Abstract
Although dyspnea is frequently encountered in the palliative care setting, its optimal management remains uncertain. Clinical approaches begin with accurate assessment, as delineated in part one of this two-part series. Comprehensive dyspnea assessment, which encompasses the physical, emotional, social, and spiritual aspects of this complex symptom, guide the clinician in choosing therapeutic approaches herein presented as part two. Global management of dyspnea is appropriate both as complementary to disease-targeted treatments that target the underlying etiology, and as the sole focus when the symptom has become intractable, disease is maximally treated, and goals of care shift to comfort and quality of life. In this setting, current evidence supports the use of oral or parenteral opioids as the mainstay of dyspnea management, and of inhaled furosemide and anxiolytics as adjuncts. Nonpharmacologic interventions such as acupuncture and pulmonary rehabilitation have potential effectiveness, although further research is needed, and use of a simple fan warrants consideration given its potential benefit and minimal burden and cost.
Introduction
Dyspnea is one of the most common symptoms reported by patients with advanced disease who are nearing the end of life. Part one of this two-part series on dyspnea for the palliative care professional describes the burden and measurement of dyspnea.1 Because of its complex biopsychosocial etiology and manifestations, dyspnea presents a particularly challenging symptom to manage—yet it is one which, nonetheless, requires an evidence-based symptom management approach. An armamentarium of both restorative and global therapies is available to address the modifiable and fixed components to dyspnea. In this article, we review the goals of therapy, and the pharmacologic, nonpharmacologic, and surgical options for treating dyspnea to provide an evidence-based approach to dyspnea management in the palliative care setting.
Goals of Therapy
The management of dyspnea seeks to concurrently address the symptom while identifying and treating underlying causes. When those causes are no longer reversible, however, symptom relief becomes the main objective of therapy. In palliative care, thus, the clinician first determines whether or not the underlying disease has been maximally treated without alleviating dyspnea and, if so, focuses on the symptom itself. Global management approaches to dyspnea, with or without disease-focused interventions, are fundamental elements in the palliative care toolbox.
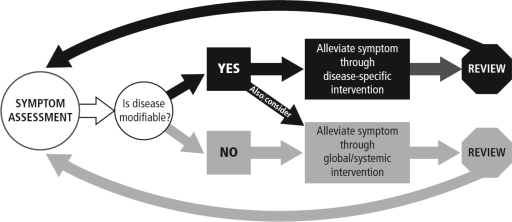
Because patients do not experience dyspnea in isolation but rather in conjunction with other symptoms, concomitant stressors, and spiritual or existential distress, dyspnea cannot be fully addressed unless these physical and nonphysical factors are understood. The clinician can set the stage for successful symptom management in the setting of advancing disease by outlining expectations for efficacy with dyspnea management, dispelling common misconceptions about dyspnea-relieving medications, and establishing a plan to continuously reevaluate the patient's dyspnea. Success is most likely when as many as possible of the patient's individual dyspnea stressors and concomitant symptoms (i.e., anxiety, depression, panic attacks) are identified and addressed. Figure 1 depicts a model for dyspnea management incorporating the principles of “total dyspnea”; the concept of total dyspnea was described in more detail in the first article in this series.
FIG. 1.
Biopsychosocial model of dyspnea management.
In this article we focus on restorative and global interventions for dyspnea management, which are intended to be used parallel to any ongoing or new disease-modifying therapies or as stand-alone therapies when modification of the underlying disease is no longer possible.
Pharmacologic Management of Dyspnea
Opioid efficacy
Opioids are the most studied and employed class of pharmacologic agents for relieving dyspnea. The effects of opioids are postulated to be secondary to their effects on ventilatory response to carbon dioxide, hypoxia, inspiratory flow resistive loading, and decreased oxygen consumption with exercise and at rest in healthy individuals. Additionally, a vasodilatory effect on pulmonary vascular pressures in animals has been demonstrated.1 Opioids have historically been used to treat anxiety and pain, which are often an integral part of the dyspnea cycle; the positive effects on these symptoms have been extensively reviewed.2 Proof-of-concept for the use of opioids in dyspnea was confirmed in a recent report of measured endogenous opioids during dyspnea. Mahler and colleagues3 showed during treadmill exercise in opioid-naïve patients with chronic obstructive pulmonary disease (COPD) the attenuation of dyspnea by endogenous, circulatory opioids and the reversal of that effect by the administration of an opioid antagonist, naloxone. The three-fold increase in endogenous opioids from rest to end-exercise suggests a mechanism by which exogenous opioids may also benefit the patient experiencing dyspnea.
Opioids, most commonly morphine, have been studied in oral, parenteral, and nebulized forms in randomized controlled trials. One systematic review and meta-analysis4 of placebo-controlled trials in dyspnea associated with any disease showed a statistically significant effect for oral or parenteral opioids only. In subgroup analysis, a positive effect of nebulized opioids was not seen, although the authors admit the available studies were of poor quality and all were very small. Two recent systematic reviews5,6 in cancer patients also looking at different modalities of opioid administration arrived at similar conclusions about the efficacy of both oral and parenteral opioids. Although one small study by Bruera and coworkers7 showed a comparable level of dyspnea relief achieved with nebulized morphine and subcutaneous morphine, seven other placebo-controlled trials have not replicated these results.
Two important clinical trials furnish the basis for recommendations for opioid dosing and titration. The single largest double-blind, controlled trial by Abernethy et al.8 enrolled 48 opioid-naïve patients with breathlessness, most of whom had COPD. In this cross-over design, participants were assigned to 20-mg once-daily sustained-release oral morphine sulfate or placebo for 4 days, followed by 4 days of the alternative. In the morphine arm, significant benefits in dyspnea and insomnia were reported. In another study in patients with prior opioid exposure, titrating to significantly higher doses (50% above baseline) conferred no additional benefit compared with increasing the dose in smaller increments (25% above baseline) for relieving persistent dyspnea.9 Thus, these authors recommend starting at 10 to 20-mg sustained-release morphine daily in divided doses, with active evaluation and gradual titration to desired effect.
Currow and colleagues recently completed a Phase II dose increment study to determine a minimum effective daily dose for opioids for dyspnea improvement and to evaluate whether or not the clinical benefit is maintained over time.10 Eighty-five participants were given escalating doses of sustained-release oral morphine, starting at 10 mg per day and increasing if they experienced less than a 10% reduction over their own baseline by 10 mg to a maximum 30 mg per day. Overall, in 65% of patients opioids reduced dyspnea by at least 10% (i.e., 65% response rate); calculated number needed to treat (NNT) was 1.5. Remarkably, for 70% of patients the beneficial dose was 10 mg per day and sustained benefit for 3 months was observed in 53% of patients at any dose. This was the first study to demonstrate that low doses of sustained opioids have a significant and persistent therapeutic effect on dyspnea.
Common nonmorphine opioids have been investigated in a limited number of studies. A small study of oral hydromorphone with 14 patients showed significant dyspnea relief at a mean dose of 2.5 mg every 6 hours.11 A recent pilot trial of nebulized or systemic hydromorphone versus nebulized saline demonstrated dyspnea improvement in all groups, suggesting the importance of a possible placebo effect for any dyspnea intervention.12 Trials of nebulized fentanyl have been plagued by slow accrual13; the only reports of efficacy come from small case series that achieved promising results using the oral, transmucosal form.14,15
Opioid safety
The usual barrier to the use of opioids as the first-line, pharmacologic treatment for dyspnea is fear of respiratory depression and accelerated death. Historically, opioids were used to alleviate dyspnea from the late-nineteenth century until the 1950s when literature highlighted concerns about the effects of opioids on respiratory depression and CO2 retention.16 This fear has been shown to be largely unfounded. Examining changes in respiratory parameters (peripheral arterial oxygen saturation [SaO2], transcutaneous arterial pressure of carbon dioxide [tcPaCO2], respiratory rate, and pulse rate) in dyspneic palliative care patients, Clemens et al.17,18 demonstrated significant decrease in respiratory rate and improvement in dyspnea with titration with morphine or hydromorphone but no significant changes in other respiratory parameters, indicating no opioid-induced respiratory depression. The studies administered oral opioids in modest doses titrated to dyspnea relief. The authors concluded that, with proper titration, opioids can be used to relieve dyspnea by decreasing respiratory rate while avoiding iatrogenic hypercarbia or hypoxia. These conclusions are also supported in a recent systematic review.4 Additionally, the study by Currow et al. reviewed prospectively more than 30 patient years of data with no events of respiratory depression or cognitive impairment in a frail, older population.10 These demonstrated benefits, and the lack of evidence of accelerated death, have led the American College of Chest Physicians in its “2010 Consensus Statement on the Management of Dyspnea in Patients with Advanced Lung or Heart Disease” to recommend that physicians titrate oral and/or parental opioids for the relief of dyspnea.19
Anxiolytics
The use of benzodiazepines and selective serotonin reuptake inhibitors (SSRIs) rests on the rationale that: patients with anxiety disorders more frequently report dyspnea, patient reports of dyspnea usually cluster with the presence of anxiety or depression, and treating anxiety/depression may thus help ameliorate dyspnea. With SSRIs, there may also be a direct effect on centers that control the perception of breathlessness.
Benzodiazepines have been studied both as single agents and paired with opioids. The initial report in 1980 of diazepam efficacy was an exploratory study of four patients with severe obstructive airway disease and without severe hypoxia at rest.20 Subsequent clinical trials of clorazepate,21 alprazolam,22 and diazepam23 have failed to show any benefit when compared with placebo. One trial24 compared three arms: morphine alone, midazolam alone, and morphine plus midazolam; the study showed a modest benefit with the addition of the benzodiazepine to morphine leading to reduction in dyspnea intensity and decreased breakthrough dyspnea. Importantly, this study enrolled advanced cancer patients with a life expectancy of less than one week (30% of patients in each arm died during the study) and would be difficult to generalize to the majority of the palliative care population. Although the use of midazolam may raise concerns about adverse events and difficulty of administration, a recent publication by Navigante and coworkers25 demonstrates both the equal efficacy and the overall safety of oral midazolam versus oral opioid. Sixty-three patients with severe dyspnea (mean dyspnea >8.5 on a 0 to 10 numerical rating scale) were randomized to either oral morphine or oral midazolam at starting doses of 3 mg and 2 mg, respectively. Doses were increased to an effective dose using a fast-titration schedule over 2 hours; patients were then followed daily for 5 days. At least 50% of both patient groups had dyspnea alleviated during the 2-hour titrating phase, with no significant difference between agents. During the 5-day follow-up phase, midazolam proved superior to morphine in controlling both baseline and breakthrough dyspnea. The most common adverse event, with no significant difference between the two agents, was mild somnolence that did not interfere with further medical workup. This recent report is the first to show efficacy of a benzodiazepine in an outpatient setting, with reasonable reported safety profiles. However, many questions about the role of midazolam remain; the duration of the Navigante studies are very short and the population severely dyspneic, making assessments of safety and generalizability of findings difficult.26 The model of titration also presupposes that the dose received in rapid titration is related to the maintenance dose. Compatibility of the doses of opioids and benzodiazepine chosen has not been demonstrated.
Inhaled furosemide
Furosemide has been postulated to reduce dyspnea because of its inhibitory effect on the cough reflex, preventive effect on bronchoconstriction in asthma, and possible indirect actions on sensory nerve endings in the airway epithelium. Inhaled furosemide has been studied in patients with cancer27,28 and COPD,29 as well as in normal participants.30 In placebo-controlled studies in COPD patients, Ong et al.29 and Jensen et al.31 both showed a significant improvement in dyspnea scores with exercise; the latter study also showed a benefit in exercise endurance time. A recent double-blind study of 15 patients (primarily lung cancer) randomized participants to receive either nebulized furosemide 40 mg, nebulizer 0.9% saline, or no treatment in random order over 3 consecutive days. Measured outcomes included a number reading test (mean numbers read during one breath) and arm exercise test (responses measured on modified Borg scale). Six of the 15 patients reported dyspnea relief with any nebulized treatment, but there remained no statistical superiority of either the saline or furosemide. Although seemingly small in its total sample size, this represents the largest controlled study in cancer patients and reproduces findings from another study with seven cancer patients that investigated a smaller 20-mg furosemide dose.32 To date most reports of inhaled furosemide benefits in cancer patients are limited to case reports and case series28,33,34; the varying dosing and delivery methods limit cross-report comparisons.
Oxygen
Supplemental oxygen is one of the interventions most frequently requested by patients35 and implemented by hospitals to relieve dyspnea.36 Studies in COPD patients have demonstrated both survival and quality-of-life advantages with oxygen therapy in the presence of significant hypoxemia. Two landmark trials from almost 30 years ago demonstrate a clear survival advantage with continuous or nocturnal oxygen in hypoxemic COPD patients whose PaO2 assessments were ≤55 mm Hg or <60 mm Hg in the setting of cor pulmonale or other evidence of end-organ damage due to hypoxia.37,38 A recent study observed that, with ambulatory oxygen therapy in patients without resting hypoxemia but with oxygen desaturation during activity, 68% of COPD patients reported improved health-related quality of life and 35% reported less dyspnea.39
Palliative oxygen treatment is usually considered to be oxygen therapy administered specifically for the relief of dyspnea when PaO2 is <55 mm Hg, and the reimbursement criteria for long-term home oxygen therapy are not met. Cranston and colleagues40 recently published a Cochrane review of palliative oxygen therapy in adult patients with chronic terminal illness in nonacute settings. Eight randomized controlled trials measuring dyspnea by an ordinal scale (Visual Analog Scale, Borg or modified Borg, or 0 to 10 Numerical Rating Scale) in patients with cancer, heart failure, and kyphoscoliosis, but not COPD alone, were included. Individually, all of the included studies had small study populations and most were underpowered to detect a 25% difference in dyspnea with the given interventions. Conflicting findings made the overall results inconclusive. In cancer dyspnea, studies examining palliative oxygen therapy use both at rest and with activity reported differing results; in cardiac dyspnea, high-flow but not low-flow oxygen provided relief during exercise; and in kyphoscoliosis, one study reported significant improvement in dyspnea and hypoxia with exercise. The authors of the systematic review ultimately concluded that the available studies fail to demonstrate a consistent effect of palliative oxygen for dyspnea, but that there remain certain populations of patients that significantly benefit from this intervention. Other systematic reviews in cancer dyspnea5,41 have suggested that oxygen benefit may be seen only in patients with more severe hypoxemia.
To clarify the role of palliative oxygen for refractory dyspnea, Abernethy and colleagues conducted a large, international, randomized, controlled, double-blind study of palliative oxygen versus medical air (i.e., room air with ambient partial pressure of oxygen) for nonhypoxemic patients (PaO2>55 mm Hg).47 Participants received either gas via concentrator through nasal cannulae at 2 L per minute and were asked to use it for more than 15 hours per day for 7 days. Participants rated their breathlessness and other measures twice per day. Neither gas demonstrated superiority in improving quality of life or relieving the sensation of breathlessness. Interestingly, both dyspnea and quality of life improved over the study period in both arms, suggesting that patients may experience benefit derived from the sensation of moving air alone, rather than from the properties of a specific gas such as oxygen. Further analysis suggested that patients with higher baseline dyspnea derived more benefit preferentially from palliative oxygen than did patients with lower baseline dyspnea, and that most benefit from the intervention occurred in the first 48 hours, with nearly all symptomatic and functional improvements manifesting in the first 3 days. The study clearly calls into question the common palliative care practice of prescribing oxygen therapy for refractory dyspnea; if medical gas is prescribed, then patients should be monitored closely and the intervention discontinued if no benefit is realized after 3 days. Because some patients who might benefit from oxygen therapy may not want to receive it42 and because the data on dyspneic patients' treatment preferences are not conclusive, palliative oxygen should be delivered only with careful consideration of the intervention's potential benefit versus patient burden and costs43; this conversation should include the patient and caregiver, whenever possible. The utility of an “N of 1” trial to address this cannot be overemphasized.44
An interesting head-to-head comparison of opioid versus oxygen therapy in a German palliative care unit was recently reported.45 To investigate the comparative effect of these two interventions on respiratory rate, dyspnea intensity, SaO2, and PaCO2, this study enrolled 46 terminally ill patients with baseline hypoxemia (<90% SaO2) or normoxemia but without uncontrolled symptoms. Patients received either 4 L of supplemental oxygen via nasal cannula, or titrated basal opioids, with the option for breakthrough opioids for symptom relief. Patients receiving opioids were more likely to have dyspnea intensity reduced. The study demonstrated no benefit of oxygen at rest in either hypoxemic or normoxemic patients. Additionally, no increased hypercarbia was seen in the opioid group versus the oxygen supplementation group.
Nonpharmacologic Management of Dyspnea
Results of the palliative oxygen trial by Abernethy et al.,46 described above, suggest that simple interventions based on the movement of air may relieve dyspnea for certain patients in a safe, cost-effective manner. A randomized, cross-over trial of a hand-held electric fan directed toward the face versus toward the leg for 5 minutes showed significant decrease in dyspnea when the moving air was directed toward the face. Participants had advanced disease but were not receiving supplemental oxygen. This study of 50 patients also demonstrated continued benefit in some patients, and new benefit in others, during the 10-minute washout period after cessation of the fan intervention.47
Pulmonary rehabilitation may be beneficial for patients with stage 3 or 4 COPD by GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria or for patients with severe dyspnea out of proportion to the severity of the disease.48 The most common model for pulmonary rehabilitation in the United States is a multidisciplinary, hospital-based, outpatient program, but the service may also be provided in home-based, community-based, or inpatient settings. These often consist of supervised 3- or 4-hour sessions of low- or high-intensity aerobic exercise, three times per week for 6 to 12 weeks. Many clinical trials have demonstrated this intervention's benefits: improvement in exercise capacity (in incremental, constant work rate, and timed walking tests), reduction in severity of dyspnea, and increase in health-related quality of life.49 Dyspnea in COPD patients is thought to be a consequence of dynamic hyperinflation resulting from increased ventilatory demand, and inadequate time allowed for expiration, when patients are active. Exercise mitigates this process by lowering ventilatory demand, resulting in a slowing of respiration at a given level of exercise. The resulting longer expiratory time produces less dynamic hyperinflation and ultimately less dyspnea.
In a prospective study of 45 people with lung cancer, most with resectable disease and good performance status, a program of aerobic exercise for 30 minutes per day significantly decreased “dyspnea” and “coughing” scores over a 4-week period.50 Similarly, most studies of exercise for dyspnea in cancer patients demonstrate benefits in patients who are either awaiting or recovering from lung resection. A retrospective review of cancer patients who were not in the perioperative setting and who were referred to a pulmonary rehabilitation center over a one-year period, most of whom had COPD as a comorbid condition, showed improved distance with the 6-minute walk test but no improvement in perceived dyspnea at rest or after the 6-minute walk.51 Although some palliative care patients may benefit from pulmonary rehabilitation, its significant cost ($2200 per participant in a model 8-week program),52 time commitment, and unproven durability of benefit in people who are deteriorating systemically49 make its use not generalizable to all palliative care populations.
For a comprehensive review and discussion of nonpharmacologic management of dyspnea, the reader is referred to a recently published Cochrane review.53
Surgical/Procedural Interventions for Dyspnea
Thoracic malignancies can cause dyspnea from obstruction of airways by mass lesions or lung parenchyma through pleural effusions. Symptomatic pleural effusions can be addressed by many surgical/procedural approaches including mechanical and chemical pleurodesis, pleural tunneled catheter placement, and open or video-assisted thoroscopic surgery (VATS) pleurectomy. Tunneled pleural catheters such as the PleurX® (Care Fusion, San Diego, CA) catheter have gained recent favor because of their ease of placement and low complication rate. Recently Monsky and coworkers54 reported in a 31-patient case series an increase in quality of life and improvement of symptoms and comfort after catheter placement in patients with end-stage malignancies. Furthermore, Olden et al. recently conducted a decision analysis to assess cost-effectiveness of the PleurX® catheter versus talc pleurodesis for malignant pleural effusion. Results showed similar effectiveness, with a distinct cost-effectiveness advantage for pleurodesis by about 0.006 quality-adjusted life years (QALYs) at an $840 lower cost. Possibly due to lower supply costs, cost-effectiveness increased (reducing cost to $100,000/QALY) with catheters for patients with a prognosis of less than 6 weeks.55 A prospective study of interventional bronchoscopy to relieve dyspnea and improve quality of life in patients with malignant central respiratory obstructions showed dyspnea improvement in 85% of patients; approximately half reported an improvement in overall quality of life.56
Lung volume reduction surgery (LVRS) is considered in patients with severe COPD who are symptomatic despite maximal medical therapy and pulmonary rehabilitation. LVRS plus optimal medical therapy is superior to medical therapy alone in treating certain subsets of patients with severe emphysema. In patients with predominantly upper lobe emphysema and low exercise capacity, LVRS improves dyspnea and exercise tolerance and confers a survival advantage.57 Furthermore, LVRS has been shown to be superior in reducing need for supplemental oxygen up to 2 years postprocedure58 and decreasing frequency of COPD exacerbations.59 The procedure, however, has limitations: 90-day postoperative mortality is approximately 5%, and major pulmonary and cardiac postoperative morbidity can exceed 20%.60
Emerging and Complementary Therapeutics
Heliox is a mixture of oxygen (generally 20% to 28%) and helium (72% to 80%). Helium, a less dense gas than the nitrogen that is naturally occurring in ambient air, is thought to produce less airway resistance when inhaled with oxygen. Compared with oxygen alone, Heliox has been shown to increase exercise tolerance in mildly hypoxic patients with moderate to severe COPD,61 and to increase SaO2, improve exercise tolerance, and decrease dyspnea scores in lung cancer patients.62 Its widespread use remains limited by its expense ($30 to $70 for 8 hours of use),63 cumbersome logistics (use requires a nonrebreathing mask; gas is delivered in large tanks), lack of routine availability in most medical centers, and lack of guidelines for patient selection.
Acupuncture, which involves carefully positioned insertion and manipulation of filiform needles,also has been studied as a minimally invasive approach to dyspnea. A randomized controlled trial64 of 24 COPD patients (mean age 64 years) with disabling dyspnea compared 13 sessions of true acupuncture versus sham acupuncture (i.e., placebo in which needles are placed at points not corresponding to acupuncture points) for 3 weeks. Patients treated with true acupuncture had less subjective breathlessness and performed better in a 6-minute walk test than did those receiving sham acupuncture. A more recent placebo-controlled study of 36 patients, mostly with COPD, found a significant improvement in VAS scores, observed in both acupuncture and placebo (mock transcutaneous electrical nerve stimulator) groups with no significant between-group difference.65 A prospective study of 20 patients with cancer-related dyspnea at rest treated with acupuncture reported that 70% of participants experienced significant dyspnea improvement; benefit peaked at 90 minutes and lasted up to 6 hours.66 Although more recent studies are now being reported demonstrating mixed results on its effect on dyspnea, the latest systematic review5 and Cochrane Database review53 found inadequate evidence to recommend acupuncture as a routine intervention for dyspnea control in cancer patients. Conclusions about its efficacy in COPD and other primary respiratory diseases are premature; further studies are needed to fill this gap.
Supplemental nutrition has been studied to counteract the muscle wasting and weight loss that are common in patients with COPD. These changes adversely affect respiratory muscle function, health status, and exercise capacity67 and may result from inadequate dietary intake68 relative to an increase in resting energy expenditure (REE)69 or imbalance in protein synthesis and turnover.70,71 Several overviews, including a recent meta-analysis,72 Cochrane Database review,73 and systematic review74 have all concluded that nutritional supplementation alone does not change meaningful outcomes in patients with COPD. A recent trial randomized 32 patients with moderate to severe COPD to exercise with breath retraining, upper and lower limb exercises, respiratory muscle stretching and exercises, and walking daily, supplemented with additional 400 kcal per day through a sports drink, versus dyspnea education and normal diet. The authors found that adding low-intensity regular exercise training to nutritional supplementation did result in a modest benefit in weight gain, increased exercise capacity, and improved health-related quality of life, including dyspnea intensity.75 Further studies using co-interventions with nutritional supplementation are needed to expand patient-controlled options for dyspnea control.
Tris-hydroxymethyl aminomethane (THAM), an intravenous compound that works as a hydrogen ion acceptor, has been shown in experimental models to reduce minute ventilation and subjective dyspnea in healthy subjects.76 The idea that artificially inducing a metabolic alkalosis and decreasing respiratory drive for acidosis compensation, as previously shown with sodium bicarbonate,77 is intriguing but requires further study.
Summary
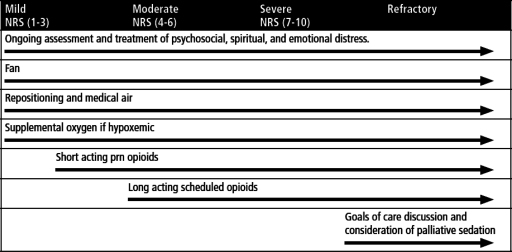
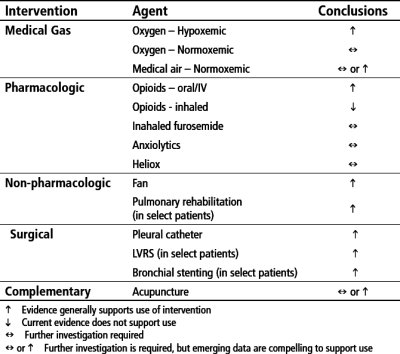
Treatment goals for dyspnea center on identifying reversible anatomic and physiologic causes, intervening upon those, and in parallel, implementing global therapies for dyspnea management (Fig. 2). Although the evidence base continues to build to support widespread use of stalwart therapies including opioids, oxygen in hypoxemic patients, and pulmonary rehabilitation, recent studies with benzodiazepines, medical air in normoxemic patients, and acupuncture are adding to our understanding of how to incorporate other agents into a comprehensive dyspnea management plan. Figure 3 summarizes options and conclusions on efficacy from the current literature base.
FIG. 2.
Global therapies for dyspnea management.
FIG. 3.
Treatment options for dyspnea.
Conclusion
Dyspnea is a significant and disabling symptom experienced by many people during the end of life. Palliative care providers must be comfortable in developing a systematic treatment approach that includes strategies aimed at reversing anatomic and physiologic causes and global therapies when underlying causes cannot be modified. Oral or parenteral opioids remain the standard initial therapy, whereas anxiolytics or inhaled furosemide are important adjuncts to consider as more data become available. Although oxygen is called upon often to alleviate dyspnea, its use should not be considered automatic and certainly should not be continued if patients do not experience clinically relevant relief in a brief time period with its use. Several nonpharmacologic options should also be considered. Further study, especially investigating interventions for the psychosocial, spiritual, and existential components that contribute to “total dyspnea,” is needed to provide evidence-based interventions for a true multidisciplinary approach to refractory dyspnea.
Author Disclosure Statement
Amy Abernethy receives research funding from the Agency for Healthcare Quality and Research, National Cancer Institute, Nation Institute of Nursing Research (National Institutes of Health [NIH]), National Institute of Aging (NIH), and Robert Wood Johnson Foundation. She receives industry funding for clinical research from Pfizer, Lilly, Bristol Myers Squibb, Helsinn, Amgen, Kanglaite, and Abbott Laboratories. She is a consultant (<$10,000/year) for Helsinn, Proventys, and GlaxoSmithKline. Jane Wheeler, Arif Kamal, Jennifer Maguire, and David Currow have no funding to disclose.
References
- 1.Kaye AD. Hoover JM. Ibrahim IN. Phelps J. Baluch A. Fields A. Huffman S. Analysis of the effects of fentanyl in the feline pulmonary vascular bed. Am J Ther. 2006;13:478–484. doi: 10.1097/01.mjt.0000178338.43545.3a. [DOI] [PubMed] [Google Scholar]
- 2.Tenore PL. Psychotherapeutic benefits of opioid agonist therapy. J Addict Dis. 2008;27:49–65. doi: 10.1080/10550880802122646. [DOI] [PubMed] [Google Scholar]
- 3.Mahler DA. Murray JA. Waterman LA. Ward J. Kraemer WJ. Zhang X. Baird JC. Endogenous opioids modify dyspnoea during treadmill exercise in patients with COPD. Eur Respir J. 2009;33:771–777. doi: 10.1183/09031936.00145208. [DOI] [PubMed] [Google Scholar]
- 4.Jennings AL. Davies AN. Higgins JP. Gibbs JS. Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57:939–944. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Aharon I. Gafter-Gvili A. Paul M. Leibovici L. Semmer SM. Interventions for alleviating cancer-related dyspnea: A systematic review. J Clin Oncol. 2008;26:2396–2404. doi: 10.1200/JCO.2007.15.5796. [DOI] [PubMed] [Google Scholar]
- 6.Viola R. Kiteley C. Lloyd NS. Mackay JA. Wilson J. Wong RK. The management of dyspnea in cancer patients: A systematic review. Support Care Cancer. 2008;16:329–337. doi: 10.1007/s00520-007-0389-6. [DOI] [PubMed] [Google Scholar]
- 7.Bruera E. Sala R. Spruyt O. Palmer JL. Zhang T. Willey J. Nebulized versus subcutaneous morphine for patients with cancer dyspnea: A preliminary study. J Pain Symptom Manage. 2005;29:613–618. doi: 10.1016/j.jpainsymman.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Abernethy AP. Currow DC. Frith P. Fazekas BS. McHugh A. Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327:523–528. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allard P. Lamontagne C. Bernard P. Tremblay C. How effective are supplementary doses of opioids for dyspnea in terminally ill cancer patients? A randomized continuous sequential clinical trial. J Pain Symptom Manage. 1999;17:256–265. doi: 10.1016/s0885-3924(98)00157-2. [DOI] [PubMed] [Google Scholar]
- 10.Currow DC. McDonald C. Oaten S. Kenny B. Allcroft P. Frith P. Briffa M. Johnson MJ. Abernethy AP. Once-daily opioids for chronic dyspnea: A dose increment and pharmacovigilance study. J Pain Symptom Manage. 2011;42:388–389. doi: 10.1016/j.jpainsymman.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Clemens KE. Klaschik E. Effect of hydromorphone on ventilation in palliative care patients with dyspnea. Support Care Cancer. 2008;16:93–99. doi: 10.1007/s00520-007-0310-3. [DOI] [PubMed] [Google Scholar]
- 12.Charles MA. Reymond L. Israel F. Relief of incident dyspnea in palliative cancer patients: A pilot, randomized, controlled trial comparing nebulized hydromorphone, systemic hydromorphone, and nebulized saline. J Pain Symptom Manage. 2008;36:29–38. doi: 10.1016/j.jpainsymman.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Smith TJ. Coyne P. French W. Ramakrishnan V. Corrigan P. Failure to accrue to a study of nebulized fentanyl for dyspnea: Lessons learned. J Palliat Med. 2009;12:771–772. doi: 10.1089/jpm.2009.0113. [DOI] [PubMed] [Google Scholar]
- 14.Benitez-Rosario MA. Martin AS. Feria M. Oral transmucosal fentanyl citrate in the management of dyspnea crises in cancer patients. J Pain Symptom Manage. 2005;30:395–397. doi: 10.1016/j.jpainsymman.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Gauna AA. Kang SK. Triano ML. Swatko ER. Vanston VJ. Oral transmucosal fentanyl citrate for dyspnea in terminally ill patients: An observational case series. J Palliat Med. 2008;11:643–648. doi: 10.1089/jpm.2007.0161. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RH. Hoseth W. Dempsey ME. Respiratory acidosis. I. Effects of decreasing respiratory minute volume in patients with severe chronic pulmonary emphysema, with specific reference to oxygen, morphine and barbiturates. Am J Med. 1954;17:464–470. doi: 10.1016/0002-9343(54)90121-7. [DOI] [PubMed] [Google Scholar]
- 17.Clemens KE. Quednau I. Klaschik E. Is there a higher risk of respiratory depression in opioid-naive palliative care patients during symptomatic therapy of dyspnea with strong opioids? J Palliat Med. 2008;11:204–216. doi: 10.1089/jpm.2007.0131. [DOI] [PubMed] [Google Scholar]
- 18.Clemens KE. Klaschik E. Symptomatic therapy of dyspnea with strong opioids and its effect on ventilation in palliative care patients. J Pain Symptom Manage. 2007;33:473–481. doi: 10.1016/j.jpainsymman.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Mahler DA. Selecky PA. Harrod CG. Benditt JO. Carrieri-Kohlman V. Curtis JR. Manning HL. Mularski RA. Varkey B. Campbell M. Carter ER. Chiong JR. Ely EW. Hansen-Flaschen J. O'Donnell DE. Waller A. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137:674–691. doi: 10.1378/chest.09-1543. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell-Heggs P. Murphy K. Minty K. Guz A. Patterson SC. Minty PS. Rosser RM. Diazepam in the treatment of dyspnoea in the 'Pink Puffer' syndrome. Q J Med. 1980;49:9–20. [PubMed] [Google Scholar]
- 21.Eimer M. Cable T. Gal P. Rothenberger LA. McCue JD. Effects of clorazepate on breathlessness and exercise tolerance in patients with chronic airflow obstruction. J Fam Pract. 1985;21:359–362. [PubMed] [Google Scholar]
- 22.Man GC. Hsu K. Sproule BJ. Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary disease. Chest. 1986;90:832–836. doi: 10.1378/chest.90.6.832. [DOI] [PubMed] [Google Scholar]
- 23.Woodcock AA. Gross ER. Geddes DM. Drug treatment of breathlessness: Contrasting effects of diazepam and promethazine in pink puffers. Br Med J (Clin Res Ed) 1981;283:343–346. doi: 10.1136/bmj.283.6287.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navigante AH. Cerchietti LC. Castro MA. Lutteral MA. Cabalar ME. Midazolam as adjunct therapy to morphine in the alleviation of severe dyspnea perception in patients with advanced cancer. J Pain Symptom Manage. 2006;31:38–47. doi: 10.1016/j.jpainsymman.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Navigante AH. Castro MA. Cerchietti LC. Morphine versus midazolam as upfront therapy to control dyspnea perception in cancer patients while its underlying cause is sought or treated. J Pain Symptom Manage. 2010;39:820–830. doi: 10.1016/j.jpainsymman.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Currow DC. Abernethy AP. Potential opioid-sparing effect of regular benzodiazepines in dyspnea: Longer duration of studies needed. J Pain Symptom Manage. 2010;40:e1–e2. doi: 10.1016/j.jpainsymman.2010.07.002. author reply e2–e4. [DOI] [PubMed] [Google Scholar]
- 27.Wilcock A. Walton A. Manderson C. Feathers L. El Khoury B. Lewis M. Chauhan A. Howard P. Bell S. Frisby J. Tattersfield A. Randomised, placebo controlled trial of nebulised furosemide for breathlessness in patients with cancer. Thorax. 2008;63:872–875. doi: 10.1136/thx.2007.091538. [DOI] [PubMed] [Google Scholar]
- 28.Kohara H. Ueoka H. Aoe K. Maeda T. Takeyama H. Saito R. Shima Y. Uchitomi Y. Effect of nebulized furosemide in terminally ill cancer patients with dyspnea. J Pain Symptom Manage. 2003;26:962–967. doi: 10.1016/s0885-3924(03)00322-1. [DOI] [PubMed] [Google Scholar]
- 29.Ong KC. Kor AC. Chong WF. Earnest A. Wang YT. Effects of inhaled furosemide on exertional dyspnea in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:1028–1033. doi: 10.1164/rccm.200308-1171OC. [DOI] [PubMed] [Google Scholar]
- 30.Nishino T. Ide T. Sudo T. Sato J. Inhaled furosemide greatly alleviates the sensation of experimentally induced dyspnea. Am J Respir Crit Care Med. 2000;161:1963–1967. doi: 10.1164/ajrccm.161.6.9910009. [DOI] [PubMed] [Google Scholar]
- 31.Jensen D. Amjadi K. Harris-McAllister V. Webb KA. O'Donnell DE. Mechanisms of dyspnoea relief and improved exercise endurance after furosemide inhalation in COPD. Thorax. 2008;63:606–613. doi: 10.1136/thx.2007.085993. [DOI] [PubMed] [Google Scholar]
- 32.Stone P. Rix Kurowska A. Tookman: Re: nebulized furosemide for dyspnea in terminal cancer patients. J Pain Symptom Manage. 2002;24:274–275. doi: 10.1016/s0885-3924(02)00479-7. author reply 275–276. [DOI] [PubMed] [Google Scholar]
- 33.Shimoyama N. Shimoyama M. Nebulized furosemide as a novel treatment for dyspnea in terminal cancer patients. J Pain Symptom Manage. 2002;23:73–76. doi: 10.1016/s0885-3924(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 34.Stone P. Kurowska A. Tookman A. Nebulized frusemide for dyspnoea. Palliat Med. 1994;8:258. doi: 10.1177/026921639400800315. [DOI] [PubMed] [Google Scholar]
- 35.Roberts CM. Short burst oxygen therapy for relief of breathlessness in COPD. Thorax. 2004;59:638–640. doi: 10.1136/thx.2003.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escalante CP. Martin CG. Elting LS. Cantor SB. Harle TS. Price KJ. Kish SK. Manzullo EF. Rubenstein EB. Dyspnea in cancer patients. Etiology, resource utilization, and survival-implications in a managed care world. Cancer. 1996;78:1314–1319. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1314::AID-CNCR21>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 38.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1:681–686. [PubMed] [Google Scholar]
- 39.Eaton T. Lewis C. Young P. Kennedy Y. Garrett JE. Kolbe J. Long-term oxygen therapy improves health-related quality of life. Respir Med. 2004;98(4):285–293. doi: 10.1016/j.rmed.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Cranston JM. Crockett A. Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Database Syst Rev. 2008;(3):CD004769. doi: 10.1002/14651858.CD004769.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Uronis HE. Currow DC. McCrory DC. Samsa GP. Abernethy AP. Oxygen for relief of dyspnoea in mildly- or non-hypoxaemic patients with cancer: A systematic review and meta-analysis. Br J Cancer. 2008;98:294–299. doi: 10.1038/sj.bjc.6604161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Currow DC. Fazekas B. Abernethy AP. Oxygen use—patients define symptomatic benefit discerningly. J Pain Symptom Manage. 2007;34:113–114. doi: 10.1016/j.jpainsymman.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Uronis HE. Currow DC. Abernethy AP. Palliative management of refractory dyspnea in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:289–304. doi: 10.2147/copd.2006.1.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nonoyama ML. Brooks D. Guyatt GH. Goldstein RS. Effect of oxygen on health quality of life in patients with chronic obstructive pulmonary disease with transient exertional hypoxemia. Am J Respir Crit Care Med. 2007;176:343–349. doi: 10.1164/rccm.200702-308OC. [DOI] [PubMed] [Google Scholar]
- 45.Clemens KE. Quednau I. Klaschik E. Use of oxygen and opioids in the palliation of dyspnoea in hypoxic and non-hypoxic palliative care patients: A prospective study. Support Care Cancer. 2009;17:367–377. doi: 10.1007/s00520-008-0479-0. [DOI] [PubMed] [Google Scholar]
- 46.Abernethy AP. McDonald CF. Frith PA. Clark K. Herndon JE., 2nd Marcello J. Young IH. Bull J. Wilcock A. Booth S. Wheeler JL. Tulsky JA. Crockett AJ. Currow DC. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: A double-blind, randomised controlled trial. Lancet. 2010;376:784–793. doi: 10.1016/S0140-6736(10)61115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galbraith S. Fagan P. Perkins P. Lynch A. Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage. 2010;39:831–838. doi: 10.1016/j.jpainsymman.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 48.Casaburi R. ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:1329–1335. doi: 10.1056/NEJMct0804632. [DOI] [PubMed] [Google Scholar]
- 49.Ries AL. Bauldoff GS. Carlin BW. Casaburi R. Emery CF. Mahler DA. Make B. Rochester CL. Zuwallack R. Herrerias C. Pulmonary rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 50.Riesenberg H. Lubbe AS. In-patient rehabilitation of lung cancer patients—a prospective study. Support Care Cancer. 2010;18:877–882. doi: 10.1007/s00520-009-0727-y. [DOI] [PubMed] [Google Scholar]
- 51.Morris GS. Gallagher GH. Baxter MF. Brueilly KE. Scheetz JS. Ahmed MM. Shannon VR. Pulmonary rehabilitation improves functional status in oncology patients. Arch Phys Med Rehabil. 2009;90:837–841. doi: 10.1016/j.apmr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Fan VS. Giardino ND. Blough DK. Kaplan RM. Ramsey SD. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008;5:105–116. doi: 10.1080/15412550801941190. [DOI] [PubMed] [Google Scholar]
- 53.Bausewein C. Booth S. Gysels M. Higginson I. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev. 2008;(2):CD005623. doi: 10.1002/14651858.CD005623.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Monsky WL. Yoneda KY. MacMillan J. Deutsch LS. Dong P. Hourigan H. Schwartz Y. Magee S. Duffield C. Boak T. Cernilia J. Peritoneal and pleural ports for management of refractory ascites and pleural effusions: Assessment of impact on patient quality of life and hospice/home nursing care. J Palliat Med. 2009;12:811–817. doi: 10.1089/jpm.2009.0061. [DOI] [PubMed] [Google Scholar]
- 55.Olden AM. Holloway R. Treatment of malignant pleural effusion: PleuRx catheter or talc pleurodesis? A cost-effectiveness analysis. J Palliat Med. 2010;13:59–65. doi: 10.1089/jpm.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amjadi K. Voduc N. Cruysberghs Y. Lemmens R. Fergusson DA. Doucette S. Noppen M. Impact of interventional bronchoscopy on quality of life in malignant airway obstruction. Respiration. 2008;76:421–428. doi: 10.1159/000152832. [DOI] [PubMed] [Google Scholar]
- 57.Naunheim KS. Wood DE. Mohsenifar Z. Sternberg AL. Criner GJ. DeCamp MM. Deschamps CC. Martinez FJ. Sciurba FC. Tonascia J. Fishman AP. National Emphysema Treatment Trial Research Group: Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg. 2006;82:431–443. doi: 10.1016/j.athoracsur.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 58.Snyder ML. Goss CH. Neradilek B. Polissar NL. Mosenifar Z. Wise RA. Fishman AP. Benditt JO. National Emphysema Treatment Trial Research Group: Changes in arterial oxygenation and self-reported oxygen use after lung volume reduction surgery. Am J Respir Crit Care Med. 2008;178:339–345. doi: 10.1164/rccm.200712-1826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Washko GR. Fan VS. Ramsey SD. Mohsenifar Z. Martinez F. Make BJ. Sciurba FC. Criner GJ. Minai O. Decamp MM. Reilly JJ. National Emphysema Treatment Trial Research Group: The effect of lung volume reduction surgery on chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;177:164–169. doi: 10.1164/rccm.200708-1194OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naunheim KS. Wood DE. Krasna MJ. DeCamp MM., Jr Ginsburg ME. McKenna RJ., Jr Criner GJ. Hoffman EA. Sternberg AL. Deschamps C. National Emphysema Treatment Trial Research Group: Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg. 2006;131:43–53. doi: 10.1016/j.jtcvs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Chiappa GR. Queiroga F., Jr Meda E. Ferreira LF. Diefenthaeler F. Nunes M. Vaz MA. Machado MC. Nery LE. Neder JA. Heliox improves oxygen delivery and utilization during dynamic exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1. 2009;179:1004–1010. doi: 10.1164/rccm.200811-1793OC. [DOI] [PubMed] [Google Scholar]
- 62.Ahmedzai SH. Laude E. Robertson A. Troy G. Vora V. A double-blind, randomised, controlled Phase II trial of Heliox28 gas mixture in lung cancer patients with dyspnoea on exertion. Br J Cancer. 2004;90:366–371. doi: 10.1038/sj.bjc.6601527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manthous C. Morgan SM. Pohlnian A. Hall JB. Heliox in the treatment of airflow obstruction: A critical review of the literature. Respiratory Care. 1997;42:1034–1042. [Google Scholar]
- 64.Jobst K. Chen JH. McPherson K. Arrowsmith J. Brown V. Efthimiou J. Fletcher HJ. Maciocia G. Mole P. Shifrin K. Lane D. Controlled trial of acupuncture for disabling breathlessness. Lancet. 1986;2:1416–1419. doi: 10.1016/s0140-6736(86)92732-7. [DOI] [PubMed] [Google Scholar]
- 65.Lewith GT. Prescott P. Davis CL. Can a standardized acupuncture technique palliate disabling breathlessness: A single-blind, placebo-controlled crossover study. Chest. 2004;125:1783–1790. doi: 10.1378/chest.125.5.1783. [DOI] [PubMed] [Google Scholar]
- 66.Filshie J. Penn K. Ashley S. Davis CL. Acupuncture for the relief of cancer-related breathlessness. Palliat Med. 1996;10:145–150. doi: 10.1177/026921639601000209. [DOI] [PubMed] [Google Scholar]
- 67.Wilson DO. Rogers RM. Wright EC. Anthonisen NR. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis. 1989;139:1435–1438. doi: 10.1164/ajrccm/139.6.1435. [DOI] [PubMed] [Google Scholar]
- 68.Creutzberg EC. Wouters EF. Mostert R. Weling-Scheepers CA. Schols AM. Efficacy of nutritional supplementation therapy in depleted patients with chronic obstructive pulmonary disease. Nutrition. 2003;19:120–127. doi: 10.1016/s0899-9007(02)00841-9. [DOI] [PubMed] [Google Scholar]
- 69.Creutzberg EC. Schols AM. Bothmer-Quaedvlieg FC. Wouters EF. Prevalence of an elevated resting energy expenditure in patients with chronic obstructive pulmonary disease in relation to body composition and lung function. Eur J Clin Nutr. 1998;52:396–401. doi: 10.1038/sj.ejcn.1600571. [DOI] [PubMed] [Google Scholar]
- 70.Engelen MP. Deutz NE. Wouters EF. Schols AM. Enhanced levels of whole-body protein turnover in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1488–1492. doi: 10.1164/ajrccm.162.4.2002045. [DOI] [PubMed] [Google Scholar]
- 71.Morrison WL. Gibson JN. Scrimgeour C. Rennie MJ. Muscle wasting in emphysema. Clin Sci (Lond) 1988;75:415–420. doi: 10.1042/cs0750415. [DOI] [PubMed] [Google Scholar]
- 72.Ferreira IM. Brooks D. Lacasse Y. Goldstein RS. Nutritional support for individuals with COPD: a meta-analysis. Chest. 2000;117:672–678. doi: 10.1378/chest.117.3.672. [DOI] [PubMed] [Google Scholar]
- 73.Ferreira IM. Brooks D. Lacasse Y. Goldstein RS. White J. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;(2):CD000998. doi: 10.1002/14651858.CD000998.pub2. [DOI] [PubMed] [Google Scholar]
- 74.Puhan MA. Scharplatz M. Troosters T. Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality—a systematic review. Respir Res. 2005;6:54. doi: 10.1186/1465-9921-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sugawara K. Takahashi H. Kasai C. Kiyokawa N. Watanabe T. Fujii S. Kashiwagura T. Honma M. Satake M. Shioya T. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir Med. 2010;104:1883–1889. doi: 10.1016/j.rmed.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 76.Nishino T. Iiyori N. Isono S. Shinozuka N. Taguchi N. Ishikawa T. THAM improves an experimentally induced severe dyspnea. J Pain Symptom Manage. 2009;37:212–219. doi: 10.1016/j.jpainsymman.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 77.Taguchi N. Ishikawa T. Sato J. Nishino T. Effects of induced metabolic alkalosis on perception of dyspnea during flow-resistive loading. J Pain Symptom Manage. 1996;12:11–17. doi: 10.1016/0885-3924(96)00043-7. [DOI] [PubMed] [Google Scholar]