Abstract
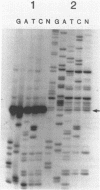
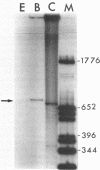
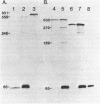
The 5'-terminal sequences of the virion protein mRNAs of ononis yellow mosaic and kennedya yellow mosaic tymoviruses were determined, and also the positions in the genomes of the transcription initiation sites of those mRNAs. Comparisons of the available genomic sequences of tymoviruses revealed two conserved regions, one at the initiation site and another longer sequence of sixteen nucleotides to the 5' side of it. The longer sequence, which we call the tymobox, was tested as a target for a designed ribozyme, which cleaved appropriate genomic fragments of three tymoviruses. A synthetic oligonucleotide with sequence complementary to the tymobox was shown to be a tymovirus-specific probe for diagnosing and identifying tymoviruses, except for wild cucumber mosaic tymovirus. The tymobox sequence was also used as a primer for the second strand DNA synthesis of dsDNA representing the virion protein gene of cacao yellow mosaic tymovirus, a tymovirus with unknown sequence. Thus, the tymobox is a useful tool in molecular studies of tymoviruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel P. P., Nelson R. S., De B., Hoffmann N., Rogers S. G., Fraley R. T., Beachy R. N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986 May 9;232(4751):738–743. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- Blok J., Gibbs A., Mackenzie A. The classification of tymoviruses by cDNA-RNA hybridization and other measures of relatedness. Arch Virol. 1987;96(3-4):225–240. doi: 10.1007/BF01320962. [DOI] [PubMed] [Google Scholar]
- Ding S. W., Keese P., Gibbs A. Nucleotide sequence of the ononis yellow mosaic tymovirus genome. Virology. 1989 Oct;172(2):555–563. doi: 10.1016/0042-6822(89)90198-0. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- French R., Ahlquist P. Characterization and engineering of sequences controlling in vivo synthesis of brome mosaic virus subgenomic RNA. J Virol. 1988 Jul;62(7):2411–2420. doi: 10.1128/jvi.62.7.2411-2420.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargouri R., Joshi R. L., Bol J. F., Astier-Manifacier S., Haenni A. L. Mechanism of synthesis of turnip yellow mosaic virus coat protein subgenomic RNA in vivo. Virology. 1989 Aug;171(2):386–393. doi: 10.1016/0042-6822(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Goldbach R. Genome similarities between plant and animal RNA viruses. Microbiol Sci. 1987 Jul;4(7):197–202. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guilley H., Briand J. P. Nucleotide sequence of turnip yellow mosaic virus coat protein mRNA. Cell. 1978 Sep;15(1):113–122. doi: 10.1016/0092-8674(78)90087-9. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Keese P., Mackenzie A., Gibbs A. Nucleotide sequence of the genome of an Australian isolate of turnip yellow mosaic tymovirus. Virology. 1989 Oct;172(2):536–546. doi: 10.1016/0042-6822(89)90196-7. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Iwai S., Ohtsuka E. Construction of a series of several self-cleaving RNA duplexes using synthetic 21-mers. FEBS Lett. 1988 Feb 15;228(2):228–230. doi: 10.1016/0014-5793(88)80004-8. [DOI] [PubMed] [Google Scholar]
- Marsh L. E., Dreher T. W., Hall T. C. Mutational analysis of the core and modulator sequences of the BMV RNA3 subgenomic promoter. Nucleic Acids Res. 1988 Feb 11;16(3):981–995. doi: 10.1093/nar/16.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. A., Dreher T. W., Hall T. C. Synthesis of brome mosaic virus subgenomic RNA in vitro by internal initiation on (-)-sense genomic RNA. Nature. 1985 Jan 3;313(5997):68–70. doi: 10.1038/313068a0. [DOI] [PubMed] [Google Scholar]
- Morch M. D., Boyer J. C., Haenni A. L. Overlapping open reading frames revealed by complete nucleotide sequencing of turnip yellow mosaic virus genomic RNA. Nucleic Acids Res. 1988 Jul 11;16(13):6157–6173. doi: 10.1093/nar/16.13.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Keese M. E., Keese P., Gibbs A. Nucleotide sequence of the genome of eggplant mosaic tymovirus. Virology. 1989 Oct;172(2):547–554. doi: 10.1016/0042-6822(89)90197-9. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Rice C. M., Dalgarno L., Strauss E. G., Strauss J. H. Sequence studies of several alphavirus genomic RNAs in the region containing the start of the subgenomic RNA. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5235–5239. doi: 10.1073/pnas.79.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Strauss E. G., Strauss J. H. Comparative studies of the 3'-terminal sequences of several alpha virus RNAs. Virology. 1981 Mar;109(2):281–289. doi: 10.1016/0042-6822(81)90499-2. [DOI] [PubMed] [Google Scholar]
- Pleij C. W., Neeleman A., van Vloten-Doting L., Bosch L. Translation of turnip yellow mosaic virus RNA in vitro: a closed and an open coat protein cistron. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4437–4441. doi: 10.1073/pnas.73.12.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Shukla D. D., Ward C. W. Structure of potyvirus coat proteins and its application in the taxonomy of the potyvirus group. Adv Virus Res. 1989;36:273–314. doi: 10.1016/s0065-3527(08)60588-6. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer N. E., O'connell K. M., Nelson R. S., Sanders P. R., Beachy R. N., Fraley R. T., Shah D. M. Expression of alfalfa mosaic virus coat protein gene confers cross-protection in transgenic tobacco and tomato plants. EMBO J. 1987 May;6(5):1181–1188. doi: 10.1002/j.1460-2075.1987.tb02352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]