Abstract
Back ground and the purpose of study
Many drug substances and variety of naturally occurring dietary or herbal components are capable of interaction with the CYP enzyme system. The aim of the study was to investigate the effect of pomegranate juice pretreatment on the bioavailability of buspirone in rabbits.
Methods
White New Zealand rabbits weighing 2.1±0.13 Kg were selected for study. The bioavailability of buspirone after pre-treatment with pomegranate juice (10 ml Kg−1 for seven days) was compared with an oral solution of 10 mg kg−1 of buspirone in distilled water. Animals were allowed free access to food and water, until night prior to dosing and were fasted for 10 hrs. In the first phase oral solution (10 mg kg−1) was administered through feeding tube followed by rinsing with 10 ml of water. In the second phase, the group was pretreated with pomegranate juice for 7 days and study was conducted after 15 days of washout period.
Results and conclusion
The results showed that there was a significant (p<0.05) difference in the bioavailability of buspirone after pre-treatment with pomegranate juice.This increase in bioavailability might be due to inhibition of CYP3A4. Further studies are required to prove this mechanism in humans.
Keywords: CYP3A, Pharmacokinetic parameters, Cmax, Tmax, Inhibition
INTRODUCTION
A variety of dietary or herbal components may interact with CYP enzyme system and induce or inhibit one or more CYP iso-forms that may increase or decrease plasma drug concentration. The CYP3A family of enzymes constitutes the most predominant phase-I drug metabolizing enzymes estimated to metabolize between 50–70 % of currently administered drugs. CYP3A4 is the most abundant form which is primarily present in the hepatocytes and enterocytes (1). Simultaneous consumption of grapefruit juice with a number of therapeutic agents that are subject to first pass intestinal/hepatic metabolism have been resulted in higher plasma levels with subsequent adverse effects due to inhibition of intestinal CYP3A4 (2,3).
Pomegranate (Punica granatum) fruits are globally consumed fresh and processed forms as juice, jam, wine, oil and in extract supplements (4). They contain diverse range of phytochemicals polyphenols punicalagin (PA), ellagic acid (EA), gallotannins, anthocyanins and other flavonoids (5). PA is the most abundant of these polyphenols, and EA has been previously shown to exhibit anti-carcinogenic properties, through induction of cell cycle arrest as well as apoptosis and inhibition of tumor formation and growth in animals (6). Pomegranate has been used in folk medicine for a wide variety of therapeutic purposes. High pomegranate consumption results in increased possibility of pomegranate-drug interaction.
Buspirone is an anxiolytic drug from the azapirone class of compounds (8) which undergoes extensive first-pass metabolism in humans, resulting in a bioavailability of less than 5%, although it is almost completely absorbed after a single oral administration (9,10). The metabolites of buspirone are mostly inactive and only oxidative dealkylation produces 1-(2–pyrimidinyl)-piperazine which is about 20 to 25% as potent as parent drug (11). The aim of the present study was to investigate the effect of pomegranate juice pretreatment on the bioavailability of buspirone in rabbits.
On the basis of report on the inhibition of CYP3A activity (7) by pomegranate, it appeared of interest to investigate the possibility of this interaction further.
MATERIAL AND METHODS
Buspirone was gifted by Sun Pharmaceuticals (Simvasa, India). Methanol and Acetonitrile were HPLC grade from E. Merck Ltd (Mumbai, India). All other chemicals used were of AR grade.
Methodology
Preparation of pomegranate juice
Pomegranates (Punica granatum) were cut into pieces, the rind was removed and the seeds were grounded in mixer (Remi, Mumbai, India) and the freshly prepared juice was administered to rats.
In vivo bioavailability study in rabbits
The animal study protocol was reviewed and approved by the institutional animal ethical committee, University College of Pharmaceutical Sciences, Kakatiya University, India. White New Zealand rabbits weighing 2.1±0.13 Kg were selected for study. The bioavailability of buspirone after pre-treatment with Pomegranate juice (10 ml Kg−1 for seven days) was compared with an oral solution of 10 mg kg−1 of in buspirone in distilled water. Animals were allowed free access to food and water, until night prior to dosing and fasted for 10 hrs. In the first phase an oral solution of buspirone (2.5 mg ml−1) was administered through feeding tube followed by rinsing with 10 ml of water. In the second phase the group was pretreatment with pomegranate juice for 7 days and study was conducted after 15 days of washout period. Blood samples (1.5 ml) from marginal ear vein were collected at pre-set intervals of 0.0, 0.5, 1, 2, 4, 8, 12, and 24 hrs respectively, after administration of oral solution and also pretreatment with pomegranate juice. All blood samples were allowed to clot and centrifuged for 10 min at 4000 rpm. The serum was separated and transferred into clean microcentrifuge tubes and stored at −20°C until HPLC analysis. The amount of buspirone in the samples was estimated using HPLC (12).
Preparation of calibration curve
Primary stock solutions of each of buspirone and diltiazem hydrochloride (Internal standard) were prepared in methanol at a concentration of 1.0 mg ml−1. The working solutions of 10µg ml−1 and 1.5 µg ml−1 were prepared by appropriately diluting the stock solutions of buspirone and diltiazem hydrochloride, respectively. Different concentrations (1, 5, 10, 50, 100, 500, 1000, 2000 and 3000 ng/ml) of buspirone in serum were prepared for calibration curve. The samples were treated as stated in extraction procedure. The peak area ratios obtained by examination of different concentrations of the drug and internal standard were plotted against the concentration of drug. The slope of the plot was determined by the method of least square regression analysis and was used to calculate the buspirone concentration in the unknown sample. The calibration curve in the range of 1–3000 ng ml−1 resulted in the regression equation y=0.0066x–0.1098 (R2=0.9971) in serum.
Extraction and sample preparation
Aliquot (0.5 ml) of the rabbit serum containing buspirone was pipetted into screw capped tubes and 100 µl of an internal standard (1500 ng ml−1 of internal standard) was added and vortexed for 2 min. Phosphate buffer (500 mM of potassium dihydrogen phosphate) saturated with sodium chloride solution of 250 µl was added, vortexed for 3 min and treated with 5 ml of dichloromethane. Vortexed again for 5 min and, centrifuged at 5000 rpm for 15 min. The dichloromethane layer (4.5 ml) was separated and allowed to evaporate under vacuum oven. The evaporated residue was re-constituted with 150 µl of mobile phase and 50 µl of the re-constituted sample was injected in to the HPLC system.
Pharmacokinetic analysis
Pharmacokinetic parameters of buspirone before and after pre-treatment with pomegranate juice were estimated in each rabbit using a computer program, KINETICA 2000. Non-compartmental analysis with three terminal points were selected for calculation of the pharmacokinetic parameters Cmax, Tmax and area under the curve (AUC). Cmax (ng ml−1) and Tmax (h) were the observed maximal drug concentration and its time, respectively.
Statistical analysis
Statistical comparisons were made using Student's t-test using Sigmastat software (Jandel Corp., CA, USA). Results were considered significant at 95% confidence interval (p<0.05).
RESULTS AND DISCUSSION
All rabbits tolerated the treatments well and there was no case of severe adverse affects during the study period. There was a statistically significant difference in pharmacokinetic parameters, Cmax, T1/2, AUC0-∞ and AUC0–24. No statistically significant difference was observed in Tmax pharmacokinetic parameter.
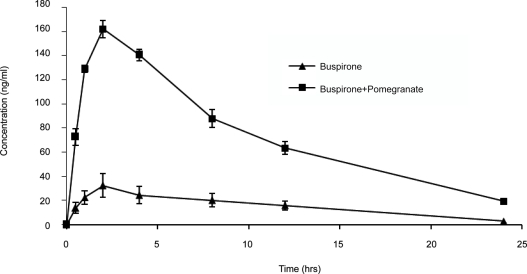
After pretreatment with pomegranate juice Cmax increased from 32.39±7.55 to 161.89±6.71 ng/ml, AUC0-∞ increased from 373.45±96.99 to 1908.26±77.25 ng-hr/ ml. AUC0–24 increased from 349.26±85.82 to 1708.77±49.81 ng – hr/ ml, T ½ increased from 5.48±1.15 to 7.15±0.51 hrs and Tmax increased from 1.67±0.5 to 2 hrs (Table1 & Fig. 1). Pomegranate juice treatment increased the Cmax, AUC0–∞, AUC0–24 and t1/2 by 4.99 (P<0.001), 5.1 (P<0.001), 4.89 (P<0.001) and 1.4 (P<0.001) times respectively. Tmax also increased but not significantly.
Table 1.
Pharmacokinetic parameters of buspirone in control and pomegranate treated rabbits.
| Control | R1 | R2 | R3 | R4 | R5 | R6 | Mean ± SD |
|---|---|---|---|---|---|---|---|
| Cmax (ng/ml) | 26.5 | 43.5 | 37.5 | 38.4 | 34.3 | 23.6 | 32.39±7.5 |
| Tmax (hr) | 1.0 | 2.0 | 2.0 | 2.0 | 2.0 | 1.0 | 1.67±0.51 |
| AUC0–24(ng-hr/ml) | 259.8 | 445.9 | 406.9 | 348.2 | 395.4 | 230.7 | 349.26±5.82 |
| AUC0–8(ng-hr/ml) | 280.5 | 470.5 | 448.9 | 358.4 | 444.4 | 239.1 | 373.45±96.99 |
| T1/2 (hr) | 5.9 | 5.1 | 6.5 | 4.1 | 7.0 | 4.4 | 5.48±1.14 |
| Treated | |||||||
| Cmax (ng/ml) | 170.8 | 164.516 | 151.88 | 157.08 | 160.9 | 166.22 | 161.89±6.7 |
| Tmax (hr) | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| AUC0–24(ng-hr/ml) | 1766.7 | 1687.1 | 1644.2 | 1671.8 | 1763.8 | 1712.9 | 1708.77±49.81 |
| AUC0–8(ng-hr/ml) | 1955.7 | 1853.11 | 1806.1 | 1886.2 | 2023.1 | 1929.4 | 1908.26±77.25 |
| T1/2 (hr) | 6.81 | 6.67 | 6.65 | 7.41 | 7.87 | 7.53 | 7.15±0.51 |
Figure 1.
Effect of Pomegranate juice pre-treatment on bioavailability of buspirone.
From the pharmacokinetic studies it appears that there is a statistically significant change in AUC0–∞, AUC0–24, T½ and Cmax after pretreatment with pomegranate juice. There was a considerable increase in the bioavailability of buspirone, after the pretreatment with pomegranate juice. This fact is also substantiated by the rise in Cmax value from 32.39 to 161.89ng/ml. Though this work was carried out in rabbits but the same results may also be expected in humans. Consumption of pomegranate juice should be avoided when patient is on therapy as pomegranate juice inhibits multiple enzymes like CYP 3A4 and CYP 2C9. A word of caution may be advised to patients about the possible drug-fruit interactions and to avoid consumption of these fruits in large quantities during the therapy with drugs which are substrates of CYP3A and P-gp.
CONCLUSION
From the results of this study it may concluded that pomegranate juice might be in enzyme inhibitor since buspirone is extensively metabolized by CYP3A4.
REFERENCES
- 1.Watkins P.B. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv. Drug Deliv. Rev. 1997;27:161–170. doi: 10.1016/s0169-409x(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 2.Bailey DG, Malcolm J, Arnold O, Spence JD. Grapefruit juice-drug interactions. Br. J. Clin. Pharmacol. 1998;46:101–110. doi: 10.1046/j.1365-2125.1998.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo LQ, Taniguchi M, Xiao YQ, Baba K, Ohta T, Yamazoe Y. Inhibitory effect of natural furanocoumarins on human microsomal cytochrome P450 3A activity. Jpn. J. Pharmacol. 2000;82:122–129. doi: 10.1254/jjp.82.122. [DOI] [PubMed] [Google Scholar]
- 4.Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 5.Cerda B, Llorach R, Ceron JJ, Espin JC, Tomas-Barberan FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr. 2003;42:18–28. doi: 10.1007/s00394-003-0396-4. [DOI] [PubMed] [Google Scholar]
- 6.Adams LS, Seeram NP, Aggarwal BB, Takada YS, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric Food Chem. 2006;54:980–985. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- 7.Hidaka M, Okumura M, Fujita K, Ogikubo T, Yamasaki K, Iwakiri T, Setoguchi N, Arimori K. Effects of pomegranate juice on human cytochrome p450 3A (cyp3A) and carbamazepine pharmacokinetics in rats. Drug Metab Dispos. 2005;33:644–648. doi: 10.1124/dmd.104.002824. [DOI] [PubMed] [Google Scholar]
- 8.Fulton B, Brogden RN. Buspirone: an updated review of its clinical pharmacology and therapeutic applications. CNS Drugs. 1997;7:68–88. [Google Scholar]
- 9.Mayol RF, Adamson DS, Gammans RE, Labudder JA. Pharmacokinetic disposition of 14C-buspirone HCl after intravenous and oral dosing in man. Clin Pharmacol Ther. 1985;37:210. [Google Scholar]
- 10.Gammans RE, Mayol RF, Labudder JA. Metabolism and disposition of buspirone. Am J Med. 1986;80:41–51. doi: 10.1016/0002-9343(86)90331-1. [DOI] [PubMed] [Google Scholar]
- 11.Iftekhar M, Chandra S. Clinical pharmacokinetics and pharmacodynamics of buspirone an anxiolytic drug. Clin Pharmacokinet. 1999;36:277–287. doi: 10.2165/00003088-199936040-00003. [DOI] [PubMed] [Google Scholar]
- 12.Ramesh G, Shravan KY, Chinna RP, Vamshi VY, Harshini K, Madhusudan Rao Y. Development of high performance liquid chromatography method for buspirone in rabbit serum: application to pharmacokinetic study. Anal Chim Acta. 2009;647:226–230. [Google Scholar]