Abstract
Background and the purpose of the study
Fruits of Rhus coriaria L. (Anacardiaceae) are traditionally used as a table spice in Iran and are highly recommended for diabetic patients.
The purpose of this study was to determine the antidiabetic properties of the ethanolic extract of Rhus coriaria fruits and also its mechanisms of action.
Methods
The effects of ethanolic extract of Rhus coriaria fruits were measured on blood glucose, lipids and antioxidant enzymes by commercial kits. mRNA levels of insulin (INS) and glucose transporter type-4 (GLUT-4) genes were investigated by RT-PCR (Reverse transcription- polymerase chain reaction) technique. Moreover, its effects on intestinal a-glucosidases was measured using an in vitro method.
Results and Conclusion
Following a single dose administration of the extract it was found that extract could significantly reduce postprandial blood glucose by 24% (at 5 hrs). In the long term experiment, on the day of 21, postprandial blood glucose (PBG) was found to be significantly lower (by 26%) compared to diabetic control group. The plant extract raised markedly serum high-density lipoprotein (HDL) by 34% and also reduced low-density lipoprotein (HDL) by 32%. Also it had noticeable antioxidant effects by elevating superoxide dismutase (SOD) and catalase(CAT) activities by 46% and 77%, respectively. However it did not show a strong effect on glutathione peroxidase (GPX) activity. The extract inhibited maltase and sucrase activities by 44% and 27%, respectively. However it made no changes in the transcript levels of INS and GLUT-4 genes.
It can be concluded that constituents of Rhus coriaria fruits have effective components which can be utilized as useful herb for alleviation of diabetes complications.
Keywords: Antioxidant, Blood Glucose, α-Glucosidase, Lipids
INTRODUCTION
Type 2 diabetes is a major public health concern, causing tens of millions of chronic illnesses and a significant number of deaths worldwide each year (1). It is characterized by hyperglycemia and profound alterations in the plasma lipids and lipoproteins profiles (2). Also antioxidant defense system which normally modulates the level of oxidative stress (3) is altered in diabetes (4) and it involves in the pathogenesis of diabetes and its complications (5). Therefore glycemic, lipids profiles and oxidative stress control is fundamental for the management of diabetes (6).
In Iran, Rhus coriaria is traditionally used as a table spice especially along with rich dishes and is highly recommended for adjustment of the blood lipids in diabetic patients.
Previous studies on this plant have shown that its fruits (7) and leaves (8) have in vitro antioxidant properties. Also it has already been reported that in vitro hypoglycemic activity of the methanolic extract of fruits of Rhus coriaria is due to inhibition of a-amylase (87% inhibition at 50 g/ml) (9).
In this study the antihyperglycemic, hypolipidemic and anioxidant activities of the ethanolic extract of Rhus coriaria fruits on diabetic male Wistar rats was investigated in order to verify the use of this herbal medicine in treatment of type 2 diabetes.
MATERIAL AND METHODS
Chemicals and reagents
DEPC Water, Taq polymerase and RNA extraction kit were purchased from CinnaGen (Tehran, Iran). DNase I, RNase free kit was from Fermentas (Ontario, Canada), RT kit and Primers were from Bioneer (Daejon, Korea), dNTPs were from BioFlux (Tokyo, Japan). The kits for determination of glucose, triglyceride (TG), total cholesterol (TC), high density lipoprotein (HDL) were from Chem Enzyme (Tehran, Iran). Hemoglobin Reagent Set was from Ziest Chem (Tehran, Iran), SOD and GPX kits were from Randox (Antrim, UK). All other chemicals and solvents were of the highest commercial grade from Merck (KGaA, Germany) or Sigma (St Louis, MO, USA).
Preparation of Rhus coriaria fruits ethanolic extract(RE)
Fruits of R. Coriaria were collected from Markazi province of Iran and were authenticated by Professor Ahmad Qahraman and a Voucher specimen (No. 5084) was deposited in the herbarium of University of Tehran (Tehran, Iran). The air dried fruits of R. coriaria were finely powdered (400 g) and extracted three times with fresh 96% ethanol at room temperature for 12 hrs. The ethanolic solution after removal of solids was concentrated at 40°C by rotary evaporator and then lyophilized. The percentage yield based on the dried starting material was 16%. The powder was stored at dark at 4°C for subsequent experiments.
Preparation of Alloxan-induced diabetic wistar rats
Male Wistar rats (Rattus norvegicus) weighing 200–250 g were used in this study (Pasteur Institute, Tehran, Iran). Animals were housed six per standard rat cage, in a room with a 12:12 hrs light/dark cycle and controlled temperature (22±1 °C). Diabetes was induced in overnight fasted rats by subcutaneous injection of alloxan monohydrate (100 mg/kg, Sigma, St Louis, MO, USA), dissolved in citrate buffer (pH=4.5), according to a previously described method (10).
Methods
The rats were divided into five groups of six each. Group I (NC): normal rats were treated with vehicle alone; group II (DC): diabetic rats were treated with vehicle alone; group III (REa+D): diabetic rats were treated with RE at doses of 200 mg/kg BW; group IV (REb+D): diabetic rats were treated with RE at doses of 400 mg/kg BW. Group V (Ac+D in the1st phase or Met+D in the 2nd phase): diabetic rats were treated with acarbose (20 mg/kgBW) or metformin (100mg/kg BW). The doses were selected from pervious publications (11).
In the first phase of this study, following 1, 3, 5, 8 and 24 hrs of oral administration of a single dose of samples to rats, PBG levels were estimated using blood obtained from tail vein and a glucometer (On Call Now, San Diego, USA). After two days, Oral Glucose Tolerance Test (OGTT) was carried out for all rats. In this phase acarbose was used as the reference drug.
In the second phase, two days after OGTT, all rats were administered once a day with samples for 21 days. In this phase metformin was used as the reference drug. PBG levels were estimated at the end of one, two and three weeks of treatments. At the end of treatment period, rats were anesthetized by ether and their bloods were collected. Serum total cholesterol, triglycerides and high density lipoprotein levels and the activities of SOD (EC: 1.15.1.1) and GPX (EC: 1.11.1.9) were measured using commercial kits. Low density lipoprotein and very low density lipoprotein were calculated by formula (12) and CAT (EC: 1.11.1.6) activity was measured by method of Aebi (13).Then the animals were killed and their pancreases and hearts were removed promptly for the estimation of insulin (INS) and glucose transporter-4 (GLUT-4) mRNA expression.
In a separate experiment the inhibitory effects of plant extract on intestinal a-Glucosidases (sucrase and maltase) was measured by an in vitro method.
Measurement of RE effects on oral glucose tolerance
NC and DC Groups received distilled water orally. Animals of REa+D and REb+D groups received orally RE, 200 and 400 mg/kg BW respectively.To group Ac+D was given the reference drug, acarbose, (20 mg/kg BW). Thirty min later, each rat received orally a carbohydrate solution (equal proportion of maltose and sucrose) (2 g/kg BW). PBG of each animal was determined at 0 min, just before carbohydrate solution loading, and at 30, 60 and 120 min after that, using a glucometer (On Call Now, San Diego, USA).
Gene expression analysis
Pancreas and heart left ventricle tissues of DC, NC and REb+D groups were dissected from rats immediately after sacrificing, placed in liquid nitrogen, and then stored at -70°C until used. Total RNA of tissues were extracted by RNX- Plus kit according to its manual. The DNA free RNA was prepared prior to RT-PCR using DNase I, RNase-free kit. For reverse transcription to obtain cDNA, AccuPower RT PreMix kit, 50 ng/l template RNA and 25 ng/l oligo dT18 were used. The primers were as follows: INS F, 5'-TTCTTCTACACACCCAAG-3'; INS R, 5'- GCAGTAGTTCTCCAGTTG-3' (155-bp) GLUT-4 F, 5'-AGGCACCCTTACCCTTTT-3'; GLUT-4 R 5'-GACAGAAGGGCAACAGAAGC-3' (318-bp); and B-act F, 5'-AGCCATGTACGTAGCCATCC- 3'; B-act R, 5'-TCTCAGCTGTGGTGGTGAAG-3' (248-bp). For PCR reaction, 500 ng of the cDNA was added to a PCR reaction mixture consisting of 10X PCR buffer (2.5 l), 50 MmMgCl2 (0.75 l), 10 mM dNTPs (0.5 l), 10 pM of paired primers (0.5 l of each), 0.25 units of Taq polymerase and distilled water in a total volume of 25 l. The reaction mixture was overlaid in a PCR thermal cycler for 35 cyclic reactions. PCR products were run on 1.5% agarose gels, stained with ethidium bromide and photographed. Images of radiographs were analyzed with TotalLab v1.10 using 1D analysis.
In vitro inhibition assay for rat intestinal sucrase and maltase activities
The inhibitory effects of RE on rat intestinal sucrase and maltase activities were determined using a literature method (14).
Statistical analyses
All data are presented as means±S.D. for six rats in each group. Comparison between groups and between time points was made by one-way analysis of variance (ANOVA) followed by Duncan's test using SPSS (SPSS Inc, Chicago, USA).
RESULTS AND DISCUSSION
Short-term effects of RE on PBG levels
Administration of the extract at the dose of 400 mg/kg reduced the glucose level at 5 hrs time point compared to both initial level and DC group (Table 1).
Table 1.
Acute effect of the ethanolic extract of Rhus coriaria fruits on postprandial blood glucose levels in Alloxan-diabetics rats.
| Groups | Dose (ms/ks BW) | Postprandial blood glucose (mg/dl) Time (h) after a single dose administration of drugs | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 8 | 24 | ||
| NC | - | 69.2±2.3d | 72±3.5cd | 74.4±3.4cd | 71.2±6.5cd | 64.8±2.9cd | 75.2±4.5cd |
| DC | - | 334.8±6.3 | 335±8.6c | 345.2±7.19c | 340.2±3.9c | 343.8±6.9c | 346±9.9c |
| REa+D | 200 | 338.2±42.2f | 331.8±11.8cf | 329.6±12.5cf | 316±10.8be | 346.8±7.6cf | 359.6±6.8bf |
| REb+D | 400 | 325.2±5.2f | 319±6.7cf | 302.8±15.9cd | 289.2±12.3bd | 294.6±21bd | 313.6±13.6cd |
| Ac+D | 20 | 318.2±10.2e | 265.2±7ad | 256.2±7.3ad | 186.2±14.8ad | 199.4±7ad | 266.8±7.8ad |
NC indicates Normal Control; DC, Diabetic Control; RE+D, Diabetic rats treated with ethanolic extract of Rhus coriaria fruits Ac+D, Diabetic rats treated with Acarbose.
p<0.0001
p<0.007 and
p>0.096 vs. Time 0
p<0.0001
p<0.006 and
p>0.058 vs. DC.
Effects of RE on oral glucose tolerance
As shwon in Table 2, diabetic rats treated with 200 and 400 mg/kg RE showed remarkable decrease in blood glucose at the first hour after carbohydrate solution loading compared to DC group;however they were not as effective as acarbose.
Table 2.
Effect of the ethanolic extract of Rhus coriaria fruits on Oral Glucose Tolerance Test in Alloxan-diabetic rats.
| Groups | Dose (mg/kg BW) | Blood Glucose (mg/dl) minutes after administration a single dose of drugs | |||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 120 | ||
| NC | - | 72.8±2.8a | 133.8±31.1a | 126.6±28.5a | 90.6±6.1a |
| DC | - | 157.6±10 | 347.2±19.9 | 308.6±12.1 | 247±9.3 |
| REa+D | 200 | 155.4±8.4b | 318.4±18.3b | 257.6±14.7a | 236.1±12.8b |
| REb+D | 400 | 175±9.7c | 328.8±13.3a | 223.2±10.8a | 232±16b |
| Ac+D | 20 | 154.4±6.8b | 234.4±10.5a | 172±6.2a | 162.8±5.6a |
NC indicates Normal Control; DC, Diabetic Control; RE+D, Diabetic rats treated with ethanolic extract of Rhus coriaria fruits; Ac+D, Diabetic rats treated with Acarbose.
p<0.0001
p>0.106 and
p>0.065 vs. DC.
Long-term effects of RE on PBG
The RE at the dose of 400 mg/kg induced significant reductions in PBG levels in diabetic rats, after 7, 14 and 21 days of treatment (Table 3), compared to initial time point. The data of REb+D and Met+D groups were significantly different from those of DC group (p<0.0001).
Table 3.
Glycemic control by ethanolic extract of Rhus coriaria fruits in Alloxan- diabetic rats after 3 weeks treatment.
| Groups | Dose (mg/kg BW) | Postprandial blood glucose (mg/dl) Days after single dose treatment daily | |||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | ||
| NC | - | 76.8±2.8d | 82±5cd | 77±6cd | 83.5±5cd |
| DC | - | 324.8±7.2 | 347±10.3b | 364±8.1a | 353.3±11.1b |
| REa+D | 200 | 337.4±15.4f | 319.2±11.9cf | 311.6±19.4ce | 314.7±22.7cf |
| REb+D | 400 | 331.4±19.6f | 282.2±17.7bd | 269.8±16.3ad | 261.6±22.4ad |
| Met+D | 100 | 318.2±10.2f | 251.3±6.7bd | 213.1±5.9ad | 190.2±8.2ad |
NC indicates Normal Control; DC, Diabetic Control; RE+D, Diabetic rats treated with ethanolic extract of Rhus coriaria fruits; Met+D, Diabetic rats treated with Metformin.
p<0.0001
p<0.004 and
p>0.141 vs. Time 0
p<0.0001
p<0.001 and
p>0.065 vs. DC.
Effects of RE on rat intestinal sucrase and maltase activities
The crude ethanolic extract of Rhus coriaria fruits showed strong inhibitory effects for both sucrase and maltase activities,25.38%(±2.05) and 44.33%(±1.14) respectively.
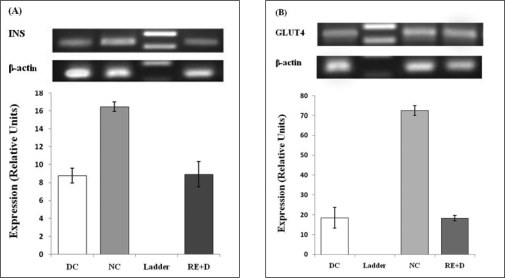
Effects of RE on INS and GLUT-4 genes expression
Densitometric scanning revealed no increase in INS (p>0.156) and GLUT-4 (p>0.564) transcripts by RE, as compared to DC group.
Effects of RE on blood lipids
Treatment with RE (400 mg/kg) strongly increased (Table 4) the level of HDL, in a way that it was comparable to NC rats (p>0.988). Also it reduced the level of LDL significantly (33%, p<0.012) compared to DC group.
Table 4.
Effects of the ethanolic extract of Rhus coriaria fruit on plasma lipids of Alloxan-diabetic rats, after 21 days of treatment.
| Groups | Dose (mg/kg BW) | Serum lipids (mg/dl) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TG | TC | HDL | LDL | VLDL | ||||||
| NC | - | 82.2±5.6a | 81.4±2.8a | 45.8±3.2be | 19.2±4a | 16.4±1.1a | ||||
| DC | - | 144.2±5.3 | 112.6±9 | 35±5.6 | 48.8±13.8 | 28.8±1 | ||||
| REa+D | 200 | 141.6±7.5d | 111.7±4.8d | 40.8±8.3d | 42.6±9.51d | 28.3±1.5d | ||||
| Met+D | 100 | 131.4±3.3c | 102.1±6.7d | 37.1±3.7d | 38.3±9.8d | 26.3±0.6d | ||||
DNC indicates Normal Control; DC, Diabetic Control; RE+D, Diabetic rats treated with ethanolic extract of Rhus coriaria fruits ; Met+D, Diabetic rats treated with Metformin.
p<0.0001
p<0.001
p<0.031 and
p>0.086 vs. DC
p>0.988 vs. NC.
Effects of RE on antioxidant enzymes activities
As presented in Table 5, the RE-treated groups showed no remarkable change in GPX activity, however a strong increase was observed in SOD and CAT activities at the dose of 400 mg/kg compared to DC group.
Table 5.
Changes in the activities of superoxide dismutase, glutathione peroxidase and catalase of erythrocytes of Alloxan-diabetic rats made ethanolic extract of Rhus coriaria fruits.
| Groups | Dose (mg/kg BW) | Blood antioxidant enzymes | ||
|---|---|---|---|---|
| SOD (U/g Hb) | GPX (U/g Hb) | CAT (K/g Hb) | ||
| NC | - | 2978±95.2a | 55.67±2.21a | 1.441±0.09a |
| DC | - | 1916.8±22.1 | 31.91±5.54 | 0.508±0.031 |
| REa+D | 200 | 2455.7±83.4a | 30.84±6.62 | 0.755±0.053a |
| REb+D | 400 | 2793.8±90.2a | 35.45±1.7c | 0.901±0.069a |
| Met+D | 100 | 2048.1±19.8c | 39.33±4.59b | 0.533±0.034c |
NC indicates Normal Control; DC, Diabetic Control; RE+D, Diabetic rats treated with ethanolic extract of Rhus coriaria fruits; Met+D, Diabetic rats treated with Metformin.
p<0.0001
p<0.004 and
p>0.107 vs. DC
DISCUSSION
Previous findings demonstrated that some genus of Rhus like Rhus chirindensis (15) and Rhus chinensis (16) have hypoglycemic properties.
In the Alloxan-induced diabetic rats, administration of the extract of Rhus coriaria fruits produced a statistically significant acute and long-term decrease in postprandial blood glucose concentration, which were not comparable to the effects of the reference drugs. It has already been reported that rhus coriaria might have hypoglycemic activity by inhibition of alpha-amylase, a glycoside hydrolase (9).Results of this study showed no change in the INS and GLUT- 4 genes expression, so antihyperglycemic effects of Rhus coriaria fruits may be related to modulation of insulin secretion or action (17). OGTT results and the ability of inhibiting the a-glucosidases activities by the extract indicate that control of postprandial glucose level might be mediated through the inhibition of carbohydrate digestion or absorption (16).
Oral administration of ethanolic extract of Rhus coriaria fruits increased SOD and CAT levels of the red blood cells. This finding upholds the in vitro antioxidant activity of Rhus coriaria demonstrated perviously (7). Antioxidant activity of Rhus coriaria could also be useful for prevention of diabetes complications due to hyperglycemia (11)Figure 1.
Figure 1.
Changes in insulin and cardiac glucose transporter-4 mRNA expression profile in rats.
(A) Analysis of INS transcripts (186 bp) in pancreas tissue in RE treated diabetic rats showed no elevated levels of INS transcripts compared with DC rats (p>0.156). (B) Analysis of GLUT-4 transcripts (449 bp) in heart tissue in RE treated diabetic rats showed no elevated levels of GLUT-4 transcripts compared with DC rats (p>0.564). The data represent the average of three or four samples (only one image is shown). NC: Normal Control; DC: Diabetic Control; RE+D: Diabetic rats treated with Rhus coriaria fruits ethanolic extract.
This study aside from Rhus coriaria antihyper- glycemic and antioxidant activities indicated that extract may also reverse dyslipidemia associated with diabetes by increasing the HDL level and decreasing the LDL level similar to some other herbs previously described (18).
In conclusion, the use of Rhus coriarisa in Iranian folk medicine as a hypolipidemic agent has a significant correlation with the scientific data generated in this study. Also the present study explains two other properties of Rhus coriaria fruits on diabetic rats which can improve the life of type 2 diabetic patients: mild antihyperglycemic and potent antioxidant peroperties. Further studies are required to show the active constituent(s) of Rhus coriaria.
ACKNOWLEDGMENTS
The authors would like to thank the Cellular and Molecular Department of University of Tehran for financial support.
REFERENCES
- 1.Roglic G, Unwin N, Bennett P, Mathers HC, Tuomilehto J, Nag S, Connolly V, King H. The Burden of Mortality Attributable to Diabetes. Diabetes Care. 2005;28:2130–2135. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- 2.Betteridge J. Lipid disorders in diabetes mellitus. In: Pickup JC, Williams G, editors. Textbook of Diabetes. London: Blackwell Science; 1997. pp. 1–35. [Google Scholar]
- 3.Saxena AK, Srivastava P, Kale RK, Baquer NZ. Impaired antioxidant status in diabetic rat liver: Effect of vanadate. Biochemical pharmacology. 1993;45:539–542. doi: 10.1016/0006-2952(93)90124-f. [DOI] [PubMed] [Google Scholar]
- 4.Maritim AC, Sanders RA, Watkins JB. Effect of alpha lipoic acid on biomarkers of oxidative stress in streptozotoc in-induced diabetic rats. Journal of Nutritional Biochemistry. 2003;14:288–294. doi: 10.1016/s0955-2863(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 5.Rahimi R, Nikfar Sh, Larijani B, Abdollahi M. Dossier: Antioxidants in the prevention of human diseases. A review on the role of antioxidants in the management of diabetes and its complications. Biomedicine & Pharmacotherapy. 2005;59:365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.ADA (American Diabetes Association) Standards of Medical Care in Diabetes. Diabetes Care. 2007;30:4–41. [Google Scholar]
- 7.Kosar M, Bozan B, Temelli F, Baser KHC. Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.) extracts. Food Chemistry. 2006;103:952–959. [Google Scholar]
- 8.Ozcan M. Antioxidant activities of rosemary, sage, and sumac extracts and their combinations on stability of natural peanut oil. J Med Food. 2003;6:267–270. doi: 10.1089/10966200360716698. [DOI] [PubMed] [Google Scholar]
- 9.Giancarlo S, Rosa LM, Nadjafi F, Francesco M. Hypoglycaemic activity of two spices extracts: Rhus coriaria L. and Bunium persicum Boiss. Natural Product Research. 2006;20:882–886. doi: 10.1080/14786410500520186. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Kumar D, Deshmukh RR, Lokhande PD, More SN, Rangari VD. Antidiabetic potential ofPhyllanthus reticulatus in Alloxan-induced diabetic mice. Fitoterapia. 2008;79:21–23. doi: 10.1016/j.fitote.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Hasani-Ranjbar SH, Larijani B, Abdollahi M. A Systematic Review of the Potential Herbal Sources ofFuture Drugs Effective in Oxidant-Related Diseases. Inflammation & Allergy-Drug Targets. 2009;8:2–10. doi: 10.2174/187152809787582561. [DOI] [PubMed] [Google Scholar]
- 12.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of LDL-C in plasma withoutuse of the preparative ultracentrifuge. Clinical Chemistry. 1972;18:449–502. [PubMed] [Google Scholar]
- 13.Aebi H. Catalase in Method. In: Vergmeyer HU, editor. Enzymatic Analysis. New York: Academic Press; 1974. p. 673. [Google Scholar]
- 14.Bhandaria MR, Jong-Anurakkuna N, Honga G, Kawabata J. a-Glucosidase and a-amylase inhibitoryactivities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.) Food Chemistry. 2008;106:247–252. [Google Scholar]
- 15.Ojewole JAO. Analgesic, anti-inflammatory and hypoglycaemic effects of Rhus chirindensis (BakerF.) [Anacardiaceae] stem-bark aqueous extract in mice and rats. Journal of Ethnopharmacology. 2007;113:338–345. doi: 10.1016/j.jep.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park IS, Kim BH. Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. Journal of Etnhopharmacology. 2003;85:283–287. doi: 10.1016/s0378-8741(02)00370-7. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz-Andrade RR, Garcia-Jimenez S, Castillo-España P, Ramírez-Ávila G, Villalobos-Molina R, Estrada- Soto S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: an anti-hyperglycemic agent. Journal of Ethnopharmacology. 2007;109:48–53. doi: 10.1016/j.jep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Hasani-Ranjbar SH, Larijani B, Abdollahi M. Clinical research, A systematic review of Iranian medicinalplants useful in diabetes mellitus. Archives of Medical Science. 2008;3:285–292. [Google Scholar]