysts.
It is most commonly found in the head and neck region, less commonly in axilla, mediastinum, groin and retroperitoneal region.[4] About 75% of cystic hygromas occur in neck and 20% in axilla. Typically, the swelling occupies lower third of the neck, and as it enlarges it passes toward the ear; often it is the posterior triangle of neck that is mainly involved. Cheek is a less frequent site for the occurrence of cystic hygroma.[6] It may occur in association with chromosomal anomalies, cardiovascular, pulmonary and musculoskeletal disorders.[10]
The management is quite difficult and an accurate diagnosis coupled with anatomic localization is essential. The diagnosis of cystic hygroma is suggested by history and clinical examination.[10] These lesions are evaluated by US, computerized tomography (CT) and MRI. US is useful for characterizing superficial lesions but is poor when there is extension into deep structures of the neck, thoracic cavity or retroperitoneum.[11] CT with intravenous contrast is better at defining anatomic locations with respect to relationship to blood vessels but is associated with bone artifacts from base of skull, vertebra and shoulder, and may be equivocal when the lesion is surrounded by soft tissues of similar accentuation. The use of MRI in cystic hygroma can facilitate accurate diagnosis and assist in the preoperative planning, thereby contributing to the successful treatment of this lesion.
MRI has the benefits of superior multiplanar capabilities, lack of bony artifacts and of ionizing radiations. Typical MRI findings of cystic hygroma include high signal intensity on T2 weighed image (T2W1), low signal intensity on T1 weighed image and multiple cysts with well-demarcated margins on T2W1. They can be diagnosed during prenatal period using ultrasonography in first trimester.[12]
Treatment is very challenging due to thin wall and its multicystic nature. The various treatment modalities are: conservative treatment like injection of sclerosing agents (OK-432, bleomycin, corticosteroids and 50% dextrose), repeated aspiration of the contents, radiotherapy, enucleation, spontaneous regression without any form of treatment and intralesional injection of triamcinolone followed by surgical excision at a second stage.[13] Over recent years, intralesional injections of sclerosing agents have been reported as an alternative treatment with promising results.
Surgical excision is the treatment of choice, but complete removal is difficult due to the infiltrative nature of these lesions and presence of thin wall of endothelium which can easily be torn. Recurrence (clinical obvious disease) after macroscopically complete surgical excision is 17%.[14] One of the methods for achieving complete surgical excision is injecting hydrocolloid impression material (agar) into the lesion by a syringe and simultaneous aspiration of cystic fluid by another syringe. As the cystic space is filled with the impression material, it becomes elastic and the posterior wall of the tumor is exposed, thereby helping in the complete removal of lesion in one piece. This method is a therapeutic option as long as the tumor does not involve major vessels and nerves.[15] Aspiration is not a treatment modality but may be useful for emergency decompression.[14]
Treatment of cystic hygroma with sclerosing agents is based on the fact that they are congenital malformations of lymphatic system with large cystic spaces lined by endothelium. This endothelial lining of the lesion is vulnerable to insults through infection and/or chemical irritants. Thus, infections can result in spontaneous reduction in the size of lesion; but this is a rare finding. Ogita et al[16] evaluated the mechanism of OK-432 (lyophilized incubation mixture of group A Streptococcus pyogenes of human origin) therapy in six patients with cystic hygroma and found that it induced and activated white blood cells (WBC) which produce cytokines. These cytokines increase the endothelial permeability and thus accelerate lymph drainage and increased lymph flow leads to shrinkage of cystic spaces.[16] Neilson et al[17] found that OK-432 is superior to surgery in the treatment of cystic hygroma. Additionally, the short-term outcome of OK-432 in large cystic lymphatic malformations is uniformly positive and without serious or permanent deleterious effects.[17]
Orford et al[18] used intralesional bleomycin as a sclerosant in 16 patients and found excellent (complete clinical resolution) response in 44% cases, good response (more than 50% response) in 44% cases and poor or no response in 12% cases. This therapy was without serious adverse effects. The only side effects which were observed were fever, vomiting, cellulitis and skin discoloration, and that too in a very few cases. So, bleomycin therapy is effective with a response rate comparable to that of surgical removal, and with the advantage of avoiding inadvertent nerve damage and scarring.[18] Pulmonary toxicity is the most serious potential side effect of bleomycin therapy. The risk is related to the total dose of more than 400 units or a single dose of more than 30 mg/m2.
Farmand and Kutterberger[13] reported that intralesional corticosteroid injection primarily and subsequent excision secondarily is an effective and promising modality of treating infantile cystic hygroma. It has the advantage of being a minor procedure and can be performed early in life, is safe, and in certain cases no subsequent surgical intervention is necessary. The few minor complications are fever for 2–3 days and local inflammatory reaction for 3–4 days at the injection site.[13]
Some authors have recommended conservative management with observation only in asymptomatic patients. The rationale for this management is based on a few documented cases of spontaneous regression.[9] Spontaneous regression of cystic hygroma without any treatment is occasionally reported and occurs with a frequency of 1.6–16%.[4] Alqahtaein et al. in their study on lymphangioma observed that spontaneous regression of the lesion is infrequent and is seen more often with recurrent lesions. They observed 10 patients of spontaneous regression out of 191 cases. Among these cases, five did not undergo any intervention earlier and five were cases of recurrence.[14] The case cited in this report was a recurrent lesion which regressed spontaneously, as going by its clinical course and history it was believed to be prudent to wait and watch. However, surgical excision is still considered as the best treatment modality for the lesion.
CONCLUSION
The behavior of cystic hygroma during infancy is variable; sometimes its growth is very rapid and precipitates respiratory difficulty, a condition that demands immediate aspiration of much of the contents of cysts and possible tracheostomy. At other times, as a result of nasopharyngeal infection, the swelling becomes inflamed and spontaneous regression of cystic hygroma may occur. Complete surgical excision of the lesion at an early age is the treatment of choice. A preliminary injection of sclerosing solution at weekly intervals reduces the size and makes the walls of cysts more fibrous and thus facilitates dissection.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bill AH, Jr, Sumner DS. A unified concept of lymphangioma and cystic hygroma. Surg Gynecol Obstet. 1965;120:79–86. [PubMed] [Google Scholar]
- 2.Brock ME, Smith RJ, Parey SE, Mobley DL. Lmphangioma: An otolaryngologic perspective. Int J Pediatr Otorhinolaryngol. 1987;14:133–40. doi: 10.1016/0165-5876(87)90024-3. [DOI] [PubMed] [Google Scholar]
- 3.Zadvinskis DP, Benson MT, Kerr HH, Mancuso AA, Cacciarelli AA, Madrazo BL, et al. Congenital malformations of the cervicothoracic lymphatic system: Embryology and pathogenesis. Radiographics. 1992;12:1175–89. doi: 10.1148/radiographics.12.6.1439020. [DOI] [PubMed] [Google Scholar]
- 4.Broomhead IW. Cystic hygroma of the neck. Br J Plast Surg. 1964;17:225–44. doi: 10.1016/s0007-1226(64)80039-4. [DOI] [PubMed] [Google Scholar]
- 5.Osborne TE, Haller JA, Levin LS, Little BJ, King KE. Submandibular cystic hygroma resembling a plunging ranula in a neonate: Review and report of a case. Oral Surg Oral Med Oral Pathol. 1991;71:16–20. doi: 10.1016/0030-4220(91)90513-c. [DOI] [PubMed] [Google Scholar]
- 6.Russell RC, Williams NS, Bulstrode . In: Bailey and Love's Short Practice of Surgery. 23rd ed. Arnold, editor. London and USA Oxford University Press; 2000. pp. 700–1. [Google Scholar]
- 7.Shahriari A, Odell JA. Cervical and thoracic components of multiorgan lymphangiomatosis managed surgically. Ann Thorac Surg. 2001;71:694–6. doi: 10.1016/s0003-4975(00)02353-5. [DOI] [PubMed] [Google Scholar]
- 8.Pachter MR, Latters R. Mesenchymal tumours of the mediastinum. III. Tumours of lymph vascular origin. Cancer. 1963;16:108–17. doi: 10.1002/1097-0142(196301)16:1<108::aid-cncr2820160112>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Schloss MD, Sweet RC, Blais C, Tewfik TL. Lymphangioma in children. J Otolaryngol. 1984;13:95–8. [PubMed] [Google Scholar]
- 10.Fisher R, Partington A, Dykes E. Cystic hygroma: Comparison between prenatal and postnatal diagnosis. J Pediatr Surg. 1996;31:473–6. doi: 10.1016/s0022-3468(96)90477-7. [DOI] [PubMed] [Google Scholar]
- 11.Sheth S, Nussbaum AR, Hutchins GM, Sanders RC. Cystic hygromas in children: Sonographic-pathologic correlation. Radiology. 1987;162:821–4. doi: 10.1148/radiology.162.3.3544038. [DOI] [PubMed] [Google Scholar]
- 12.Fung K, Poenaru D, Soboleski DA, Kamal IM. Impact of magnetic resonance imaging on the surgical management of cystic hygromas. J Pediatr Surg. 1998;33:839–41. doi: 10.1016/s0022-3468(98)90654-6. [DOI] [PubMed] [Google Scholar]
- 13.Farmand M, Kuttenberger JJ. A new therapeutic concept for the treatment of cystic hygroma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:389–95. doi: 10.1016/s1079-2104(96)80013-8. [DOI] [PubMed] [Google Scholar]
- 14.Alqahtani A, Nguyen LT, Flageole H, Shaw K, Laberge JM. 25 years’ experience with lymphangiomas in children. J Pediatr Surg. 1999;34:1164–8. doi: 10.1016/s0022-3468(99)90590-0. [DOI] [PubMed] [Google Scholar]
- 15.Katsuno S, Ezawa S, Minemura T. Excision of cervical cystic lymphangioma using injection of hydrocolloid dental impression material. A technical case report. Int J Oral Maxillofac Surg. 1999;28:295–6. [PubMed] [Google Scholar]
- 16.Ogita S, Tsuto T, Nakamura K, Deguchi E, Tokiwa K, Iwai N. OK-432 therapy for lymphangioma in children: Why and how does it work? J Pediatr Surg. 1996;31:477–80. doi: 10.1016/s0022-3468(96)90478-9. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen LH, Charabi B, Jensen F, Claesson G, Bretlau P. Cystic hygroma: OK-432 is superior to surgery. Int Congr Ser. 2003;1254:519–22. [Google Scholar]
- 18.Orford J, Barker A, Thonell S, King P, Murphy J. Bleomycin therapy for cystic hygroma. J Pediatr Surg. 1995;30:1282–7. doi: 10.1016/0022-3468(95)90485-9. [DOI] [PubMed] [Google Scholar]


