Abstract
Aim:
The ameloblastoma is a benign odontogenic tumor of epithelial origin that exhibits a locally aggressive behavior with a high level of recurrence, being believed theoretically to come from dental lamina remains, the enamel organ in development, epithelial cover of odontogenic cysts or from the cells of the basal layer of the oral mucosa. Especially larger, aggressive lesions require a more radical surgical approach resulting in large jaw defects. This paper discusses our experiences in the management of ameloblastoma tumor in 20 such patients.
Materials and Methods:
A review of 20 cases of ameloblastoma (6 in the maxillary and 14 in the mandibular region) is presented. The lesions were between 4 and 8 cm in diameter. The methods of treatment consisted of radical surgery (i.e., segmental resection) and conservative treatments (i.e., enucleation with bone curettage). Half the cases were treated conservatively and others surgically.
Results:
Enucleation with curettage was done in 10 cases, out of which six (60%) showed recurrence, whereas one (10%) case in the surgical group showed recurrence. Relatively higher tendencies of recurrence were observed in the cases treated conservatively. The aesthetic and functional outcomes were satisfying in all patients.
Conclusion:
According to our opinion, radical surgical resection of ameloblastoma is the treatment of choice, followed by the reconstruction of the defects, allowing good functional and aesthetic outcome.
Keywords: Ameloblastoma, enucleation, resection
INTRODUCTION
Odontogenic tumors comprise of a complex group of lesions of diverse histopathological types and clinical behavior.[1] Of all swellings of the oral cavity, 9% are odontogenic tumors and within this group, ameloblastoma accounts for 1% of lesions. WHO defines it as a locally invasive polymorphic neoplasia that often has a follicular or plexiform pattern in a fibrous stroma. Its behavior has been described as being benign but locally aggressive.[2] In 20% of all cases the tumor can be found in the upper jaw, predominantly in the canine or molar region. Within the mandible, 70% are located in the molar region or the ascending ramus, 20% in the premolar region and 10% in the anterior part.[3] Ameloblastomas occur with equal frequency in both sexes.[4,5]
The age range is usually between the first and the seventh decade of life with a mean in the fourth decade.[6] Clinically, ameloblastomas can be classified into 4 groups: unicystic, solid or multicystic, peripheral, and malignant. The unicystic ameloblastoma usually appears as a “cystic” lesion with either an intraluminal or an intramural proliferation of the cystic lining.[7] Radiographically, it may resemble a well-circumscribed slow-growing radiolucency. Multicystic ameloblastoma can infiltrate into the adjacent tissue and has the ability to recur and even metastasize. Its prevalence is a slightly older age group than the unicystic ameloblastoma. Radiographically, the appearance is generally unilocular or multilocular.[8] Peripheral ameloblastoma mostly appears in the alveolar mucosa. It is a soft-tissue version of an ameloblastoma but can also involve the underlying bone.[9] The malignant ameloblastoma is a rare entity. It is defined as an ameloblastoma that has already metastasized but still maintains its classical microscopic features.[10]
A histological classification subdivides into follicular, plexiform, acanthomatous and granular ameloblastoma. In most cases the tumor is asymptomatic, presenting as an incidental finding on orthopantomography. The most common symptoms are facial swelling, pain, malocclusion, loosening of teeth, ill-fitting dentures, periodontal diseases or ulceration, oroantral fistulas and nasal airway obstruction.[11] Literature basically describes two therapy strategies: a conservative way of treatment and radical procedures. While smaller lesions are generally treated by a less aggressive approach, larger lesions require a radical surgical tumor ablation resulting in large defects making reconstruction difficult.
Management of ameloblastoma has been controversial because of the unique biological behavior of this disease as a slow-growing, locally invasive tumor with a high rate of recurrence.[12–17] Recurrence rates of ameloblastoma are reportedly as high as 15-25% after radical treatment[13–15] and 75-90% after conservative treatment.[13–17] Therefore, wide resection of the jaw in accordance with the treatment of malignant tumors is usually recommended for ameloblastomas. Recent advancements in understanding the biological behaviors of ameloblastoma have led to more rational surgical approaches.[18–20]
MATERIALS AND METHODS
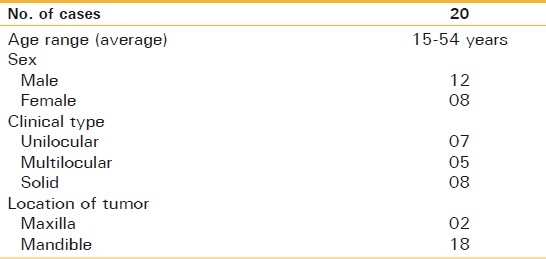
In total, 20 patients with primary ameloblastoma treated during the period from 2003 to 2008 were available for this study. Clinical information and radiographs were obtained from the records of the Department of Oral and Maxillofacial Surgery, Institute of Dental Sciences, Bareilly. The patient age ranged from 15 to 60 years, with a sex distribution of 12 males and eight females. According to the clinical and radiographical features, tumors were classified as three types: unicystic, multicystic or solid. The distribution of patients of each type was seven unicystic, five multicystic and eight solid types. Two cases occurred in the maxilla and 18 in the mandible and the tumors were often located in the molar to the ramus region [Table 1].
Table 1.
Clinical features
All patients were diagnosed with ameloblastoma by means of histological examination of biopsy specimens. Histological diagnosis and classification were based on the criteria defined by the World Health Organization (WHO) histological classification. The methods of treatment consisted of radical surgery (segmental resection) and conservative treatments (enucleation with bone curettage). Radical surgery was defined as the procedure in which the ameloblastoma was resected, with a safety margin of at least 2 cm of normal bone, with or without a continuity defect.
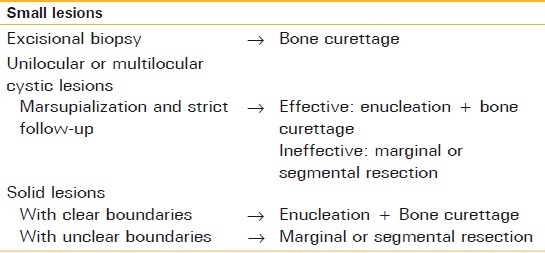
Conservative treatment has been carried out in accordance with our comprehensive conservative treatment protocol for ameloblastomas since the 1980s [Table 2]. Small lesions are submitted to excisional biopsy, and once the ameloblastoma has been diagnosed, the lesion is enucleated and curetted, including the surrounding healthy bone. Unilocular or multilocular cystic lesions are usually marsupialized before surgery. Lesions of solid-type tumor with clear margin viewed by means of radiographical examination are usually curetted extensively, and lesions with unclear margins, such as those with a soap-bubble appearance, or those with ineffective marsupialization are subjected to marginal or segmental resection depending on their size and location.
Table 2.
Comprehensive treatment protocol of ameloblastoma
Enucleation with bone curettage was defined as the procedure in which the ameloblastoma was enucleated in conjunction with excision of the overlying mucosa and, subsequently, sufficient bone curettage. While curetting the bone, 2.5% gentian violet was used on the surface of adjacent healthy bone to ensure removal of the tumor. The colored bone was usually curetted three or four times, for more than 5 mm in depth, by using a large round bur. If an isolated tumor nest was recognized in the cancellous bone during this procedure, additional curettage was performed. When the nerve was exposed in the surgical field, it was lifted out from the bony canal when curetting the bone to avoid damage to the nerve. After the surgical treatment, the patients had clinical and radiographical examinations every year for at least 5 years.
The effects of resection on the recurrence data after a follow-up period of at least 5 years were evaluated. Furthermore, these recurrence data were analyzed with respect to clinical types and WHO patterns.
RESULTS
Out of the 20 ameloblastomas, 18 were located in the mandible (90%) and two in the maxillary region (10%). Twelve of the mandibular ameloblastomas (66.6%) were found in the corpus- and mandibular-angle region, both of them reaching the ascending ramus. In six cases (33.3%) the tumor was located in the anterior part of the mandible (symphysis). The size of the lesions varied between 4 and 8 cm in diameter, as is seen in Figures 1 and 2.
Figure 1.
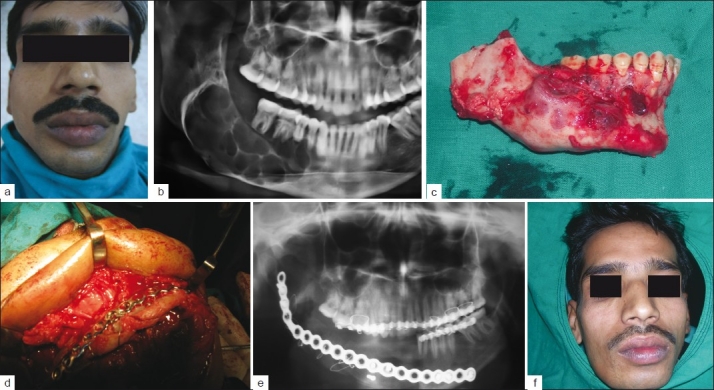
(a) Preoperative frontal view, (b) Multilocular radiolucency involving right body and angle of mandible, (c) Resected part of mandible involving 2 cm of normal bone, (d) Reconstruction of mandible with iliac crest graft, (e) Postoperative X-ray, (f) Postoperative frontal view
Figure 2.
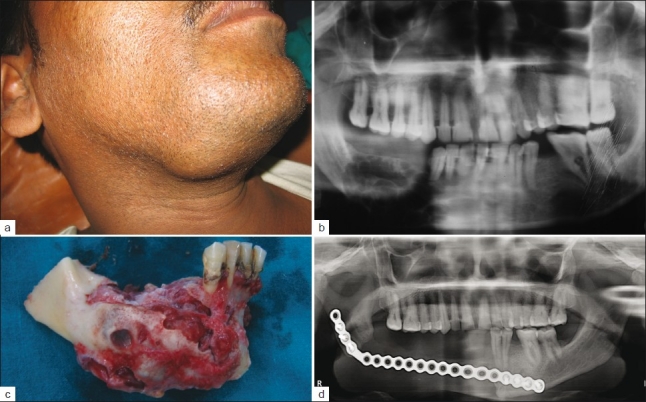
(a) Ameloblastoma of right body of mandible causing expansion, (b) OPG showing multilocular lesion at right body of mandible, (c) Resected part of mandible involving 2 cm of normal bone, (d) Postoperative X-ray
Ten patients underwent enucleation with bone curettage, including those with six unicystic and four multicystic types of ameloblastoma. The effect of the marsupialization was graded radiographically as follows: extremely effective, the lesion almost disappeared; effective, the lesion decreased to less than half its initial size; ineffective, the lesion did not decrease remarkably or tended to grow further. The effect of enucleation with bone curettage was evaluated as extremely effective in one case, effective in six cases and ineffective in three cases. Extremely effective cases were observed only in the unicystic type, whereas effective and ineffective cases were included in both the unicystic and the multicystic types. The effective rate, including extremely effective and effective cases, was 70% (07/10) in total [Table 3].
Table 3.
Effect of enucleation and curettage for cystic ameloblastoma

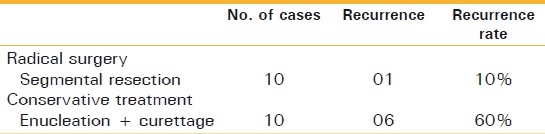
Types of surgical treatment and recurrence data for primary ameloblastoma are shown in Table 4. Radical surgery and conservative treatment were performed in 10 patients each. Recurrences were observed in seven patients; these included one case with segmental resection and six cases with enucleation and bone curettage. In terms of comparison of recurrence rates of different surgical modalities, relatively high recurrence rates were observed in the patients treated by enucleation with bone curettage (60%). Recurrence rate after radical surgery is 10%.
Table 4.
Types of surgical modalities for primary ameloblastoma
Wound healing disturbances occurred in only one patient after mandibular reconstruction. This was a result of heavy smoking in combination with poor oral hygiene.
DISCUSSION
Wide resection of the jaw is usually the recommended treatment for ameloblastoma, should priority be given to the recurrence rate. However, radical surgery often means that the patients have serious complications including facial deformity, masticatory dysfunction, and abnormal jaw movement. Considering the characteristics of ameloblastoma as a locally invasive but slow-growing and extremely rare metastasizing benign tumor, the priority of the treatment method should be discussed from the points of morbidity and quality of life of the patients, noting that the recurrence rate is not always the primary factor.
Two therapy strategies are mentioned in literature: a conservative way of treatment and radical procedures. Non-radical surgical procedures like enucleation and curettage, combined with liquid nitrogen spray cryosurgery, or just drilling of the perilesional bone are mentioned to be useful in unicystic ameloblastomas, especially in children and young patients. Other authors show high rates of reccurence of ameloblastoma after conservative treatment protocols and therefore recommend radical surgical treatment.[5] Authors suggests a “rational radical conservative” resection of the mandible with preservation of the lower border of the mandible to maintain the continuity of the lower jaw and the facial contours.
In the previous reports, conservative treatments for ameloblastoma appeared to have failed to control local recurrences. Sehdev et al,[15] reported recurrence after the conservative approach (curettage) in more than 90% of 92 ameloblastomas. Shatkin and Hoffmeister[13] reported that 86% of 20 mandibular ameloblastomas recurred after curettage compared with a 14% recurrence rate after en bloc resection. Other authors have reported a series of 84 ameloblastomas in which they found a 52% rate of recurrence in patients treated conservatively and a 25% rate of recurrence in patients with primary tumor treated by the radical approach. However, extensive tumors require a more radical approach. The amount of resection is variable and depends on the site and extension of the tumor. Patients included in our study all presented with locally advanced tumors already infiltrating the surrounding soft tissue. According to our opinion, conservative ways of treatment in these cases are not appropriate and will surely result in local recurrent tumors making further surgical treatment even more complicated resulting in cosmetic and speech deficits.
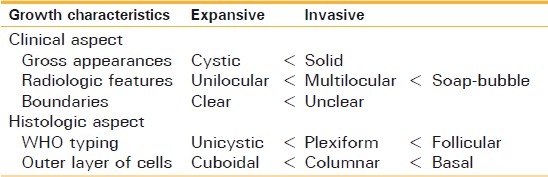
When planning the treatment of ameloblastoma, it is important to understand the growth characteristics and to remove the full extent of the tumor, including the surrounding tissues. Otherwise, the remaining tumor cells may lead to multiple morbidities of recurrence. Recent advancements in the understanding of the biological behaviors of ameloblastoma have revealed that unicystic lesions are well-localized by the fibrous capsule of the cyst, with few tumors broaching peripheral tissues, whereas multicystic and solid lesions are characterized by an aggressive infiltration to adjacent tissue.[20] Gardner[20] discussed the treatment of ameloblastoma on the basis of pathological and anatomical considerations. He stated that the recommended treatment for solid and multicystic ameloblastoma was radical treatment, whereas unicystic ameloblastoma was usually cured by curettage. Table 5 shows our guideline for evaluation of the growth characteristics of ameloblastoma.
Table 5.
Guideline for evaluation of growth characteristics of tumors
Postsurgical defects in the maxillary region predispose the patient to hypernasal speech, fluid leakage into the nasal cavity, impaired masticatory function, and in some patients, various degrees of cosmetic deformity. Mandibular resection can also prove devastating to mastication, deglutition, phonation, and oral competence. Moreover, the mandible frames the lower third of the face and represents a major component of the human appearance. Satisfactory reconstruction of complex jaw defects, especially in a single-step procedure, is therefore a surgical challenge. For benign tumors, the bone grafts have become a reliable source during the last few years in osseous reconstruction. The fibula, scapula and iliac crest are the commonly chosen donor sites to reconstruct mandibular or maxillary defects. For reconstruction of defects in the mandible we preferred iliac crest bone grafts as a good quality of bone is provided in sufficient amount.
Furthermore, the natural curvature of the iliac bone is ideally suited for mandibular reconstruction, using the ipsilateral or contralateral crest, depending on which segment of the mandible needs rebuilding. Postoperative morbidity after iliac crest bone grafts is a well-known fact. The donor defect should be carefully closed, since a potential for abdominal herniation still remains. Some authors recommend using nonvascularized grafts for reconstruction of mandibular continuity defects less than 9 cm in length. According to the authors this technique allows better results concerning facial esthetics and implant insertion. The success rate, however, was low and patients receiving nonvascularized grafts had to undergo an average of one more surgical procedure for total reconstruction. According to our opinion free flaps with well vascularized soft tissue provide reliable wound healing, reducing the risk of plate exposure. However, these flaps are frequently overcontoured, impairing speech and swallowing.
It is a well-known fact that nonvascularized bone transplants show high resorption rates resulting in severe bone loss after a few months because of lack of physiological stress. In our case the quality and height of bone after removal of the osteosynthesis material was fairly well. In our follow-up regime, patients were scheduled for clinical and radiological examination twice a year for the first 5 years and after that only once a year. We suggest a long follow-up period for at least 10 years as recurrence may also appear years after primary surgery.
CONCLUSIONS
Ameloblastoma has a high rate of local recurrence if it is not adequately removed. In our opinion, radical surgical resection of ameloblastoma is the treatment of choice. Especially in cases of large, expansive tumors a radical surgical protocol is a very good option to prevent relapse of the tumor on a long-term basis. Reconstruction of the defects with bone graft material allows good functional and esthetic outcome and decreases the number of surgeries. For reconstructing the mandible we prefer bone grafts from the iliac crest. The natural curvature and variable bone height offers the possibility of exact reconstruction of the defect.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Neville BW, Damn DD, Allen CM, Bouqout JK, editors. 2nd ed. Philadelphia: WB Saunders Co; 2002. Oral and maxillofacial pathology; pp. 610–5. [Google Scholar]
- 2.Lagares DT, Cossío PI, José M, Pérez JUL. Mandibular ameloblastoma. Med Oral Patol Oral Cir Bucal (Ed.impr.) Valencia mayo. 2005;10:231–8. [PubMed] [Google Scholar]
- 3.Torres-Lagares D, Infante-Cossío P, Hernández-Guisado JM, Gutiérrez-Pérez JL. Mandibular ameloblastoma. A review of the literature and presentation of six cases. Med Oral Patol Oral Cir Bucal. 2005;10:231–8. [PubMed] [Google Scholar]
- 4.Mehlisch DR, Dahlin DC, Masson JK. Ameloblastoma: A clinicopathologic report. J Oral Surg. 1972;30:9–22. [PubMed] [Google Scholar]
- 5.Pinsolle J, Michelet V, Coustal B, Siberchicot F, Michelet FX. Treatment of ameloblastoma of the jaws. Arch Otolaryngol Head Neck Surg. 1995;121:994–6. doi: 10.1001/archotol.1995.01890090036007. [DOI] [PubMed] [Google Scholar]
- 6.Kameyama Y, Takehana S, Mizohata M, Nonobe K, Hara M, Kawai T, et al. A clinicopathological study of ameloblastomas. Int J Oral Maxillofac Surg. 1987;16:706–12. doi: 10.1016/s0901-5027(87)80057-7. [DOI] [PubMed] [Google Scholar]
- 7.Robinson L, Martinez MG. Unicystic ameloblastoma: A prognostically distict entity. Cancer. 1977;40:2278–82. doi: 10.1002/1097-0142(197711)40:5<2278::aid-cncr2820400539>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Williams TP. Management of ameloblastoma: A changing perspective. J Oral Maxillofac Surg. 1993;51:1064–70. doi: 10.1016/s0278-2391(10)80440-9. [DOI] [PubMed] [Google Scholar]
- 9.Stanley HR, Jr, Krogh HW. Peripheral ameloblastoma; Report of a case. Oral Surg Oral Med Oral Pathol. 1959;12:760–5. doi: 10.1016/0030-4220(59)90124-0. [DOI] [PubMed] [Google Scholar]
- 10.Corio RL, Goldblatt LI, Edwards PA, Hartman KS. Ameloblastom carcinoma: A clinicopathologic study and assessment of eight cases. Oral Surg Oral Med Oral Pathol. 1987;64:570–6. doi: 10.1016/0030-4220(87)90063-6. [DOI] [PubMed] [Google Scholar]
- 11.Adekeye EO. Ameloblastoma of the jaws: A survey of 109 Nigerian patients. J Oral Surg. 1980;38:36–41. [PubMed] [Google Scholar]
- 12.Cawson RA, Binnie WH, Speight PM, Barrett AW, Wright JM, Thorogood P. 5th ed. London: Churchill Livingstone; 1998. Lucas's pathology of tumors of the oral tissues; pp. 25–44. [Google Scholar]
- 13.Shatkin S, Hoffmeister FS. Ameloblastoma: A rational approach to therapy. Oral Surg Oral Med Oral Pathol. 1965;20:421–35. doi: 10.1016/0030-4220(65)90231-8. [DOI] [PubMed] [Google Scholar]
- 14.Mehlisch DR, Dahlin DC, Masson JK. Ameloblastoma: A clinicopathologic report. J Oral Surg. 1972;30:9–22. [PubMed] [Google Scholar]
- 15.Sehdev MK, Huvos AG, Strong EW, Gerold FP, Willis GW. Ameloblastoma of maxilla and mandible. Cancer. 1974;33:324–33. doi: 10.1002/1097-0142(197402)33:2<324::aid-cncr2820330205>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Tsaknis PJ, Nelson JF. The maxillary ameloblastoma: An analysis of 24 cases. J Oral Surg. 1980;38:336–42. [PubMed] [Google Scholar]
- 17.Vedtofte P, Hjorting-Hansen E, Jensen BN, Roed-Peterson B. Conservative surgical treatment of mandibular ameloblastomas. Int J Oral Surg. 1978;7:156–61. doi: 10.1016/s0300-9785(78)80018-0. [DOI] [PubMed] [Google Scholar]
- 18.Robinson L, Martinez MG. Unicystic ameloblastoma: A prognostically distinct entity. Cancer. 1977;40:2278–85. doi: 10.1002/1097-0142(197711)40:5<2278::aid-cncr2820400539>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Gardner DG, Corio RL. Plexiform unicystic ameloblastoma.A variant of ameloblastoma with a low-recurrence rate after enucleation. Cancer. 1984;53:1730–5. doi: 10.1002/1097-0142(19840415)53:8<1730::aid-cncr2820530819>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Gardner DG. A pathologist's approach to the treatment of ameloblastoma. J Oral Maxillofac Surg. 1984;42:161–6. doi: 10.1016/s0278-2391(84)80026-9. [DOI] [PubMed] [Google Scholar]


