Abstract
Traumatic brain injury (TBI) is associated with cognitive deficits, memory impairment, and epilepsy. Previous studies have reported neuronal loss and neuronal hyperexcitability in the post-traumatic hippocampus. A-type K+ currents (IA) play a critical role in modulating the intrinsic membrane excitability of hippocampal neurons. The disruption of IA is reportedly linked to hippocampal dysfunction. The present study investigates the changes of IA in the hippocampus after TBI. TBI in rats was induced by controlled cortical impact. The impact induced a reproducible lesion in the cortex and an obvious neuronal death in the ipsilateral hippocampus CA3 region. At one week after TBI, immunohistochemical staining and Western blotting showed that the expression of IA channel subunit Kv4.2 was markedly decreased in the ipsilateral hippocampus, but remained unchanged in the contralateral hippocampus. Meanwhile, electrophysiological recording showed that IA currents in ipsilateral CA1 pyramidal neurons were significantly reduced, which was associated with an increased neuronal excitability. Furthermore, there was an increased sensitivity to bicuculline-induced seizures in TBI rats. At 8 weeks after TBI, immunohistochemical staining and electrophysiological recording indicated that IA returned to control levels. These findings suggest that TBI causes a transient downregulation of IA in hippocampal CA1 neurons, which might be associated with the hyperexcitability in the post-traumatic hippocampus, and in turn leads to seizures and epilepsy.
Key words: controlled cortical impact, hyperexcitability, IA, Kv4.2, Kv4.3, post-traumatic epilepsy
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability among young people in the United States (Bruns and Hauser, 2003). Survivors of TBI are often left with neurological dysfunctions including cognitive deficits, memory impairment, and epilepsy (Bales et al., 2009; D'Ambrosio and Perucca 2004; Millis et al., 2001). These outcomes are associated with abnormalities of hippocampal structure and function, as the hippocampus plays an important role in cognitive function and is a frequent locus of seizure generation. Clinical and pre-clinical studies have indicated that the hippocampus is particularly vulnerable to TBI. In rat TBI model, neurons in the CA3 and hilar regions of the hippocampus generally exhibit the greatest sensitivity to TBI (Hicks et al., 1996; Lowenstein et al., 1992). The neuronal death and de-afferentation occurring in the hippocampal formation after TBI frequently result in an aberrant increase of neuronal excitability (D'Ambrosio et al., 1999; Griesemer and Mautes, 2007; McKinney et al., 1997; Santhakumar et al., 2003). The mechanisms causing the neuronal hyperexcitability after TBI are still not well understood.
A-type K+ currents (IA) are voltage-dependent, transient outward K+ currents that activate rapidly upon depolarization (threshold ∼−50 mV), inactivate quickly, and recover fast from inactivation (Jerng et al., 2004). IA channels are composed of four α-subunits that come together as either homotetrameric or heterotetrameric channels. Furthermore, the α-subunits are typically associated with auxiliary subunits, including Kvβ (Rettig et al., 1994), K+ channel interacting proteins (KChIPs) (An et al., 2000), and dipeptidyl aminopeptidase (DPPX) (Nadal et al., 2003), which modulate their functional properties and surface expression. Among the α-subunits, Kv4 subunits (Kv4.1, Kv4.2, and Kv4.3) are the major subunits that give rise to somatodendritic IA currents in hippocampal neurons. Immunohistochemical studies have shown that Kv4.1 is expressed at very low levels in the hippocampus. Kv4.2 is robustly detected on the soma and dendrites of CA1-CA3 pyramidal neurons and granule cells in dentate gyrus (DG), whereas Kv4.3 is present in interneurons, dentate granule cells, and CA3 pyramidal cells (Serodio and Rudy, 1998).
IA plays a crucial role in regulating neuronal excitability. IA on the soma and dendrites controls action potential patterns, influences excitatory postsynaptic potentials, and modulates resting membrane potential (Andrasfalvy et al., 2008; Cai et al., 2004; Kim et al., 2005). IA has also been recognized as a major player in long-term plasticity, which is the fundamental mechanism in learning and memory (Chen et al., 2006; Frick et al., 2004). Given that IA contributes significantly to dampen neuronal excitability, disruptions in IA play a critical role in epileptogenesis. It is well known that seizures can be induced by selective pharmacological blockers of IA, for example 4-AP (Gandolfo et al., 1989). Disruptions of IA have been described in many nonhuman animal models of epilepsy (Bernard et al., 2004; Lugo et al., 2008; Monaghan et al., 2008) and in human temporal lobe epilepsy (TLE) (Singh et al., 2006). In addition, it has been reported that Kv4.2-/- mice demonstrate a decreased seizure and status epilepticus latency compared to wild-type mice in the kainic acid model of epilepsy (Barnwell et al., 2009). In the present study, we investigate the alterations of IA in the hippocampus of the rat brain at 1 and 8 weeks following controlled cortical impact (CCI). Our results indicate that IA is suppressed in CA1 pyramidal neurons of the hippocampus in the early stage of TBI.
Methods
Animals
Adult male Sprague–Dawley rats (250–300g) were used in the study. The rats were purchased from Harlan (Indianapolis, IN) and housed in pairs with a 12-h light/dark cycle. All rats had access to food and water ad libitum throughout the study. Kv4.2-/- mice were originally obtained from Dr. Jeanne Nerbonne, Washington University Medical School (Guo et al., 2005). Experimental protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery
TBI was induced using the CCI model (Dixon et al., 1991). The rats were anesthetized with a mixture of 1-2% isoflurane (Butler, Dublin, OH) in 33% O2 and 66% N2 via a nasal mask, and the volume of gas was adjusted to maintain the PCO2 and PO2 at ∼40 and 120 mm Hg, respectively. The rat's head was placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). A 7-mm craniotomy was performed via a midline incision with a dental drill between lambda and bregma and centered over the right frontoparietal cortex lateral to the central suture. The dura was kept intact over the cortex. The impact device (Benchmark Stereotaxic Impactor; Myneurolab, St. Louis, MO) was mounted on the right side at an angle of 25° from vertical. Rats were subjected to a right frontoparietal cortex impact with a velocity of 4.0 m/sec, tissue deformation of 2.5 mm, and impact duration of 100 ms with a 5-mm impactor tip. A total of 69 rats were injured. Experiments were performed at 1 and 8 weeks after TBI.
Western blotting
Brain slices were prepared using procedures similar to those previously described (Deng et al., 2009). Briefly, the animals were anesthetized with ketamine-HCl (80 mg/kg, intraperitoneally) and decapitated. The brains were quickly removed and immersed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 130 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose (pH 7.4, 295-305 mOsm/L). Transverse hippocampus slices of 400-μm thickness were cut using a vibratome (VT 1000; Leica, Nussloch, Germany). Subsequently, the regions of CA1 and CA3 were microdissected under a surgical microscope (Bausch & Lomb, Rochester, NY) and frozen in liquid nitrogen. Tissues were lysed with ice-cold radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]; Boston BioProducts, Worcester, MA) supplemented with a protease inhibitor cocktail (Roche, Indianapolis, IN) and incubated an additional 30 min on ice. After brief sonication on ice, cell lysates were centrifuged at 12,000g for 20 min at 4°C to pellet nuclei and debris, and the resulting supernatants were collected for analysis. Protein concentration was determined by BCA protein assay (Bio-Rad, Hercules, CA). Protein samples were boiled in 2 × SDS gel-loading buffer (Invitrogen, Carlsbad, CA) prior to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins (20 μg) were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes (Millipore, Bedford, MA). The membranes were rinsed with distilled water, blocked with 1% bovine serum albumin (BSA; Sigma, St Louis, MO) in TBS-0.1% Tween 20 (TBST) for 1 h, and then incubated with primary antibodies overnight in blocking buffer at 4°C. We used rabbit polyclonal anti-Kv4.2 (1:1,000; Chemicon, Temecula, CA) or mouse monoclonal anti-β-actin antibodies (1:20,000; Sigma). The membranes were washed with TBST, and incubated at room temperature for 1 h with horseradish peroxidase (HRP)-conjugated anti-rabbit (1:5,000; Chemicon) or anti-mouse secondary antibodies (1:20,000; Chemicon). Bands were detected by the enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ) and visualized by exposing the membrane to X-ray films (Fuji, Tokyo, Japan). Band densitometry analysis of the membrane was performed using scanned images of unsaturated immunoblot films, using NIH ImageJ 1.37 analysis software (Lei et al., 2010).
The Western analyses were performed as described in our previous studies (Lei et al. 2008, 2010). The following protocol was used for experimental treatments. Each gel that we used in the experiments had 10 wells. The first well was loaded with protein marker. The left nine wells were loaded in sequence with control (sample 1), TBI-contralateral (sample 1), TBI-ipsilateral (sample 1), control (sample 2), TBI-contralateral (sample 2), TBI-ipsilateral (sample 2), control (sample 3), TBI-contralateral (sample 3), TBI-ipsilateral (sample 3). It was not possible to analyze all six different samples of each group on one Western blot; therefore, we analyzed them using two blots (three samples per blot). We normalized all Western signals of Kv4.2 to those of β-actin and then expressed all values as “percent of control” using the controls from a specific blot to normalize only those signals from the same blot. This provided us with a series of “percent of control” values for control, TBI-contralateral, and TBI-ipsilateral groups.
Immunocytochemical staining
The rats were deeply anesthetized and perfused through the ascending aorta with a solution of phosphate-buffered saline (PBS) (0.01 M, pH 7.4) for ∼5 min, followed by 4% paraformaldehyde in PBS for 20–30 min. Brains were removed and postfixed in 4% paraformaldehyde at 4°C overnight. Sets of coronal sections containing the hippocampus were cut (40 μm) with a vibratome (Technical Products International, St. Louis, MO) and collected in PBS. One set of sections was stained with hematoxylin & eosin (H&E, Fisher Scientific, Pittsburg, PA). The sections from control and TBI groups were stained together in each immunohistochemical session. After rinse 3×5 min in PBS, the sections were incubated for 30 min in 0.3% H2O2 diluted in PBS to quench the endogenous peroxidase activity. Subsequently, sections were blocked and permeabilized in permeabilization solution (10% goat or horse serum, 0.1% Triton X-100 in PBS) for 1 h at room temperature. Thereafter, the sections were incubated with rabbit polyclonal anti-Kv4.2 antibody (1:1,000; Chemicon), or mouse monoclonal anti-Kv4.3 antibody (1:1,000; UC Davis/NIH NeuroMab Facility, Davis, CA) in permeabilization solution overnight at 4°C. Following overnight incubation in the primary antibody, the sections were washed in PBS for 3×10 min, incubated with biotinylated goat anti-rabbit or horse anti-mouse IgG (1:200; Vector Laboratories, Temecula, CA) in blocking solution (10% goat or horse serum in PBS) for 1 h at room temperature. Following incubation, the sections were then washed 3×10 min in PBS and processed according to the avidin–biotin–peroxidase complex method by using Vector kits (Vector Laboratories). After rinses 3×10 min in PBS, immunoreactivity was visualized using 0.05% diaminobenzidine tetrahydrochloride (DAB; Sigma) in PBS containing 0.015% H2O2 for 1–2 min at room temperature. All sections within the reaction were exposed to DAB for the exact same time. The sections were mounted onto slides, air dried, dehydrated in graded series of ethanol, infiltrated in xylene, and embedded in paraffin. The slides were then examined with a microscope (BX50; Olympus, Tokyo, Japan). Images were acquired with a digital camera coupled to control software (DP70-BSW; Olympus) at 40 and 200 × magnification. The settings were kept constant throughout all experiments.
Electrophysiological recording
Hippocampal slices were prepared as described previously and then incubated in ACSF (continuously equilibrated with 95% O2-5% CO2) for >1 h at room temperature (∼24°C) before being transferred to the recording chamber. Recording electrodes were prepared from borosilicate glass (Warner Instruments, Hamden, CT) using a horizontal electrode puller (Sutter Instruments, Novato, CA). Electrodes had resistance of 2-4 MΩ when filled with an intracellular solution containing (in mM): 120 KMeSO4, 12 KCl, 1 MgCl2, 1 ethylene glycol tetraacetic acid (EGTA), 0.2 CaCl2, 10 HEPES, and 2 Mg-ATP, pH 7.3, 290-295 mOsm/L. Oxygenated ACSF was used as extracellular solution and the flow rate was adjusted to 2–3 mL/min. Recordings were carried out at room temperature. Cells were visualized with an infrared-differential interference contrast microscope (BX50WI; Olympus) and a CCD camera. Whole-cell patch-clamp recordings were performed with an Axopatch 200B amplifier (Molecular Devices, Palo Alto, CA). After tight-seal (>1 GΩ) formation, the electrode capacitance was compensated. The membrane capacitance, series resistance and input resistance of the recorded neurons were measured by applying a 5 mV (10 ms) hyperpolarizing voltage pulse from a holding potential of −60 mV. The series resistance was 8-15 MΩ. Cells with a series resistance >10% of the input resistance was discarded. The membrane capacitance reading was used as the value for whole cell capacitance. During the experiment, the membrane capacitance and series resistance were periodically monitored. Cells with a series resistance change >20% during the experiment were excluded from the analysis. Signals were filtered at 2 kHz and digitized at a sampling rate of 5 kHz using a data-acquisition program (Axograph 4.6; Molecular Devices).
At a holding potential of −60 mV, the voltage-dependent outward potassium currents were evoked by voltage steps (from −80 mV to +70 mV in 10 mV increments, 400 ms) following a 300 ms hyperpolarizing pulse of −120 mV in the presence of TTX (1 μM) and CdCl2 (300 μM) to block voltage-activated Na+ and Ca2+ currents, as well as Ca2+-activated potassium currents. Taking advantage of the rapid inactivation at depolarized membrane potential, IA was isolated by subtracting the currents evoked after depolarized pre-pulses (10 mV, 100 ms) from those evoked without depolarized pre-pulses.
The current density of IA for each neuron was obtained by dividing the membrane capacitance from current amplitude. The current amplitude of IA was measured at the peak of each current (∼4 ms after the onset of the command pulses). The steady-state activation curves were established similarly to those previously reported (Deng et al., 2004). Briefly, the conductance (G) was calculated using the following equation: G=I/(Vm−Vk), where I was the current amplitude, Vm was the command potential and Vk was the reversal potential of potassium (Vk=−98 mV). The conductance was then normalized with respect to the maximum value and plotted as a function of the membrane potential during the test pulse. The resulting activation curves were fitted with a normalized Boltzmann distribution: G/Gmax=1/[1+exp(Vm−V1/2)/Vc], where Gmax was the maximum conductance at +70 mV, V1/2 was the membrane voltage at which the current amplitude was half-maximum, and Vc was the slope factor. The steady-state inactivation properties of IA were determined by measuring the current availability with a testing pulse of +10 mV following 2-sec pre-pulse between −120 mV and 0 mV in 10 mV increments. The plot of mean normalized peak currents as a function of pre-pulse voltage was fitted with a normalized Boltzmann distribution: I/Imax=1/[1+exp(V1/2−Vm)/Vc], where Imax was the maximum current at+10 mV following the pre-pulse of −130 mV.
For whole cell current-clamp recording, the fast I-clamp mode was used. Depolarizing current pulses (800 ms, 20∼180 pA) were applied to evoke either a single firing or repetitive firings. The spike latency was measured from the onset of the current injection to the peak of the first action potential. The spike width was measured at the spike base (Deng et al., 2009).
Bicuculline-induced seizures
(+) bicuculline (Sigma) was dissolved in 0.1 N HC1 and adjusted to pH 6 - 6.5 with 1 N NaOH. Rats were injected with (+) bicuculline (2.0 mg/kg, i.p.). After injection of bicuculline, rats were placed in a clear cage. The rats' behavior was videotaped and observed for a period of 30 min. Bicuculline-induced seizures have two typical phases: clonic and tonic–clonic (Schindler et al., 2004). Clonic seizures were defined by rhythmic movements of the head and forelimbs. Tonic–clonic seizures began with wild running and jumping, and then developed into a short-lasting tensing of all muscles (tonus) and a long-lasting clonus. Clonic seizures could appear alone or followed by tonic–clonic seizures.
Data analysis
The values were presented as mean±SEM. The results were analyzed using χ2 test, student's t test or one-way ANOVA followed by post-hoc Scheffé test (StatView 5.0; Abacus Concepts, Berkeley, CA). Changes were considered significant if p<0.05.
Results
Selective neuronal death in the hippocampus after TBI
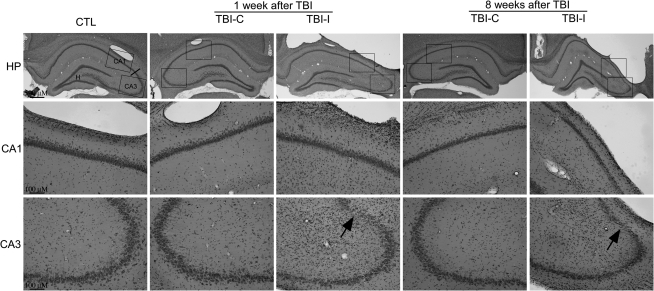
Some rats had a brief period of apnea immediately following injury. After a rapid and uneventful recovery from anesthesia, all TBI rats displayed normal exploratory, grooming, and feeding behavior in their home cages. A lesion (including cavity) was found at the temporal cortices ipsilateral to the site of impact at 1 week after CCI (Fig. 1). H&E staining indicated that a pattern of selective neuronal death in the hippocampus was evident. The 5-mm impact caused substantial neuronal injury in the CA3 region. By contrast, the morphology of the CA1 region on the ipsilateral side of the impact site was normal. At 8 weeks after TBI, there was gross tissue loss in the ipsilateral cortex and distortions in the morphology of the ipsilateral hippocampus. In addition to the neuronal loss in the CA3 region, thinning of the CA1 stratum oriens was apparent. Cortical and hippocampal tissue contralateral to the impact site revealed no discernible histological alterations at 8 weeks post-TBI.
FIG. 1.
Representative H&E photomicrographs showing cellular degeneration and loss in vulnerable hippocampal regions at 1 and 8 weeks after TBI. Low power (upper panel, original magnification x40) and higher power (lower two panels, original magnification x 200) photomicrographs illustrated a cavitary cortical lesion and significant neuronal loss in the CA3 region of the ipsilateral hippocampus (TBI-I) at 1 and 8 weeks after TBI. Compared with control (CTL), there was no obvious neuronal loss in the ipsilateral CA1 region and the contralateral hippocampus (TBI-C) by 8 weeks after TBI. Arrows indicate regions undergoing cell death. Note an obvious thinning of the CA1 stratum oriens in the ipsilateral hippocampus at 8 weeks post-TBI.
Kv4.2 expression is altered after TBI
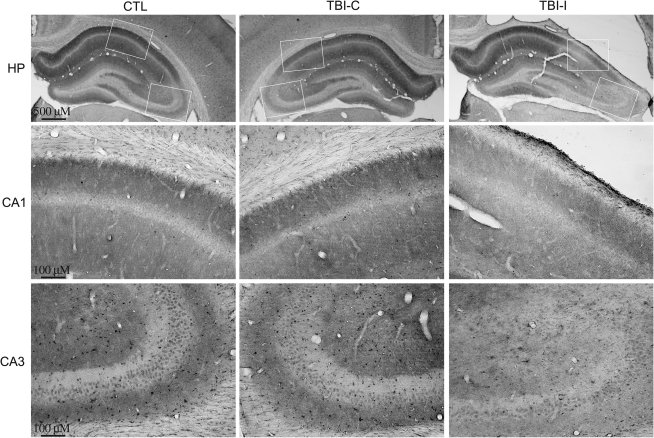
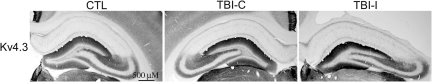
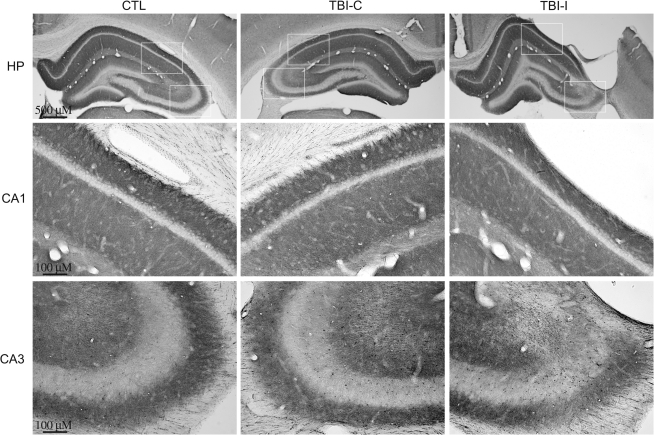
To examine the patterns of IA channel expression after TBI, immunohistochemistry was performed with subunit-specific antibodies. Kv4.2 is the major subunit giving rise to somatodendritic IA currents in hippocampal neurons. In the control hippocampus, Kv4.2 was found on the soma and dendrites of pyramidal neurons in the CA1 and CA3 regions and dentate granule cells (Fig. 2). At 1 week after TBI, a dramatic decrease of Kv4.2 immunoreactivity was observed in the ipsilateral CA1 region under the impact site. Intensity of Kv4.2 immunostaining was decreased mainly in dendritic layers (i.e., stratum radiatum, lucidum, and oriens) of the ipsilateral CA1 region. Meanwhile, a profound reduction of Kv4.2 immunoreactivity was found in the CA3 region of the ipsilateral hippocampus. Compared with the control hippocampus, there were no obvious changes of Kv4.2 immunoreactivity in the contralateral hippocampus. Kv4.3 is another important subunit responsible for somatodendritic IA currents in hippocampal neurons. In the control hippocampus, immunoreactivity for Kv4.3 is concentrated in CA3 pyramidal cell dendrites, as well as in the somata and dendrites of interneurons throughout the hippocampus. In contrast to Kv4.2, Kv4.3 immunoreactivity remained unchanged in the hippocampus at 1 week after TBI (Fig. 3). To investigate the temporal profile of alteration of Kv4.2 expression after TBI, immunoreactivity of Kv4.2 was further examined at 8 weeks after TBI. The Kv4.2 immunoreactivity in the ipsilateral CA1 region returned to control levels at 8 weeks post-TBI (Fig. 4). However, the reduction of Kv4.2 immunoreactivity in the ipsilateral CA3 region was still observed at 8 weeks after TBI.
FIG. 2.
Kv4.2-immunoreactive profiles in the hippocampus of control and TBI rats at 1 week after TBI. Photomicrographs of horizontal sections were taken to show the distribution of Kv4.2 in the hippocampus of a control rat, and rat 1 week post-TBI. Kv4.2 immunoreactivity was dramatically decreased in the ipsilateral CA1 and CA3 regions (TBI-I), compared with the control CA1 and CA3 regions (CTL). The reduction of Kv4.2 immunoreactivity was predominately in dendritic layers (i.e., stratum radiatum, lucidum, and oriens) of the ipsilateral CA1 region. There were no obvious changes of Kv4.2 immunoreactivity in the contralateral CA1 and CA3 regions (TBI-C).
FIG. 3.
Kv4.3-immunoreactive profiles in the hippocampus of control and TBI rats at 1 week after TBI. Photomicrographs of horizontal sections were taken to show the distribution of Kv4.3 in the hippocampus of a control rat, and rat 1 week post-TBI. Kv4.3 immunoreactivity was unchanged in both the ipsilateral (TBI-I) and contralateral (TBI-C) hippocampus, compared with the control hippocampus (CTL).
FIG. 4.
Kv4.2-immunoreactive profiles in the hippocampus of control and TBI rats at 8 weeks after TBI. Photomicrographs of horizontal sections were taken to show the distribution of Kv4.2 in the hippocampus of a control rat, and rat 8 weeks post-TBI. In the ipsilateral hippocampus (TBI-I), Kv4.2 immunoreactivity in CA1 recovered to control levels at 8 weeks after injury, whereas Kv4.2 immunoreactivity in CA3 still remained at lower levels. There were no obvious changes of Kv4.2 immunoreactivity in the contralateral hippocampus (TBI-C) at 8 weeks after TBI.
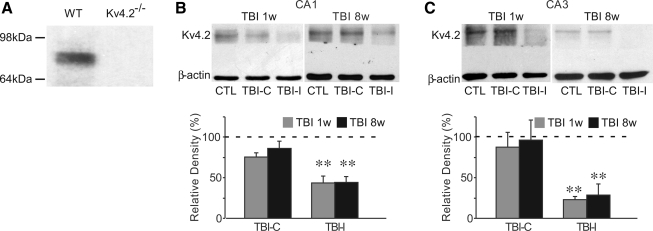
For quantization of protein expression, Western blotting analysis was performed on hippocampi from control and TBI rats. The Kv4.2 band recognized by the polyclonal anti-Kv4.2 antibody was at 75 kDa (Fig. 5). In order to discriminate the specificity of the antibody for Kv4.2, brain extracts derived from Kv4.2-/- mice were used. The Kv4.2 antibody completely failed to recognize its 75-kDa product in Kv4.2-/- extracts (Fig. 5A). These data provided definitive proof of Kv4.2 antibody specificity. At 1 week after TBI, consistent with immunohistochemical results, Western blotting analysis indicated a dramatic reduction of total Kv4.2 protein levels in the ipsilateral CA1 (43.6±8.4% of control, n=6, p<0.01) and CA3 (22.9±3.6% of control, n=6, p<0.01), but not in the contralateral CA1 (75.4±5.5% of control, n=6, p>0.05) and CA3 (88.0±17.9% of control, n=6, p>0.05). The total Kv4.2 remained at the lower levels in the ipsilateral hippocampus at 8 weeks after TBI (Fig. 5). The total Kv4.2 levels were 44.2±7.3% of control (n=6, p<0.01) in the ipsilateral CA1 region, and 28.3±14.0% of control (n=6, p<0.01) in the ipsilateral CA3 region.
FIG. 5.
Western blotting of Kv4.2 in the hippocampus of control and TBI rats at 1 and 8 weeks after TBI. Brain extracts from Kv4.2−/− mice confirmed the Kv4.2 antibody specificity (A). Western blotting using polyclonal rabbit anti-Kv4.2 antibody indicated the absence of Kv4.2 protein (Mw ∼75 kDa) in brain extracts from Kv4.2−/− mice. The CA1 and CA3 regions were dissected at 1 and 8 weeks after TBI. Total Kv4.2 protein levels were significantly reduced within the CA1 (B) and CA3 (C) regions from the ipsilateral hippocampus (TBI-I), which was still observed at 8 weeks after injury. There was no significant difference in total Kv4.2 protein levels within isolated CA1 and CA3 regions from the contralateral hippocampus (TBI-C). β-actin worked as a loading control. Blots are representative of six independent experiments. Data are presented as mean±SEM. Statistical analysis was performed by one way ANOVA. ** p<0.01 versus control (CTL).
TBI induced a decrease of IA currents and an increase of neuronal excitability
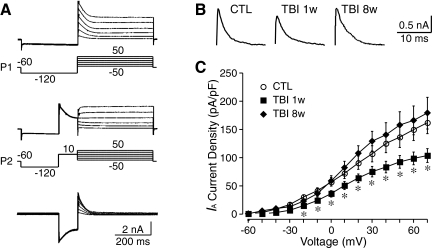
Kv4.2 underlies the majority of somatodendritic IA currents in CA1 pyramidal neurons. Deletion of the Kv4.2 gene and a loss of Kv4.2 protein result in a near-complete elimination of somatodendritic IA currents in CA1 pyramidal neurons (Chen, et al., 2006). To address whether TBI altered IA currents, we compared IA currents in CA1 pyramidal neurons from control and TBI rats. The transient IA was separated from the sustained Ikd by taking advantage of the different voltage dependence of these types of K+ currents. To evoke total outward K+ currents including IA, membrane potential was held at −60 mV and stepped voltage command pulses (−80 to +70 mV, 10mV/step, 400 ms) were applied following a conditioning voltage step (−120 mV, 300 ms, protocol 1). IA was inactivated by a 100-ms pre-pulse at +10 mV (protocol 2). IA currents were isolated by subtracting Ikd evoked by protocol 2 from the total K+ currents evoked by protocol 1 (Fig. 6A). As shown in Figure 6B and C, IA currents were significantly decreased in CA1 pyramidal neurons from the ipsilateral hippocampus at 1 week post-TBI. The current density of IA in CA1 pyramidal neurons was 106.5±14.3 pA/pF (n=10, at +30 mV) in control and 74.2±9.1 pA/pF (n=13, p<0.05) in TBI neurons. The current density of IA returns to 128.8±17.4 pA/pF (n=5, p>0.05) at 8 weeks after TBI. TBI had no influence on the voltage-dependent kinetics of IA currents (data not shown).
FIG. 6.
Changes of IA currents in CA1 pyramidal neurons at 1 and 8 weeks after TBI. (A) Representative traces of K+ currents recorded from CA1 pyramidal neurons. IA was isolated by the subtraction of the currents evoked by voltage protocol 2 (P2) from those evoked by protocol 1 (P1). (B) Representative traces of IA of CA1 pyramidal neurons from control (CTL) and the ipsilateral hippocampus at 1 (TBI 1w) and 8 (TBI 8w) weeks after TBI. (C) Pooled data showing the significant decrease of the current density of IA in CA1 pyramidal neurons at 1 week after TBI, which returned to the control levels at 8 weeks after TBI. Data are presented as mean±SEM. Statistical analysis was performed by one way ANOVA. *p<0.05 versus control.
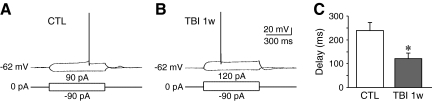
Because IA is important for regulating neuronal excitability, we compared the voltage responses to current injection of CA1 pyramidal neurons from control and TBI rats at 1 week after injury when IA was reduced. We found that TBI neurons exhibited a significant decrease in the delay of the first action potential (control: 238.3±34.6 ms, n=7; TBI: 130.0±24.0 ms, n=7, p<0.05, Fig. 7). These data indicate that TBI leads to an increased of excitability in CA1 pyramidal neurons.
FIG. 7.
Alterations of neuronal excitability in CA1 pyramidal neurons at 1 week after TBI. (A) Representative traces showing the voltage responses to current pulses in control neurons (CTL). (B) Representative traces showing the voltage responses to current pulses in neurons at 1 week after TBI (TBI 1w). (C) Pooled data showing that TBI led to a decrease of the latency to the first spike. Data are presented as mean±SEM. Statistical analysis was performed by one way ANOVA. *p<0.05 versus control.
Susceptibility to bicuculline-induced seizures is increased at 1 week after TBI
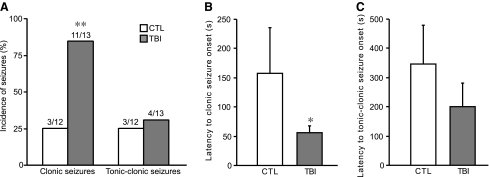
TBI rats did not develop spontaneous behavioral seizures up to 8 weeks after injury. However, TBI increased the susceptibility to bicuculline-induced seizures when tested at 1 week after surgery. As shown in Figure 8A, bicuculline at a dose of 2.0 mg/kg (i.p.) produced a significantly higher incidence of clonic seizures in TBI rats than in the control ones (84.6% vs. 25%, p<0.01). Furthermore, the latency of clonic seizures was significant decreased in TBI rats (56.2±11.8 sec, n=11), as compared with control rats (157.7±78.1 sec, n=3, p<0.05, Fig. 8B). There was a tendency of decrease of the latency of clonic–tonic seizures in TBI rats (control: 347.3±132.2 sec, n=3; TBI: 201.8±78.9 sec, n=4, Fig. 8C), despite that the difference was not statistically significant.
FIG. 8.
Susceptibility to seizures induced by bicuculline at 1 week after TBI. (A) Occurrence rate of clonic and tonic–clonic seizures induced by 2.0 mg/kg (+)-bicuculline. TBI significantly increased occurrence rate of bicuculline-induced clonic seizures. (B) Latency to clonic seizure onset. TBI rats showed a dramatic decrease of latency to clonic seizure onset. (C) Latency to tonic–clonic seizure onset. A decrease of clonic–tonic seizure latency was observed in TBI rats, although no statistical significance was detected. Data are presented as mean±SEM. Statistical analysis was performed by χ2test (A) or Student's t test (B and C). *p<0.05, **p<0.01 versus control (CTL).
Discussion
The principal findings of the present study are that TBI leads to a significant reduction in Kv4.2 expression and IA currents in the hippocampus at 1 week post-injury. The decrease of IA is associated with an increase of excitability of CA1 pyramidal neurons, which might contribute, at least in part, to the seizures/epilepsy following TBI.
In the present study, results from both immunohistochemical staining and whole-cell recording indicated that IA in CA1 neurons was decreased 1 week after TBI, but returned to the control levels by 8 weeks. Whereas the Western blotting data showed a sustained reduction of Kv4.2 protein levels up to 8 weeks after TBI. The discrepancy between the Western blotting data and the immunohistochemical and electrophysiological data might be the result of the post-translational modification after the insult and/or the substitution of other subunits that contribute to the IA currents. In addition to Kv4.2, other Kv α-subunits, including Kv1.4, Kv4.1, and Kv4.3, can give rise to IA currents (Serodio et al., 1994, 1996). It has been reported that an upregulation of Kv1 subunits in CA1 pyramidal neurons compensates for the lack of Kv4.2 in knockout mice (Chen et al., 2006). Therefore, the substitution of other Kv α-subunits, such as Kv1.4, for the loss of Kv4.2, might be responsible for the recovery of IA currents in CA1 neurons at 8 weeks after TBI, but fail to be detected by the Western blotting technique. In the CA3 region, both immunochemical staining and Western blotting studies showed a long-lasting reduction of Kv4.2 expression levels. The decreased expression of Kv4.2 in CA3 might be attributable to the long-term brain malfunction. In contrast to Kv4.2, Kv4.3 expression levels in CA3 were unchanged after TBI. One possible explanation is that Kv4.2 and Kv4.3 have different sensitivity to insults. It is well known that the synthesis and degradation of different ion channel subunits have different responses to brain injuries. For example, Kv1.2 and Kv4.2 mRNAs are reduced following seizures, whereas Kv1.1 mRNA remains unchanged (Tsaur et al., 1992). It has also been shown that the Kv4.2 subunit is selectively reduced in glutamate-induced toxicity (Lei et al., 2010).
The underlying mechanisms of the neuronal hyperexcitability following brain injury are not fully understood. In the central nervous system (CNS), IA plays a central role in dampening neuronal excitability. By limiting excitability of individual neurons, IA may prevent hyperexcitability of the nervous system as a whole. Kv4.2 is widely believed to be the molecular counterpart to the somatodendritic IA in hippocampal neurons. The inhibition of somatodendritic IA and the deficiency of Kv4.2 facilitate the generation of first action potential and increase the burst firing probability (Andrasfalvy et al., 2008; Kim et al. 2005). Therefore, the reductions of IA might be responsible for the post-traumatic hyperexcitability in CA1 pyramidal neurons. Consistent with our finding, a recent study also reported a transient increase of excitability of CA1 pyramidal neurons after TBI (Griesemer and Mautes, 2007).
TBI is recognized as a major cause of epilepsy in the young, adults, and the elderly (Agrawal et al., 2006). Clinic studies indicate that 35–62% of patients with post-traumatic epilepsy (PTE) have TLE (Diaz-Arrastia et al., 2000; Hudak et al., 2004). Animal studies have also shown spontaneous seizures identified using video-ECoG/EEG recordings (D'Ambrosio et al., 2009; Kharatishvili and Pitkanen, 2010; Nilsson et al., 1994). An increased seizure susceptibility to chemical convulsants, such as bicuculline, has been reported in hippocampal slices from TBI rats (Santhakumar et al., 2001). It is also reported that TBI results in a persistently increased susceptibility to pentylenetetrazole-induced seizures in vivo (Golarai et al., 2001). Likewise, we observed a reduced seizure threshold to bicuculline-evoked behavioral seizures at 1 week after TBI in this study. As discussed previously, IA channels contribute significantly to the regulation of neuronal excitability; therefore, disruptions in IA are involved in the development of epileptic seizures. Indeed, Kv4.2 truncation mutation lacking the last 44 amino acids in the carboxyl terminal is reported in a patient with TLE (Singh et al., 2006). In addition, the physiological relevance of disruption of IA to seizures and epilepsy has been explored in various experimental animal studies. For example, seizure activity causes a reduction of Kv4.2 mRNAs in the dentate granule cells of the hippocampus in pentylenetetrazole-treated rats (Tsaur et al., 1992). A reduction in Kv4.2 mRNA levels is also observed in dentate granule cells at 3 h post-seizure in the rat kainic acid epilepsy model (Francis et al., 1997). Moreover, an immunohistochemistry study reports altered expression and localization of hippocampal IA channels (Kv1.4, Kv4.2, Kv4.3, KChIPl, and KChIP2) 4 and 12 weeks after pilocarpine treatment (Monaghan et al., 2008). With the use of whole-cell dendritic recording in hippocampal CA1 pyramidal neurons, it is found that the excitability of hippocampal neuron dendrites is increased because of a decreased availability of IA channels in the rat pilocarpine model of TLE (Bernard et al., 2004). In addition to the changes of intrinsic neuronal excitability, other mechanisms have been proposed to be associated with the occurrence of seizures after TBI. These mechanisms include mossy fiber sprouting (Golarai et al., 2001; Kharatishvili et al., 2006) and alterations in the excitatory and inhibitory networks (Santhakumar et al., 2001; van den Pol et al., 1996).
The pathophysiological sequelae following TBI are believed to be mediated by overactivation of NMDA receptors and sequential Ca2+ overload (Arundine and Tymianski, 2004; Nadler et al., 1995). Calpain is a neutral, Ca2+-activated protease that regulates numerous downstream targets and is linked to pathological conditions including TBI (Goll et al., 2003). Our previous studies have described a NMDA and Ca2+ dependent downregulation of Kv4.2 and IA (Lei, et al. 2008). In addition, the downregulation of Kv4.2 and IA induced by glutamate is mediated by the activation of NR2B-containing NMDA receptors and calpain proteolysis (Lei et al., 2010). Intriguingly, a transient activation of calpain is also noted in the hippocampus after CCI (Pike et al., 1998). At 24 h post-CCI, a fivefold increase in the calpain activity is evident in the ipsilateral hippocampus, which is maintained up to 1 week and returns to near-basal levels at 2 weeks post-CCI. This time course of calpain activation in the hippocampus post-CCI coincides with the changes of IA observed in the present study. Therefore, it is likely that increased calpain-mediated degradation of Kv4.2 after TBI in the hippocampus might contribute to the downregulation of IA in hippocampal neurons.
Acknowledgments
This work was supported by grants from National Institute of Neurological Disorders and Stroke (NINDS) NS071238, American Heart Association (AHA) 10GRNT4500000, and Indiana Spinal Cord and Brain Injury Research Fund ISDH/A70-0-079212.
The monoclonal antibody Kv4.3 was obtained from the University of California, Davis/National Institutes of Health (NIH) NeuroMab Facility, supported by NIH grant U24NS050606 and maintained by the Department of Neurobiology, Physiology and Behavior, College of Biological Sciences, University of California, Davis, CA 95616.
Author Disclosure Statement
No competing financial interests exist.
References
- Agrawal A. Timothy J. Pandit L. Manju M. Post-traumatic epilepsy: an overview. Clin. Neurol. Neurosurg. 2006;108:433–439. doi: 10.1016/j.clineuro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- An W.F. Bowlby M.R. Betty M. Cao J. Ling H.P. Mendoza G. Hinson J.W. Mattsson K.I. Strassle B.W. Trimmer J.S. Rhodes K.J. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Andrasfalvy B.K. Makara J.K. Johnston D. Magee J.C. Altered synaptic and non-synaptic properties of CA1 pyramidal neurons in Kv4.2 knockout mice. J. Physiol. 2008;586:3881–3892. doi: 10.1113/jphysiol.2008.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M. Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol. Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales J.W. Wagner A.K. Kline A.E. Dixon C.E. Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci. Biobehav. Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell L.F. Lugo J.N. Lee W.L. Willis S.E. Gertz S.J. Hrachovy R.A. Anderson A.E. Kv4.2 knockout mice demonstrate increased susceptibility to convulsant stimulation. Epilepsia. 2009;50:1741–1751. doi: 10.1111/j.1528-1167.2009.02086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C. Anderson A. Becker A. Poolos N.P. Beck H. Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- Bruns J., Jr. Hauser W.A. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl. 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- Cai X. Liang C.W. Muralidharan S. Kao J.P. Tang C.M. Thompson S.M. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Chen X. Yuan L.L. Zhao C. Birnbaum S.G. Frick A. Jung W.E. Schwarz T.L. Sweatt J.D. Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J. Neurosci. 2006;26:12,143–12,151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R. Hakimian S. Stewart T. Verley D.R. Fender J.S. Eastman C.L. Sheerin A.H. Gupta P. Diaz–Arrastia R. Ojemann J. Miller J.W. Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain. 2009;132:2805–2821. doi: 10.1093/brain/awp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R. Maris D.O. Grady M.S. Winn H.R. Janigro D. Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J. Neurosci. 1999;19:8152–8162. doi: 10.1523/JNEUROSCI.19-18-08152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R. Perucca E. Epilepsy after head injury. Curr. Opin. Neurol. 2004;17:731–735. doi: 10.1097/00019052-200412000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P. Pang Z. Zhang Y. Xu Z.C. Developmental changes of transient potassium currents in large aspiny neurons in the neostriatum. Brain Res. Dev. Brain Res. 2004;153:97–107. doi: 10.1016/j.devbrainres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Deng P. Pang Z.P. Lei Z. Xu Z.C. Excitatory roles of protein kinase C in striatal cholinergic interneurons. J. Neurophysiol. 2009;102:2453–2461. doi: 10.1152/jn.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz–Arrastia R. Agostini M.A. Frol A.B. Mickey B. Fleckenstein J. Bigio E. Van Ness P.C. Neurophysiologic and neuroradiologic features of intractable epilepsy after traumatic brain injury in adults. Arch. Neurol. 2000;57:1611–1616. doi: 10.1001/archneur.57.11.1611. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Francis J. Jugloff D.G. Mingo N.S. Wallace M.C. Jones O.T. Burnham W.M. Eubanks J.H. Kainic acid-induced generalized seizures alter the regional hippocampal expression of the rat Kv4.2 potassium channel gene. Neurosci. Lett. 1997;232:91–94. doi: 10.1016/s0304-3940(97)00593-4. [DOI] [PubMed] [Google Scholar]
- Frick A. Magee J. Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat. Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Gandolfo G. Gottesmann C. Bidard J.N. Lazdunski M. Subtypes of K+ channels differentiated by the effect of K+ channel openers upon K+ channel blocker-induced seizures. Brain Res. 1989;495:189–192. doi: 10.1016/0006-8993(89)91236-5. [DOI] [PubMed] [Google Scholar]
- Golarai G. Greenwood A.C. Feeney D.M. Connor J.A. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J. Neurosci. 2001;21:8523–8537. doi: 10.1523/JNEUROSCI.21-21-08523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll D.E. Thompson V.F. Li H. Wei W. Cong J. The calpain system. Physiol. Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Griesemer D. Mautes A.M. Closed head injury causes hyperexcitability in rat hippocampal CA1 but not in CA3 pyramidal cells. J. Neurotrauma. 2007;24:1823–1832. doi: 10.1089/neu.2006.0237. [DOI] [PubMed] [Google Scholar]
- Guo W. Jung W.E. Marionneau C. Aimond F. Xu H. Yamada K.A. Schwarz T.L. Demolombe S. Nerbonne J.M. Targeted deletion of Kv4.2 eliminates I(to,f) and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ. Res. 2005;97:1342–1350. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- Hicks R. Soares H. Smith D. McIntosh T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol. 1996;91:236–246. doi: 10.1007/s004010050421. [DOI] [PubMed] [Google Scholar]
- Hudak A.M. Trivedi K. Harper C.R. Booker K. Caesar R.R. Agostini M. Van Ness P.C. Diaz–Arrastia R. Evaluation of seizure-like episodes in survivors of moderate and severe traumatic brain injury. J. Head Trauma Rehabil. 2004;19:290–295. doi: 10.1097/00001199-200407000-00003. [DOI] [PubMed] [Google Scholar]
- Jerng H.H. Pfaffinger P.J. Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol. Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I. Nissinen J.P. McIntosh T.K. Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I. Pitkanen A. Association of the severity of cortical damage with the occurrence of spontaneous seizures and hyperexcitability in an animal model of posttraumatic epilepsy. Epilepsy Res. 2010;90:47–59. doi: 10.1016/j.eplepsyres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Kim J. Wei D.S. Hoffman D.A. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J. Physiol. 2005;569:41–57. doi: 10.1113/jphysiol.2005.095042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z. Deng P. Li Y. Xu Z.C. Downregulation of Kv4.2 channels mediated by NR2B-containing NMDA receptors in cultured hippocampal neurons. Neuroscience. 2010;165:350–362. doi: 10.1016/j.neuroscience.2009.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z. Deng P. Xu Z.C. Regulation of Kv4.2 channels by glutamate in cultured hippocampal neurons. J. Neurochem. 2008;106:182–192. doi: 10.1111/j.1471-4159.2008.05356.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein D.H. Thomas M.J. Smith D.H. McIntosh T.K. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo J.N. Barnwell L.F. Ren Y. Lee W.L. Johnston L.D. Kim R. Hrachovy R.A. Sweatt J.D. Anderson A.E. Altered phosphorylation and localization of the A-type channel, Kv4.2 in status epilepticus. J. Neurochem. 2008;106:1929–1940. doi: 10.1111/j.1471-4159.2008.05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney R.A. Debanne D. Gahwiler B.H. Thompson S.M. Lesion-induced axonal sprouting and hyperexcitability in the hippocampus in vitro: implications for the genesis of posttraumatic epilepsy. Nat. Med. 1997;3:990–996. doi: 10.1038/nm0997-990. [DOI] [PubMed] [Google Scholar]
- Millis S.R. Rosenthal M. Novack T.A. Sherer M. Nick T.G. Kreutzer J.S. High W.M., Jr. Ricker J.H. Long-term neuropsychological outcome after traumatic brain injury. J. Head Trauma Rehabil. 2001;16:343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Monaghan M.M. Menegola M. Vacher H. Rhodes K.J. Trimmer J.S. Altered expression and localization of hippocampal A-type potassium channel subunits in the pilocarpine-induced model of temporal lobe epilepsy. Neuroscience. 2008;156:550–562. doi: 10.1016/j.neuroscience.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal M.S. Ozaita A. Amarillo Y. Vega–Saenz de Miera E. Ma Y. Mo W. Goldberg E.M. Misumi Y. Ikehara Y. Neubert T.A. Rudy B. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37:449–461. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- Nadler V. Biegon A. Beit–Yannai E. Adamchik J. Shohami E. 45Ca accumulation in rat brain after closed head injury; attenuation by the novel neuroprotective agent HU-211. Brain Res. 1995;685:1–11. doi: 10.1016/0006-8993(95)00367-y. [DOI] [PubMed] [Google Scholar]
- Nilsson P. Ronne–Engstrom E. Flink R. Ungerstedt U. Carlson H. Hillered L. Epileptic seizure activity in the acute phase following cortical impact trauma in rat. Brain Res. 1994;637:227–232. doi: 10.1016/0006-8993(94)91237-8. [DOI] [PubMed] [Google Scholar]
- Pike B.R. Zhao X. Newcomb J.K. Posmantur R.M. Wang K.K. Hayes R.L. Regional calpain and caspase-3 proteolysis of alpha-spectrin after traumatic brain injury. Neuroreport. 1998;9:2437–2442. doi: 10.1097/00001756-199808030-00002. [DOI] [PubMed] [Google Scholar]
- Rettig J. Heinemann S.H. Wunder F. Lorra C. Parcej D.N. Dolly J.O. Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Santhakumar V. Ratzliff A.D. Jeng J. Toth Z. Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann. Neurol. 2001;50:708–717. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- Santhakumar V. Voipio J. Kaila K. Soltesz I. Post-traumatic hyperexcitability is not caused by impaired buffering of extracellular potassium. J. Neurosci. 2003;23:5865–5876. doi: 10.1523/JNEUROSCI.23-13-05865.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C.J. Slamberova R. Vathy I. Cholera toxin B decreases bicuculline seizures in prenatally morphine- and saline-exposed male rats. Pharmacol. Biochem. Behav. 2004;77:509–515. doi: 10.1016/j.pbb.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Serodio P. Kentros C. Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. J. Neurophysiol. 1994;72:1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- Serodio P. Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J. Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- Serodio P. Vega–Saenz de Miera E. Rudy B. Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. J. Neurophysiol. 1996;75:2174–2179. doi: 10.1152/jn.1996.75.5.2174. [DOI] [PubMed] [Google Scholar]
- Singh B. Ogiwara I. Kaneda M. Tokonami N. Mazaki E. Baba K. Matsuda K. Inoue Y. Yamakawa K. A Kv4.2 truncation mutation in a patient with temporal lobe epilepsy. Neurobiol. Dis. 2006;24:245–253. doi: 10.1016/j.nbd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Tsaur M.L. Sheng M. Lowenstein D.H. Jan Y.N. Jan L.Y. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992;8:1055–1067. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- van den Pol A.N. Obrietan K. Chen G. Excitatory actions of GABA after neuronal trauma. J. Neurosci. 1996;16:4283–4292. doi: 10.1523/JNEUROSCI.16-13-04283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]