Abstract
Tea is one of the most popular beverages in the world and has been studied extensively as a health-promoting beverage that may act to prevent a number of chronic diseases and cancers. (-)-Epigallocatechin gallate [(-)-EGCG], a major component in green tea, is unstable under physiological conditions and methylation of (-)-EGCG by catechol-O-methyltransferase (COMT) is a modification that reduces the biological activity of (-)-EGCG. In the current study, we hypothesized that suppression of COMT activity in human breast cancer cells could increase the proteasome-inhibitory potency of (-)-EGCG and therefore enhance its tumor cell growth-inhibitory activity. We first determined the COMT genotype and basal levels of COMT activity in various human breast cancer cell lines. Furthermore, when breast cancer MDA-MB-231 cells containing high COMT activity were tested, the diminished COMT activity apparently increased the effectiveness of (-)-EGCG via augmented proteasome inhibition and apoptosis induction. This study supplements the previous findings that methylated (-)-EGCG is less bioactive and supports the notion that COMT inhibition may increase the anti-cancer properties of tea polyphenols and the combination may serve as a novel approach or supplemental treatment for breast cancer chemotherapy.
Keywords: epigallocatechin gallate, catechol-O-methyltransferase, proteasome inhibition, apoptosis, breast cancer
Introduction
The anti-cancer and cancer-preventive effects of green tea and its main constituent (-)-epigallocatechin gallate [(-)-EGCG] are well documented in literature including cell culture, animal, epidemiological, and clinical studies (1–7). We previously reported that (-)-EGCG potently and specifically inhibits the chymotrypsin-like activity of the proteasome in vitro (IC50 = 86–194 nM), and induces tumor cell growth arrest in the G1 phase of the cell cycle (8). Furthermore, we have established that an ester bond within (-)-EGCG is critical for its proteasomal inhibitory properties (8).
The ubiquitin/proteasome system controls the turnover of critical regulatory proteins involved in several cellular processes such as cell cycle and apoptosis (9,10). The eukaryotic proteasome contains at least three known catalytic activities: chymotrypsin-like, trypsin-like, and caspase-like or peptidyl-glutamyl peptide-hydrolyzing (PGPH)-like activities (11). Our laboratory and others have reported that inhibition of the proteasome chymotrypsin-like activity is associated with induction of apoptosis in tumor cells (12,13). Since proteasome activity is essential for tumor cell proliferation and drug resistance development, the proteasome-mediated protein degradation pathway has been considered an important target for cancer prevention and therapy. In fact, the proteasome inhibitor Bortezomib (Velcade, PS-341) has been approved by the US Food and Drug Administration (FDA) and its anti-tumor activity has been reported in a variety of tumor models in clinical trials (14–16). Although the toxicity of bortezomib is predictable (17,18), there is still a need for the use of other proteasome inhibitors with less or no toxic side effects.
The caveat to the use of (-)-EGCG in cancer prevention and therapy is its low bioavailability due to its instablity under neutral or alkaline conditions (i.e. physiologic pH) and to biologically inactivating processes that include methylation (19–21). Methylation of (-)-EGCG and other tea catechins occurs by catechol-O-methyltransferase (COMT) enzyme activity (22). COMT is widely distributed throughout the body and functions as a detoxifying enzyme to eliminate biologically active or toxic catechols found in tissues (22). In humans, a single gene for COMT encodes both a soluble COMT (S-COMT) and a membrane-bound COMT (MB-COMT) that are transcribed by activation of two separate promoters. A single nucleotide polymorphism (G to A) in codon 108 (S-COMT) or 158 (MB-COMT) results in a valine to methionine (Val to Met) substitution in both forms of COMT proteins (23). This polymorphism is responsible for a high- [Val/Val (H/H)], intermediate- [Val/Met (H/L)], or low-activity [Met/Met (L/L)] form of COMT and correlates with a 3–4-fold difference in enzyme activity between the high- and low-activity expressed genes (23,24).
The potential for COMT to diminish the cancer preventive effects of (-)-EGCG in vivo was highlighted in a case-control study of breast cancer in Asian-American women (25). The study revealed a reduced risk of breast cancer in women who consumed green tea and who also carried the low activity COMT polymorphism. In contrast, breast cancer risk did not differ between tea drinkers and non-tea drinkers among those who were homozygous for the high activity COMT allele. These data suggest that methylated tea polyphenols may be less cancer-protective. While a molecular mechanism for the findings was not identified, a seeming predictor of (-)-EGCG potency in vivo may inversely correlate with individual COMT activity status. The reduced incidence of breast cancer in individuals who consumed green tea and harbored the low COMT activity polymorphism may be the result of less (-)-EGCG methylation which may allow for (-)-EGCG to act as a proteasome inhibitor more effectively. Furthermore, pharmacologically induced COMT inhibition may also decrease (-)-EGCG methylation thereby increasing its pro-teasome inhibitory potency. A variety of COMT inhibitors are commercially available including 3,5-dinitrocatechol (DNC). This potent, selective, and orally active COMT inhibitor offers a tool for interfering in the metabolism of various COMT substrates including (-)-EGCG.
In the current study, it was hypothesized that breast cancer cells expressing COMT-H/H would be more affected by the proteasome inhibitory activity of (-)-EGCG if they were also exposed to a COMT inhibitor than COMT-H/H cells exposed to (-)-EGCG alone. COMT status (L/L, H/L, and H/H) in various breast cancer cells lines was determined by genotyping. MDA-MB-231 breast cancer cells were found to possess the high COMT activity allele and were utilized to analyze proteasome activity and mechanisms of apoptosis after exposure to DNC and (-)-EGCG. Not only was DNC found to decrease COMT activity in various breast cancer cells, but co-treatment with DNC and (-)-EGCG was able to reduce cellular proliferation/viability. Furthermore, morphological changes and apoptotic nuclei were present in MDA-MB-231 cells and correlated with an increase in caspase-3/-7 activity after the co-treatment. Finally, the apoptotic changes observed after the co-treatment were associated with a decrease in proteasome activity. Therefore, the diminished COMT activity apparently increased the effectiveness of (-)-EGCG via mechanisms such as augmented proteasome inhibition and resulted in the induction of apoptosis. This study supplements the previous findings that methylated (-)-EGCG is less bioactive and provides valuable information for defining clinical criteria in cancer prevention and treatment.
Materials and methods
Materials
3,5-Dinitrocatechol (DNC), esculetin, S-(5′-adenosyl)-L-methionine (SAM), Bisbenzimide Hoechst no. 33258 stain, 3-[4,5-dimethylthiazol-2-yl]-2.5-diphenyl-tetrazolium bromide (MTT), DMSO, and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640, penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA). Fluorogenic peptide substrates Suc-LLVY-AMC (for the proteasomal chymotrypsin-like activity) and N-acetyl-DEVD-AMC (for caspase-3/-7 activity) were from Calbiochem (San Diego, CA, USA).
Cell culture and cell extract preparation
MCF10A (normal-MCF10), MCF10AT1K clone 2 [premalignant-MCF10 (abbreviated Kcl2)], and MCF10dcis.com [malignant-MCF10 (abbreviated DCIS)] breast cells were cultured as described previously (26). Briefly, MCF10A and Kcl2 cells were cultured in 1:1 F12/DMEM supplemented with 5.26% (v/v) horse serum, 100 U/ml of penicillin, 100 μg/ml of streptomycin, 100 ng/ml of cholera endotoxin, 10 μg/ml insulin, 10 ml of 1 M NaHCO3 in 500 ml medium, 20 ng/ml of epidermal growth factor, and 0.5 μg/ml hydrocortisone. DCIS cells were cultured in 1:1 F12/DMEM media supplemented with 5.26% (v/v) horse serum, 10 ml of 1 M NaHCO3, 100 U/ml of penicillin, and 100 μg/ml of streptomycin.
Human breast cancer MDA-MB-231 cells were grown in RPMI-1640 supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. ZR-75-1 cells were grown in RPMI with 2 mM L-glutamine adjusted to contain 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10 mM HEPES, 1.0 mM of 90% sodium pyruvate, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 10% fetal bovine serum. MCF-7 cells were grown in DMEM containing 10% fetal calf serum, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 0.01 mg/ml insulin. All cells were maintained at 37°C in a humidified incubator with an atmosphere of 5% CO2. A whole cell extract was prepared as described previously (10). Briefly, cells were harvested, washed with PBS and homogenized in a lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% NP-40, 0.5 mM phenylmethylsulfonyl fluoride, and 0.5 mM dithiothreitol) for 30 min at 4°C. Afterwards, the lysates were centrifuged at 12,000 x g for 12 min at 4°C and the supernatants collected as whole-cell extracts.
Cell viability/proliferation assay
The MTT assay was used to determine the effects of various compounds on proliferation of MDA-MB-231 breast cancer cells. Cells were plated onto a 96-well plate and grown to 70–80% confluency followed by addition of 10 μM of DNC and 50 μM of (-)-EGCG for 24 h incubation at 37°C. All co-treatments of DNC and (-)-EGCG were conducted such that DNC was incubated for 30 min before the addition of (-)-EGCG. Inhibition of cell proliferation was measured as previously described (27). All samples were assayed in triplicate in three independent experiments, and the mean value for each experiment was calculated. The results are displayed as mean (± standard deviation) and are expressed as percentage of the control, which was considered to be 100%.
Proteasome activity assay
The chymotrypsin-like proteasome activity assay fluorometrically measures proteasome activity in cells treated with a potential proteasome inhibitor. At the endpoint of these experiments, the amount of fluorescence measured correlates to the amount of proteasome activity. MDA-MB-231 breast cancer cells were grown to 30–40% confluency, treated daily with 50 μM of the indicated compound for 24 h, harvested, and used for whole cell extract preparation. Whole cell extracts (10 μg) were incubated with Suc-Leu-Leu-Val-Tyr-AMC (40 μM) fluorogenic substrate at 37°C in 100 μl of assay buffer (50 mM Tris-HCl, pH 8.0) for 2.5 h. After incubation, production of free hydrolyzed 7-amino-4-methylcoumarin (AMC) groups liberated by substrate hydrolysis was fluorometrically measured using a Victor 3 multilabel counter with an excitation filter of 380 nm and an emission filter of 460 nm (PerkinElmer, Boston, MA, USA).
Cellular and nuclear morphology analysis
A Zeiss (Thornwood, NY) Axiovert 25 microscope was used for all microscopic imaging with either phase contrast for cellular morphology or fluorescence for nuclear morphology with Hoechst staining, as previously described (27).
Caspase-3 activity assay
The caspase-3/-7 activity assay fluorometrically measures caspase activation in cells treated with a given compound. At the endpoint of these experiments, the amount of fluorescence measured correlates to the amount of caspase-3/-7 activity. Cells were treated with 50 μM of each compound, harvested, and lysed as described above. Ac-DEVD-AMC (40 μM) was then incubated with the prepared cell lysates (20 μg of protein) at 37°C in 100 μl of assay buffer (50 mM Tris-HCl, pH 7.5) for 2.5 h. The caspase-3 activity was measured by the production of free AMC groups liberated by substrate hydrolysis using a Victor 3 multilabel counter with an excitation filter of 380 nm and an emission filter of 460 nm as previously described (28).
COMT activity assay
The COMT activity assay was utilized based on previously published work (29). Briefly, esculetin (40 μM) and (-)-EGCG (50 μM) were dissolved in DMSO and diluted with aqueous buffer solution (100 mM phosphate, 5 mM MgCl2, 20 mM L-cysteine, pH 7.4) in 100 μl of reaction mixture which included 20 μg protein lysate (cell lysis is described above). The plate was placed on ice, and 20 μg/ml of protein from cell lysate was added. A preincubation period of 5 min was performed by placing the plate at 37°C. The reaction was initiated by the addition of 200 μM SAM, at 37°C and the reaction was followed for 60 min using a Victor 3 multilabel counter with an excitation filter of 380 nm and an emission filter of 460 nm. The change in fluorescence caused by enzymatic O-methylation of esculetin to scopoletin was used to describe inhibitory activity. Controls without SAM were also included.
COMT sequencing
The 5′-nuclease assay using TaqMan, (Applied Biosystems, University Park, IL, USA) was used to detect the COMT rs4680 polymorphism. Oligonucleotide primers and probes were designed using Primer Express software (Applied Biosystems). Standard TaqMan conditions using 1X Universal PCR mix (Applied Biosystems), 25 ng DNA, COMT-F (CGACTGTGCCGCCATCA) and COMT-R (AACGGGTCAGGCATGCA) primers, and COMTGwt (6FAM-CTTGTCCTTCACGCCAGCGAAAT-TAMRA) and COMTA (VIC-CCTTGTCCTTCATGCCAGCGAA-TAMRA) probes. An Applied Biosystems 7900 was used for the amplification and detection of products. For quality control, 5% of the products were sequenced and 10% were directly repeated.
Results
COMT activity and genotypes varies in different human breast cell lines
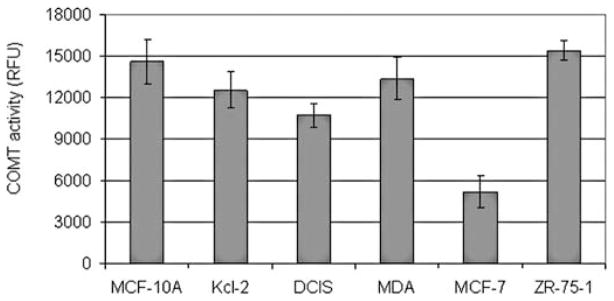
Six human breast cell lines including MCF10A, Kcl-2, DCIS, MDA-MB-231, ZR-75-1 and MCF-7 were analyzed to determine their COMT status. COMT genotyping revealed that all cell lines, except the MCF-7 cell line, expressed the COMT-H allele (Table I). These findings correlate with a previous study in which MCF10A and ZR-75-1 cells exhibited the COMT-H encoding allele and MCF-7 the COMT-L allele (30). Additionally, a fluorogenic assay was performed using whole cell lysates to test COMT enzymatic activity. COMT activity was lowest in MCF-7 cells compared to all other cell lines (Fig. 1).
Table I.
Genotype and corresponding activity of human breast normal and cancer cell lines.
| Human breast cell lines | Polymorphism of the gene | Corresponding COMT activity |
|---|---|---|
| MCF 10A | G/G | High |
| MCF 10AT1K clone 2 | G/G | High |
| MCF 10DCIS.com | G/G | High |
| MDA-MB-231 | G/G | High |
| ZR-75-1 | G/G | High |
| MCF-7 | A/A | Low |
Figure 1.
COMT activity in human normal and breast cancer cell lines. COMT activity in whole cell extracts was measured using a fluorogenic activity assay as described in the Materials and methods. MCF-7 cells displayed low activity compared to all other cell lines.
COMT inhibition enhances proteasome inhibition and apoptosis induction by (-)-EGCG in MDA-MB-231 cells
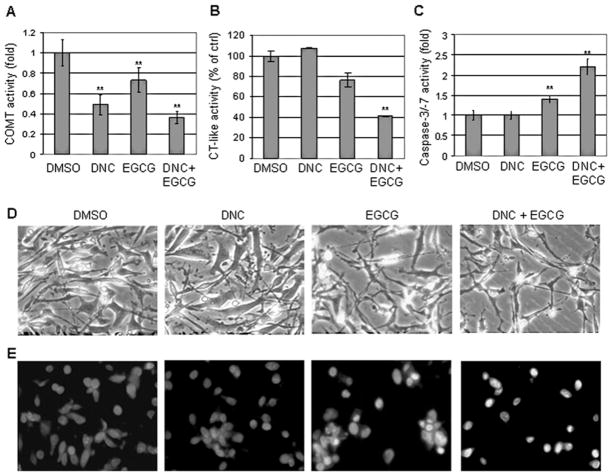
It was surmised that (-)-EGCG would be less bio-effective in cells harboring high COMT activity and that a COMT inhibitor could rescue the effectiveness of (-)-EGCG in these cells. To test this hypothesis, we first examined COMT activity after exposure to (-)-EGCG and the COMT inhibitor, DNC. We performed analysis with various concentrations of DNC on all cell lines described herein (data not shown) and observed COMT inhibition without cell death. We chose the concentration of DNC that provided optimum COMT inhibition for these studies. MDA-MB-231 cells, possessing high COMT activity (Table I), were treated with either 10 μM of DNC, 50 μM of (-)-EGCG, or a combination of both for 24 h, followed by measurement of COMT activity, proteasomal chymotrypsin-like activities, and apoptosis induction. In MDA-MB-231 cells treated with DNC, COMT activity was inhibited by ~50% (Fig. 2A). (-)-EGCG also apparently inhibited COMT enzymatic activity, which is consistent with the notion that (-)-EGCG is a COMT substrate (31). Furthermore, the co-treatment with both DNC and (-)-EGCG decreased COMT activity by >60%, indicating additive inhibition by the two compounds (Fig. 2A).
Figure 2.
Inhibition of COMT activity enhances proteasome inhibition and apoptosis induction by (-)-EGCG in breast cancer cells. MDA-MB-231 cells were treated with either 10 μM of the COMT inhibitor (DNC), 50 μM of (-)-EGCG, or a co-treatment with both DNC and (-)-EGCG for 24 h, followed by measuring COMT activity (A), proteasomal chymotrypsin (CT)-like activity (B), caspase-3/-7 activation (C), cellular morphological changes (D), and Hoechst nuclear staining (E). DMSO was used as solvent control. bars, SD; *p<0.05; **p<0.01. Magnification, x40.
Since COMT activity could be suppressed in cells possessing high activity, we presumed that proteasome inhibition would be enhanced upon co-treatment with (-)-EGCG and DNC. The proteasomal chymotrypsin (CT)-like activity in MDA-MB-231 cells was assessed next. DNC had no effect on proteasome activity and treatment with 50 μM (-)-EGCG exhibited only ~23% inhibition after 24 h treatment (Fig. 2B). However, after co-treatment with DNC and (-)-EGCG, 60% inhibition in the proteasomal chymotrypsin-like activity was observed (Fig. 2B). These findings indicate that (-)-EGCG appears to be a more potent proteasome inhibitor after COMT inhibition.
To determine whether (-)-EGCG could induce more cell death when combined with DNC, caspase-3/-7 activation, morphological changes, and Hoechst nuclear staining were examined in MDA-MB-231 cells. Treatment with DNC had no effect on caspase-3/-7 activation and (-)-EGCG treatment alone increased caspase-3/-7 activities by ~1.5 fold (Fig. 2C). The (-)-EGCG and DNC combination treatment resulted in a 2.1-fold increase of caspase-3/-7 activities. Cells treated with (-)-EGCG alone displayed minimal effect on cell morphology while co-treatment with DNC and (-)-EGCG produced morphology indicative of cellular stress (Fig. 2D). Furthermore, Hoechst staining revealed that cells co-treated with DNC and (-)-EGCG resulted in punctuate, brightly stained apoptotic nuclei (Fig. 2E). These data suggest that suppressed COMT activity could enhance the apoptosis inducing effect of (-)-EGCG.
COMT inhibition enhances growth inhibition by (-)-EGCG in human cancer but not normal, nontransformed breast cells
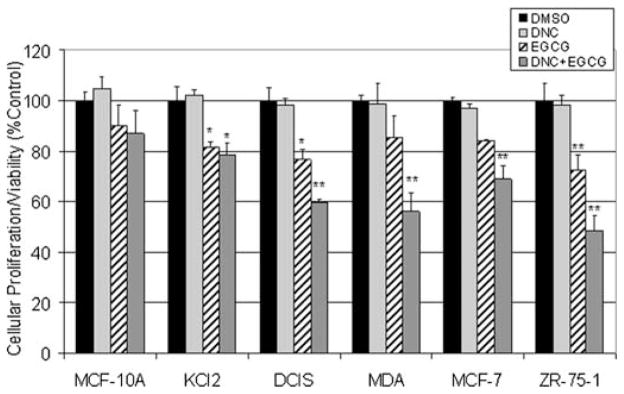
Finally, inhibition of cell proliferation by (-)-EGCG after COMT inhibition was evaluated using the MTT assay in various human cancer and nontranformed breast cell lines. DNC was used to compare the effects of pharmacological COMT inhibition vs. native COMT activity in various breast cancer cell lines. In the MCF10A series of tumor cells, the ‘normal’ MCF10A cells were minimally affected by any of the treatments. Co-treatment with DNC and (-)-EGCG reduced cell viability by only 12% (Fig. 3). As the tumor cell lines increased in aggressiveness, a decrease in cell viability was observed after co-treatment with DNC and (-)-EGCG, indicated by 21 and 40% inhibition in cell viability in Kcl2 and DCIS cells, respectively (Fig. 3). Furthermore, MDA-MB-231 cells exhibited a 45% decrease in cell viability after co-treatment with DNC and (-)-EGCG and only 15% decrease in cell viability after (-)-EGCG treatment alone (Fig. 3). This 3-fold difference in cellular viability is most likely due to the increased effectiveness of (-)-EGCG upon inhibition of the enzymatic O-methylation activity of COMT.
Figure 3.
Human breast cancer or nontransformed cell proliferation/viability after exposure to DNC and (-)-EGCG. An MTT assay was used to detect cell proliferation/viability in indicated breast cancer cells or nontransformed MCF-10A cells, as described in the Materials and methods. Treatments included either 10 μM of the COMT inhibitor (DNC), 50 μM of (-)-EGCG, or a co-treatment with both DNC and (-)-EGCG for 24 h. The DMSO control was set at the volume corresponding to the co-treatment. bars, SD; *p<0.05; **p<0.01.
Discussion
Proteasome inhibition by (-)-EGCG could serve as a non-toxic chemopreventive strategy and has been shown to impart selective activity against rapidly proliferating tumor cells over non-transformed or normal cells (8,32,33). However, reduced biological activity of (-)-EGCG is observed in the presence of O-methylations corresponding to reduced proteasome-inhibitory activities (20,21,34–36). Therefore, manipulating the activity of COMT to limit its methylating activity toward polyphenolic catechins could increase the effectiveness of these proteasome inhibitors.
Not only does COMT catalyze the methylation of (-)-EGCG, but also of both 2-OH and 4-OH catechol estrogens to methoxy estrogens, which reduces their potential for DNA damage (37) and increases the concentration of 2-methoxy-estradiol, an anti-proliferative metabolite for human breast cancer, lung cancer, and leukemia cells (38–40). Since COMT is expressed ubiquitously, it is likely that the COMT activity level influences the amount of circulating catecholamines, catechol estrogens, and polyphenolic catechins throughout the body. Therefore, the purported anti-cancer effects of methoxy estrogens would be considerably less in individuals who harbor the low COMT activity allele. In fact, numerous studies have indicated that COMT-L is associated with an increased risk of breast cancer (41–43). Likewise, COMT-L was found to be associated with progression and lymph node metastasis of breast cancer in Japanese women (44). Others have indicated a non-significant or limited association with COMT-L and breast cancer risk, but preclude their findings by stratifying data according to menopausal status (45,46).
While an increased risk of breast cancer may be associated with COMT-L, other studies have indicated that consumption of green tea may be protective in individuals carrying the genotype. Indeed, a study conducted by Wu et al indicated that green tea consumption was a factor for breast cancer risk reduction (6). In a later study, the same group specified that women who drank green tea and carried the COMT-L allele had a statistically significant reduced risk of breast cancer compared to those individuals who carried the high activity allele and displayed neither reduced nor increased risk of breast cancer (25). Furthermore, a decrease in plasma estrone and estradiol were found to be the result of green tea consumption (47). Collectively, these studies suggest that the carcinogenic activity of estrogens is modulated not only by green tea consumption, but also by COMT status. Therefore, modulating the effects of COMT in breast cancer by green tea consumption or exposure to (-)-EGCG may correlate with a decrease in disease incidence, progression, or recurrence.
Since low COMT activity and consumption of tea polyphenols have been linked to a decreased risk in breast cancer, this study was focused on an examination of COMT status in breast cancer cell lines and manipulation of COMT activity in those cells. It was hypothesized that suppression of COMT in cells expressing COMT-H would be more affected by the proteasome inhibitory activity of (-)-EGCG than COMT-H cells with unaltered COMT activity. The COMT-H expressing MDA-MB-231 breast cancer cells were studied extensively in this study and were utilized to detect proteasome activity and mechanisms of apoptosis after COMT manipulation and exposure to (-)-EGCG.
A panel of breast cancer cell lines was assayed via DNA sequencing to determine their COMT genotype. A previous study indicated that MCF-10A and ZR-75-1 cells possessed the COMT-H encoding allele while MCF-7 cells possessed the COMT-L encoding allele (30). The findings presented herein are in agreement with this previous study (Table I). However, until now, no other study has examined the COMT status in other cell lines such as Kcl2, DCIS, and MDA-MB-231, all of which were found in this work to be homozygous for COMT-H (Table I).
A COMT activity assay was used to correlate the genotyping results with the biological COMT activities of the breast cancer cell lines. Similar to the genotyping, the breast cancer cells exhibited differences in their basal COMT activity. In cells that were found by genotyping to be COMT-H expressing, the COMT activity assay indicated higher activity by as much as 3-fold compared to the MCF-7 cells that were expressing the COMT-L phenotype (Fig. 1). This is consistent with a previous study that described MCF-7 cells that expressed COMT activity 2–3-fold lower than ZR-75-1 cells (48). Because of the lack of evidence for MDA-MB-231 cells in the literature and because these COMT activity results were so promising, a focused examination of MDA-MB-231 cells for COMT manipulation in additional experiments was warranted.
DNC was used to pharmacologically inhibit COMT activity in MDA-MB-231 cells by 50% (Fig. 2A). While more potent COMT inhibition would be ideal, this reduction in activity enhanced the proteasome inhibitory ability of (-)-EGCG in a chymotrypsin-like assay (Fig. 2B). Protea-some activity was reduced by 60% in MDA-MB-231 cells co-treated for 24 h with DNC and (-)-EGCG compared to the control while the chymotrypsin-like activity was only reduced by 23% in cells treated with (-)-EGCG alone. Therefore, COMT inhibition by DNC treatment appeared to increase (-)-EGCG stability and proteasome inhibitory activities.
Proteasome inhibition is well documented to activate apoptosis. The brightly stained, apoptotic nuclei indicative of apoptosis after Hoechst staining and, caspase-3/-7 activition were most apparent after co-treatment with the DNC and (-)-EGCG (Fig. 2C and E). The apoptotic changes observed in these MDA-MB-231 cells were presumably in correlation with proteasome inhibition after this co-treatment.
In summary, pharmacologically diminished COMT activity increases the effectiveness of (-)-EGCG as a proteasome inhibitor and an anti-cancer agent. The data presented in this study support the hypothesis that methylating events limit the cancer-preventative and therapeutic advantages of tea polyphenols. Finally, in the clinical setting, COMT status could be used to determine which individuals would benefit from green tea consumption/treatment in an attempt to reduce the onset and recurrence of certain cancers, including breast cancer.
Acknowledgments
We thank the Karmanos Cancer Institute Genomics Core Facility for assisting in the COMT genotyping and Dr Fred Miller for providing the MCF10A series of cell lines (MCF-10A, Kcl-2 and DCIS). This study was partially supported by research grants (1R01CA120009 and 3R01CA120009-04S1) from the National Cancer Institute (to Q.P.D.).
References
- 1.Yang CS. Tea and health. Nutrition. 1999;15:946–949. doi: 10.1016/s0899-9007(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 2.Fujiki H. Two stages of cancer prevention with green tea. J Cancer Res Clin Oncol. 1999;125:589–597. doi: 10.1007/s004320050321. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Ahmad N, Mohan RR, et al. Prostate cancer chemo-prevention by green tea: In vitro and in vivo inhibition of testosterone-mediated induction of ornithine decarboxylase. Cancer Res. 1999;59:2115–2120. [PubMed] [Google Scholar]
- 4.Kazi A, Smith DM, Daniel K, et al. Potential molecular targets of tea polyphenols in human tumor cells: Significance in cancer prevention. In Vivo. 2002;16:397–403. [PubMed] [Google Scholar]
- 5.Liao S, Umekita Y, Guo J, et al. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995;96:239–243. doi: 10.1016/0304-3835(95)03948-v. [DOI] [PubMed] [Google Scholar]
- 6.Wu AH, Yu MC, Tseng CC, et al. Green tea and risk of breast cancer in Asian Americans. Int J Cancer. 2003;106:574–579. doi: 10.1002/ijc.11259. [DOI] [PubMed] [Google Scholar]
- 7.Shanafelt TD, Call TG, Zent CS, et al. Phase I trial of daily oral polyphenon E in patients with asymptomatic rai stage 0 to II chronic lymphocytic leukemia. J Clin Oncol. 2009;27:3808–3814. doi: 10.1200/JCO.2008.21.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 9.Besche HC, Peth A, Goldberg AL. Getting to first base in proteasome assembly. Cell. 2009;138:25–28. doi: 10.1016/j.cell.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeller D, Dikic V. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458:438–444. doi: 10.1038/nature07960. [DOI] [PubMed] [Google Scholar]
- 11.Seemuller E, Lupas A, Stock D, et al. Proteasome from thermoplasma acidophilum: A threonine protease. Science. 1995;268:579–582. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- 12.An B, Goldfarb RH, Siman R, et al. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 13.Lopes UG, Erhardt P, Yao R, et al. P53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 14.Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7:9–16. doi: 10.1634/theoncologist.7-1-9. [DOI] [PubMed] [Google Scholar]
- 15.Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals) IDrugs. 2002;5:828–834. [PubMed] [Google Scholar]
- 16.Kane RC, Farrell AT, Sridhara R, et al. United States food and drug administration approval summary: Bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12:2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 17.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 18.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Zhu QY, Tsang D, et al. Degradation of green tea catechins in tea drinks. J Agric Food Chem. 2001;49:477–482. doi: 10.1021/jf000877h. [DOI] [PubMed] [Google Scholar]
- 20.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug Metab Dispos. 2003;31:572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 21.Okushio K, Suzuki M, Matsumoto N, et al. Methylation of tea catechins by rat liver homogenates. Biosci Biotechnol Biochem. 1999;63:430–432. doi: 10.1271/bbb.63.430. [DOI] [PubMed] [Google Scholar]
- 22.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 23.Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: Catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Wu AH, Tseng CC, Van Den Berg D, et al. Tea intake, COMT genotype, and breast cancer in asian-american women. Cancer Res. 2003;63:7526–7529. [PubMed] [Google Scholar]
- 26.Santner SJ, Dawson PJ, Tait L, et al. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res Treat. 2001;65:101–110. doi: 10.1023/a:1006461422273. [DOI] [PubMed] [Google Scholar]
- 27.Daniel KG, Kuhn DJ, Kazi A, et al. Anti-angiogenic and anti-tumor properties of proteasome inhibitors. Curr Cancer Drug Targets. 2005;5:529–541. doi: 10.2174/156800905774574075. [DOI] [PubMed] [Google Scholar]
- 28.Chen D, Daniel KG, Chen MS, et al. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Kurkela M, Siiskonen A, Finel M, et al. Microplate screening assay to identify inhibitors of human catechol-O-methyltransferase. Anal Biochem. 2004;331:198–200. doi: 10.1016/j.ab.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Goodman JE, Jensen LT, He P, et al. Characterization of human soluble high and low activity catechol-O-methyltransferase catalyzed catechol estrogen methylation. Pharmacogenetics. 2002;12:517–528. doi: 10.1097/00008571-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Zhu BT, Patel UK, Cai MX, et al. O-methylation of tea polyphenols catalyzed by human placental cytosolic catechol-O-methyltransferase. Drug Metab Dispos. 2000;28:1024–1030. [PubMed] [Google Scholar]
- 32.Landis-Piwowar KR, Kuhn DJ, Wan SB, et al. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (-)-EGCG analogs and their prodrugs. Int J Mol Med. 2005;15:735–742. [PubMed] [Google Scholar]
- 33.Smith DM, Wang Z, Kazi A, et al. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol Med. 2002;8:382–392. [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel KG, Landis-Piwowar KR, Chen D, et al. Methylation of green tea polyphenols affects their binding to and inhibitory poses of the proteasome β5 subunit. Int J Mol Med. 2006;18:625–632. [PubMed] [Google Scholar]
- 35.Landis-Piwowar KR, Wan SB, Wiegand RA, et al. Methylation suppresses the proteasome-inhibitory function of green tea polyphenols. J Cell Physiol. 2007;213:252–260. doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Q, Zhang A, Tsang D, et al. Stability of green tea catechins. J Agric Food Chem. 1997;45:4624–4628. [Google Scholar]
- 37.Cavalieri EL, Stack DE, Devanesan PD, et al. Molecular origin of cancer: Catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci USA. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lakhani NJ, Sarkar MA, Venitz J, et al. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23:165–172. doi: 10.1592/phco.23.2.165.32088. [DOI] [PubMed] [Google Scholar]
- 39.Lottering ML, Haag M, Seegers JC. Effects of 17 beta-estradiol metabolites on cell cycle events in MCF-7 cells. Cancer Res. 1992;52:5926–5932. [PubMed] [Google Scholar]
- 40.Michnovicz JJ, Hershcopf RJ, Naganuma H, et al. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med. 1986;315:1305–1309. doi: 10.1056/NEJM198611203152101. [DOI] [PubMed] [Google Scholar]
- 41.Huang CS, Chern HD, Chang KJ, et al. Breast cancer risk associated with genotype polymorphism of the estrogen-metabolizing genes CYP17, CYP1A1, and COMT: A multigenic study on cancer susceptibility. Cancer Res. 1999;59:4870–4875. [PubMed] [Google Scholar]
- 42.Thompson PA, Shields PG, Freudenheim JL, et al. Genetic polymorphisms in catechol-O-methyltransferase, menopausal status, and breast cancer risk. Cancer Res. 1998;58:2107–2110. [PubMed] [Google Scholar]
- 43.Yim DS, Parkb SK, Yoo KY, et al. Relationship between the Val158Met polymorphism of catechol-O-methyltransferase and breast cancer. Pharmacogenetics. 2001;11:279–286. doi: 10.1097/00008571-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Matsui A, Ikeda T, Enomoto K, et al. Progression of human breast cancers to the metastatic state is linked to genotypes of catechol-O-methyltransferase. Cancer Lett. 2000;150:23–31. doi: 10.1016/s0304-3835(99)00368-7. [DOI] [PubMed] [Google Scholar]
- 45.Hamajima N, Matsuo K, Tajima K, et al. Limited association between a catechol-O-methyltransferase (COMT) polymorphism and breast cancer risk in Japan. Int J Clin Oncol. 2001;6:13–18. doi: 10.1007/pl00012073. [DOI] [PubMed] [Google Scholar]
- 46.Hu Z, Song CG, Lu JS, et al. A multigenic study on breast cancer risk associated with genetic polymorphisms of ER Alpha, COMT and CYP19 gene in BRCA1/BRCA2 negative Shanghai women with early onset breast cancer or affected relatives. J Cancer Res Clin Oncol. 2007;133:969–978. doi: 10.1007/s00432-007-0244-7. [DOI] [PubMed] [Google Scholar]
- 47.Wu AH, Arakawa K, Stanczyk FZ, et al. Tea and circulating estrogen levels in postmenopausal Chinese women in Singapore. Carcinogenesis. 2005;26:976–980. doi: 10.1093/carcin/bgi028. [DOI] [PubMed] [Google Scholar]
- 48.Dawling S, Roodi N, Mernaugh RL, et al. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: Comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61:6716–6722. [PubMed] [Google Scholar]