Abstract
Background and the purpose of the study
Studies show that chitosan nanoparticles increase mucoadhesivity and penetration of large molecules across mucosal surface. The aim of the present study was to investigate the use of thiolated chitosan in the development of polysaccharide-coated nanoparticles in order to confer specific functionality to the system.
Methods
Methyl methacrylate nanoparticles were coated with thiolated chitosan using a radical polymerization method. Thiolation was carried out using glutathione (GSH) to improve mucoadhesivity and permeation enhancing properties of chitosan. Mucoadhesion studies were carried out by calculating the amount of mucin adsorbed on nanoparticles in a specific period of time. Complement consumption was assessed in human serum (HS) by measurement of the hemolytic capacity of the complement system after contact with nanoparticles.
Results
The FT-IR and 1HNMR spectra both confirmed the synthesis and showed the conjugation of thiolated chitosan to methyl methacrylate (MMA) homopolymer. Nanoparticles were spherical having a mean diameter within the range of about 334–650 nm and their positive zeta potential values indicated the presence of the cationic polysaccharide at the nanoparticle surface. Increasing the amount of thiolated chitosan led to mucoadhesivity and complement activation. However there was not dose dependent correlation between these phenomenons and the absence of thiolated chitosan led to particles with larger size, and without ability to activate complement process.
Major conclusion
It can be concluded that nanoparticles could be used for the mucosal delivery of peptides and proteins. Results show that the thiolated chitosan had higher mucoadhesion and complement activation than unmodified chitosan.
Keywords: Thiolated chitosan, Complement consumption, Glutathione, Mucosal drug delivery, Nanoparticles
INTRODUCTION
Surface modified nanoparticles of polymers have received much attention as drug carriers. Such nanoparticles have been modified for specific objectives. The shell characteristics of core-shell nanoparticles are more important than those of the cores, as the shell layer directly contacts body fluids and organs. Generally the nanoparticles are coated with hydrophilic polymer in order to give long-lasting half-lives in circulation, and are usually conjugated with functional ligands or proteins for site specific delivery (1). In this view, methods for the synthesis of nanoparticles composed of a polyalkylcyanoacrylate core coated with polysaccharides are particularly attractive because both the amphiphilic copolymer and the nanoparticles can be obtained in a single step. This can be achieved by the formation of a free radical at the end of the polysaccharide chain through oxidation of the reaction with Cerium (IV) ions in aqueous acidic medium. The polymerization of alkylcyanoacrylate (ACA) monomers is then initiated by these radicals, leading to linear block copolymers that undergo spontaneous auto-association to form nanoparticles with a hydrophobic core coated by the hydrophilic polysaccharide. This radical polymerization method has been tested using different polysaccharides including dextran, dextran sulfate, chitosan and in all cases have provided very suitable suspensions of nanoparticles (2, 3).
It is anticipated that with further characterization and adjustments these nanoparticles can deliver drugs to their targets. In this article a polymethylmethacrylate coated with thiolated chitosan conjugates is described. Thiolated polymers (thiomers) improve the uptake of hydrophilic macromolecules from the GI tract, and it is hoped that this product to be tested for carrying capacity, size, and future utility (3, 4).
Chitosan, a natural-based polymer obtained by alkaline deacetylation of chitin, is nontoxic, biocompatible and biodegradable (3, 5–8). Thiolation of chitosan has been shown to improve the polymer intrinsic mucoadhesivity property due to formation of disulphide bonds with cysteine-rich domains of mucus glycoproteins (2). Permeation enhancing as well as cohesive properties that increase the stability of a delivery system are also achieved (9). The purpose of the present study was to synthesize and characterize poly–methyl-methacrylate (PMMA) nanoparticles coated with Chitosan-glutathione (GSH) conjugate and to investigate the effects of different parameters such as thiolated chitosan, methylmethacrylate, and different amounts of initiators on the size, zeta potential and shape, and in vitro mucoadhesive properties of nanoparticles (10). Also human complement activation was used to investigate in vivo fate of the nanoparticles after administration. The complement system is a key inflammatory mediator in immune complex diseases and complement sensitization of red blood cells (RBCs) result in hemolysis. Sheep erythrocytes were sensitized with hemolysine and after addition of nanoparticles, the complement consumption was assessed in human serum (HS) by measurement of the hemolytic capacity of the complement system after contact with nanoparticles.
MATERIAL AND METHODS
Materials
Chitosan (low molecular weight, degree of deacetylation 98%) (Primex, Island), L-Cysteine HCl, L-Glutathione reduced form, Cerium ammonium nitrate (IV) and Monomer Methylmethacrylate (MMA) were purchased from Merck (Germany) 1-Ethyl-3-(3-Dimethylaminopropyl Carbodiimide hydrochloride (EDAC), Ellman's reagent (5-5)-dithiobis (2-nitrobenzoic acid), N-Hydroxysuccinimide (NHS) and mucin type II (1% sialic acid) were purchased from Sigma-Aldrich (USA). All other chemicals were reagent grade and used as purchased.
Synthesis of chitosan conjugates
Glutathione covalently was attached to chitosan according the methods described previously (5, 9).
Determination of the thiol group content
The amount of thiol groups immobilized on chitosan-GSH conjugate was determined spectrophotometrically using Ellman's reagent for quantification of free thiol groups (11). The amount of thiol moieties was calculated from the corresponding standard curve elaborated under the same conditions with L-cysteine HCl solutions (0–0.2 µmol/ml) (r2=0.9994).
Thiolated chitosan characterization
FT-IR (Nicolet 550 FT-IR Spectrophotometer, U.S.A) and HNMR (Bruker 500 Ultrashield-MHZ, Germany) were used to show the formation of amide bonds between chitosan and glutathione.
Preparation of thiolated chitosan coated nanoparticles of PMMA
Nanoparticles were prepared by radical emulsion polymerization, according to method described before (2). Briefly different amounts of solution of thiolated chitosan (0.069g and 0.276 g) in nitric acid were mixed with solution of cerium IV ammonium nitrate (1 ml, 2 ml) and MMA (1 ml, 0.25 ml) at 40°C for 40 min. The medium was purified by dialysis against acetic acid solution in Milli-Q water twice for 90 min and once overnight. The prepared formulations are listed in table 1.
Table 1.
Formulations of prepared nanoparticles with different amounts of thiolated chitosan, MMA and Initiator
| Parameters | |||
|---|---|---|---|
| Formulation | Amount of Initiator (ml) | Amount of MMA (ml) | Thiolated Chitosan (g) |
| A | 2.00 | 0.50 | 0.138 |
| B | 2.00 | 0.50 | 0.000 |
| C | 2.00 | 0.50 | 0.069 |
| D | 2.00 | 0.50 | 0.276 |
| E | 2.00 | 0.25 | 0.138 |
| F | 2.00 | 1.00 | 0.138 |
| G | 1.00 | 0.50 | 0.138 |
Nanoparticle characterization
The techniques of FT-IR and HNMR were used to display conjugation of thiolated chitosan to methylmethacrylate homopolymer. Nanoparticles were dissolved in D2O containing 0.1 M of DCl (Deuterochloric acid.)
Samples were diluted in Milli-Q water. The hydrodynamic mean diameter, the size distribution and the zeta potential of all formulations were determined at 20–25°C using Nano-Zeta Sizer (Malvern Instruments, UK). The electric surface charge of the polymer particles was deduced from the electrophoretic mobility of the particles. Results corresponded to the average of three determinations.
All formulated suspensions were successively diluted in Milli-Q water to 1/100 (v/v). The dilutions were spread on an aluminum disc and dried at room temperature in a desiccator. The dried nanoparticles were observed under gold-plate under a scanning electron microscope (Philips XL30 Netherlands). Transmission electron microscopy was performed using a transmission electron microscope (TEM-CEM902A-ZWEISS Germany).
Bioadsorption and mucin assay study
The amount of mucin adsorbed by 2.0 mg of nanoparticles in a certain period of time was used to determine mucoadhesion (12). At first, solution (0.5 mg/ml) of the type II of mucin was mixed with nanoparticle solution (4 mg/ml) and the resulting solution was agitated with vortex and incubated at 37°C for 16–18 hrs. Then the process was carried out for ten minutes using centrifugation at 10,000 rpm. Colorimetric method with Periodic Acid Schiff (PAS) Staining (Schiff Reagent with final concentration of 1.0%) was used in order to measure free mucin in the supernatant (13). Eighty milligram of sodium bisulphate was added to 5 ml of Schiff reagent and solution was incubated at 37°C until it became colorless or pale yellow. Ten microliter of 50% periodic acid was added to 7 ml of 7% acetic acid to prepare freshly Periodic Acid Schiff Solution. Supernatants were added to the diluted periodic acid and incubated for 2 hrs at 37°C. Then 100 µl of Schiff reagent was added at room temperature and absorbance was measured at 560 nm after thirty minutes. Concentration of mucin in solution before and after adsorption by the chitosan nanoparticles was measuresd to determine mucoadhesion. Mucin standards (0.1, 0.25 and 0.5 mg/ml) were measured by the same procedure.
Complement consumption studies
Complement consumption was assessed according to the described method (14) in human serum (HS) by measurement of the hemolytic capacity of the complement system after contact with nanoparticles. Briefly 100 µl of HS was added to various amounts of the nanoparticle suspensions (50, 100, 125, 150, 200 and 250 µl and the suspensions were incubated at 37°C for 60 min. Simultaneously, sheep erythrocytes were sensitized with hemolysine diluted at 1:1, 600 (v/v) in VBS2+ and suspended to a final concentration of 1.108 cells/ml. After incubation, different amounts of the mixture of nanoparticles/serum (0.2, 0.25, 0.3, 0.35, 0.4 and 0.6 ml) were diluted with VBS2+to reach a total volume of 0.8 ml. The sensitized sheep erythrocytes (0.2 ml) were also added and the samples were incubated at 37°C for 45 min. Following addition of 2 ml of NaCl, unlysed erythrocytes were separated by centrifugation and lyses of cells were measured at 405 nm. Results were expressed as CH50 units consumption(11). (The CH50 unit represents the concentration of hemolytic complement units per ml of serum able to cause 50% hemolysis of a fixed volume of these sheep red cells.)
RESULTS
Characterization of chitosan-GSH conjugate
Chitosan-GSH conjugate was synthesized by the amide bond formation between carboxylic acid groups of glycine carboxylic acid groups of glutathione and amine groups of chitosan in the presence of EDAC (15, 16).
The by-products during cross-linking of EDAC/NHS are water-soluble and can be easily removed by rinsing. The content of immobilized thiol groups strongly depends on the EDAC/NHS concentration and the molar ratio used. The optional coupling conditions were defined: Polymer/GSH ratio of 1:5, pH 5.5–6.0, and a reaction time of 7 hrs.
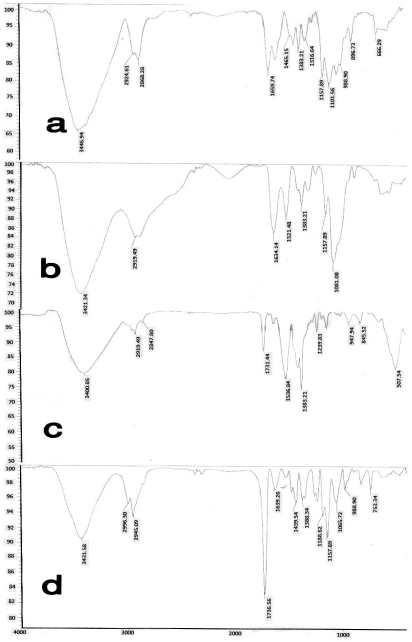
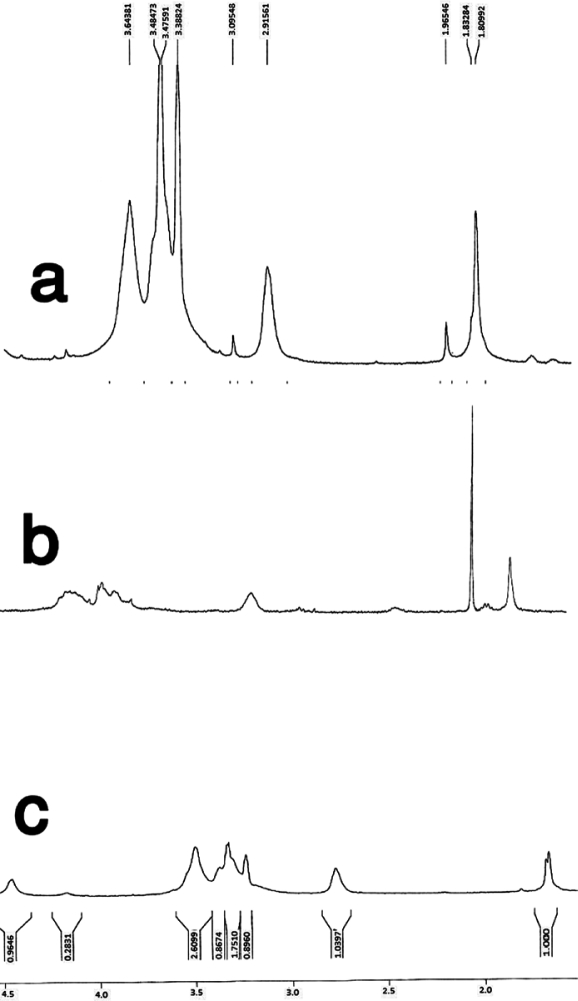
The conjugate exhibited 239.8 µmol of immobilized free thiol group per gram of polymer. FT-IR and 1HNMR spectra of chitosan and thiolated chitosan (Figs 1 and 2) confirmed the presence of thiol group at the surface of chitosan (Fig. 2).
Figure 1.
FT-IR spectra of (a) chitosan, (b) thiolated chitosan, (c) PMMA, (d) polymethylmethacrylate nanoparticles coated with thiolated chitosan
Figure 2.
1HNMR spectra of (a) chitosan, (b) thiolated chitosan, (c) PMMA, (d) polymethylmethacrylate nanoparticles coated with thiolated chitosan.
Determination of the nanoparticle characteristics
IR spectrum of the thiolated chitosan-poly-methylmethacrylate conjugate showed the carbonyl absorption peak at 1731.44/cm−1 (Fig. 1, Part C) for the homopolymer poly-methylmethacrylate (PMMA). In the conjugate co-polymer the presence of the carbonyl absorption peak at 1736.56 cm−1 confirmed the conjugation reaction between thiolated chitosan and PMMA (Fig. 1). The peaks of 1.66 ppm and 1.68 ppm in 1HNMR spectrum of thiolated chitosan-PMM conjugate is related to the methylene and methyl groups of methylmethacrylate. In addition the lack of the peak around 3.64 ppm (located at 3.4–3.64 ppm) related to the O-methyl group of methyl methacrylate in thiolated chitosan, but present at the PMM spectrum also confirmed the formation of the copolymer (of methylmethacrylate coated with thiolated chitosan) as shown in figure 2 (17).
Nanoparticle characteristics
As shown in table 2, the particles ranging from 334 to 940 nm were obtained for thiolated chitosan formulations. In the absence of thiolated chitosan (formulation B) larger particles (P 0.0001) were formed. On the other hand when the amount of thiolated chitosan and MMA were 0.069 g and 0.5 ml respectively (Formulation C), the size of particles increased, occurring at around 388.7 nm (P<0.05). The increase in thiolated chitosan to two-folds leads to an increase in nanoparticle size (formulation D: 2 ml initiator, 0.50 ml MMA, at 0.276 g chitosan, 924.30 nm) (P<0.0001).
Table 2.
Effect of the amount of thiolated chitosan on the size of nanoparticles (n=3±SD). (Amount of MMA=0.5 ml and Initiator=2 ml)
| Amount of thiolated chitosan (g) | Size of Nanoparticles (nm)±SD |
|---|---|
| 0.00 (Formulation B) | 2155.30±42 |
| 0.07 (Formulation C) | 388.70±8 |
| 0.14 (Formulation A) | 334.00±12 |
| 0.28 (Formulation D) | 924.30±18 |
Table 3 shows the effects of different amounts of MMA on particle size (at 0.138 g thiolated chitosan). When the amount of MMA was 0.25 (formulation E) and 0.5 ml (formulation A), the particle size were 382.23 and 334 nm, respectively (P<0.05). The increase of MMA to 1ml (formulation F) led to larger particles of 570 nm (P<0.001). As for the effect of different amounts of initiator, it may only assumed a lack of predictability with the initiator, showing it only catalyzes without being dose-dependent (data not shown).
Table 3.
Effect of the amount of MMA on the size of nanoparticles (n=3±SD). (0.138 g thiolated chitosan, 2 ml initiator)
| Amount of MMA (ml) | Size of Nanoparticles (nm)±SD |
|---|---|
| 0.25 (Formulation E) | 382.23±12 |
| 0.5 (Formulation A) | 334±12 |
| 1 (Formulation F) | 570±19 |
The zeta potential for all formulations was positive, indicating that the cationic polysaccharide had indeed covered the surface of the nanoparticles. There was a significant difference between formulations A and B, due to a reduction in the zeta potential of formulation B (P<0.001). The PMM with thiolated chitosan clearly increased Zeta potential (59.13 mV) while the PMM not covered with chitosan (without thiol addition) showed marked reduction in Zeta potential (18.026 mV).
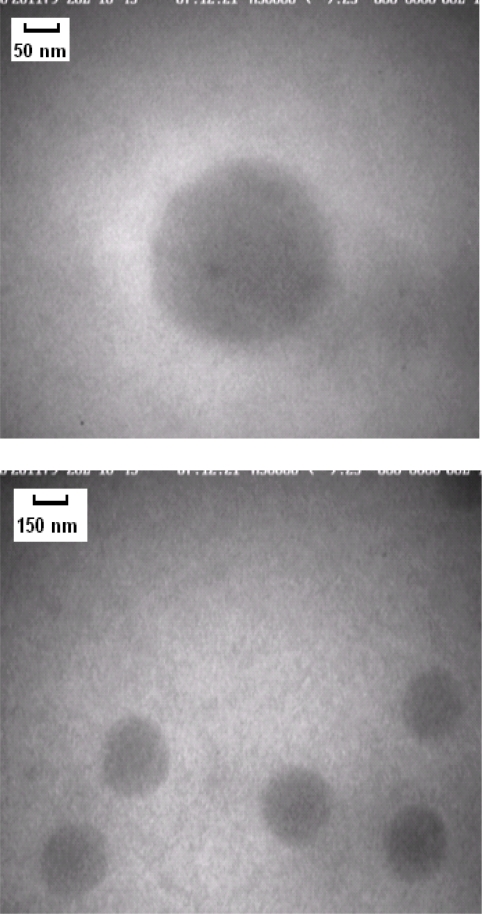
In figures 3 and 4 SEM and TEM micrographs showed populations of spherical nanoparticles for all formulations except formulation B.
Figure 3.
Scanning eletron microscopy image of polymethylmethacrylate nanoparticles coated with thiolated chitosan.
Figure 4.
Transmission electron microscopy image of polymethylmethacrylate nanoparticles coated with thiolated.chitosan
Nanoparticle mucoadhesion
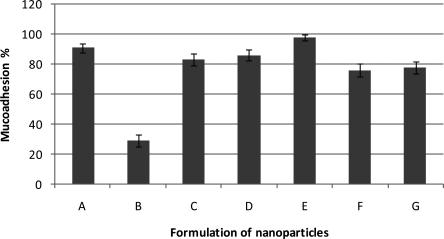
Mucin, a glycoprotein, is the major component of the mucus that coats the cells lining, the surfaces of the respiratory, digestive and urogenital tracts (18). Mucoadhesiveness of nanoparticles was calculated as the amount of mucin adsorbed by 2 mg of thiolated chitosan nanoparticles in a certain period of time (n=2). Absence of thiolated chitosan (formulation B) led to lowest mucoadhehesion (29%). As shown in figure 5 when the amount of MMA was 0.5 ml and thiolated chitosan was 0.069 g (formulation C), 0.138 g (formulation A) and 0.276 g (formulation D), mucoadhesivity were 83, 91 and 86%, respectively.
Figure 5.
Effects of different amounts of MMA and thiolated chitosan on mucoadhesion (A=0.138g thiolated chitosan, 0.50 ml MMA; B=0.000g thiolated chitosan, 0.50 ml MMA; C=0.069g thiolated chitosan, 0.50 ml MMA; D=0.276g thiolated chitosan, 0.50 ml MMA; E=0.138g thiolated chitosan, 0.25 ml MMA; F=0.138g thiolated chitosan, 1.00 ml MMA; G=0.138g thiolated chitosan, 0.50 ml MMA) (n=2±SD).
Figure 6.
Schematic representation of designed nanoparticle form
 Hydrophilic polymeric chain
Hydrophilic polymeric chain
 Hydrophobic core
Hydrophobic core
The highest value of the mucoadhesivity (98%) was obtained for formulation E. According to figure 5, there is not any direct correlation between the amount of thiolated chitosan and mucoadhesivity.
Complement consumption studies
Consumption of CH50 units in the presence of particles was assessed at a constant incubation time. The results are the mean of two determinations with a standard deviation of approximately 7%. Strong complement activation occurred in the presence of formulation C (98%). On the contrary, formulation B did not induce any system response (25%). The formulations D and A also did not induce any strong complement activation respectively (45%, 50%), indicating that only the presence of chitosan tightly bound to thiol groups induces a full complement response. As shown in table 4 formulation G (0.5 ml MMA, 0.138 g thiolated chitosan) led to 60% complement activatioin, whereas formulation F (1 ml MMA, 0.138 g thiolated chitosan) led to 95% complement activation. On the other hand there was not any direct correlation between the amount of thiolated chitosan and complement activation.
Table 4.
Effect of different formulations on human complement activation. (n=2±SD).
| Formulation | Amount of Initiator (ml) | Amount of MMA (ml) | Thiolated Chitosan (g) | CH50 units consumed % (±SD) |
|---|---|---|---|---|
| A | 2.000 | 0.500 | 0.138 | 50±7.07 |
| B | 2.000 | 0.500 | 0.000 | 25±7.07 |
| C | 2.000 | 0.500 | 0.069 | 98±0 |
| D | 2.000 | 0.500 | 0.276 | 45±7.07 |
| E | 2.000 | 0.250 | 0.138 | 82.5±3.53 |
| F | 2.000 | 1.000 | 0.138 | 95±1.41 |
| G | 1.000 | 0.500 | 0.138 | 60±7.07 |
DISCUSSION
Over the years, mucoadhesive polymers have shown to be able to adhere to various mucosal membranes. Moreover, using such polymers, the degradation of orally administered peptide drugs by intestinal enzymes can be avoided and permeation enhancing effects (or the inhibition of brush border membrane-bound enzymes) become feasible (19). Within recent years, the deacetylated form of chitin – chitosan has received considerable attention as a novel incipient in drug delivery systems (20,21). Trimethylated chitosan, mono-N-carboxymethyl chitosans (22), N-sulfo-chitosan (23) or chitosan-EDTA conjugates (24) were synthesized to improve the properties of the polymer (21).
Thiolated polymers (thiomers) improve the uptake of hydrophilic macromolecules from the GI tract. Thiol groups inhibit protein tyrosine phosphatase, and is involved in the closing process of tight junctions, via a GSH-mediated mechanism (25). Moreover, focusing on the structure of GSH as a non-toxic ligand, because of the presence of the thiol group in the tripeptide structure and its high negative redox potential, the Chitosan-GSH exhibited higher permeation-enhancing properties compared to other thiomers and so the Chitosan-GSH conjugate is more stable (9). The presence of thiol groups in the modified polymers promote the formation of inter-and intra-chain disulfide bonds.
For the preparation of nanoparticles in the present study a method called free radical emulsion polymerization was used. Nano-sized particles with a central core composed of polymethylmethacrylate and a surface covered by high-polar macromonomer chains of thiolated chitosan were obtained as shown in figure 7. Nanoparticles are formed simultaneously by polymerization of methylmethacrylate, and the process promotes at one stage spontaneously. Cerium (IV) ammonium nitrate is used as the initiator of this process.
The molecular weight of polymeric chains has an impact on the mucoadhesive properties. Tensile studies with chitosan-TBA conjugates have indicated that medium molecular mass thiolated chitosans display relatively highest mucoadhesiveness (26).
The particle size in this study was found to be around 334–650 nm. When the amount of reactants, including thiolated chitosan, methylmethacrylate monomer, and initiator amount, were 0.138 g, 0.5 ml, 2 ml respectively (formulation A), the particle size was around 334 nm and the shape was spherical. The absence of thiolated chitosan led to the formation of particles with larger size (formulation B). It may be also supposed that without chitosan nanoparticles become obsolete. Hence the presence of thiolated chitosan is required for the synthesis of nanoparticles, but acts as a dose dependent parameter i.e. in lowest or the highest concentrations larger particles are obtained (as shown in formulations C, D).
The zeta potential for all formulations was positive and in the same range, except for “formulation B: which had no thiolated chitosan”. The particle sizes of all nanoparticles measured by a zetasizer nanosizer were larger than those obtained by quantitative analysis of the transmission electron micrographs or scanning electron micrographs. This is because the contrast of the TEM pictures allows only the visualization of nanoparticle core, whereas hydrodynamic radiuses of the particles are measured by the zeta sizer (27).
Studies show that chitosan acts as a promising bioadhesive material. It has been suggested that amino groups of chitosan interact with negatively charged group in biological membrane and increase mucoadhesivity (28). It has also been reported that the covalent attachment of thiol groups to polymers greatly increases mucoadhesiveness and permeation properties without affecting biodegradability (4, 29). From the results of this study it is hypothesized that the absorption of the drugs through mucosa could be enhanced by administration of thiolated chitosan nanoparticles because of their greater mucoadhesiveness and permeability properties. Results show that thiolated chitosan nanoparticles bind mucin II (containing 1% sialic acid), and this is shown by increased mucoadhesiveness in the presence of chitosan Fig. 5. In this reaction, positive charges of thiolated chitosan can adhere to the negatively charged mucus layer by establishment of electrostatic interactions (30). According to the figure 5, amount of thiolated chitosan did not have significant impact on mucoadhesivity and there was not any direct correlation with the amount of thiolated chitosan and mucoadhesivity.
Another useful technique for nanoparticle surface characterization is complement activation. This method was found to give a good predictive result of the in vivo fate of the nanoparticles after administration, especially regarding their uptakes by the macrophages (MPS). It has been demonstrated that surface properties of nanoparticles are the key factors that determine their in vivo fates (31–33).
In this study the degree of complement activation was studied by hemolytic complement assay. Complement activation led to the massive production of complement molecules that act as enzymes for subsequent chemical reactions, resulting in cell lysis. As it is shown in table 4 the presence of thiolated chitosan affected nanoparticle solution by the activation of complement in a dose dependent manner. Absence of thiolated chitosan led to particles (even those with a larger size) unable to activate the complements. On the other hand increasing the amount of thiolated chitosan led to complement activation. The remaining formulations induced complement activation as follows: F: 95%, E: 82.5%, G: 60%. Studies show that activation of bioadhesive material. It has been suggested that complement has correlation with free NH2 groups of chitosan (34).
CONCLUSIONS
Within this study, a new effective radical polymerization method was used to synthesize and conjugate thiolated chitosan to methyl methacrylate homopolymers (in this case PMMA) and tested to confirm their effectiveness in (hydrophilic polymer mucous membrane) gastrointestinal passage and nanoparticle half-life efficiency (as well as other properties). The advantage of this method is that during synthesis the PMMA core coated with polysaccharides a free radical is formed that polymerizes with methyl methacrylate (MMA) monomers to form nanoparticles suspension all in one step. Thiolation of chitosan was demonstrated to improve the mucoadhesivity property due to formation of disulphide bonds with cysteine-rich domains of mucus glycoproteins as well as to increase complement activation.
REFERENCES
- 1.Choi S W, Kim W S, Kim J H. Surface modification of functional nanoparticles for controlled drug delivery. J Dispers Sci Technol. 2003;24:475–487. [Google Scholar]
- 2.Bravo-Osuna I, Schmitz T, Bernkop-Schnürch A, Vauthier C, Ponchel G. Elaboration and characterization of thiolated chitosan-coated acrylic nanoparticles. Int J Pharm. 2006;316:170–175. doi: 10.1016/j.ijpharm.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 3.Moghaddam F A, Atyabi F, Dinarvand R. Preparation and in vitro evaluation of mucoadhesion and permeation enhancement of thiolated chitosan-pHEMA core-shell nanoparticles. Nanomed-Nanotechnol. 2009;5:208–215. doi: 10.1016/j.nano.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Bernkop-Schnürch A, Hornof M, Zoidl T. Thiolated polymers-Thiomers: Synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int J Pharm. 2003;260:229–237. doi: 10.1016/s0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]
- 5.Atyabi F, Moghaddam F A, Dinarvand R, Zohuriaan-Mehr M J, Ponchel G. Thiolated chitosan coated poly hydroxyethyl methacrylate nanoparticles: Synthesis and characterization. Carbohydr Polym. 2008;74:59–67. [Google Scholar]
- 6.Atyabi F, Talaie F, Dinarvand R. Thiolated Chitosan nanoparticles as an oral delivery system for amikacin: In vitro and ex vivo evaluations. J Nanosci Nanotechnol. 2009;9:4593–4603. doi: 10.1166/jnn.2009.1090. [DOI] [PubMed] [Google Scholar]
- 7.Avadi M R, Sadeghi A M M, Mohammadpour N, Abedin S, Atyabi F, Dinarvand R, Rafiee-Tehrani M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomed-Nanotechnol. 2010;6:58–63. doi: 10.1016/j.nano.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Saremi S, Atyabi F, Akhlaghi S P, Ostad S N, Dinarvand R. Thiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: Preparation, in vitro and ex vivo evaluation. Int J Nanomedicine. 2011;6:119–128. doi: 10.2147/IJN.S15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kafedjiiski K, Föger F, Werle M, Bernkop-Schnürch A. Synthesis and in vitro evaluation of a novel chitosan-glutathione conjugate. Pharm Res. 2005;22:1480–1488. doi: 10.1007/s11095-005-6248-6. [DOI] [PubMed] [Google Scholar]
- 10.Atyabi F, Majzoob S, Dorkoosh F, Sayyah M, Ponchel G. The impact of trimethyl chitosan on in vitro mucoadhesive properties of pectinate beads along different sections of gastrointestinal tract. Drug Dev Ind Pharm. 2007;33:291–300. doi: 10.1080/03639040601085391. [DOI] [PubMed] [Google Scholar]
- 11.Bernkop-Schnürch A, Schwarz V, Steininger S. Polymers with thiol groups: A new generation of mucoadhesive polymers? Pharm Res. 1999;16:876–881. doi: 10.1023/a:1018830204170. [DOI] [PubMed] [Google Scholar]
- 12.Lee D W, Shirley S A, Lockey R F, Mohapatra S S. Thiolated chitosan nanoparticles enhance anti-inflammatory effects of intranasally delivered theophylline; p. 7.p. 7. Respir Res, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He P, Davis S S, Illum L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int J Pharm. 1998;166:75–88. [Google Scholar]
- 14.Peracchia M T, Vauthier C, Passirani C, Couvreur P, Labarre D. Complement consumption by poly(ethylene glycol) in different conformations chemically coupled to poly(isobutyl 2-cyanoacrylate) nanoparticles. Life Sci. 1997;61:749–761. doi: 10.1016/s0024-3205(97)00539-0. [DOI] [PubMed] [Google Scholar]
- 15.Krȩżel A, Bal W. Structure-function relationships in glutathione and its analogues. Org Biomol Chem. 2003;1:3885–3890. doi: 10.1039/b309306a. [DOI] [PubMed] [Google Scholar]
- 16.Grabarek Z, Gergely J. Zero-length crosslinking procedure with the use of active esters. Anal Biochem. 1990;185:131–135. doi: 10.1016/0003-2697(90)90267-d. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda A, Kobayashi H, Itoh S, Kataoka K, Tanaka J. Immobilization of laminin peptide in molecularly aligned chitosan by covalent bonding. Biomaterials. 2005;26:2273–2279. doi: 10.1016/j.biomaterials.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Ferez-Vilar J, Hill R L. The structure and assembly of secreted mucins. J Biol Chem. 1999;274:31751–31754. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- 19.Bernkop-Schnürch A. Mucoadhesive polymers: Strategies, achievements and future challenges. Adv Drug Delivery Rev. 2005;57:1553–1555. doi: 10.1016/j.addr.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–1331. doi: 10.1023/a:1011929016601. [DOI] [PubMed] [Google Scholar]
- 21.Thanou M, Florea B I, Langemeÿer M W E, Verhoef J C, Junginger H E. N-trimethylated chitosan chloride (tmc) improves the intestinal permeation of the peptide drug buserelin in vitro (caco-2 cells) and in vivo (rats) Pharm Res. 2000;17:27–31. doi: 10.1023/a:1007558206506. [DOI] [PubMed] [Google Scholar]
- 22.Thanou M, Nihot M T, Jansen M, Verhoef J C, Junginger H E. Mono-N-carboxymethyl chitosan (MCC), a polyampholytic chitosan derivative, enhances the intestinal absorption of low molecular weight heparin across intestinal epithelia in vitro and in vivo. J Pharm Sci. 2001;90:38–46. doi: 10.1002/1520-6017(200101)90:1<38::aid-jps5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Baumann H, Faust V. Concepts for improved regioselective placement of O-sulfo, N-sulfo, N-acetyl, and N-carboxymethyl groups in chitosan derivatives. Carbohydr Res. 2001;331:43–57. doi: 10.1016/s0008-6215(01)00009-x. [DOI] [PubMed] [Google Scholar]
- 24.Bernkop-Schnürch A, Scerbe-Saiko A. Synthesis and in vitro evaluation of chitosan-EDTA-protease-inhibitor conjugates which might be useful in oral delivery of peptides and proteins. Pharm Res. 1998;15:263–269. doi: 10.1023/a:1011970703087. [DOI] [PubMed] [Google Scholar]
- 25.Bernkop-Schnürch A, Kast C E, Guggi D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: Thiomer/GSH systems. J Control Release. 2003;93:95–103. doi: 10.1016/j.jconrel.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Bernkop-Schnürch A, Hornof M, Guggi D. Thiolated chitosans. Eur J Pharm Biopharm. 2004;57:9–17. doi: 10.1016/s0939-6411(03)00147-4. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann F, Cinatl J, Jr, Kabičková H, Cinatl J, Kreuter J, Stieneker F. Preparation, characterization and cytotoxicity of methylmethacrylate copolymer nanoparticles with a permanent positive surface charge. Int J Pharm. 1997;157:189–198. doi: 10.1016/s0378-5173(97)00242-1. [DOI] [PubMed] [Google Scholar]
- 28.Mishra D N, R M G Design and characterization of bioadhesive in-situ gelling ocular inserts of gatifloxacin sesquihydrate. Daru. 2008;16:1–8. [Google Scholar]
- 29.Kast C E, Valenta C, Leopold M, Bernkop-Schnürch A. Design and in vitro evaluation of a novel bioadhesive vaginal drug delivery system for clotrimazole. J Control Release. 2002;81:347–354. doi: 10.1016/s0168-3659(02)00077-9. [DOI] [PubMed] [Google Scholar]
- 30.Dünnhaupt S, Barthelmes J, Hombach J, Sakloetsakun D, Arkhipova V, Bernkop-Schnürch A. Distribution of thiolated mucoadhesive nanoparticles on intestinal mucosa. Int J Pharm. 2011;408:191–199. doi: 10.1016/j.ijpharm.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 31.Kreuter J. Nanoparticles as adjuvants for vaccines. Pharm Biotechnol. 1995;6:463–472. doi: 10.1007/978-1-4615-1823-5_19. [DOI] [PubMed] [Google Scholar]
- 32.Béduneau A, Saulnier P, Anton N, Hindré F, Passirani C, Rajerison H, Noiret N, Benoit J P. Pegylated nanocapsules produced by an organic solvent-free method: Evaluation of their stealth properties. Pharm Res. 2006;23:2190–2199. doi: 10.1007/s11095-006-9061-y. [DOI] [PubMed] [Google Scholar]
- 33.Passirani C, Barratt G, Devissaguet J P, Labarre D. Interactions of nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate) with the complement system. Life Sci. 1998;62:775–785. doi: 10.1016/s0024-3205(97)01175-2. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki Y, Miyatake K, Okamoto Y, Muraki E, Minami S. Influence of the chain length of chitosan on complement activation. Carbohydr Polym. 2003;54:465–469. [Google Scholar]