Abstract
Background and the purpose of the study
Amphotericin B (AmB) which is an appropriate antibiotic for the treatment of mycosis has many toxic effects including nephrotoxicity. Recently preparation of a new drug loaded nanoparticles for the reduction of toxicity and increase in the effectiveness of AmB has been reported. The objective of this study was to prepare and evaluate in vitro and in vivo efficacy of the spray-dried AmB-loaded nanospheres.
Methods
AmB-loaded nanospheres was prepared by means of nanoprecipitation method. The spray-dried nanospheres was prepared by using aerosil and AmB entrapment efficacy was measured by HPLC method. Minimum inhibitory concentration (MIC) of AmB-loaded nanospheres against Candida albicans (ATCC 90028) was determined by using microdilution method and its in vitro haemolytic effect and antifungal efficacy on infected rabbits was also analyzed.
Results
The entrapment efficacy for AmB loaded nanospheres was 65.2%±3. The MIC of AmB-loaded nanospheres against C. albicans compared to the free antibiotic was lower significantly. Also, the AmB-loaded nanospheres found to be 9.5 times less toxic than free AmB on human red blood cells. In vivo testing indicated that AmB-loaded nanospheres have a stronger protective effect against candidiasis compared to the free AmB.
Conclusion
Results of this study suggest that prepared spray-dried AmB-loaded nanospheres would be a good choice for the treatment of mycosis because of low toxicity and high stability and effectiveness.
Keywords: Amphotricin B, Nanosphere, Antifungal activity, Candidiasis
INTRODUCTION
Amphotericin B (AmB) is a suitable antibiotic for the treatment of systemic mycosis (1). However, this drug has low aqeous solibility and is poorly absorbed in gastro-intestinal tract which limit its uses for the treatment of systemic fungal infections (2, 3). In recent years, various types of drug formulations for AmB such as micellar solution of AmB with deoxycholate having serious nephrotoxicity and lipid-based nanoparticulate formulations of low nephrotoxicity have been investigated (4, 5). In addition these formulations are quite expensive. In this field polymeric nanoparticle delivery systems has been extensively investigated (6, 7). Nanoparticles, as a polymeric sub micron carriers have a general name to describe nanocapsules and nanospheres (8). According to the literature, the nanospheres have a polymeric wall envelope and an oil core matrix (9). Nanosphere suspensions have been developed as drug targeting delivery system, using polyesters, poly (alklylcyanoacrylate), poly (lactic acid) and other polymers (10, 11). The development of these systems for industrial objectives are limited due to low maintaining stability of suspensions for a prolonged time period and usually, prepared nanoparticles tend to aggregate because nanoparticles sediment slowly and seperated by severe mixing (12, 13). However, aggregation of nanoparticles in suspension can occur (4). Spray-drying has been shown as a good method for drying of nanoparticles and to increase the stability of drug-loaded nanoparticles (8, 13). The aim of the present study was to apply the spray-dried technique for drying of the prepared AmB-loaded nanospheres using poly[(±)-lactid-co-glycolid] (PLGA) and evaluation of the stability and in vitro hemolytic effect and in vivo therapeutic efficacy of the prepared nanoparticles.
MATERIAL AND METHODS
PLGA (Capped, alkylester terminated, M.W.: 76000-115000 and Ratio of lactid to glycolid of 75:25), sorbitan monostearate, RPMI 1640 and AmB were obtained from Sigma (St. Louis, USA). Colloidal silicon dioxide (aerosil 200) was purchased from Degussa (Frankfurt, Germany).
Preparation of the spray-dried AmB-loaded nanospheres
All samples were prepared by nanoprecipitation of PLGA by the reported method (14). Briefly, the AmB-loaded nanosphers suspension was prepared by dissolving the PLGA (l g), AmB (0.150 g) and the sorbitan monostearate (0.766 g) in acetone (270 ml) and polysorbate 80 (0.766 g). Then, the prepared suspension was spray-dried by the reported method (13). In brief, aerosil 200 [3.0% (w/v)] was added to the nanospheres suspension and the mixture was fed into a mini-spray-dryer Büchi MSD 190 (Flawil, Switzerland).
In vitro dissolution studies
AmB-loaded nanospheres containing 30 µg of AmB were placed into 20 ml of the dissolution medium [1% (v/v) of Tween 80 in 10mM HEPES buffer, pH 7.4] at 37°C. Then at pre-determined time intervals of 1, 2 and 3 months the amount of released AmB was determined using HPLC method (7).
Determination of yield and entrapment efficiency
The respective percentage of yield was calculated using the following equation:
For determination of entrapment efficiency, the content of AmB in loaded nanospheres was determined by HPLC as described previously (7). Then, the percentage of drug entrapped was calculated as:
Particle size and zeta-potential measurement
Mean particle size and zeta-potential of the prepared nanospheres before and after drying process were evaluated by the reported method using Malvern zetasizer (Malvern instrument Ltd., Worcestershire, UK) apparatus (14, 15).
In vitro toxicity
In vitro toxicity of the free and prepared nanospheres containing AmB was determined by the reported method (16). Briefly, blood samples were collected from rabbits in the presence of the heparin. Then, 0.2 ml of PBS containing various amounts of free and nanosphere forms of AmB (0-400 µg AmB/ml) were mixed with 0.8 ml of 0.1% red blood cells (vol. /vol.) suspension and in vitro haemolytic effects of the dose levels of AmB was determined by spectrophotometer (model 1700, shimadzu, Japan).
Antifungal susceptibility testing
The MICs of AmB in free and nanospheres loaded forms were determined by microdilution methodology, according to Clinical and Laboratory Standards Institute (17). Briefly, Candida albicans (ATCC 90028) cell suspensions of ∼1×106 cells/ml were diluted 1:50 in RPMI- 1640 growth medium and dispensed (100 µl) into a microtiter tray containing serial two-fold dilutions of AmB. The tray was then incubated for 48 hrs at 37°C. The MIC was recorded to be the lowest concentration of the AmB in free and nanosphere forms that prevented visible growth of C. albicans and expressed in µg/ml.
In vivo study
In vivo therapeutic efficacy of the prepared nanoparticles was tested by a described method (16), with some modification. Forty male white rabbits (2.5-3 kg body weight) obtained from the National Institute of Pasture (Iran) were handled according to the national guidelines of the laboratory animals (18). Animals were infected with 0.25 ml of Candida albicans cell suspension (7×106 cells/ml) in normal saline via the caudal vein and divided into 4 groups. All groups received the drug intravenously as follows: Group 1 received free AmB (1mg/kg); groups 2 received AmB-loaded nanospheres (1 mg/kg); group 3 received empty nanospheres (1mg/kg) and group 4 received physiological saline (1ml/kg). All groups were treated as described above for 12 days starting from the 3rd day of infection. Two days after the last dose the survived rabbits were anesthetized and sacrificed. Then fungal colony forming units (CFU) in lung, liver, kidney, spleen and brain was determined.
Statistical analyses
All data were expressed as mean±S.E.M. The CFU values were statistically evaluated by analysis of variance of one-way classification with unequal frequencies from three separate experiments. The percentage of survival rabbits were determined by using Chi-squared with Yates correction and by Fisher's exact test.
RESULTS AND DISCUSSION
Yield and Entrapment Efficiency
The results showed that percentage of yield and AmB entrapment efficiency were 57.9%±8 and 65.2%±3, respectively.
Particle size and zeta-potential analysis
Table 1, shows mean particle size and zeta-potential of empty and AmB-loaded nanospheres before and after drying process. Size homogeneity of empty and AmB-loaded nanospheres which was suggested that AmB was entrapped into nanoparticle core, according to the previous studies (5, 6). Also, the high negative zeta-potential of the prepared nanospheres revealed that the nanoparticles have appropriate stability in aqueous dispersion (14).
Table 1.
Particle size and zeta-potential of the empty and AmB-loaded nanospheres before and after spray-drying.
| Formulations | Mean particle size (nm) | zeta-potential (mV) |
|---|---|---|
| Before spray-drying | ||
| Empty nanospheres | 110±2 | −33.6±5 |
| AmB-loaded nanospheres | 118±5 | −31.1±7 |
| After spray-drying | ||
| Empty nanospheres | 92±3 | −29.3±2 |
| AmB-loaded nanospheres | 96±1 | −27.9±4 |
In vitro dissolution studies
The percentage of AmB recoveries for nanospheres after 3 months was 90%±2 (Fig. 1). This phenomenon suggested that majority of AmB incorporated into nanoparticles. This result is not in accordance with the previous reports that have indicated within 5-30 minutes most of AmB were released from nanoparticles, because AmB was attached to the outer surface of the nanoparticles (3, 14).
Figure 1.
Percentage of AmB recovery from nanospheres prepared with PLGA after 3 months of storage at 37°C.
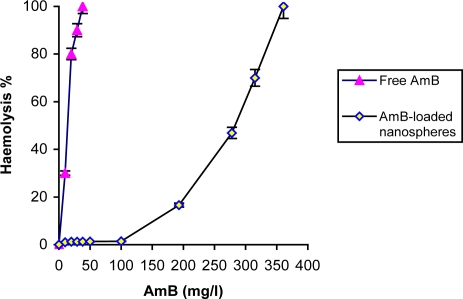
In vitro toxicity study
The hemolytic ability of free AmB and AmB-loaded nanospheres is shown in figure 2. The maximum hemolytic result (100% lysis) for AmB-loaded nanospheres was estimated to be 361 µg/ml. Under all conditions, by increasing AmB doses (0-250 µg AmB/ml), the percentage of haemolytic effect increased. AmB-loaded nanospheres showed mild toxicity (1.2% lysis of erythrocytes) at concentration of 38 µg/ml as compared to the free drug. Data of this study are in accordance with results of another report (3).
Figure 2.
Percentage of haemolysis with AmB in free and entrapment in nanosphere forms. The data was expressed as mean±S.E.M. from three separate experiments.
Fungal susceptibilities
The MIC of AmB-loaded nanospheres against C. albicans was reduced 3-fold compared with free AmB Table.2. This result indicates that prepared AmB-loaded nanospheres like NS-718, LNS-AmB and LNPS-AmB (lipid nanoparticles incorporated with AmB) have high therapeutic efficacy and are useful for the treatment of mycosis, including candidiasis (9, 11, 19).
Table 2.
In vitro antifungal activities of AmB in either free or nanosphere forms against Candida albicans (ATCC 90028).
| Minimum Inhibitory Concentration (MIC,µg/ml) Drugs | ||
|---|---|---|
| Drugs | Free AmB | AmB-loaded nanospheres |
| Microorganism | ||
| Candida albicans (ATCC 90028) | 0.5 | 0.125 |
Therapeutic efficacy of AmB-loaded nanospheres on candidiasis rabbit model
The treatment of Candida albicans-infected rabbits with AmB-loaded nanospheres as compared with control animals showed significant reduction in CFU values in evaluated organs especially in kidney and spleen Table.3. It was found that mortality of Candida albicans-infected rabbits as control (without AmB administrated) was 100% after 15 days, whereas rabbits treated with free AmB and AmB-loaded nanospheres showed increase in survival rate of 20 and 90%, respectively. One of the reasons for the differences in the antifungal potencies and toxicities among free AmB and prepared AmB formulations could be due to high stability of AmB-loaded nanoparticles (6, 20).
Table 3.
The percentage of survived rabbits and colony-forming units (CFU) in different organs of infected-rabbits.
| Treatment | Tissue/Organ | Log CFU Gram tissue (n=3) | Percentage of survival 15 day after the therapy (n=10) |
|---|---|---|---|
| Lung | 4.312±0.5 | ||
| Liver | 4.801±0.4 | ||
| Control without drug administration | Kidney | 4.923±1.1 | None survived |
| (received physiological saline) | Spleen | 4.742±1.3 | |
| Brain | 4.221±1.6 | ||
| Lung | 4.310±0.3 | ||
| Liver | 4.843±0.7 | ||
| Empty nanospheres | Kidney | 4.901±0.8 | None survived |
| (1 mg Kg−1, I.V.) | Spleen | 4.698±0.9 | |
| Brain | 4.250±1.1 | ||
| Lung | 4.017±0.03 | ||
| Free AmB | Liver | 4.216±0.13 | |
| (1 mg Kg−1, I.V.) | Kidney | 4.736±0.19 | 20 |
| Spleen | 4.380±1.1 | ||
| Brain | 3.510±0.20 | ||
| Lung | 1.193±0.04 * | ||
| AmB-loaded nanospheres | Liver | 1.189±0.03 * | |
| (1 mg Kg−1, I.V.) | Kidney | Nil ** | 90 |
| Spleen | Nil ** | ||
| Brain | 1.116±0.03 * |
The values are expressed as mean±S.E.M. from three separate experiments. Analysis of variance of one-way classification between the treatment means was heterogeneous and the t-test values (two-tailed) were significant
p < 0.05
p < 0.001
CONCLUSION
The results of in the present study indicated that the prepared spray-dried AmB-loaded nanospheres may represent a more appropriate choice for treatment of mycose because efficacy and toxicity of this formulation was balanced and probably in future it may serve as an effective novel antifungal drug delivery system for treatment of patients with fungal infections.
ACKNOWLEDGMENTS
This study was supported by Islamic Azad University, Borujerd Branch, Iran. The authors would like to acknowledge staffs of university.
REFERENCES
- 1.Falahati M, Shabani M, Rodaki M, Jahaniani F, Porshang Bagheri K, Ebrahimi SA. Interaction between ketoconazole, amphotericin B and terbinafin and three diazenumdiolates in concomitant uses against some fungal species. DARU. 2006;14:87–92. [Google Scholar]
- 2.Espada R, Valdespina S, Alfonso C, Rivas G, Ballesteros MP. Effect of aggregation state on the toxicity of defferent amphotericin B preparations. Int. J. Pharm. 2008;361:64–69. doi: 10.1016/j.ijpharm.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Tiyaboonchai W, Limpeanchob N. Formulation and characterization of amphotericin B–chitosan–dextran sulfate nanoparticles. Int. J. Pharm. 2007;329:142–149. doi: 10.1016/j.ijpharm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm. Res. 2006;23:1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 5.Pestana KC, Formariz TP, Franzini CM, Sarmento VH, Chiavacci LA, Scarpa MV, Egito ES, Oliveira AG. Oil-in-water lecithin-based microemulsions as a potential delivery system for amphotericin B. Colloid Surf. B: Biointerfaces. 2008;66:253–259. doi: 10.1016/j.colsurfb.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Amaral AC, Boca AL, Ribeiro AM, Nunes J. Amphotericin B in poly (lactic-co-glycolic acid) (PLGA) and dimercaptosuccinic acid (DMSA) nanoparticles against paracoccidioidomycosis. J. Anti. Chem. 2009;63:529–533. doi: 10.1093/jac/dkn539. [DOI] [PubMed] [Google Scholar]
- 7.Lemke A, Kiderlen AF, Petri B, Kayser O. Delivery of amphotericin B nanosuspensions to the brain and determination of activity against Balamuthia mandrillaris amebas . Nanomedicine. 2010;34:232–238. doi: 10.1016/j.nano.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Darbandi MA, Rouholamini Najafabadi A, Gilani K, Tajerzadeh H. The effect of vehicles on spray drying of rifampicin inhalable microparticles: In vitro and in vivo evaluation. DARU. 2008;16:128–135. [Google Scholar]
- 9.Herbrecht R, Natarajan-Ame S, Nivoix Y. The lipid formulations of amphotericin B. Expert Opin. Pharmacother. 2003;4:1277–1287. doi: 10.1517/14656566.4.8.1277. [DOI] [PubMed] [Google Scholar]
- 10.Khandare J, Minko T. Polymer–drug conjugates: progress in polymeric prodrugs. Prog. Polym. Sci. 2006;31:359–397. [Google Scholar]
- 11.Jung SH, Lim DH, Jung SH, Lee JE, Jeong KS, Seong H, Shin BC. Amphotericin B-entrapping lipid nanoparticles and their in vitro and in vivo Characteristics. Eur. J. Pharm. Sci. 2009;37:313–320. doi: 10.1016/j.ejps.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003;42:463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 13.Pohlmann AR, Weiss V, Mertins O. Spray-dried indomethacin-loaded polyester nanocapsules and nanospheres development, stability evalution and nanostructure models Eur. Pharm. Sci. 2002;16:305–312. doi: 10.1016/s0928-0987(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 14.Das S, Suresh PK, desmukh R. design of eudragit RL 100 nanoparticles by nanoparticipation method for ocular drug delivery. Nanomedicine. 2010;6:318–323. doi: 10.1016/j.nano.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Tamaddon AM, Hosseini-Shirazi F, Moghimi HR. Preparation of oligodeoxynucleotide encapsulated cationic liposomes and release study with models of cellular membranes. DARU. 2007;15:61–70. [Google Scholar]
- 16.Chakraborty KK, Naik SR. Therapeutic and hemolytic evaluation of in –situ liposomal preparation containing amphotericin B complexed with different chemically modified Beta cyclodextrins. J. Pharm. Sci. 2003;6:231–237. [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. References method for broth dilution antifungal susceptibility testing of filamentous fungi: Approved standard NCCLS document M38-A; Wayne (Pa): National Committee for Clinical Laboratory Standards; 2002. [Google Scholar]
- 18.Committe on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources. Commission on Life Sciences National Research Council. Guide for the care and use of laboratory animals; Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 19.Fukui H, Koike T, Saheki A, Sonoke S, Tomii Y, Seki J. Evaluation of the efficacy and toxicity of amphotericin B incorporated in lipid nano-sphere (LNS) Int. J. Pharm. 2003;263:51–60. doi: 10.1016/s0378-5173(03)00342-9. [DOI] [PubMed] [Google Scholar]
- 20.Espuelas MS, Legrand P, Campanero MA, Appel M, Chéron M, Gamazo C, Barratt G, Irache JM. doi: 10.1093/jac/dkg351. [DOI] [PubMed] [Google Scholar]