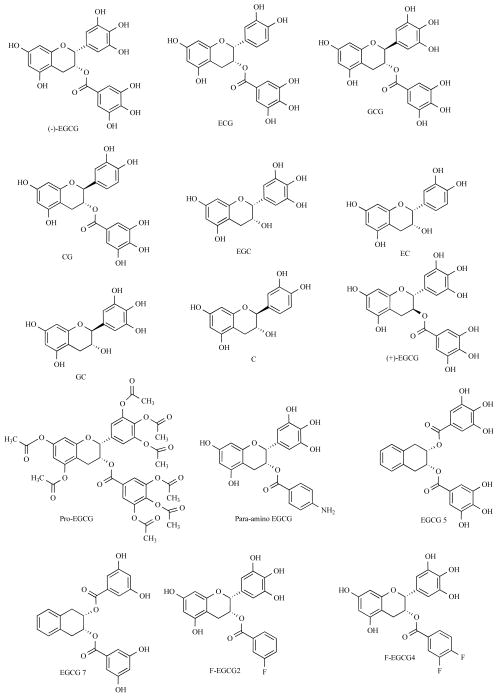
Abstract
Next to water, tea is the most popular beverage in the world. The most abundant and active compound in green tea is (−)-epigallocatechin-3-gallate (EGCG), which is extensively studied for its cancer-preventive and anti-cancer activities as well as its cellular targets. One potential molecular target of EGCG is the proteasome. While molecular docking and structure-activity relationship (SAR) analysis suggests that the ester carbon of EGCG is important for mediating its proteasome-inhibitory activity, EGCG is very unstable under physiological conditions. Therefore, a series of analogs were synthesized aiming to improve stability and bioavailability of EGCG. Among them, peracetate-protected or the prodrug of EGCG was found to have increased bioavailability, stability, and proteasome-inhibitory activities against various human cancer cells and tumors compared to EGCG, suggesting its potential use for cancer prevention and treatment. Epidemiological studies have indicated that green tea consumption is associated with the reduced risk of cancers, especially associated with the reduced risk of late stage of cancers. This risk reduction may be attributed not only to proteasome inhibition, but also to numerous other intracellular molecules targeted by EGCG that are involved in cell cycle regulation, apoptosis, angiogenesis, and metastasis.
Keywords: Proteasome inhibitors, drug discovery, chemoprevention, targeted therapy
INTRODUCTION
Targeting the proteasome has become an attractive approach in cancer prevention and cancer therapy because most of the intracellular proteins involved in carcinogenesis and tumor progression are degraded through the ubiquitin-proteasome pathway [1]. Ubiquitin-proteasome degradation system involves two successive steps, enzymatic ubiqutination followed by proteolysis through the 26S proteasome. Ubiquitination is a process of conjugating multiple ubiquitin molecules to the protein substrate. Ubiquitin is a highly conserved 76-amino acid protein that becomes covalently ligated to a target protein by a multi-enzymatic system consisting of Ub-activating (E1), Ub-conjugating (E2), and the Ub-ligating (E3) enzymes, which act in a sequential manner. Ubiquitinated proteins are recognized and selectively degraded by the 26S proteasome, the largest protease complex present in the nucleus and cytosol of a eukaryotic cell. The ubiquitin is then released and recycled. The proteolytic core of this catalytic machine, the 20S proteasome, is composed of 28 subunits arranged in four heptameric, tightly stacked rings (α7, β7, β7, α7) to form a barrel-shaped structure [2]. The proteolytic activities are confined to the β-subunits conferring the unique and distinguishing proteasome feature of multiple peptidase activities that include chymotrypsin-like (cleavage after hydrophobic side chains, mediated by the β5 subunit), peptidylglutamyl peptide hydrolyzing (PGPH)-like (cleavage after acidic side chains, mediated by the β1 subunit), and trypsin-like (cleavage after basic side chains, mediated by the β2 subunit) activities [3]. The entrance of substrate proteins to the active site of the complex is guarded by the α-subunits that allow access only to unfolded and extended polypeptides.
The ubiquitin-proteasome pathway plays a critical role in regulation of essential cellular processes (such as cell cycle progression, proliferation, and apoptosis) and abolishment of unwanted or abnormal proteins that result from oxidative damage and mutations. Furthermore, proteins identified as proteasome substrates include cyclins A, B, D and E, tumor suppressor protein p53, pro-apoptotic protein Bax [4], cyclin-dependent kinase inhibitor p27 [5], and the NF-κB inhibitor, IκB-α [6]. Because tumor cells appear to rely more heavily on high proteasome activity than normal cells, pro-teasome inhibition could contribute to a novel, selective strategy for cancer prevention and therapy [7].
THE PROTEASOME AS A POTENTIAL MOLECULAR TARGET OF GREEN TEA POLYPHENOLS
Tea (Camellia sinensis) is the most popular beverage, next to water, consumed in the world. Assumption of green tea, a focus of this review article, is suggested to bring about cancer-preventive and anti-oxidant effects from various green tea polyphenols including (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epigallocatechin-3-gallate (EGCG) (Fig. 1) [8]. Among them, EGCG is the most abundant and potent constituent.
Fig. 1.
Chemical structures of EGCG and its synthetic analogs and prodrugs.
We first reported that ester bond-containing tea polyphenols potently and selectively inhibit the proteasomal chymotrypsin-like but not the trypsin-like activity [9]. Among the green tea polyphenols examined, EGCG showed the strongest inhibitory activity against purified 20S proteasome, 26S proteasome of tumor cell extracts, and 26S proteasome in intact tumor cells. EGCG inhibits the chymotrypsin-like activity of the proteasome in vitro with an IC50 value around 86 to 194 nM and in vivo at 1–10 μM [9]. These in vivo EGCG concentrations are comparable with the concentrations found in the serum of green tea drinkers. Furthermore, the inhibition of the proteasome in vivo was able to accumulate the natural proteasome substrates p27Kip1 and IκB-α as well as induce the arrest of tumor cells in the G1 phase. A green or black tea extract, which contains significant portions of EGCG (51.5 and 19.7%, respectively) and ECG (14.7 and 14.9%, respectively), also strongly inhibited the chymotrypsin-like activity of the 20S proteasome (IC50 values were 0.1 and 0.3 μg/ml, respectively) [9]. EGCG not only inhibits proteasomal chymotrypsin-like activity, but also inhibits immunoproteasome BrAAP activity mediated by the β5i subunit, as shown by other researchers [10, 11].
Structural-Activity Relationship of EGCG Analogs
To determine the potential binding site of EGCG for its mediated proteasome inhibition, cell-free proteasome activity in the presence of naturally occurring green tea polyphenols has been examined. The ester bond-containing tea poly-phenols, such as ECG, gallocatechin-3-gallate (GCG), and catechin-3-gallate (CG) (Fig. 1), as well as EGCG were all strong inhibitors for the chymotrypsin-like activity of the purified 20S proteasome with IC50 values at nM ranges [9]. In contrast, the polyphenols without the gallate ester function, such as EGC, EC, gallocatechin (GC), and catechin (C) (Fig. 1) could not inhibit the proteasomal chymotrypsin-like activity [9]. These results indicate that the ester bonds contained in green tea polyphenols are essential for potent inhibition of the proteasomal chymotrypsin-like activity [9].
Consistently, the ester bond carbon of EGCG was found to have the highest susceptibility toward a nucleophilic attack by the Thr1 of the β5 subunit of the proteasome among all the other atoms with a value of 0.7, similar to other ester bond-containing polyphenols (ECG, GCG, and CG) [9], whereas low nucleophilic susceptibility was found in non-ester bond-containing polyphenols (EGC EC, GC, and C with values of 0.2~0.3). Thus, the nucleophilic susceptibility of EGCG toward the β5 subunit correlates with the biological ability of EGCG to inhibit proteasomal chymotrypsin-like activity. These data support the essential role of poly-phenol ester bonds in the inhibition of the proteasome activity [9].
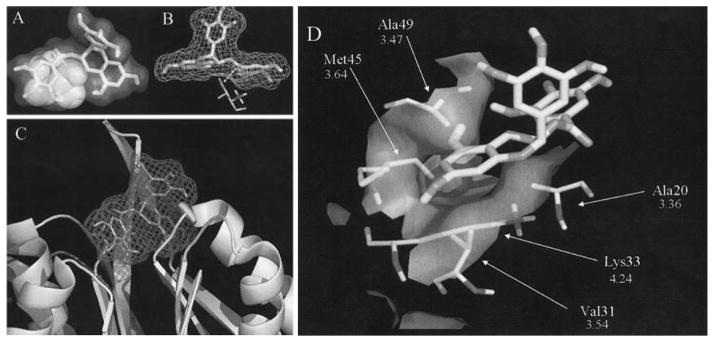
Our subsequent structure-activity relationship studies showed that the A ring and gallate ester/amide bond were essential for the proteasome-inhibitory function of EGCG [12]. In a mechanistic model, with in silico docking calculations, to account for proteasome inhibition, the polyphenol gallate ester, for example, of EGCG was found to bind the active site of the β5 subunit of 20S proteasome. The A ring of EGCG acts as the phenyl ring of a phenylalanine mimic, binding to the hydrophobic S1 pocket of the β5 subunit (Fig. 2). The ester bond of EGCG is then in reasonable proximity (about 3.18 Ǻ) from the Thr 1 OH, which is responsible for the proteasomal catalytic activity (Fig. 2). Inhibition is then due to the irreversible transfer of the gallate moiety from EGCG to the hydroxy oxygen of Thr 1 [13]. This model was further supported by the results from natural polyphenols and their respective enantiomers [14]. The unnatural enantiomers were equal potent to, or more potent than the natural compounds in inhibiting the chymotrypsin-like activity of the proteasome [14] because they were also able to dock into the S1 pocket. For example, (+)-EGCG (Fig. 1) was oriented in the proteasome β5 subunit, with the A–C rings in the S1 pocket and the B ring in solvent. In order for the gallate group of (+)-EGCG to bind in the same relative position to the hydroxyl of Thr 1 as natural (−)-EGCG, the A–C rings of (+)-EGCG had to flip 180° to attain similar orientation/conformation [14]. Similar molecular docking explanations were applied to the homology modeled active subunits of the IFN-gamma inducible proteasome [15].
Fig. 2. Docking of EGCG to the chymotrypsin site of the yeast 20S proteasome.
A stick figure of EGCG with a transparent surface is used to show the proximity between EGCG and Thr 1, represented by a space-filling model (A). The dotted line represents a distance of 3.18 A from the hydroxyl of Thr 1 to the carbonyl carbon of EGCG. EGCG filled most of the binding cleft, which was seen by drawing a water-accessible mesh surface around EGCG (B) when docked into the binding site as depicted by a ribbon structure of the β5 subunit (C). The S1 pocket of the β5 subunit is defined by the hydrophobic residues, Ala 20, Val 31, Ile 35, Met 45, Ala 49, and Gln 53. The hydrophobic portion of A ring on EGCG is oriented in the middle of the S1 pocket between the side-chains of Ala 49, Ala 20, and Lys 33 (D). This conformation would allow the hydrophilic hydroxyls of the A ring to project out of the two sides of the S1 hydrophobic pocket and participate in H-bonding. In addition, the sidewalls of the S1 pocket that interact with EGCG are created by Met 45 and Val 31 (D). Reproduced with permission from Proteins: Structure, Function, and Bioinformatics.
Structure activity relationships of Bn-EGCG, an EGCG analog synthesized with all eight hydroxyls protected by benzyl groups have also been studied. The benzyl analog theoretically eliminated any hydrogen bonding created by the hydroxyls in EGCG while maintaining the integrity of the ester bond. The protection of the hydroxyl groups on EGCG renders the compound completely inactive to inhibit either purified 20S proteasome or cellular 26S proteasome, indicating the hydroxyl groups on EGCG are important for mediating proteasome inhibition [16]. In silico docking was performed to elucidate the role of the EGCG hydroxyl groups and it was determined that the two hydroxyl groups at the C5 and C7 positions of the A ring on EGCG binding to Lys 32 and Ala 146 in the hydrophobic S1 pocket of the β5 subunit of the proteasome. This binding allows for the electrophilic carbonyl carbon of EGCG to be oriented in a suitable position for nucleophilic attack by the hydroxyl group of N-terminal Thr 1 of the β5 subunit of the proteasome, inhibiting the proteasomal chymotrypsin-like activity [17]. In addition, the hydroxyl groups on the D ring forms hydrogen bonds with Gly 47 and Ser 131 of the proteasome that further contributes to binding stability of EGCG to the proteasome [17].
Detailed structure–activity relationship analysis by using a number of synthetic green tea polyphenol analogs involving modifications of the EGCG A ring and B ring revealed that a decrease in the number of B ring OH groups led to decreased potency. Introduction of a hydrophobic benzyl group into the 8 position of the A ring did not significantly affect the proteasome-inhibitory potency [17].
Synthetic EGCG Analogs and Prodrugs
Green tea has been shown to affect a variety of biological systems, including metabolism, angiogenesis, and cell proliferation. Unfortunately, the most abundant green tea polyphenol, EGCG, is very unstable in neutral or alkaline conditions leading to low bioavailability. To find more stable and more potent green tea polyphenols that target the proteasome, several novel EGCG analogs have been synthesized following strategies that include hydroxyl group removal in various positions, acetate protection, para-amino group addition, and fluoro-substitutions.
EGCG Peracetate as Prodrug
EGCG analogs with the OH groups eliminated from the B and/or D rings as well as their putative prodrugs with OH groups protected as the acetates, have been tested in vitro and in whole cells [18, 19]. Compared to non-protected EGCG analogs, acetate-protected analogs exhibit greater potency to inhibit proliferation and induce apoptosis in human leukemic, prostate, and breast cancer cells [20]. HPLC analysis elucidated that the acetate protected EGCG analog (Pro-EGCG) (Fig. 1) is six times more stable than natural EGCG when incubated with RPMI 1640 culture medium at 37 °C. Various bioassays indicate that an intercellular conversion, from the acetate-protected to the parent unprotected hydroxyl form occurs within cells. These assays showed that the Pro-EGCG has no inhibitory activity against a purified 20S proteasome in vitro, as expected, but exhibits increased proteasome-inhibitory activity associated with cell death in intact leukemic cells over natural EGCG. Furthermore, in human breast MDA-MB-231 xenograft nude mice treated with Pro-EGCG or with EGCG it was found that Pro-EGCG resulted in much more significant inhibition of tumor growth than unprotected EGCG (56% vs. 23%), associated with increased levels of proteasome inhibition and tumor cell apoptosis in vivo [21]. These results indicate that Pro-EGCG may function as a viable and novel proteasome inhibitor prodrug.
D-Ring Analogs
Additional EGCG analogs containing only a para-hydroxybenzoate ester group (Para-OH EGCG) [22] or para-aminobenzoate group to replace the gallate ester have also been examined. We have compared the IC50 of EGCG with para-OH EGCG, which is a naturally-occurring catechin isolated from Cistus salvifolius, for their inhibitory activity against the chymotrypsin-like activity of a purified protea-some in vitro. It was found that the loss of the two OH groups in the D-ring of para-OH EGCG led to much reduced inhibitory activity of about 200-fold. On the other hand, EGCG analogs possessing a p-NH2 (Para-amino EGCG) (Fig. 1) or p-NHBoc (Boc; tert-butoxycarbonyl) D ring act as novel proteasome inhibitors and apoptosis inducers with potency similar to pro-EGCG in Raji B cells [23]. The para-aminobenzoate moiety is a normal constituent of folic acid and may offer the possibility of novel and powerful anticancer agents.
Several novel fluoro-substituted benzoates of EGC, or EGCG analogs with OH groups eliminated from the D ring and replaced with one or two fluorine(s) called F-EGCGs (Fig. 1), and their acetate protected counterparts have also been analyzed [24]. Compared to acetate protected EGCG, acetate protected 3,4-difluorobenzoate EGCG (Pro-F-EGCG4) exhibited greater potency against proteasomal chymotrypsin-like activity, suppressed cell proliferation, and induced apoptosis in human leukemia Jurkat T cells [24]. Administration of Pro-F-EGCG4 or acetate protected fluoro-substituted benzoates of EGCG at the meta position (Pro-F-EGCG2) in human breast cancer MDA-MB-231 xenografts showed that the two fluro-substituted EGGC analogs inhibited tumor growth with similar potency to that of the acetate protected EGCG. MDA-MB-231 tumors treated with each fluoro-substituted EGCG analog showed proteasome inhibition and apoptotic cell death, suggesting that the proteasome might be one of the cellular targets of fluoro-EGCGs and that proteasome inhibition is partially responsible for the observed antitumor activity [25]. Similar to the previously mentioned EGCG analogs, the EGCG acetate-protected, fluoro-substituted benzoates also have potential as novel anti-cancer and cancer-preventive agents.
C-Ring Carbocyclic Analogs
We have found that the unnatural enantiomer (+)-EGCG (Fig. 1) as well as other catechin gallates were equal potent to, or more potent than the natural compounds in inhibiting the chymotrypsin-like activity of the proteasome [14, 16]. In silico studies suggested that they were also docked to the S1 pocket in the proteasome β5 subunit, with the A–C rings of (+)-EGCG flipped by 180° to attain similar orientation/conformation [13]. This suggested that the binding site may be pseudo-symmetrical and may be amenable to binding to analogs which are symmetrical. We have thus synthesized the EGCG analog which has a carbocyclic B ring as well as simpler analogs [12]. The IC50 of this EGCG analog was found to be almost as potent as EGCG for their inhibitory activity against the chymotrypsin-like activity of a purified proteasome in vitro. Recently, naphthalene-2,3-diyl digallates and analogs had been synthesized and reported to have cancer cell cytotoxicity and postulated to function as tea polyphenol analogs [26]. Their inhibitory action against proteasome had not been determined however.
Biotransforming Modifications of EGCG
Following absorption EGCG is subjected to modification through major biotransformation reactions including methylation, glucuronidation, and sulfonation, resulting in reduced biological activities of EGCG in vivo [27, 28]. Methylation of EGCG occurs via catechol-O-methyltransferase (COMT) activity, an enzyme ubiquitinously distributed in the body. In humans, a single gene for COMT encodes both a soluble COMT (S-COMT) and a membrane-bound COMT (MB-COMT). A single nucleotide polymorphism (G to A) in codon 108 (S-COMT) or 158 (MB-COMT) results in a valine to methionine (Val to Met) substitution that encodes for a high- (Val/Val [H/H]), intermediate- (Val/Met [H/L]), or low-activity (Met/Met [L/L]) form of COMT [29]. There is a 3~4-fold difference in enzyme activity between the high- and low-activity expressed genes [30]. One group reported that women who carried at least one low-activity COMT allele and were tea drinkers had a significantly reduced risk of breast cancer compared with non-tea drinkers [31]. In contrast, risk of breast cancer did not differ between tea drinkers and non-tea drinkers among those who were homozygous for the high-activity COMT allele [31]. These results suggested that high COMT activity could methylate and inactivate EGCG and other green tea polyphenols, resulting in non-benefit of tea drinking in high COMT patients.
Recently, methylated EGCG and ECG analogs that are metabolites or potential metabolites of green tea polyphenols EGCG and ECG were synthesized and studied for their structure-activity relationships (SARs) using a purified 20S proteasome [32]. The addition of a single methyl group on EGCG or ECG led to decreased proteasome inhibition and, as the number of methyl groups increased, the inhibitory potencies further decreased [33]. These SARs were supported by our findings from in silico docking analysis, showing that methylation has no effect on nucleophilic susceptibility of EGCG and ECG, but it may disrupt the ability of these polyphenols to interact with Thr 1 of the proteasome β5 subunit [34]. In silico docking shows that methylation results in the ester carbon being moved away or blocked entirely from Thr 1 and that methylation impairs the ability of EGCG and ECG to dock in a consistent low energy pose. Such observations indicating no change in nucleophilic susceptibility upon moving or blocking the ester carbon from Thr 1, and lack of a consistent docking pose, suggest that methylation disrupts the ability of EGCG and ECG to bind to the proteasome β5 subunit, which may then diminish their proteasomal chymotrypsin-inhibitory and, therefore, other biological activities [34].
Biological data support the in silico findings, which show that acetate-protected, monomethylated EGCG induces greater cellular proteasome inhibition and apoptosis than acetate-protected, trimethylated EGCG, consistent with the potencies of the parent methylated analogs against a purified 20S proteasome. Therefore, methylation on green tea polyphenols under physiological conditions could decrease their proteasome-inhibitory activity, contributing to decreased cancer-preventive effects of tea [32].
In a recent study, we hypothesized that suppression of COMT activity in human breast cancer cells could increase the proteasome-inhibitory potency of EGCG and therefore enhance its tumor cell growth-inhibitory activity. We first determined the COMT genotype and basal levels of COMT activity in various human breast cancer cell lines. Furthermore, when breast cancer MDA-MB-231 cells containing high COMT activity was tested, the diminished COMT activity with the potent COMT inhibitor dinitrocatechol (DNC) apparently increased the effectiveness of EGCG via augmented proteasome inhibition and apoptosis induction [35]. This study supplements the previous findings that methylated EGCG is less bioactive and supports the notion that COMT inhibition may increase the anti-cancer properties of green tea polyphenols and the combination may serve as a novel approach or supplemental treatment for breast cancer chemotherapy.
Toward the goal of targeting the problem of EGCG biological methylation in vivo, a novel EGCG analog devoid of the o-catechol structure (EGCG 7) (Fig. 1) that should not be a substrate of COMT [36] has been examined. While the proteasome-inhibitory activity of this EGCG analog 7 was not affected by the addition of DNC, proteasome inhibition by EGCG 7 partner, EGCG 5 (Fig. 1) as well as EGCG was enhanced. Similarly, addition of DNC potently enhanced the antiproliferative activities of acetate protected EGCG, but not the acetate protected form of this novel synthetic compound in MDA-MB-231 breast cancer cells expressing high COMT activity [36].
Although EGCG 7, presumably resistant to COMT-mediated methylation and inactivation in cells, was able to inhibit the activity of purified 20S proteasome and cellular 26S proteasome [36], the involved molecular mechanism is unknown. Most recently, we applied computational solution to understand the possible interaction between EGCG analogs including EGCG 7 and the proteasome β5 subunit [37] which is responsible for the chymotrypsin-like activity. We found that the ester carbonyls at C2 and C3 carbon atoms may be targets for nucleophilic attack in EGCG 7 and 5 (Fig. 1). The meta-diol structure in presumably COMT-resistant 7 gives more stable conformation and lower docked free energy than other EGCG analogs.
These studies suggest that COMT is a critical factor in mediating the antiproliferative activities of EGCG and EGCG analogs, especially in cells expressing high COMT activity, such as MDA-MB-231 breast cancer cells.
EPIDEMIOLOGICAL AND CLINICAL STUDIES OF GREEN TEA
Green tea consumption may be promising as an activity against the development of cancer without inducing major toxicities. While a variety of studies shows that the risk of many types of cancers is reduced upon green tea consumption, highlighted herein are studies suggesting a reduced risk of prostate, breast, gastrointestinal, lung and skin cancer. It should be noted that cancer risk reduction is not conclusive in tea drinkers and the opposing evidence is also included.
Green Tea and Prostate Cancer
In a pilot study to investigate the effects of green tea on premalignant lesions before prostate cancer development, 60 patients with high-grade prostate intraepithelial neoplasia were administered a daily dose of 600 mg green tea in capsule form or placebo for one year. The results showed a significant reduction in the incidence of prostate cancer (3% incidence among the 30 green tea-treated subjects compared with 30% incidence among the 30 placebo-treated subjects). No significant side or adverse effects were documented [38].
While a number of case-control studies have indicated that general tea consumption was found to be associated with a decreased risk of prostate cancer [39–41], other case-control and prospective studies have concluded otherwise [42–46]. In fact, one study suggested that prostate cancer risk was increased with green tea consumption in a cohort of men of Japanese ancestry in Hawaii [47]. On the other hand, a recent large prospective study conducted in Japan showed that green tea consumption was associated with a decrease risk of advanced prostate cancer, but not localized prostate cancer [48]. These results suggest that some well-designed intervention studies could provide a clear demonstration of the cancer-preventive and anti-cancer activities of green tea polyphenols.
Green Tea and Breast Cancer
Similar to prostate cancer, epidemiologic studies have shown inconsistent results on association between green tea consumption and risk of breast cancer. For example, one meta-analysis included 13 papers that examined populations in eight countries and indicated a lower risk for breast cancer with green tea consumption [49]. Another meta-analysis indicated that consumption of 5 or more cups of green tea a day resulted in a non-statistically significant trend towards the prevention of breast cancer development [50]. However, recently, a large nested case-control study also demonstrated no overall association between plasma green tea polyphenols and the risk of breast cancer in Japan [51].
While some studies point toward no risk-reducing benefit of breast cancer in tea drinkers, numerous studies indicate quite the opposite. In fact, green tea protection against breast cancer was observed among carriers of low-activity COMT alleles but not among those who possessed high-activity COMT alleles in Asian-American women (discussed in section 2.3) [31]. Likewise, a low risk of breast cancer among women with higher green tea intake and the low-activity genotype of angiotensin-converting enzyme gene was observed among Singapore Chinese women [52] and regular consumption of green tea is associated with improved prognosis of breast cancer at early stages (I and II) [53, 54]. Other studies conducted in China indicated that drinking green tea is associated with a decreased risk for breast cancer compared to non-drinkers [55, 56]. Results showed that breast cancer risk was reduced in association with years of green tea drinking and with the amount of tea consumed per month [56].
Green Tea and Gastrointestinal Cancer
Green tea consumption has also been shown to be protective against the development of colon cancer and gastric cancer, yet some controversial data still persists. After the ingestion of a single dose of green tea, basal levels of prostaglandin E2 (PGE2), a biomarker of colorectal carcinogenesis, was reduced, suggesting green tea as a colorectal chemopreventive agent [57]. An analysis of six cohort studies that measured green tea consumption revealed a significantly decreased risk of gastric cancer in women whose intake was greater than or equal to five cups a day [58] and in women whose plasma levels of EGCG were the highest [59]. However, a large Phase II trial investigating the chemopreventive effects of decaffeinated green tea on esophageal squamous carcinogenesis in China concluded that decaffeinated green tea intervention for one year was not sufficient to alleviate esophageal precancerous lesions [60]. Furthermore, a meta-analysis to determine the association between the consumption of green tea and the risk of stomach cancer in cohort studies found no preventive effect [61].
Green Tea and Lung Cancer
Studies investigating the association of green tea consumption with lung cancer risk have reported inconsistent findings. In support of green tea consumption on reduced lung cancer risk, a meta-analysis was conducted by a literature search in PubMed from 1966 to 2008. The overall evaluation of 22 relevant studies suggests that high consumption of green tea but not black tea may be related to the reduction of lung cancer risk [62]. On the other hand, a large population-based prospective cohort study in Japan found no evidence that the consumption of green tea reduces lung cancer incidence [63].
Green Tea and Skin Cancer
Epidemiological evidence and laboratory studies demonstrate that solar ultraviolet (UV) radiation is the major reason for the initiation of skin cancer [64]. In animal models, oral administration or topical treatment of green tea provided significant protection against UV radiation-induced skin tumorigenesis (reviewed in [65]). Green tea administration in the drinking water to SKH-1 hairless mice was found to protect against UVB radiation-induced tumorigenesis [66, 67]. Green tea was also shown inhibitory effects at the stage of skin cancer progression; for example, it caused partial regression of established skin papillomas in mice [68].
In human, green tea drinking or topical treatment were shown to protect against the harmful effects of solar UV radiation. The topical application of green tea on the skin prior to UV irradiation apparently reduced the UV-induced erythema response, the number of sunburned cells as well as UV-induced DNA damage [69]. The topical treatment of human skin with green tea or EGCG resulted in inhibition of UVB-induced myeloperoxidase activation and prostaglandin metabolites production, which play a critical role in tissue infiltration and inflammatory disorders [70].
For each of the various types of cancers described above some controversy exists for risk reduction in association with green tea consumption. However, the overwhelming evidence suggests that the purified form of the most prevalent green tea polyphenol, EGCG, acts as an anticancer agent in tumor cells (for prostate cancer alone: [71–75]). Finally, it should be noted that consumption of green tea products should probably be contraindicated during therapy for multiple myeloma with bortezomib [76]. It appears that EGCG antagonizes the cell-killing efficacy of bortezomib by reacting directly with it. Therefore the potential nutrition–drug interactions should also be kept in mind when investigating the combinational therapies with natural compounds.
MOLECULAR MECHANISMS OF GREEN TEA POLYPHENOL EGCG
Green tea polyphenols exert chemopreventive effects through induction of cell cycle arrest and apoptosis or suppression of angiogenesis and metastasis.
Green Tea Polyphenols Induce Tumor Cell Cycle Arrest
Green tea polyphenol EGCG inhibits cell proliferation, blocks cell cycle progression, and induces apoptosis in a variety of cancer cell lines [77–81]. EGCG significantly increases the expression of tumor-suppressor proteins p53, p21, Fas/APO-1, and Bax in p53-positive Hep G2 cells [78] and similar results were observed in human prostate cancer cells [82]. In fact, when LNCaP cells with wild-type p53 were used as a model to study the role of p53 and NF-κB in EGCG-induced growth arrest and apoptosis, it was found that EGCG could stabilize p53 protein via phosphorylation of critical serine residues on p53 and modulation of MDM2-p14ARF pathway. Furthermore the results demonstrated that activation of p53-dependent downstream targets p21 and Bax and down-regulation of NF-κB-dependent Bcl-2 resulted in growth arrest and apoptosis [82]. Another study showed that in Hep G2 cells, EGCG blocked cell cycle progression at G1 by p53 expression and p21 up-regulation [83].
Green Tea Polyphenols Induce Tumor Cell Apoptosis
Regarding to apoptosis induction, we have found that growth-arrested prostate cancer cells express high levels of hyperphosphorylated Bcl-XL in mitochondria and that treatment with green tea polyphenols or EGCG block expression of the hyper-, but not hypo-phosphorylated Bcl-XL, accompanied by cytochrome c release, caspase activation, and apoptosis [84]. Furthermore, EGCG has been shown to induce cell death via activation of caspase-8 and down-regulation of Bid in MIA PaCa-2 cells while a dominant negative caspase-8 variant of the same cells abrogates EGCG-induced apoptosis [85]. A similar analysis of acute myeloid leukemia cells showed an increase of death-associated protein kinase 2 (DAPK2) levels and cell death after treatment with EGCG while cell death was significantly reduced in DAPK2 silenced cells [86].
Green Tea Polyphenols Inhibit Tumor Angiogenesis
The process of tumor vessel formation, migration, and metastasis is required for tumor cell survival in which vascular endothelial growth factor (VEGF) plays a critical role. EGCG exhibits anti-angiogenic activities in various experimental studies [87, 88], which is associated with a decrease of VEGF production [89–92], expression [93–95], binding activity [96] and phosphorylation [97]. VEGF is a downstream target of hypoxia-inducible factor-1 (HIF-1), which is a transcription factor of oxygen-regulated genes that are involved in cell proliferation, cell survival, and angiogenesis [98]. EGCG significantly inhibits the protein level of transcription factor HIF-1α, and the mechanism may involve an increase in HIF-1α protein degradation [94]. Additionally, signal transducer and activator of transcription 3 (STAT3) regulates the transcriptional activation of genes involved in angiogenesis such as VEGF [99] and EGCG treatment has been shown to markedly reduce activation of STAT3, resulting in reduced VEGF protein expression both in vitro and in vivo [95].
Green Tea Polyphenols Suppresse Cancer Metastasis
Matrix metalloproteinases represent a class of proteases that are involved in tumor cell invasion and metastasis [100] and active matrix metalloproteinase (MMP), such as MMP-2 and MMP-9, are generated through cleavage of inactive forms by membrane-type 1 (MT1-MMP). EGCG has been shown to reduce MMP-2 and MMP-9 expression and enzymatic activities [101, 102] via inhibition of MT1-MMP [103]. Reversion-inducing, cysteine-rich protein with Kazal motifs (RECK) is a newly identified tumor suppressor gene that negatively regulates MMPs. Treatment of oral cancer cells with EGCG partially reversed the hypermethylation status of the RECK gene and significantly enhanced the expression level of RECK mRNA and correlated with the inhibition of MMP-2 and MMP-9 levels after treatment with EGCG [104]. Finally, EGCG also inhibits the activation of β1 integrin expression and activation of its downstream molecules such as focal adhesion kinase (FAK), AKT and extracellular signal-regulated kinase (ERK), resulting in inhibition of invasion and migration in salivary gland carcinoma cells [102].
CONCLUSION
Green tea or its active constituent is widely investigated in various cancers and multiple investigations indicate that green tea consumption is associated with reduced cancer risk although human epidemiological studies still remain inconsistent. Well-controlled clinical studies are required to clarify which populations will reap the greatest health benefits from green tea consumption and the amount of green tea to be consumed. Green tea polyphenols target a wide range of molecules that influence cell proliferation, cell death, angio-genesis and metastasis. While the chemopreventive mechanism of green tea cannot be limited to a specific pathway, protein, or gene, its use as a therapeutic agent has limitless possibilities in its natural and analog forms and should continue to be pursued in future studies.
Acknowledgments
This work is supported in part by research grants from the National Cancer Institute-National Institutes of Health (1R01CA120009, 3R01CA120009-04S1, to QPD).
ABBREVIATIONS
- Boc
tert-butoxycarbonyl
- C
catechin
- CG
catechin-3-gallate
- COMT
catechol-O-methyltransferase
- DAPK2
death-associated protein kinase 2
- DNC
3,5-dinitrocatechol
- EC
epicatechin
- ECG
epicatechin-3-gallate
- EGC
epigallocatechin
- EGCG
epigallocatechin-3-gallate
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- GC
gallocatechin
- GCG
gallocatechin-3-gallate
- MMP
matrix metalloproteinase
- Para-amino EGCG
EGCG analog possessing a p-NH2 on the D ring
- PGPH
peptidylglutamyl peptide hydrolyzing
- PGE2
prostaglandin E2
- Pro-EGCG
the acetate protected EGCG analog
- Pro-F- EGCG2
acetate protected fluoro-substituted benzoates of EGCG at the meta position
- Pro-F- EGCG4
acetate protected 3,4-difluorobenzoate EGCG
- RECK
reversion-inducing, cysteine-rich protein with Kazal motifs
- STAT
signal transducer and activator of transcription
- SAR
structure-activity relationship
- Thr 1
N-terminal threonine
- UV
ultraviolet
- VEGF
vascular endothelial growth factor
References
- 1.Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22(5):442–451. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 2.Groll M, Heinemeyer W, Jager S, Ullrich T, Bochtler M, Wolf DH, Huber R. The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proc Natl Acad Sci (USA) 1999;96(20):10976–10983. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals) IDrugs. 2002;5(8):828–34. [PubMed] [Google Scholar]
- 4.Li B, Dou QP. Bax degradation by the ubiquitin/proteasomedependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci (USA) 2000;97(8):3850–3855. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269(5224):682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 6.Perkins ND. The Rel/NF-kappa B family: friend and foe. Trends Biochem Sci. 2000;25(9):434–440. doi: 10.1016/s0968-0004(00)01617-0. [DOI] [PubMed] [Google Scholar]
- 7.Orlowski RZ. Proteasome inhibitors in cancer therapy. Methods Mol Biol. 2005;301:339–350. doi: 10.1385/1-59259-895-1:339. [DOI] [PubMed] [Google Scholar]
- 8.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85(13):1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 9.Nam S, Smith DM, Dou QP. Ester bond-containing tea poly-phenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276(16):13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 10.Bonfili L, Cecarini V, Amici M, Cuccioloni M, Angeletti M, Keller JN, Eleuteri AM. Natural polyphenols as proteasome modulators and their role as anti-cancer compounds. FEBS J. 2008;275(22):5512–5526. doi: 10.1111/j.1742-4658.2008.06696.x. [DOI] [PubMed] [Google Scholar]
- 11.Pettinari A, Amici M, Cuccioloni M, Angeletti M, Fioretti E, Eleuteri AM. Effect of polyphenolic compounds on the proteolytic activities of constitutive and immuno-proteasomes. Antioxid Redox Signal. 2006;8(1–2):121–129. doi: 10.1089/ars.2006.8.121. [DOI] [PubMed] [Google Scholar]
- 12.Kazi A, Wang Z, Kumar N, Falsetti SC, Chan TH, Dou QP. Structure-activity relationships of synthetic analogs of (−)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Res. 2004;24(2B):943–954. [PubMed] [Google Scholar]
- 13.Smith DM, Daniel KG, Wang Z, Guida WC, Chan TH, Dou QP. Docking studies and model development of tea polyphenol proteasome inhibitors: applications to rational drug design. Proteins Struct Funct Bioinformatics. 2004;54(1):58–70. doi: 10.1002/prot.10504. [DOI] [PubMed] [Google Scholar]
- 14.Wan SB, Chen D, Dou QP, Chan TH. Study of the green tea polyphenols catechin-3-gallate (CG) and epicatechin-3-gallate (ECG) as proteasome inhibitors. Bioorg Med Chem. 2004;12(13):3521–3527. doi: 10.1016/j.bmc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Mozzicafreddo M, Cuccioloni M, Cecarini V, Eleuteri AM, Angeletti M. Homology modeling and docking analysis of the interaction between polyphenols and mammalian 20S proteasomes. J Chem Inf Model. 2009;49(2):401–409. doi: 10.1021/ci800235m. [DOI] [PubMed] [Google Scholar]
- 16.Smith DM, Wang Z, Kazi A, Li LH, Chan TH, Dou QP. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol Med. 2002;8(7):382–392. [PMC free article] [PubMed] [Google Scholar]
- 17.Wan SB, Landis-Piwowar KR, Kuhn DJ, Chen D, Dou QP, Chan TH. Structure-activity study of epigallocatechin gallate (EGCG) analogs as proteasome inhibitors. Bioorg Med Chem. 2005;13(6):2177–2185. doi: 10.1016/j.bmc.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 18.Lam WH, Kazi A, Kuhn DJ, Chow LM, Chan AS, Dou QP, Chan TH. A potential prodrug for a green tea polyphenol proteasome inhibitor: evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG] Bioorg Med Chem. 2004;12(21):5587–5593. doi: 10.1016/j.bmc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn DJ, Lam WH, Kazi A, Daniel KG, Song S, Chow LM, Chan TH, Dou QP. Synthetic peracetate tea polyphenols as potent proteasome inhibitors and apoptosis inducers in human cancer cells. Front Biosci. 2005;10:1010–1023. doi: 10.2741/1595. [DOI] [PubMed] [Google Scholar]
- 20.Landis-Piwowar KR, Kuhn DJ, Wan SB, Chen D, Chan TH, Dou QP. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (−)-EGCG analogs and their pro-drugs. Int J Mol Med. 2005;15(4):735–742. [PubMed] [Google Scholar]
- 21.Landis-Piwowar KR, Huo CD, Chen D, Cui QC, Minic V, Shi GQ, Chan TH, Dou QP. A novel pro-drug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anti-cancer agent. Cancer Res. 2007;67(9):4303–4310. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 22.Osanai K, Huo C, Landis-Piwowar KR, Dou QP, Chan TH. Synthesis of (2R,3R)-epigallocatechin-3-O-(4-hydroxybenzoate): a novel catechin from Cistus salvifolius, and evaluation of its proteasome inhibitory activities. Tetrahedron. 2007;63:7565–7570. doi: 10.1016/j.tet.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osanai K, Landis-Piwowar KR, Dou QP, Chan TH. A para-amino substituent on the D-ring of green tea polyphenol epigallocatechin-3-gallate as a novel proteasome inhibitor and cancer cell apoptosis inducer. Bioorg Med Chem. 2007;15(15):5076–5082. doi: 10.1016/j.bmc.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Z, Qin XL, Gu YY, Chen D, Cui QC, Jiang T, Wan SB, Dou QP. Prodrugs of fluoro-substituted benzoates of EGC as tumor cellular proteasome inhibitors and apoptosis inducers. Int J Mol Sci. 2008;9(6):951–961. doi: 10.3390/ijms9060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Sun DK, Chen D, Cui QC, Gu YY, Jiang T, Chen W, Wan SB, Dou QP. Antitumor activity of novel fluoro-substituted (−)-epigallocatechin-3-gallate analogs. Cancer Lett. 2010;292(1):48–53. doi: 10.1016/j.canlet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puig T, Turrado C, Benhamú B, Aguilar H, Relat J, Ortega-Gutiérrez S, Casals G, Marrero PF, Urruticoechea A, Haro D, López-Rodríguez ML, Colomer R. Novel Inhibitors of Fatty Acid Synthase with Anticancer Activity. Clin Cancer Res. 2009;15(24):7608–7615. doi: 10.1158/1078-0432.CCR-09-0856. [DOI] [PubMed] [Google Scholar]
- 27.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos. 2003;31(5):572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 28.Okushio K, Suzuki M, Matsumoto N, Nanjo F, Hara Y. Methylation of tea catechins by rat liver homogenates. Biosci Biotechnol Biochem. 1999;63(2):430–432. doi: 10.1271/bbb.63.430. [DOI] [PubMed] [Google Scholar]
- 29.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- 31.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63(21):7526–7529. [PubMed] [Google Scholar]
- 32.Landis-Piwowar KR, Wan SB, Wiegand RA, Kuhn DJ, Chan TH, Dou QP. Methylation suppresses the proteasome-inhibitory function of green tea polyphenols. J Cell Physiol. 2007;213(1):252–260. doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- 33.Landis-Piwowar KR, Milacic V, Dou QP. Relationship between the methylation status of dietary flavonoids and their growth-inhibitory and apoptosis-inducing activities in human cancer cells. J Cell Biochem. 2008;105(2):514–523. doi: 10.1002/jcb.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel KG, Landis-Piwowar KR, Chen D, Wan SB, Chan TH, Dou QP. Methylation of green tea polyphenols affects their binding to and inhibitory poses of the proteasome beta5 subunit. Int J Mol Med. 2006;18(4):625–632. [PubMed] [Google Scholar]
- 35.Landis-Piwowar KR, Chen D, Chan TH, Dou QP. Inhibition of catechol-O-methyltransferase activity in human breast cancer cells enhances the biological effect of the green tea polyphenol (−)-EGCG. Oncol Rep. 2010;24(2):563–569. doi: 10.3892/or_00000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huo C, Yang H, Cui QC, Dou QP, Chan TH. Proteasome inhibition in human breast cancer cells with high catechol-O-methyltransferase activity by green tea polyphenol EGCG analogs. Bioorg Med Chem. 2010;18(3):1252–1258. doi: 10.1016/j.bmc.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanwar J, Mohammad I, Yang H, Huo C, Chan TH, Dou QP. Computational modeling of the potential interactions of the proteasome β5 subunit and catechol-O-methyltransferase-resistant EGCG analogs. Int J Mol Med. 2010;26(2):209–215. doi: 10.3892/ijmm_00000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66(2):1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 39.Jain MG, Hislop GT, Howe GR, Burch JD, Ghadirian P. Alcohol and other beverage use and prostate cancer risk among Canadian men. Int J Cancer. 1998;78(6):707–711. doi: 10.1002/(sici)1097-0215(19981209)78:6<707::aid-ijc7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108(1):130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 41.Sonoda T, Nagata Y, Mori M, Kozako T, Li HC, Lema C, Yashiki S, Fujiyoshi T, Yoshinaga M, Nagata Y, Akiba S, Takezaki T, Yamada K, Sonoda S. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci. 2004;95(7):238–242. doi: 10.1111/j.1349-7006.2004.tb02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P. Tea consumption and cancer risk. Nutr Cancer. 1992;17(1):27–31. doi: 10.1080/01635589209514170. [DOI] [PubMed] [Google Scholar]
- 43.Slattery ML, West DW. Smoking, alcohol, coffee, tea, caffeine, and theobromine: risk of prostate cancer in Utah (United States) Cancer Causes Control. 1993;4(6):559–563. doi: 10.1007/BF00052432. [DOI] [PubMed] [Google Scholar]
- 44.Villeneuve PJ, Johnson KC, Kreiger N, Mao Y. Risk factors for prostate cancer: results from the Canadian National Enhanced Cancer Surveillance System. The Canadian Cancer Registries Epidemiology Research Group. Cancer Causes Control. 1999;10(5):355–367. doi: 10.1023/a:1008958103865. [DOI] [PubMed] [Google Scholar]
- 45.Allen NE, Sauvaget C, Roddam AW, Appleby P, Nagano J, Suzuki G, Key TJ, Koyama K. A prospective study of diet and prostate cancer in Japanese men. Cancer Causes Control. 2004;15(9):911–920. doi: 10.1007/s10552-004-1683-y. [DOI] [PubMed] [Google Scholar]
- 46.Kikuchi N, Ohmori K, Shimazu T, Nakaya N, Kuriyama S, Nishino Y, Tsubono Y, Tsuji I. No association between green tea and prostate cancer risk in Japanese men: the Ohsaki Cohort Study. Br J Cancer. 2006;95(3):371–373. doi: 10.1038/sj.bjc.6603230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989;49(7):1857–1860. [PubMed] [Google Scholar]
- 48.Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167(1):71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- 49.Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and breast cancer risk: a meta-analysis of epidemiological studies. Carcinogenesis. 2006;27(7):1310–1315. doi: 10.1093/carcin/bgi276. [DOI] [PubMed] [Google Scholar]
- 50.Seely D, Mills EJ, Wu P, Verma S, Guyatt GH. The effects of green tea consumption on incidence of breast cancer and recurrence of breast cancer: a systematic review and meta-analysis. Integr Cancer Ther. 2005;4(2):144–155. doi: 10.1177/1534735405276420. [DOI] [PubMed] [Google Scholar]
- 51.Iwasaki M, Inoue M, Sasazuki S, Miura T, Sawada N, Yamaji T, Shimazu T, Willett WC, Tsugane S. Plasma tea poly-phenol levels and subsequent risk of breast cancer among Japanese women: a nested case-control study. Breast Cancer Res Treat. 2010;124(3):827–834. doi: 10.1007/s10549-010-0916-x. [DOI] [PubMed] [Google Scholar]
- 52.Yuan JM, Koh WP, Sun CL, Lee HP, Yu MC. Green tea intake, ACE gene polymorphism and breast cancer risk among Chinese women in Singapore. Carcinogenesis. 2005;26(8):1389–1394. doi: 10.1093/carcin/bgi080. [DOI] [PubMed] [Google Scholar]
- 53.Nakachi K, Suemasu K, Suga K, Takeo T, Imai K, Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn J Cancer Res. 1998;89(3):254–261. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inoue M, Tajima K, Mizutani M, Iwata H, Iwase T, Miura S, Hirose K, Hamajima N, Tominaga S. Regular consumption of green tea and the risk of breast cancer recurrence: follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC): Japan. Cancer Lett. 2001;167(2):175–182. doi: 10.1016/s0304-3835(01)00486-4. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, Holman CD, Huang JP, Xie X. Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis. 2007;28(5):1074–1078. doi: 10.1093/carcin/bgl252. [DOI] [PubMed] [Google Scholar]
- 56.Shrubsole MJ, Lu W, Chen Z, Shu XO, Zheng Y, Dai Q, Cai Q, Gu K, Ruan ZX, Gao YT, Zheng W. Drinking green tea modestly reduces breast cancer risk. J Nutr. 2009;139(2):310–316. doi: 10.3945/jn.108.098699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.August DA, Landau J, Caputo D, Hong J, Lee MJ, Yang CS. Ingestion of green tea rapidly decreases prostaglandin E2 levels in rectal mucosa in humans. Cancer Epidemiol Biomarkers Prev. 1999;8(8):709–713. [PubMed] [Google Scholar]
- 58.Inoue M, Sasazuki S, Wakai K, Suzuki T, Matsuo K, Shimazu T, Tsuji I, Tanaka K, Mizoue T, Nagata C, Tamakoshi A, Sawada N, Tsugane S. Green tea consumption and gastric cancer in Japanese: a pooled analysis of six cohort studies. Gut. 2009;58(10):1323–1332. doi: 10.1136/gut.2008.166710. [DOI] [PubMed] [Google Scholar]
- 59.Sasazuki S, Inoue M, Miura T, Iwasaki M, Tsugane S. Plasma tea polyphenols and gastric cancer risk: a case-control study nested in a large population-based prospective study in Japan. Cancer Epidemiol Biomarkers Prev. 2008;17(2):343–351. doi: 10.1158/1055-9965.EPI-07-0428. [DOI] [PubMed] [Google Scholar]
- 60.Wang LD, Zhou Q, Feng CW, Liu B, Qi YJ, Zhang YR, Gao SS, Fan ZM, Zhou Y, Yang CS, Wei JP, Zheng S. Intervention and follow-up on human esophageal precancerous lesions in Henan, northern China, a high-incidence area for esophageal cancer. Gan To Kagaku Ryoho. 2002;29(Suppl):159–172. [PubMed] [Google Scholar]
- 61.Myung SK, Bae WK, Oh SM, Kim Y, Ju W, Sung J, Lee YJ, Ko JA, Song JI, Choi HJ. Green tea consumption and risk of stomach cancer: a meta-analysis of epidemiologic studies. Int J Cancer. 2009;124(3):670–637. doi: 10.1002/ijc.23880. [DOI] [PubMed] [Google Scholar]
- 62.Tang N, Wu Y, Zhou B, Wang B, Yu R. Green tea, black tea consumption and risk of lung cancer: a meta-analysis. Lung Cancer. 2009;65(3):274–283. doi: 10.1016/j.lungcan.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Li Q, Kakizaki M, Kuriyama S, Sone T, Yan H, Nakaya N, Mastuda-Ohmori K, Tsuji I. Green tea consumption and lung cancer risk: the Ohsaki study. Br J Cancer. 2008;99(7):1179–1184. doi: 10.1038/sj.bjc.6604645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA. 1991;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nichols JA, Katiyar SK. Skin photoprotection by natural poly-phenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302(2):71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang ZY, Huang MT, Ferraro T, Wong CQ, Lou YR, Reuhl K, Iatropoulos M, Yang CS, Conney AH. Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice. Cancer Res. 1992;52(5):1162–1170. [PubMed] [Google Scholar]
- 67.Mittal A, Piyathilake C, Hara Y, Katiyar SK. Exceptionally high protection of photocarcinogenesis by topical application of (−)-epigallocatechin-3-gallate in hydrophilic cream in SKH-1 hairless mouse model: relationship to inhibition of UVB-induced global DNA hypomethylation. Neoplasia. 2003;5(6):555–565. doi: 10.1016/s1476-5586(03)80039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang ZY, Huang MT, Ho CT, Chang R, Ma W, Ferraro T, Reuhl KR, Yang CS, Conney AH. Inhibitory effect of green tea on the growth of established skin papillomas in mice. Cancer Res. 1992;52(23):6657–6665. [PubMed] [Google Scholar]
- 69.Elmets CA, Singh D, Tubesing K, Matsui M, Katiyar SK, Mukhtar H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J Am Acad Dermatol. 2001;44(3):425–432. doi: 10.1067/mjd.2001.112919. [DOI] [PubMed] [Google Scholar]
- 70.Katiyar SK, Matsui M, Elmets CA, Mukhtar H. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999;69(2):148–153. [PubMed] [Google Scholar]
- 71.Duhon D, Bigelow RL, Coleman DT, Steffan JJ, Yu C, Langston W, Kevil CG, Cardelli JA. The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-Met receptor in prostate cancer cells. Mol Carcinog. 2010;49(8):739–749. doi: 10.1002/mc.20649. [DOI] [PubMed] [Google Scholar]
- 72.Luo L, Luo JH, Yu YP. (−)-Epigallocatechin-3-gallate induces Du145 prostate cancer cell death via downregulation of inhibitor of DNA binding 2 a dominant negative helix-loop-helix protein. Cancer Sci. 2010;101(3):707–712. doi: 10.1111/j.1349-7006.2009.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsieh TC, Wu JM. Targeting CWR22Rv1 prostate cancer cell proliferation and gene expression by combinations of the phyto-chemicals EGCG, genistein and quercetin. Anticancer Res. 2009;29(10):4025–4032. [PMC free article] [PubMed] [Google Scholar]
- 74.Chuu CP, Chen RY, Kokontis JM, Hiipakka RA, Liao S. Suppression of androgen receptor signaling and prostate specific antigen expression by (−)-epigallocatechin-3-gallate in different progression stages of LNCaP prostate cancer cells. Cancer Lett. 2009;275(1):86–92. doi: 10.1016/j.canlet.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas F, Patel S, Holly JM, Persad R, Bahl A, Perks CM. Dihydrotestosterone sensitises LNCaP cells to death induced by epigallocatechin-3-Gallate (EGCG) or an IGF-I receptor inhibitor. Prostate. 2009;69(2):219–224. doi: 10.1002/pros.20873. [DOI] [PubMed] [Google Scholar]
- 76.Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, Petasis NA, Chen TC, Schonthal AH. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113(23):5927–5937. doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- 77.Smith DM, Dou QP. Green tea polyphenol epigallocatechin inhibits DNA replication and consequently induces leukemia cell apoptosis. Int J Mol Med. 2001;7(6):645–652. doi: 10.3892/ijmm.7.6.645. [DOI] [PubMed] [Google Scholar]
- 78.Kuo PL, Lin CC. Green tea constituent (−)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J Biomed Sci. 2003;10(2):219–227. doi: 10.1007/BF02256057. [DOI] [PubMed] [Google Scholar]
- 79.Ahmad N, Cheng P, Mukhtar H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem Biophys Res Commun. 2000;275(2):328–334. doi: 10.1006/bbrc.2000.3297. [DOI] [PubMed] [Google Scholar]
- 80.Liang YC, Lin-Shiau SY, Chen CF, Lin JK. Inhibition of cyclindependent kinases 2 and 4 activities as well as induction of Cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (−)-epigallocatechin-3-gallate. J Cell Biochem. 1999;75(1):1–12. [PubMed] [Google Scholar]
- 81.Liberto M, Cobrinik D. Growth factor-dependent induction of p21(CIP1) by the green tea polyphenol, epigallocatechin gallate. Cancer Lett. 2000;154(2):151–161. doi: 10.1016/s0304-3835(00)00378-5. [DOI] [PubMed] [Google Scholar]
- 82.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22(31):4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 83.Huang CH, Tsai SJ, Wang YJ, Pan MH, Kao JY, Way TD. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol Nutr Food Res. 2009;53(9):1156–1165. doi: 10.1002/mnfr.200800592. [DOI] [PubMed] [Google Scholar]
- 84.Kazi A, Smith DM, Zhong Q, Dou QP. Inhibition of bcl-x(l) phosphorylation by tea polyphenols or epigallocatechin-3-gallate is associated with prostate cancer cell apoptosis. Mol Pharmacol. 2002;62(4):765–771. doi: 10.1124/mol.62.4.765. [DOI] [PubMed] [Google Scholar]
- 85.Basu A, Haldar S. Combinatorial effect of epigallocatechin-3-gallate and TRAIL on pancreatic cancer cell death. Int J Oncol. 2009;34(1):281–286. [PubMed] [Google Scholar]
- 86.Britschgi A, Simon HU, Tobler A, Fey MF, Tschan MP. Epigallocatechin-3-gallate induces cell death in acute myeloid leukaemia cells and supports all-trans retinoic acid-induced neutrophil differentiation via death-associated protein kinase 2. Br J Haema-tol. 2010;149(1):55–64. doi: 10.1111/j.1365-2141.2009.08040.x. [DOI] [PubMed] [Google Scholar]
- 87.Kavantzas N, Chatziioannou A, Yanni AE, Tsakayannis D, Balafoutas D, Agrogiannis G, Perrea D. Effect of green tea on angiogenesis and severity of atherosclerosis in cholesterol-fed rabbit. Vascul Pharmacol. 2006;44(6):461–463. doi: 10.1016/j.vph.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Q, Tang X, Lu Q, Zhang Z, Rao J, Le AD. Green tea extract and (−)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol Cancer Ther. 2006;5(5):1227–1238. doi: 10.1158/1535-7163.MCT-05-0490. [DOI] [PubMed] [Google Scholar]
- 89.Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumor growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84(6):844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2(6):350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 91.Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132(8):2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- 92.Basini G, Bianco F, Grasselli F. EGCG, a major component of green tea, inhibits VEGF production by swine granulosa cells. Biofactors. 2005;23(1):25–33. doi: 10.1002/biof.5520230104. [DOI] [PubMed] [Google Scholar]
- 93.Kojima-Yuasa A, Hua JJ, Knnedy DO, Matsui-Yuasa I. Green tea extract inhibits angiogenesis of human umbilical vein endothelial cells through reduction of expression of VEGF receptors. Life Sci. 2003;73(10):1299–1313. doi: 10.1016/s0024-3205(03)00424-7. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Q, Tang X, Lu Q, Zhang Z, Rao J, Le AD. Green tea extract and (−)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol Cancer Ther. 2006;5(5):1227–1238. doi: 10.1158/1535-7163.MCT-05-0490. [DOI] [PubMed] [Google Scholar]
- 95.Zhu BH, Zhan WH, He YL, Cai SR, Wang Z, Zhang CH. Epigallocatechin-3-gallate inhibits growth and angiogenesis of gastric cancer and its molecular mechanism. Zhonghua Wei Chang Wai Ke Za Zhi. 2009;12(1):82–85. [PubMed] [Google Scholar]
- 96.Kondo T, Ohta T, Igura K, Hara Y, Kaji K. Tea catechins inhibit angiogenesis in vitromeasured by human endothelial cell growth migration and tube formation through inhibition of VEGF receptor binding. Cancer Lett. 2002;180(2):139–144. doi: 10.1016/s0304-3835(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 97.Lamy S, Gingras D, Beliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62(2):381–385. [PubMed] [Google Scholar]
- 98.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36(1):1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 99.Chen Z, Han ZC. STAT3: a critical transcription activator in angiogenesis. Med Res Rev. 2008;28(2):185–200. doi: 10.1002/med.20101. [DOI] [PubMed] [Google Scholar]
- 100.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fassina G, Vene R, Morini M, Minghelli S, Benelli R, Noonan DM, Albini A. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin Cancer Res. 2004;10(14):4865–4873. doi: 10.1158/1078-0432.CCR-03-0672. [DOI] [PubMed] [Google Scholar]
- 102.Park JH, Yoon JH, Kim SA, Ahn SG, Yoon JH. (−)-Epigallocatechin-3-gallate inhibits invasion migration of salivary gland adenocarcinoma cells. Oncol Rep. 2010;23(2):585–590. [PubMed] [Google Scholar]
- 103.Oku N, Matsukawa M, Yamakawa S, Asai T, Yahara S, Hashimoto F, Akizawa T. Inhibitory effect of green tea polyphenols on membrane-type 1 matrix metalloproteinase, MT1-MMP. Biol Pharm Bull. 2003;26(9):1235–1238. doi: 10.1248/bpb.26.1235. [DOI] [PubMed] [Google Scholar]
- 104.Kato K, Long NK, Makita H, Toida M, Yamashita T, Hatakeyama D, Hara A, Mori H, Shibata T. Effects of green tea polyphenol on methylation status of RECK gene and cancer cell invasion in oral squamous cell carcinoma cells. Br J Cancer. 2008;99(4):647–654. doi: 10.1038/sj.bjc.6604521. [DOI] [PMC free article] [PubMed] [Google Scholar]