Abstract
Cancer-preventive effects of tea polyphenols, especially epigallocatechin-3-gallate (EGCG), have been demonstrated by epidemiological, preclinical, and clinical studies. Green tea polyphenols such as EGCG have the potential to affect multiple biological pathways, including gene expression, growth factor-mediated pathways, the mitogen-activated protein kinase-dependent pathway, and the ubiquitin/proteasome degradation pathway. Therefore, identification of the molecular targets of EGCG should greatly facilitate a better understanding of the mechanisms underlying its anticancer and cancer-preventive activities. Performing structure–activity relationship (SAR) studies could also greatly enhance the discovery of novel tea polyphenol analogs as potential anticancer and cancer-preventive agents. In this chapter, we review the relevant literature as it relates to the effects of natural and synthetic green tea polyphenols and EGCG analogs on human cancer cells and their potential molecular targets as well as their antitumor effects. We also discuss the implications of green tea polyphenols in cancer prevention.
2. Introduction
The history of tea consumption dates back around 5000 years ago in ancient China. Today, tea is the most popular beverage consumed by two-thirds of the world’s population. Green tea, black tea, and oolong tea are all derived from the Camellia sinensis plant and contain an assortment of compounds, the most significant of which are polyphenols. The differences among green, black, and oolong teas are derived from their fermentation processes. Green tea does not readily undergo fermentation, but black tea is completely fermented, while oolong tea contains partially fermented leaves. Among all teas consumed in the world, green tea is the most studied for health benefits [1], and tea polyphenols are considered to contribute to the preventive effects on various pathological disorders, including cancers.
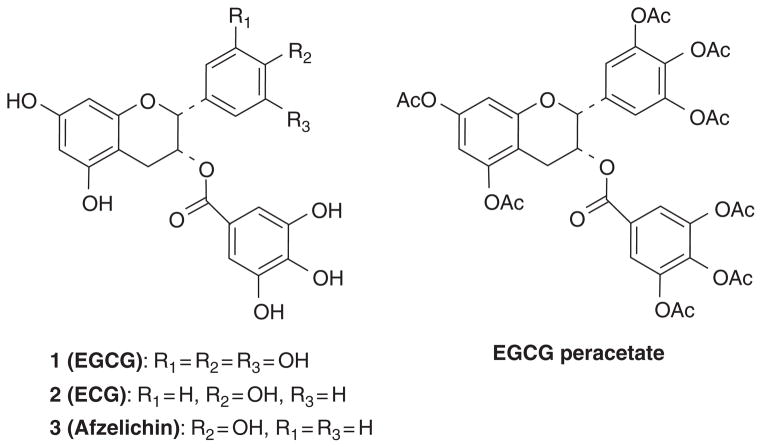
The major components of tea are catechins which contain a benzopyran skeleton with a phenyl group substituted at the 2-position and a hydroxyl (or ester) function at the 3-position. Variations to the catechin structure include the stereochemistry of the 2,3-substituents and the number of hydroxyl groups in the B- and D-ring. Belonging to the flavan-3-ol class of flavonoids, the most abundant catechins found in tea leaves include (−)-epigallocatechin-3-gallate [(−)-EGCG], (−)-epigallocatechin [(−)-EGC], (−)-epicatechin-3-gallate [(−)-ECG], (−)-epicatechin [(−)-EC], (−)-catechin-3-gallate [(−)-CG], and (−)-gallocatechin-3-gallate [(−)-GCG] (Fig. 1).
Fig. 1.
Chemical structures of major tea polyphenols and synthetic prodrug of EGCG (EGCG peracetate or Pro-EGCG).
Although the cancer-preventive effects of green tea and tea polyphenols have been reviewed previously [1–6], the current review critically analyzes the existing data relating to the chemopreventive effects of tea polyphenols, and specifically their molecular targets involved in this process. Furthermore, the derivatives and synthetic analogs and prodrugs of tea polyphenols along with their antitumor effects reported recently by our groups and other scientists are also summarized. We sincerely apologize to those authors whose work is not cited in this short review due to space limitation.
3. The Role of Tea Polyphenols in Carcinogenesis
Cancer development initiates (called the initiation stage) from changes in gene modifications such as an irreversible genetic alteration which can occur via simple mutations, transversions, transitions, and/or small deletions in DNA [7]. Alternatively, genetic alterations may result from changes in gene function, without alteration of the DNA sequence, including histone modification, transcriptional activity, and DNA methylation [8]. The second stage of carcinogenesis is promotion, involving reversible changes in the expression of the genome mediated through promoter–receptor interactions. The final stage of carcinogenesis involves progression that is characterized by karyotypic instability and malignant growth and enhanced by formation and propagation of genetic errors that occur due to increased cellular proliferation [7,9].
Chemoprevention aims to decrease the occurrence of cancer by the administration of natural or synthetic compounds [4]. Numerous studies have demonstrated the chemopreventive potential of green tea and tea polyphenols [10–13]. Dietary cancer chemopreventive agents exert their effects by modulating multiple cell signaling pathways involved in the carcinogenic process [14]. Furthermore, various studies indicate that diet-derived compounds are capable of prolonging one or more stages of the carcinogenic process [1,4,15]. For example, green tea polyphenols (GTPs) affect multiple important biological pathways and carcinogenesis. It is generally accepted that many of the cancer chemopreventive effects of green tea are mediated by its most abundant polyphenol, (−)-EGCG. In the following sections, we will summarize the biological role of green tea and (−)-EGCG as well as its synthetic analogs and prodrugs from both preclinical and clinical studies, with particular emphasis on the molecular targets involved in cancer prevention.
4. In Vitro and In Vivo Preclinical Studies on Tea Polyphenols
The antitumor effects of tea polyphenol and (−)-EGCG were discovered from various cancer cell lines, animal models, and clinical studies. For example, in over 20 tumor cell lines, as listed in Table 1, tea polyphenols and (−)-EGCG showed significant growth-inhibitory and antitumor effects. Tea inhibited carcinogenesis in various animal models bearing lung, skin, esophagus, and liver cancers [65–67]. Green tea infusion (e.g., 1.25 g of tea leaves brewed in 100 ml water), as the sole source of fluid intake in A/J mice, significantly decreased N-nitrosodiethylamine (NDEA)-induced lung tumor incidence (by 36–44%) and tumor multiplicity (by 44–60%) [68]. The inhibition of skin carcinogenesis by tea was also investigated. It was found that oral administration of GTPs reduced UVB-induced skin cancer incidence by 35%, tumor multiplicity by 63%, and tumor growth by 55% [16]. Furthermore, the inhibitory effect of GTPs was associated with reduced expression of the matrix metalloproteinases (MMP)-2, MMP-9, and CD31, which are important factors in tumor growth, metastasis, and angiogenesis [16]. In addition, topical application of GTPs (6 mg/animal) in DMBA-initiated mice prior to promotion by 12-O-tetradecanoylphorbol-13-acetate (TPA) or mezerein (MEZ) resulted in significant protection against skin tumor formation in terms of tumor incidence (by 32–60%), multiplicity (by 49–63%), and tumor volume/mouse (by 73–90%) [69]. The inhibitory effects of green tea on hepatocarcinogenesis were tested in mice, given pentachlorophenol (PCP) as a carcinogen following treatment with the initiator diethylnitrosamine (DEN). It was found that in mice that were pretreated with a green tea infusion and continuously exposed to the same infusion during the process of initiation and promotion, the incidence of hepatocellular tumors was decreased by 40% [70]. Decaffeinated green tea or decaffeinated black tea extracts as the fluid intake were also given to N-nitrosomethylbenzylamine (NMBzA)-induced esophageal tumor model of Sprague–Dawley rats, and this resulted in reduced esophageal tumor incidence and multiplicity by ~70% in both groups [17].
TABLE 1.
A Summary of the Biological Effects of Tea Polyphenols and (−)-EGCG on Various Cancer Cell Lines and Tumors
| Agents from tea | Tumors and tumor cells | References |
|---|---|---|
| Green tea | Epidemiological | [11–13] |
| Tea polyphenol | Skin tumor | [16] |
| Green and black tea | Esophageal tumor | [17] |
| Tea polyphenol and (−)-EGCG | Cervical cancer cells | [18–20] |
| Tea polyphenol and (−)-EGCG | Ovarian carcinoma | [21,22] |
| (−)-EGCG | Human epidermoid carcinoma A431 cells | [23] |
| Tea polyphenol and (−)-EGCG | Human breast cancer cells and tumors, Mouse mammary epithelial cells | [24–28] |
| Tea polyphenol and (−)-EGCG | Oral squamous carcinoma cells, Oral cancer cells, Oral epithelial cells growth in vitro, IFN-γ-stimulated human oral cancer cells | [29–32] |
| Tea polyphenol and (−)-EGCG | Human drug-resistant lung cancer cells, Human lung cancer cells | [33–37] |
| Tea polyphenol and (−)-EGCG | Human hepatocellular carcinoma cells, Phase IIa liver cancer | [38–40] |
| Tea polyphenol and (−)-EGCG | Bladder cancer cells | [41,42] |
| Tea polyphenol and (−)-EGCG | Prostate cancer | [43–49] |
| (−)-EGCG | Head and neck tumor | [50,51] |
| (−)-EGCG | Mouse embryonic fibroblast cells | [52] |
| (−)-EGCG | Human osteogenic sarcoma (HOS) cells | [53] |
| (−)-EGCG | Laryngeal squamous carcinoma cells | [54] |
| (−)-EGCG | Nasopharyngeal carcinoma cells | [55] |
| (−)-EGCG | Renal cell carcinoma | [56,57] |
| Green tea extracts and (−)-EGCG | Intestinal tumor, Colorectal cancer | [58–60] |
| (−)-EGCG | Hypopharyngeal carcinoma cells | [61] |
| (−)-EGCG | Pancreatic cancer cells | [62] |
| Tea polyphenol and (−)-EGCG | Human gastric cancer cells | [63,64] |
Several groups have reported that (−)-EGCG plays a critical role in preventing the growth of cervical cancer associated with apoptosis induction. Moreover, the growth-inhibitory effects of (−)-EGCG in cervical cancer cells may be associated with the inhibition of telomerase [18–20]. (−)-EGCG may also augment the chemotherapeutic effects by inhibiting the endothelin axis and downstream signaling pathways associated with overcoming drug resistance in ovarian carcinoma [21–23].
In our previous study, (−)-EGCG and its prodrug, (−)-EGCG peracetate or Pro-EGCG (Fig. 1), were found to potently inhibit human breast cancer cells and tumor growth [24]. The suppression of cell proliferation and gene expression by combinatorial synergy of (−)-EGCG, resveratrol, and γ-tocotrienol in estrogen receptor-positive MCF-7 breast cancer cells was also demonstrated [25]. Another investigation revealed that (−)-EGCG inhibited growth in the mouse viral mammary epithelial carcinogenesis model RIII/MG and induced apoptosis, suggesting the clinical relevance of EGCG as a chemopreventive agent [26].
Various in vitro studies demonstrated that tea polyphenols or pure (−)-EGCG inhibited oral squamous carcinoma cells and oral epithelial cell growth [29–31,71]. (−)-EGCG suppressed indoleamine 2,3-dioxygenase, an immunomodulatory protein by blocking the γ-interferon-induced JAK-PKC-δ-STAT1 signaling in human oral cancer cells [32]. A number of studies revealed that (−)-EGCG exerted inhibitory effects in a variety of cancer cells by targeting multiple cellular molecules. (−)-EGCG was found to inhibit the growth rate and induce G2-M arrest in lung cancer cells in vitro and in vivo [33–36,72,73]. (−)-EGCG was also reported to inhibit human hepatocellular carcinoma cells [38–40,74]. (−)-EGCG was found to downregulate N-cadherin and suppresses migration of bladder carcinoma cells [75]. Additionally, (−)-EGCG was found to inhibit transitional cell carcinoma of the bladder cell line T24 [41]. Preclinical evidence showed that green tea extract (GTE) induced ornithine decarboxylase and activated extracellular signal-regulated kinase in bladder carcinoma ECV304 cells [42]. In an in vivo study, it was suggested that (−)-EGCG and its prodrug (−)-EGCG peracetate could augment the anticancer effects in androgen-independent prostate cancer [43].
Preclinical studies have been demonstrated that tea polyphenol and (−)-EGCG could inhibit cell proliferation and induce cell death in a variety of cancer cells including head and neck tumor [50], lung cancer [76], prostate cancer [44–47], breast cancer [25], mouse embryonic fibroblast cell tumor [52], human osteogenic sarcoma [53], human epidermoid carcinoma [23], laryngeal squamous carcinoma cells [54], nasopharyngeal carcinoma [55], renal cell carcinoma [56,57], intestinal tumor [57,58], colorectal cancer [59,60], hypopharyngeal carcinoma [61], and pancreatic cancer cells [62]. Several studies also revealed the effects of (−)-EGCG on gastric cancer [63,64]. The apoptotic effect of (−)-EGCG on the human gastric cancer cell line MKN45 was observed via activation of the mitochondrial pathway [63].
Regarding human prostate cancer, epidemiological studies show that green tea consumption may reduce the incidence of prostate cancer. Kurahashi et al. investigated 49,920 men aged 40–69 years, who completed a questionnaire that included their green tea consumption habit and followed up the subjects more than 10 years [77]. In this group, 404 men were diagnosed with prostate cancer, of whom 114 had advanced cases, 271 were localized cancers, and 19 were of an undetermined stage. The results showed that green tea consumption was not associated with localized prostate cancer but was associated with a dose-dependent decrease in the risk of advanced prostate cancer [77]. A clinical trial was performed to determine chemopreventive effect of green tea catechins (GTCs) on human prostate cancer development. Sixty volunteers with high-grade prostate intraepithelial neoplasia (HG-PIN), who would develop prostate cancer within 1 year, were given three GTC capsules, 200 mg each (total 600 mg/day) or placebo [44]. After 1 year treatment with GTCs, only one person was diagnosed with prostate cancer among the 30 GTCs-treated men (incidence, ~3%), whereas nine cancers were found among the 30 placebo-treated men (incidence, 30%) [44]. The results demonstrate that GTCs are safe and very effective for treating premalignant lesions and preventing prostate cancer development [44]. A Phase II trial was conducted by the North Central Cancer Treatment Group to explore green tea’s antineoplastic effects in patients with androgen-independent prostate carcinoma [78]. Forty-two patients, who were asymptomatic and had progressive prostate specific antigen (PSA) elevation with hormone therapy, were administrated orally with 6 g of green tea per day [78]. Patients were monitored monthly for response and toxicity. The results showed that only one patient had shown tumor response defined by 50% decrease in PSA from baseline (229–105 ng/dl) but this decrease was not sustained beyond 2 months. The overall response rate to GTCs was only 2% [78]. Green tea toxicity was observed in 69% of patients and included nausea, emesis, insomnia, fatigue, diarrhea, abdominal pain, and confusion. This clinical trial demonstrated that green tea has limited antineoplastic activity among patients with androgen-independent prostate carcinoma [78].
In conclusion, many studies using tea polyphenol and (−)-EGCG in various cancer cell lines support their cancer-preventive effects (Table 1). Furthermore, results from Phase IIa chemoprevention trial of GTPs carried out in high-risk individuals have demonstrated the relative safety of GTP consumption in human subjects [38]. The established antitumor and cancer-preventative activities of tea polyphenol and (−)-EGCG also encourage investigators to further elucidate their essential molecular targets.
5. Tea Polyphenols and Their Molecular Targets in Cancer Cells
Tea polyphenols possess broad inhibitory activity against carcinogenesis and are effective when administered during its initiation, promotion, or progression. However, the molecular mechanisms of tea polyphenols responsible for their inhibitory actions are not fully understood. Some of the molecular mechanisms proposed include protection of DNA from damage and/or methylation in normal cells, inhibition of tumor proteasome activity, inhibition of oncogene gene expression, induction of apoptosis, cell-cycle regulation, and inhibition of cell proliferation and tumor promotion-related events, which are discussed further below.
5.1. Green Tea and (−)-EGCG Protect DNA from Methylation and Damage
Epigenetics is the study of reversible, heritable changes in gene function that occur without a change in the sequence of nuclear DNA [8]. Epigenetic mechanisms control eukaryotic development beyond DNA-stored information, but can also contribute to carcinogenesis by altering chromatin structure, histone acetylation, transcriptional activity, and DNA methylation. Epigenetic silencing by hypermethylation of tumor suppressor or DNA repair-related genes occurs most frequently during the early stages of the carcinogenesis [79]. Moreover, the epigenetic change may result in additional changes in gene constructions. It was reported that silencing of the O6-methylguanine-DNA methyltransferase gene (MGMT) resulted in cells with the ability to acquire a specific type of genetic mutation in p53 and subsequently an inability to repair DNA guanosine adducts [80].
(−)-EGCG was reported to inhibit the activity of DNA methyltransferase (DNMT), resulting in CpG demethylation and reactivation of methylation-silenced genes in human esophageal cancer KYSE 510 cells such as p16INK4a, retinoic acid receptor β (RARβ), MGMT, and human mutL homologue 1 (hMLH1) [81]. Caudal-related homeobox transcription factor 2 (Cdx2), a tumor suppressor gene is frequently inactivated by promoter hypermethylation in gastric carcinoma and colorectal cancer cells. Green tea decreased the Cdx2 methylation frequency in a dose-dependent manner, showing 10/25 (40%), 7/18 (39%), 2/8 (25%), and 0/6 (0%) of Cdx2 methylation frequency in tested groups of people who consumed three or less, four to six, seven to nine and ten cups or more a day, respectively [82].
Ultraviolet radiation (UVR) can induce DNA damage, which is one of the mechanisms of tumor formation. It was found that preincubation with (−)-EGCG significantly decreased DNA damage induced by UVR in human skin fibroblasts, lung fibroblasts, and epidermal keratinocytes cell lines [83]. Furthermore, it was observed that the peripheral blood cells from individuals who consumed green tea showed lower levels of DNA damage compared to that from controls [83].
In a Phase II random controlled tea intervention trial, 143 heavy smokers, aged 18–79 years old, were randomly grouped to drink green tea, black tea, or water for 4 months. Assessment of urinary 8-OHdG, an indicator of oxidative DNA damage after adjustment for baseline measurements and other potential confounders, revealed a significant decrease in urinary 8-OHdG (31%) after 4 months of drinking decaffeinated green tea (P = 0.002) [84], suggesting that regular green tea drinking might protect smokers from oxidative damage.
5.2. Tea Polyphenols and Inhibition of Oncogene Expression
It has been shown that various genes associated with carcinogenesis are influenced by tea polyphenols. Tea polyphenols or (−)-EGCG were found to reduce expression levels of cyclin D1 and bcl-2 and increase expression of p53 and p27, which resulted in apoptosis induction in several tumors including hepatocellular carcinoma, lung carcinogenesis, and nasopharyngeal carcinoma [35,37,55,74].
It has been reported that expression of MMP-9 gene, which is involved in cancer cell growth and metastasis, is inhibited by EGCG in macrophage-differentiated HL-60 myeloid leukemia cells [85]. Treatment with EGCG dramatically inhibited MMP-9 protein secretion by human myeloid leukemia HL-60 cells with IC50 value of 3.2 μM. EGCG also diminished MMP-9 gene expression and inhibited mRNA levels in the HL-60 cells [85].
Overexpression of the HER-2/neu receptor (HER-2) and epidermal growth factor receptor (EGFR) is frequently observed in patients with breast carcinoma and also in patients with head and neck squamous cell carcinoma (HNSCC). Masuda et al. showed that EGCG could significantly inhibit the phosphorylation of HER-2 in breast and HNSCC cell lines and also induce inhibition of Stat3 activation, inhibition of c-fos, and cyclin D1 promoter activity, and decreased cellular levels of the cyclin D1 and Bcl-XL proteins [51].
It has been reported that (−)-EGCG and GTPs inhibited TNF-α gene expression in the cells, mediated through inhibition of NF-κB activation [86]. Heterogeneous nuclear ribonucleoprotein (hnRNP) A2/B1 has been shown to be overexpressed in breast and lung tumors, and characterized as an early marker of lung cancer [87]. EGCG could potently inhibit the hnRNP A2/B1 gene expression with IC50 29 μM which resulted in growth inhibition of human lung cancer cells [72].
It was reported that EGCG suppressed growth of human lung cancer cells through inhibition of the Ras-GTPase-activating protein SH3 domain-binding protein 1 (G3BP1) [35], which is a member of enzymes associated with the heterogeneous nuclear RNA-binding proteins and is an element of the oncogenic Ras signal transduction pathway [88].
Inhibition of oncogene expression by green tea and EGCG also tested in animal studies. Hu et al. reported that high level of expression of c-myc, c-raf, and c-H-ras oncogenes in lung tissue was induced in mice by treatment with tobacco-specific nitrosamine 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK). After feeding with 2% tea extract for 4–8 weeks, 50%, 20%, and 50% inhibition of c-myc, c-raf, and c-H-ras gene expressions, respectively, were observed in lung tissue of the NNK-treated mice [89].
EGFR upregulation has been shown to be associated with a number of mechanisms relating to tumor development [90]. MMP-2 overexpression has been reported to be associated with the invasive and malignant phenotypes of many cancer cell lines in vitro and in vivo [91,92]. EGCG could downregulate mRNA expression and protein levels of EGFR in breast cancer cells [27]. Treatment of human breast cancer cells with EGCG also reduced the activity, protein expression, and mRNA expression level of MMP-2 [28].
5.3. Tea Polyphenols Inhibit Proteasome Activity in Cancer Cells
The ubiquitin/proteasome system is responsible for the degradation of regulatory proteins that are involved in critical cellular processes such as cell cycle and apoptosis [93,94]. The eukaryotic proteasome contains at least three known catalytic activities: chymotrypsin-like, trypsin-like, and caspase-like or peptidyl–glutamyl peptide-hydrolyzing (PGPH)-like activities [95]. The proteasomal activity is required for tumor cell proliferation and drug resistance development [96]. Therefore, targeting the proteasome-mediated degradation pathway has been considered as an important approach for cancer therapy and prevention. The association of proteasome inhibition and apoptosis induction has been observed in studies of ours and other groups [97,98]. Indeed, clinical trials have demonstrated that the proteasome inhibitor Bortezomib (Velcade, PS-341) has antitumor activity in multiple myeloma and other type of cancers [48,99,100].
We reported that (−)-EGCG potently and specifically inhibited the chymotrypsin-like activity of the proteasome in vitro (IC50 = 86–194 nM), and that (−)-EGCG could induce tumor cell growth arrest in G1 phase of the cell cycle [101]. For the first time, we reported that an ester bond within (−)-EGCG played a critical role in its inhibitory activity of the proteasome [101]. In addition, synthetic (−)-EGCG amides and (−)-EGCG analogs with modifications in the A-ring, C-ring, or ester bond inhibited the chymotrypsin-like activity of purified 20S proteasome with altered potencies, induced growth arrest in the G1 phase of the cell cycle in leukemia Jurkat T cells, and suppressed colony formation of human prostate cancer LNCaP cells [102].
(−)-EGCG is the most potent polyphenol in green tea, but it is unstable under neutral or alkaline conditions (i.e., physiologic pH). In an effort to discover more stable polyphenol proteasome inhibitors, we synthesized several (−)-EGCG analogs with −OH groups eliminated from the B-and/or D-rings. In addition, we also synthesized their putative prodrugs with −OH groups protected by acetate (Fig. 1) that can be removed by cellular cytosolic esterases. We first examined the structure–activity relationship (SAR) of these unprotected and protected compounds with respect to their protea-some-inhibitory potentials. We found that decreasing the number of −OH groups from either the B- or D-ring leads to diminished proteasome-inhibitory activity in vitro. However, in cultured tumor cells, the acetate protected analogs were capable of potently inhibiting the proteasomal chymotrypsin-like activity by as much as 97% [103]. Furthermore, we found that, compared to (−)-EGCG, protected analogs exhibited greater potency to inhibiting proliferation and inducing apoptosis in human leukemic, prostate, breast, and simian virus 40-transformed cells [104]. Importantly, the protected analogs were nontoxic to human normal and nontransformed cells [104].
5.4. Other Molecular Targets of Tea Polyphenols
In several reviews, multiple molecular targets of GTPs were summarized [6,105,106], such as mitogen-activated protein kinases (MAPKs), EGFR-mediated pathways, and the insulin-like growth factor (IGF)-I-mediated signal transduction pathway. Herein, we emphasize some important published findings in which cancer-specific protein targets were modulated by tea polyphenols.
(−)-EGCG could suppress heregulin-β1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-Kinase (PI3K)/Akt and MAP kinase cascade signaling [107]. (−)-EGCG also inhibited the endothelin axis and decreased ETAR-dependent activation of the p42/p44 and p38 MAPKs and phosphatidylinositol 3-kinase pathway in ovarian carcinoma [18].
Adhami et al. reported that after oral administration of GTPs for 24 weeks, the progression and invasion of prostate cancer was inhibited in a transgenic adenocarcinoma of the mouse prostate (TRAMP) model [49]. The chemoprevention effect of tea polyphenols in the TRAMP animal models was associated with decreased IGF-I level in the prostate tissue of the mice [49]. These findings were supported by another study, in which tea polyphenols could inhibit IGF-I-mediated Akt phosphorylation [108]. The data from both in vitro and in vivo preclinical studies indicated that IGF-I signal transduction pathway is one of the critical molecular targets for tea polyphenols.
Other studies revealed that tea polyphenols could target the MAPKs pathway. Opare Kennedy et al. found that GTE possessed growth-inhibitory effects toward Ehrlich ascites tumor cells associated with a cellular thiol-dependent activation of MAP kinases [109].
A poor prognosis of small-cell lung carcinoma (SCLC) is particularly due to the development of drug resistance. Sadava et al. found that EGCG could induce apoptosis in both drug-sensitive (H69) and drug-resistant (H69VP) SCLC cells with similar IC50 values (~70 μM). Treatment of both cell lines with EGCG at the concentration of IC50 values for 24 h also resulted in 50–60% reduced telomerase activity [34].
Taken together, as seen with other natural compounds, tea polyphenols play potent-inhibitory roles in different stages of carcinogenesis and toward multiple targets throughout various tumor types. We have listed some of the major molecular targets of tea polyphenols in this review, which are associated with their anticancer and cancer-preventative effects.
6. Tea Polyphenol Derivatives in Cancer Studies
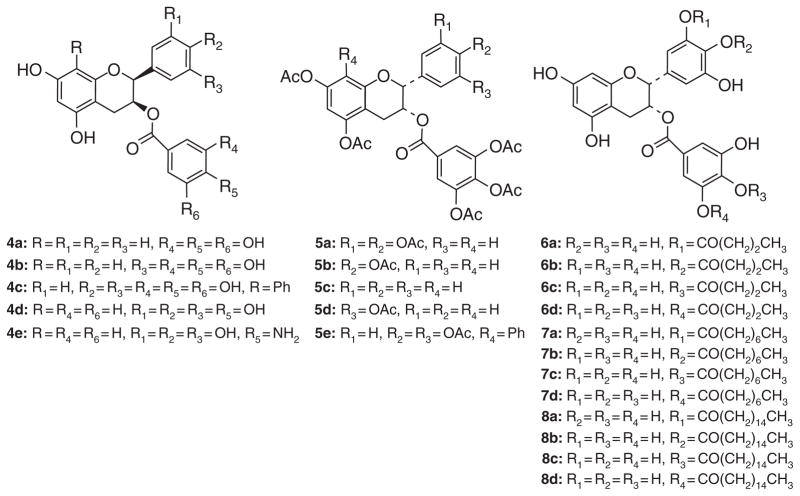
The low absorption, poor membrane-permeability, metabolic transformations, and unstability of tea polyphenols tend to restrict their clinical use. The hydroxyl groups of (−)-EGCG are subject to be modified through biotransformation reactions, including methylation, glucuronidation, and sulfation, resulting in reduced biological activities in vivo [110–112]. Many efforts have been made by chemical modification of tea polyphenols to improve their bioavailability [113,114]. In 2001, we reported the first chemical synthesis of epigallocatechin gallate (1) in an enantioselective manner providing separately the natural (−)-EGCG as well as its enantiomer [115]. This was followed by the syntheses of ECG (2), Afzelechin (as the gallate 3) [116], and a number of analogs (Fig. 1) [103,117,118]. Structure–activity studies revealed that EGCG showed the optimal activity toward proteasome inhibition among the natural GTPs and the synthetic analogs (4a–4e) (Fig. 2) [101,117,119]. Decreasing the number of −OH groups from either the A-, B-, or D-ring of EGCG was found to diminished proteasome-inhibitory activity in vitro (Fig. 2) [116,118,120]. The carbonyl function of EGCG and analogs is essential for their proteasome-inhibitory activity [101]. The ester oxygen at C-3 can be replaced by the NH isostere with only minor attenuation of activity toward purified proteasome, but enhanced potency toward cellular proteasome. This most observation is most likely due to increased stability [119].
Fig. 2.
Chemical structures of EGCG analogues 4a–8d.
In order to increase the stability of (−)-EGCG, we appended peracetate protective groups to its reactive hydroxyl groups and results showed that the EGCG peracetate could be converted into (−)-EGCG when incubated with cell extracts. Interestingly, a series of bioassays show that EGCG peracetate has no inhibitory activity against a purified 20S proteasome, but exhibits increased proteasome-inhibitory activity in intact leukemic cells over natural (−)-EGCG, indicating an intercellular conversion taking place. Therefore, our results indicate that EGCG peracetate (also named Pro-EGCG) (Fig. 1) may function as a prodrug of the GTP proteasome inhibitor (−)-EGCG [113]. In vivo studies using mice also showed a significant inhibition of breast tumor growth by Pro-EGCG, compared with (−)-EGCG, associated with increased proteasome inhibition and apoptosis induction in tumor tissues [104]. The peracetates of (−)-EGCG analogs (5a–5e), with −OH groups eliminated from the B- and/or D-rings, showed similar effects as those of the EGCG peracetate (Fig. 2) [103]. These studies disclosed that the prodrugs of (−)-EGCG and its analogs not only increased their stability but also improved their bioavailability.
In a follow-up study, (−)-(EGCG) monoesters modified with butanoyl (6a–6d) (EGCG-C4), octanoyl (7a–7d) (EGCG-C8), palmitoyl groups (8a–8d) (EGCG-C16) (Fig. 2) were synthesized by a lipase-catalyzed transesterification method, and their antitumor activities were investigated in vitro and in vivo. EGCG-C16 suppressed tumor growth in vivo in colorectal tumor-bearing mice in comparison to an untreated control, vector control (DMSO), and EGCG [121].
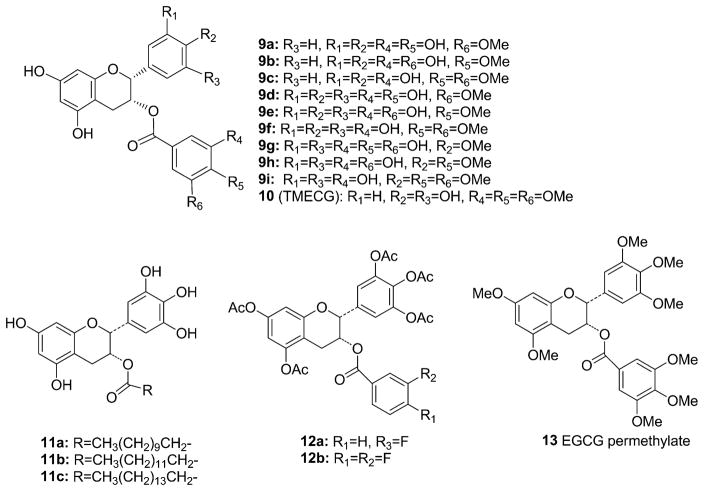
It was suggested that O-methylation of the catechins by catechol-O-methyltransferase (COMT), an enzyme ubiquitously present in humans, may reduce the cancer-preventive effects of the catechins [122]. We also synthesized nine different methylated catechins (9a–9i) (Fig. 3) which are metabolites or potential metabolites of tea catechins in biomethylation [123]. It was found that the addition of a methyl group on the B- or D-ring of (−)-EGCG or (−)-ECG led to decreased proteasome inhibition and, as the number of methyl groups increased, the inhibitory potencies further decreased [124]. Metabolic O-methylation of EGCG may indeed reduce the effectiveness of EGCG as it relates to its anticancer activity [125], lending support of the human study [122].
Fig. 3.
Chemical structures of EGCG analogues 9a–13.
Another study disclosed that 3-O-(3,4,5-trimethoxybenzoyl)-(−)-epicatechin (10) (TMECG) (Fig. 3) showed significant antiproliferative activity against several cancer cell lines, especially melanoma [126]. In an anticancer screening, 3-O-acyl and alkyl-(−)-epicatechin derivatives (11a–11c) (Fig. 3) exhibited superior anticancer activity compared to (−)-ECG. Furthermore, several compounds that were modified aliphatic chains with moderate sizes (C8–C12) showed strong anticancer activity (IC50 = 6.4–31.2 μM) [127].
More recently, we synthesized several novel fluoro-substituted (−)-EGCG analogs, namely F-EGCG analogs (12a, 12b) (Fig. 3), as well as their prodrug forms with all −OH groups protected by acetate. The prodrug form of one F-EGCG analog exhibited greater potency than the previously reported peracetate of (−)-EGCG to inhibit proteasomal activity, suppress cell proliferation, and induce apoptosis in human leukemia Jurkat T cells. These results demonstrate the potential of these compounds to be developed into novel anticancer and cancer-preventive agents [128,129].
Several studies have revealed the reversal effect of tea polyphenols and (−)-EGCG on multidrug resistance in human carcinoma cells [130]. In our recent study, it was found that permethyl (−)-EGCG and its analogs exhibited promising P-gp modulating activity in a P-gp overexpressing breast cancer cell line (LCC6MDR). One micromolar of permethyl (−)-EGCG (13) (Fig. 3) could sensitize LCC6MDR cells toward paclitaxel by 18.2-fold. These results currently await publication.
7. Conclusions
Tea polyphenols are potent bioactive compounds that possess anticarcinogenic activities. They interfere with the initiation, development, and progression of cancer by modulating critical processes of cellular proliferation, differentiation, apoptosis, angiogenesis, and metastasis. Although tea has been consumed for centuries, it has only recently been studied extensively as a health-promoting beverage that may act to prevent a number of diseases including cancer. Various studies showed that tea polyphenols potently induce apoptotic cell death and cell-cycle arrest in tumor cells but not in their normal cell counterparts, and that GTPs affect multiple biological pathways. Various animal studies have revealed that treatment with green tea inhibits tumor incidence and multiplicity in different organ sites such as skin, lung, liver, stomach, mammary gland, and colon. Recently, Phases I and II clinical trials have been conducted to explore the anticancer effects of green tea in humans. Studies focusing on the purified tea polyphenol compound (−)-EGCG should continue to provide researchers an improved understanding of the pharmacokinetics of tea polyphenols such as absorption, distribution, their role in anticancer reactions, metabolism and molecular mechanisms. Although structural modifications of (−)-EGCG have shown promising results toward their anticancer effects, work should continue on optimizing and evaluating additional analogs of GTPs in an effort to discover more potent, stable, and specific tea polyphenol analogs as potential novel anticancer agents. A major challenge of cancer prevention is to integrate new molecular findings into clinical practice. Identification of more molecular targets or biomarkers for tea polyphenols is paramount to cancer prevention and treatment by green tea/synthetic (−)-EGCG analogs, and will greatly assist in a better understanding of its anticancer mechanisms.
Acknowledgments
This work is supported in part by research grants from the National Cancer Institute–National Institutes of Health (to Q. P. D.; 1R01CA120009 and 3R01CA120009-04S1), and the Areas of Excellence Scheme established under the University Grants Committee of the Hong Kong Special Administrative Region, China (to T. H. C.; Project No. AoE/P-10/01).
References
- 1.Mukhtar H, Ahmad N. Cancer chemoprevention: future holds in multiple agents. Toxicol Appl Pharmacol. 1999;158:207–210. doi: 10.1006/taap.1999.8721. [DOI] [PubMed] [Google Scholar]
- 2.Chen D, Milacic V, Chen MS, Wan SB, Lam WH, Huo C, et al. Tea polyphenols, their biological effects and potential molecular targets. Histol Histopathol. 2008;23:487–496. doi: 10.14670/hh-23.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huo C, Wan SB, Lam WH, Li L, Wang Z, Landis-Piwowar KR, et al. The challenge of developing green tea polyphenols as therapeutic agents. Inflammopharmacol. 2008;16:248–252. doi: 10.1007/s10787-008-8031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 5.Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269–280. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitot HC. The molecular biology of carcinogenesis. Cancer. 1993;72:962–970. doi: 10.1002/1097-0142(19930801)72:3+<962::aid-cncr2820721303>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 9.Pitot HC, Dragan YP. Facts and theories concerning the mechanisms of carcinogenesis. FASEB J. 1991;5:2280–2286. [PubMed] [Google Scholar]
- 10.Fujiki H. Two stages of cancer prevention with green tea. J Cancer Res Clin Oncol. 1999;125:589–597. doi: 10.1007/s004320050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769–775. doi: 10.1006/pmed.1997.0242. [DOI] [PubMed] [Google Scholar]
- 12.Mukhtar H, Ahmad N. Mechanism of cancer chemopreventive activity of green tea. Proc Soc Exp Biol Med. 1999;220:234–238. doi: 10.1046/j.1525-1373.1999.d01-40.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakachi K, Suemasu K, Suga K, Takeo T, Imai K, Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn J Cancer Res. 1998;89:254–261. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 15.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 16.Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J Nutr. 2005;135:2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZY, Wang LD, Lee MJ, Ho CT, Huang MT, Conney AH, et al. Inhibition of N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats by green and black tea. Carcinogenesis. 1995;16:2143–2148. doi: 10.1093/carcin/16.9.2143. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi M, Yokoyama M, Watanabe S, Uchiyama M, Nakao Y, Hara K, et al. Inhibitory effect of the tea polyphenol, (−)-epigallocatechin gallate, on growth of cervical adenocarcinoma cell lines. Cancer Lett. 2006;234:135–142. doi: 10.1016/j.canlet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 19.Qiao Y, Cao J, Xie L, Shi X. Cell growth inhibition and gene expression regulation by (−)-epigallocatechin-3-gallate in human cervical cancer cells. Arch Pharm Res. 2009;32:1309–1315. doi: 10.1007/s12272-009-1917-3. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama M, Noguchi M, Nakao Y, Pater A, Iwasaka T. The tea polyphenol, (−)-epigallocatechin gallate effects on growth, apoptosis, and telomerase activity in cervical cell lines. Gynecol Oncol. 2004;92:197–204. doi: 10.1016/j.ygyno.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Spinella F, Rosano L, Di Castro V, Decandia S, Albini A, Nicotra MR, et al. Green tea polyphenol epigallocatechin-3-gallate inhibits the endothelin axis and downstream signaling pathways in ovarian carcinoma. Mol Cancer Ther. 2006;5:1483–1492. doi: 10.1158/1535-7163.MCT-06-0053. [DOI] [PubMed] [Google Scholar]
- 22.Wilson-Simpson F, Vance S, Benghuzzi H. Physiological responses of ES-2 ovarian cell line following administration of epigallocatechin-3-gallate (EGCG), thymoquinone (TQ), and selenium (SE) Biomed Sci Instrum. 2007;43:378–383. [PubMed] [Google Scholar]
- 23.Bhatia N, Agarwal C, Agarwal R. Differential responses of skin cancer-chemopreventive agents silibinin, quercetin, and epigallocatechin 3-gallate on mitogenic signaling and cell cycle regulators in human epidermoid carcinoma A431 cells. Nutr Cancer. 2001;39:292–299. doi: 10.1207/S15327914nc392_20. [DOI] [PubMed] [Google Scholar]
- 24.Dou QP. Molecular mechanisms of green tea polyphenols. Nutr Cancer. 2009;61:827–835. doi: 10.1080/01635580903285049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh TC, Wu JM. Suppression of cell proliferation and gene expression by combinatorial synergy of EGCG, resveratrol and gamma-tocotrienol in estrogen receptor-positive MCF-7 breast cancer cells. Int J Oncol. 2008;33:851–859. [PubMed] [Google Scholar]
- 26.Yanaga H, Fujii T, Koga T, Araki R, Shirouzu K. Prevention of carcinogenesis of mouse mammary epithelial cells RIII/MG by epigallocatechin gallate. Int J Mol Med. 2002;10:311–315. [PubMed] [Google Scholar]
- 27.Farabegoli F, Papi A, Orlandi M. (−)-Epigallocatechin-3-gallate downregulates EGFR, MMP-2, MMP-9 EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant cells. Biosci Rep. 2011;31:99–108. doi: 10.1042/BSR20090143. [DOI] [PubMed] [Google Scholar]
- 28.Sen T, Moulik S, Dutta A, Choudhury PR, Banerji A, Das S, et al. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009;84:194–204. doi: 10.1016/j.lfs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Hong J, Kim MR, Lee NH, Lee BH. Food Sci Biotechnol Inhibition of oral epithelial cell growth in vitro by epigallocatechin-3-gallate; its modulation by serum and antioxidant enzymes. Food Sci Biotechnol. 2009;18:971–977. [Google Scholar]
- 30.Kato K, Long NK, Makita H, Toida M, Yamashita T, Hatakeyama D, et al. Effects of green tea polyphenol on methylation status of RECK gene and cancer cell invasion in oral squamous cell carcinoma cells. Br J Cancer. 2008;99:647–654. doi: 10.1038/sj.bjc.6604521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto T, Digumarthi H, Aranbayeva Z, Wataha J, Lewis J, Messer R, et al. EGCG-targeted p57/KIP2 reduces tumorigenicity of oral carcinoma cells: role of c-Jun N-terminal kinase. Toxicol Appl Pharmacol. 2007;224:318–325. doi: 10.1016/j.taap.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Cheng CW, Shieh PC, Lin YC, Chen YJ, Lin YH, Kuo DH, et al. Indoleamine 2, 3-dioxygenase, an immunomodulatory protein, is suppressed by (−)-epigallocatechin-3-gallate via blocking of gamma-interferon-induced JAK-PKC-delta-STAT1 signaling in human oral cancer cells. J Agric Food Chem. 2010;58:887–894. doi: 10.1021/jf903377e. [DOI] [PubMed] [Google Scholar]
- 33.Okabe S, Suganuma M, Hayashi M, Sueoka E, Komori A, Fujiki H. Mechanisms of growth inhibition of human lung cancer cell line, PC-9, by tea polyphenols. Jpn J Cancer Res. 1997;88:639–643. doi: 10.1111/j.1349-7006.1997.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadava D, Whitlock E, Kane SE. The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem Biophys Res Commun. 2007;360:233–237. doi: 10.1016/j.bbrc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 35.Shim JH, Su ZY, Chae JI, Kim DJ, Zhu F, Ma WY, et al. Epigallocatechin gallate suppresses lung cancer cell growth through Ras-GTPase-activating protein SH3 domain-binding protein 1. Cancer Prev Res (Phila Pa) 2010;3:670–679. doi: 10.1158/1940-6207.CAPR-09-0185. [DOI] [PubMed] [Google Scholar]
- 36.Suganuma M, Kurusu M, Suzuki K, Tasaki E, Fujiki H. Green tea polyphenol stimulates cancer preventive effects of celecoxib in human lung cancer cells by upregulation of GADD153 gene. Int J Cancer. 2006;119:33–40. doi: 10.1002/ijc.21809. [DOI] [PubMed] [Google Scholar]
- 37.Manna S, Mukherjee S, Roy A, Das S, Panda CK. Tea polyphenols can restrict benzo [a]pyrene-induced lung carcinogenesis by altered expression of p53-associated genes and H-ras, c-myc and cyclin D1. J Nutr Biochem. 2009;20:337–349. doi: 10.1016/j.jnutbio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, et al. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–268. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu M, Shirakami Y, et al. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008;262:10–18. doi: 10.1016/j.canlet.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Shirakami Y, Shimizu M, Adachi S, Sakai H, Nakagawa T, Yasuda Y, et al. (−)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009;100:1957–1962. doi: 10.1111/j.1349-7006.2009.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin J, Xie LP, Zheng XY, Wang YB, Bai Y, Shen HF, et al. A component of green tea, (−)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem Biophys Res Commun. 2007;354:852–857. doi: 10.1016/j.bbrc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Facchini A, Zanella B, Stefanelli C, Guarnieri C, Flamigni F. Effect of green tea extract on the induction of ornithine decarboxylase and the activation of extracellular signal-regulated kinase in bladder carcinoma ECV304 cells. Nutr Cancer. 2003;47:104–110. doi: 10.1207/s15327914nc4701_13. [DOI] [PubMed] [Google Scholar]
- 43.Lee SC, Chan WK, Lee TW, Lam WH, Wang X, Chan TH, et al. Effect of a prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate on the growth of androgen-independent prostate cancer in vivo. Nutr Cancer. 2008;60:483–491. doi: 10.1080/01635580801947674. [DOI] [PubMed] [Google Scholar]
- 44.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 45.Bettuzzi S, Rizzi F, Belloni L. Clinical relevance of the inhibitory effect of green tea catechins (GtCs) on prostate cancer progression in combination with molecular profiling of catechin-resistant tumors: an integrated view. Pol J Vet Sci. 2007;10:57–60. [PubMed] [Google Scholar]
- 46.Johnson JJ, Bailey HH, Mukhtar H. Green tea polyphenols for prostate cancer chemo-prevention: a translational perspective. Phytomedicine. 2010;17:3–13. doi: 10.1016/j.phymed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez BB, Sarfaraz S, et al. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27:2055–2063. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- 48.Kane RC, Farrell AT, Sridhara R, Pazdur R. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12:2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 49.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, Khuri FR, et al. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008;123:1005–1014. doi: 10.1002/ijc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masuda M, Suzui M, Lim JT, Weinstein IB. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin Cancer Res. 2003;9:3486–3491. [PubMed] [Google Scholar]
- 52.Yagiz K, Wu LY, Kuntz CP, James Morre D, Morre DM. Mouse embryonic fibroblast cells from transgenic mice overexpressing tNOX exhibit an altered growth and drug response phenotype. J Cell Biochem. 2007;101:295–306. doi: 10.1002/jcb.21184. [DOI] [PubMed] [Google Scholar]
- 53.Ji SJ, Han DH, Kim JH. Inhibition of proliferation and induction of apoptosis by EGCG in human osteogenic sarcoma (HOS) cells. Arch Pharm Res. 2006;29:363–368. doi: 10.1007/BF02968585. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Hao MW, Dong K, Lin F, Ren JH, Zhang HZ. Apoptosis induction effects of EGCG in laryngeal squamous cell carcinoma cells through telomerase repression. Arch Pharm Res. 2009;32:1263–1269. doi: 10.1007/s12272-009-1912-8. [DOI] [PubMed] [Google Scholar]
- 55.Luo F, Hu Z, et al. Effect of tea polyphenols and EGCG on nasopharyngeal carcinoma cell proliferation and the mechanisms involved. Chin J Cancer Res. 2001;13:235–242. [Google Scholar]
- 56.Gu B, Ding Q, Xia G, Fang Z. EGCG inhibits growth and induces apoptosis in renal cell carcinoma through TFPI-2 overexpression. Oncol Rep. 2009;21:635–640. [PubMed] [Google Scholar]
- 57.Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Modulation of human renal cell carcinoma 786-0 MMP-2 and MMP-9 activity by inhibitors and inducers in vitro. Med Oncol. 2006;23:245–250. doi: 10.1385/mo:23:2:245. [DOI] [PubMed] [Google Scholar]
- 58.Suganuma M, Ohkura Y, Okabe S, Fujiki H. Combination cancer chemoprevention with green tea extract and sulindac shown in intestinal tumor formation in Min mice. J Cancer Res Clin Oncol. 2001;127:69–72. doi: 10.1007/s004320000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inaba H, Nagaoka Y, Kushima Y, Kumagai A, Matsumoto Y, Sakaguchi M, et al. Comparative examination of anti-proliferative activities of (−)-epigallocatechin gallate and (−)-epigallocatechin against HCT116 colorectal carcinoma cells. Biol Pharm Bull. 2008;31:79–84. doi: 10.1248/bpb.31.79. [DOI] [PubMed] [Google Scholar]
- 60.Kim M, Murakami A, Ohigashi H. Modifying effects of dietary factors on (−)-epigallo-catechin-3-gallate-induced pro-matrix metalloproteinase-7 production in HT-29 human colorectal cancer cells. Biosci Biotechnol Biochem. 2007;71:2442–2450. doi: 10.1271/bbb.70213. [DOI] [PubMed] [Google Scholar]
- 61.Lim YC, Park HY, Hwang HS, Kang SU, Pyun JH, Lee MH, et al. (−)-Epigallo-catechin-3-gallate (EGCG) inhibits HGF-induced invasion and metastasis in hypopharyngeal carcinoma cells. Cancer Lett. 2008;271:140–152. doi: 10.1016/j.canlet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, Desmoulin S, Banerjee S, Kong D, Li Y, Deraniyagala RL, et al. Synergistic effects of multiple natural products in pancreatic cancer cells. Life Sci. 2008;83:293–300. doi: 10.1016/j.lfs.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ran ZH, Xu Q, Tong JL, Xiao SD. Apoptotic effect of Epigallocatechin-3-gallate on the human gastric cancer cell line MKN45 via activation of the mitochondrial pathway. World J Gastroenterol. 2007;13:4255–4259. doi: 10.3748/wjg.v13.i31.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu BH, Zhan WH, Li ZR, Wang Z, He YL, Peng JS, et al. (−)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J Gastroenterol. 2007;13:1162–1169. doi: 10.3748/wjg.v13.i8.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conney AH, Lu Y, Lou Y, Xie J, Huang M. Inhibitory effect of green and black tea on tumor growth. Proc Soc Exp Biol Med. 1999;220:229–233. doi: 10.1046/j.1525-1373.1999.d01-39.x. [DOI] [PubMed] [Google Scholar]
- 66.Dreosti IE, Wargovich MJ, Yang CS. Inhibition of carcinogenesis by tea: the evidence from experimental studies. Crit Rev Food Sci Nutr. 1997;37:761–770. doi: 10.1080/10408399709527801. [DOI] [PubMed] [Google Scholar]
- 67.Yang CS, Kim S, Yang GY, Lee MJ, Liao J, Chung JY, et al. Inhibition of carcinogenesis by tea: bioavailability of tea polyphenols and mechanisms of actions. Proc Soc Exp Biol Med. 1999;220:213–217. doi: 10.1046/j.1525-1373.1999.d01-36.x. [DOI] [PubMed] [Google Scholar]
- 68.Wang ZY, Hong JY, Huang MT, Reuhl KR, Conney AH, Yang CS. Inhibition of N-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mice by green tea and black tea. Cancer Res. 1992;52:1943–1947. [PubMed] [Google Scholar]
- 69.Katiyar SK, Mohan RR, Agarwal R, Mukhtar H. Protection against induction of mouse skin papillomas with low and high risk of conversion to malignancy by green tea polyphenols. Carcinogenesis. 1997;18:497–502. doi: 10.1093/carcin/18.3.497. [DOI] [PubMed] [Google Scholar]
- 70.Umemura T, Kai S, Hasegawa R, Kanki K, Kitamura Y, Nishikawa A, et al. Prevention of dual promoting effects of pentachlorophenol, an environmental pollutant, on diethylnitrosamine-induced hepato- and cholangiocarcinogenesis in mice by green tea infusion. Carcinogenesis. 2003;24:1105–1109. doi: 10.1093/carcin/bgg053. [DOI] [PubMed] [Google Scholar]
- 71.Ho YC, Yang SF, Peng CY, Chou MY, Chang YC. Epigallocatechin-3-gallate inhibits the invasion of human oral cancer cells and decreases the productions of matrix metalloproteinases and urokinase–plasminogen activator. J Oral Pathol Med. 2007;36:588–593. doi: 10.1111/j.1600-0714.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 72.Fujimoto N, Sueoka N, Sueoka E, Okabe S, Suganuma M, Harada M, et al. Lung cancer prevention with (−)-epigallocatechin gallate using monitoring by heterogeneous nuclear ribonucleoprotein B1. Int J Oncol. 2002;20:1233–1239. [PubMed] [Google Scholar]
- 73.Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–616. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 74.Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2010;21:140–146. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Rieger-Christ KM, Hanley R, Lodowsky C, Bernier T, Vemulapalli P, Roth M, et al. The green tea compound, (−)-epigallocatechin-3-gallate downregulates N-cadherin and suppresses migration of bladder carcinoma cells. J Cell Biochem. 2007;102:377–388. doi: 10.1002/jcb.21299. [DOI] [PubMed] [Google Scholar]
- 76.Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu G, et al. Pro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010;31:902–910. doi: 10.1093/carcin/bgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- 78.Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–1446. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- 79.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 80.Esteller M, Risques RA, Toyota M, Capella G, Moreno V, Peinado MA, et al. Promoter hypermethylation of the DNA repair gene O(6)-methylguanine-DNA methyl-transferase is associated with the presence of G:C to A:T transition mutations in p53 in human colorectal tumorigenesis. Cancer Res. 2001;61:4689–4692. [PubMed] [Google Scholar]
- 81.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (−)-epigallo-catechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- 82.Yuasa Y, Nagasaki H, Akiyama Y, Sakai H, Nakajima T, Ohkura Y, et al. Relationship between CDX2 gene methylation and dietary factors in gastric cancer patients. Carcinogenesis. 2005;26:193–200. doi: 10.1093/carcin/bgh304. [DOI] [PubMed] [Google Scholar]
- 83.Morley N, Clifford T, Salter L, Campbell S, Gould D, Curnow A. The green tea polyphenol (−)-epigallocatechin gallate and green tea can protect human cellular DNA from ultraviolet and visible radiation-induced damage. Photodermatol Photoimmunol Photomed. 2005;21:15–22. doi: 10.1111/j.1600-0781.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- 84.Hakim IA, Harris RB, Brown S, Chow HH, Wiseman S, Agarwal S, et al. Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. J Nutr. 2003;133:3303S–3309S. doi: 10.1093/jn/133.10.3303S. [DOI] [PubMed] [Google Scholar]
- 85.Annabi B, Currie JC, Moghrabi A, Beliveau R. Inhibition of HuR and MMP-9 expression in macrophage-differentiated HL-60 myeloid leukemia cells by green tea polyphenol EGCg. Leuk Res. 2007;31:1277–1284. doi: 10.1016/j.leukres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Fujiki H, Suganuma M, Okabe S, Kurusu M, Imai K, Nakachi K. Involvement of TNF-alpha changes in human cancer development, prevention and palliative care. Mech Ageing Dev. 2002;123:1655–1663. doi: 10.1016/s0047-6374(02)00101-x. [DOI] [PubMed] [Google Scholar]
- 87.Zhou J, Mulshine JL, Unsworth EJ, Scott FM, Avis IM, Vos MD, et al. Purification and characterization of a protein that permits early detection of lung cancer. Identification of heterogeneous nuclear ribonucleoprotein-A2/B1 as the antigen for monoclonal antibody 703D4. J Biol Chem. 1996;271:10760–10766. doi: 10.1074/jbc.271.18.10760. [DOI] [PubMed] [Google Scholar]
- 88.Costa M, Ochem A, Staub A, Falaschi A. Human DNA helicase VIII: a DNA and RNA helicase corresponding to the G3BP protein, an element of the ras transduction pathway. Nucleic Acids Res. 1999;27:817–821. doi: 10.1093/nar/27.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu G, Han C, Chen J. Inhibition of oncogene expression by green tea and (−)-epigallo-catechin gallate in mice. Nutr Cancer. 1995;24:203–209. doi: 10.1080/01635589509514408. [DOI] [PubMed] [Google Scholar]
- 90.Pines G, Kostler WJ, Yarden Y. Oncogenic mutant forms of EGFR: lessons in signal transduction and targets for cancer therapy. FEBS Lett. 2010;584:2699–2706. doi: 10.1016/j.febslet.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo Y, Liang F, Zhang ZY. PRL1 promotes cell migration and invasion by increasing MMP2 and MMP9 expression through Src and ERK1/2 pathways. Biochemistry. 2009;48:1838–1846. doi: 10.1021/bi8020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mason DP, Kenagy RD, Hasenstab D, Bowen-Pope DF, Seifert RA, Coats S, et al. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ Res. 1999;85:1179–1185. doi: 10.1161/01.res.85.12.1179. [DOI] [PubMed] [Google Scholar]
- 93.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 94.Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 95.Seemuller E, Lupas A, Stock D, Lowe J, Huber R, Baumeister W. Proteasome from thermoplasma acidophilum: a threonine protease. Science. 1995;268:579–582. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- 96.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 97.An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 98.Lopes UG, Erhardt P, Yao R, Cooper GM. p53-Dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 99.Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7:9–16. doi: 10.1634/theoncologist.7-1-9. [DOI] [PubMed] [Google Scholar]
- 100.Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals) IDrugs. 2002;5:828–834. [PubMed] [Google Scholar]
- 101.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 102.Kazi A, Wang Z, Kumar N, Falsetti SC, Chan TH, Dou QP. Structure–activity relationships of synthetic analogs of (−)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Res. 2004;24:943–954. [PubMed] [Google Scholar]
- 103.Landis-Piwowar KR, Kuhn DJ, Wan SB, Chen D, Chan TH, Dou QP. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (−)-EGCG analogs and their prodrugs. Int J Mol Med. 2005;15:735–742. [PubMed] [Google Scholar]
- 104.Kuhn D, Lam WH, Kazi A, Daniel KG, Song S, Chow LM, et al. Synthetic peracetate tea polyphenols as potent proteasome inhibitors and apoptosis inducers in human cancer cells. Front Biosci. 2005;10:1010–1023. doi: 10.2741/1595. [DOI] [PubMed] [Google Scholar]
- 105.Clement Y. Can green tea do that? A literature review of the clinical evidence. Prev Med. 2009;49:83–87. doi: 10.1016/j.ypmed.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 106.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pan MH, Lin CC, Lin JK, Chen WJ. Tea polyphenol (−)-epigallocatechin 3-gallate suppresses heregulin-beta1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase cascade signaling. J Agric Food Chem. 2007;55:5030–5037. doi: 10.1021/jf070316r. [DOI] [PubMed] [Google Scholar]
- 108.Klein RD, Fischer SM. Black tea polyphenols inhibit IGF-I-induced signaling through Akt in normal prostate epithelial cells and Du145 prostate carcinoma cells. Carcinogenesis. 2002;23:217–221. doi: 10.1093/carcin/23.1.217. [DOI] [PubMed] [Google Scholar]
- 109.Opare Kennedy D, Kojima A, Hasuma T, Yano Y, Otani S, Matsui-Yuasa I. Growth inhibitory effect of green tea extract and (−)-epigallocatechin in Ehrlich ascites tumor cells involves a cellular thiol-dependent activation of mitogenic-activated protein kinases. Chem Biol Interact. 2001;134:113–133. doi: 10.1016/s0009-2797(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 110.Kida K, Suzuki M, Matsumoto N, Nanjo F, Hara Y. Identification of biliary metabolites of (−)-epigallocatechin gallate in rats. J Agric Food Chem. 2000;48:4151–4155. doi: 10.1021/jf000386x. [DOI] [PubMed] [Google Scholar]
- 111.Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, et al. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003;31:452–461. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- 112.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos. 2003;31:572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 113.Lam WH, Kazi A, Kuhn DJ, Chow LM, Chan AS, Dou QP, et al. A potential prodrug for a green tea polyphenol proteasome inhibitor: evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG] Bioorg Med Chem. 2004;12:5587–5593. doi: 10.1016/j.bmc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 114.Lambert JD, Sang S, Hong J, Kwon SJ, Lee MJ, Ho CT, et al. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab Dispos. 2006;34:2111–2116. doi: 10.1124/dmd.106.011460. [DOI] [PubMed] [Google Scholar]
- 115.Li L, Chan TH. Enantioselective synthesis of epigallocatechin-3-gallate (EGCG), the active polyphenol component from green tea. Org Lett. 2001;3:739–741. doi: 10.1021/ol000394z. [DOI] [PubMed] [Google Scholar]
- 116.Wan TH, Chan SB. Enantioselective Synthesis of Afzelechin and Epiafzelechin. Tetrahedron. 2004;60:8207–8211. [Google Scholar]
- 117.Smith DM, Wang Z, Kazi A, Li LH, Chan TH, Dou QP. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol Med. 2002;8:382–392. [PMC free article] [PubMed] [Google Scholar]
- 118.Wan SB, Landis-Piwowar KR, Kuhn DJ, Chen D, Dou QP, Chan TH. Structure–activity study of epi-gallocatechin gallate (EGCG) analogs as proteasome inhibitors. Bioorg Med Chem. 2005;13:2177–2185. doi: 10.1016/j.bmc.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 119.Smith DM, Daniel KG, Wang Z, Guida WC, Chan TH, Dou QP. Docking studies and model development of tea polyphenol proteasome inhibitors: applications to rational drug design. Proteins. 2004;54:58–70. doi: 10.1002/prot.10504. [DOI] [PubMed] [Google Scholar]
- 120.Osanai K, Huo C, Landis-Piwowar KR, Dou QP, Chan TH. Synthesis of (2R, 3R)-epigallocatechin-3-O-(4-hydroxybenzoate), a novel catechin from Cistus salvifolius, and evaluation of its proteasome inhibitory activities. Tetrahedron. 2007;63:7565–7570. doi: 10.1016/j.tet.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matsumura K, Kaihatsu K, Mori S, Cho HH, Kato N, Hyon SH. Enhanced anti-tumor activities of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives in vitro and in vivo. Biochem Biophys Res Commun. 2008;377:1118–1122. doi: 10.1016/j.bbrc.2008.10.128. [DOI] [PubMed] [Google Scholar]
- 122.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian–American women. Cancer Res. 2003;63:7526–7529. [PubMed] [Google Scholar]
- 123.Wan SB, Dou QP, Chan TH. Regiospecific and enantioselective synthesis of methylated metabolites of tea catechins. Tetrahedron. 2006;62:5897–5904. [Google Scholar]
- 124.Dou QP, Landis-Piwowar KR, Chen D, Huo C, Wan SB, Chan TH. Green tea polyphenols as a natural tumour cell proteasome inhibitor. Inflammopharmacol. 2008;16:208–212. doi: 10.1007/s10787-008-8017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Landis-Piwowar KR, Wan SB, Wiegand RA, Kuhn DJ, Chan TH, Dou QP. Methylation suppresses the proteasome-inhibitory function of green tea polyphenols. J Cell Physiol. 2007;213:252–260. doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- 126.Sanchez-del-Campo L, Oton F, Tarraga A, Cabezas-Herrera J, Chazarra S, Rodriguez-Lopez JN. Synthesis and biological activity of a 3,4,5-trimethoxybenzoyl ester analogue of epicatechin-3-gallate. J Med Chem. 2008;51:2018–2026. doi: 10.1021/jm701346h. [DOI] [PubMed] [Google Scholar]
- 127.Park KD, Lee SG, Kim SU, Kim SH, Sun WS, Cho SJ, et al. Anticancer activity of 3-O-acyl and alkyl-(−)-epicatechin derivatives. Bioorg Med Chem Lett. 2004;14:5189–5192. doi: 10.1016/j.bmcl.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 128.Yang H, Sun DK, Chen D, Cui QC, Gu YY, Jiang T, et al. Antitumor activity of novel fluoro-substituted (−)-epigallocatechin-3-gallate analogs. Cancer Lett. 2010;292:48–53. doi: 10.1016/j.canlet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu Z, Qin XL, Gu YY, Chen D, Cui QC, Jiang T, et al. Prodrugs of fluoro-substituted benzoates of EGC as tumor cellular proteasome inhibitors and apoptosis inducers. Int J Mol Sci. 2008;9:951–961. doi: 10.3390/ijms9060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wei D, Mei Y, Liu J. Quantification of doxorubicin and validation of reversal effect of tea polyphenols on multidrug resistance in human carcinoma cells. Biotechnol Lett. 2003;25:291–294. doi: 10.1023/a:1022343832525. [DOI] [PubMed] [Google Scholar]