Abstract
Background and the purpose of the study
One of the most common malignancies in women is breast cancer. Although several treatments for breast cancer are available, application of herbal medicine as a supplementary treatment is a new option to help curing the disease. In this study anticancer effects of Polygonum avicular herbal extract was investigated.
Methods
Polygonum avicular extract was obtained by methanol. MCF-7 cell line was treated with different concentrations of Polygonum avicular (50, 100, 150, 200, 250, 300,350 400 ng/µl) for different time lengths ( 6, 12, 24, and 48 hrs). MTT assay and Flow Cytometry were used to evaluate cell proliferation and apoptosis, respectively. RT-PCR was also carried out to evaluate the expression of apoptotic genes.
Results and Discussion
Results showed that Polygonum avicular induced cytotoxicity in MCF-7 cell line at concentrations higher than 300 ng/µl and this was confirmed by the highest rate of cell death as measured by Trypan Blue and MTT assays. RT-PCR results showed up-regulation of P53 and down-regulation of Bcl-2 proteins which implied the ability of Polygonum avicular to induce apoptosis in MCF-7 cells and confirmed its anticancer property. Further studies are required to evaluate effects of the extract on other apoptotic genes.
Keywords: Herbal medicine, Breast cancer, knotweed, cell death
INTRODUCTION
Many investigations are undergoing on herbal drugs to replace them with conventional treatments of various types of cancer and other diseases (1). In the world, breast cancer comprises 10.4% of all cancer incidences among women, making it the second most common type of non-skin cancers (after lung cancer) and the fifth most common cause of cancer death (2). Breast cancer is usually treated with surgery, chemotherapy or radiation, or both which have severe side effects or failure in treatment. Chemotherapy drugs cause DNA damage not only in cancer cells, but also in fast-growing normal cells where it results in serious side effects (3). Therefore, finding a new and safe agent (without or with fewer side effects) is a very interesting point for the researchers in this field.
One of the approaches in drug discovery is the ethnomedical data approach. Traditional healers are very useful sources of ethnomedical data. However, it is usually difficult to obtain full information from this source, because they consider such information as their sources of income, and therefore are unwilling to release it free of charge (4). In Azerbaijan province of Iran, some traditional healers use boiled Knotweed extract for treatment of cancer and they have obtained good results. However, there is not any documentary report available on the anticancer or antitumor effects of the plant. Therefore, the main aim of this study was to evaluate cytotoxic properties and anticancer effects of polygonum avicular (Knotweed) extract.
Polygunom aviculare L (Knotweed), a member of Polygonaceae, is used to treat ailments caused by high humidity and has vasorelaxant effects on pre-contracted aortic tissues of rat (5). Knotweed is a safe and effective astringent and diuretic herb that is used mainly in treatment of complaints such as dysentery and hemorrhoids (6). Phenolics and flavonoids that are found in polygonum avicular have multiple biological effects including antioxidant and antitumor activities with the capacity to scavenge free radicals. These compounds also have shown DNA protective activities (5). Therefore, it seems that application of Polygunom aviculare herbal extract might show healing effects in cancer and other diseases which are concurrent with lipid and protein peroxidation, DNA damages and high amount of free radicals (7).
Resistance to apoptosis is a hallmark of cancer and decreased sensitivity to apoptosis leads to an elevated therapeutic threshold for classical interventions such as chemotherapy or radiotherapy (8). In the present study, capability of the Polygonum avicular extract to induce apoptosis was examined by evaluation of the expression of Bcl-2 and P53 genes.
MATERIALS AND METHODS
Preparation of polygonum avicular extract
Polygonum avicular was collected from Oscow Mountains of Azarbaijan province of Iran in summer of the year 2008. Voucher specimens for this collection (AD2009-1892) was identified by Prof. A. Delazar and have been deposited in the Herbarium of the Faculty of Pharmacy, Tabriz, Iran, and Prof. A. Delazar determinate. The extraction method was maceration. Aerial parts of the plant were dried at room temperature for 2 weeks and subjected to extraction with 70% methanol at room temperature followed by evaporation of combined hydromethanolic solutions through rotatory evaporation at 50° C under low pressure to give the dried extract (11.23 g).
Cell culture
The human breast cancer cell line (MCF-7) was obtained from national cell bank of Iran (NCBI, Pasteur Institute of Iran). The cells were cultured in RPMI-1640 with 10% FBS and 100 u/ml penicillin and 100 µg/ml streptomycin in 5% CO2 at 37°C for 2 week and then were treated with herbal extract.
Assessment of cell viability
The MCF-7 cells were incubated with different concentrations (50, 100, 150, 200, 250, 300,350 and 400 ng/µl) of Polygonum avicular extract for different time lengths of 6, 12, 24, and 48 hrs. The cells were also treated with different concentrations of cisplatin as a positive control. Following incubation, the cells were harvested and counted with a hemocytometer under phase contrast microscope. The viability of the cells was examined by trypan blue dye staining (9).
Cell proliferation assay
MTT assay was performed to evaluate cell proliferation where 5×105 cells were seeded in 96 wellplates and incubated with 50, 100, 150, 200, 250, 300,350 and 400 ng/µl of Polygonum avicular or 60, 120,180, 240, 300, 360 and 420 µg/ml of cisplatin for different time lengths. Then, 15 µl MTT (0.5 mg/ml) was added to each well, and the plates were incubated for 2 hrs. Then, the medium was replaced with 200 µl of DMSO and the absorbance was recorded at 570 nm (9).
Apoptotic assay
Apoptotic and necrotic cells were differentiated after double staining of the cells with Annexin-V and Propidiumiodide (PI) using Annexin-V-FLOUS staining kit followed by flow cytometry in accordance to the manufacturer's protocol (Roche, Mannheim, Germany) (9).
RT-PCR cDNA Synthesis
The MCF-7 cells were treated with Polygonum avicular herbal extract. Then, total cell RNAs were isolated by Tripure Isolation Reagent (Roche Applied Science, Germany) according to the manufacturer's recommendation. The total RNAs were eluted in 20 µl of RNase-free water and stored at -80° C for subsequent procedures. The quantity and quality of the purified RNAs were verified by Nanodrop spectrophotometer (ND-1000, Wilmington, DE) and electrophoresis using 1% agarose gel (Cinnagene, Tehran, Iran), respectively. The purified RNA was used as template for cDNA synthesis. Reverse transcription was carried out by SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) with 500 ng of DNase I (Invitrogen, Carlsbad, CA) treated RNAs (to eliminate any contaminating chromosomal DNA) as template.
Assessment of the expression of apoptotic/antiapoptotic genes
Semiquantitative PCR of the P53 proapoptotic gene and the Bcl-2 antiapoptotic gene was performed using recombinant Taq DNA polymerase (Cinnagene, Tehran, Iran). For normalization of the RT-PCR, β-actin expression was examined. Specific primers were designed by primer3 software (version 0.4.0, http://frodo.wi.mit.edu/) based on sequences obtained from GenBank database (http://www.ncbi.nlm.nih.gov/). The designed primers were subjected to BLAST (NCBI GenBank) to ensure that they have complete homology with the mentioned genes (Table 1). Subsequent to an initial denaturation step at 94° C for 5 min, cDNA was subjected to 33 cycles of PCR consisting of denaturation at 94° C for 30 sec, annealing at 59° C for 30 sec, and extension at 72° C for 59 sec followed by a final extension step of 5 minutes at 72° C. PCR products were separated by electrophoresis in a 2 % agarose gel (Cinnagene, Tehran, Iran) to verify the successful amplification (9).
Table 1.
Primer sequences for Semi-quantitative PCR
| mRNA or Gene (Accession ID) | Oligonucleotide sequence (5'-3') | Anneling Tm(°C) | Product Length (bp) |
|---|---|---|---|
| P53 | F- GTGGAAGGAAATTTGCGTGTG | 59 °C | 180 |
| NM_001126117.1 | R- CCAGTGTGATGATGGTGAGGATG | ||
| Bcl-2 | F- TGCACCTGACGCCCTTCAC | 59 °C | 291 |
| NM_000633.2 | R- AGACAGCCAGGAGAAATCAAACAG | ||
| β-actin | F- TTCTACAATGAGCTGCGTGTGG | 59 °C | 119 |
| NM_001101.3 | R- TTCTACAATGAGCTGCGTGTGG |
Statistical analyses
Results were presented as mean ± SD in triplicate experiments. Differences were determined using ANOVA with the Tukey-Kramer multiple comparisons test.
RESULTS
Effects of Polygonum avicular extract on the cell viability and prolifration
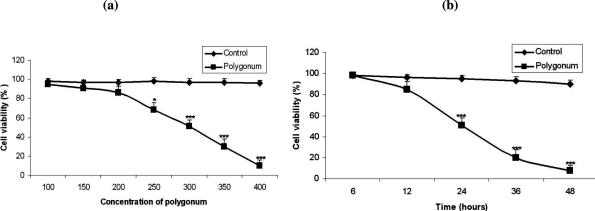
Results showed high cytotoxicity of the extract at the concentrations of 300 and 350 ng/µl after 24 hrs compared to the controls Ninety nine percent cell death occurred at the concentration of 400 ng/µl after 24 hrs (Fig. 1). As is shown in figures 2 and 3, treatment of the MCF-7 cells with Polygonum avicular inhibited cell proliferation significantly compared to the controls (P<0.001). Polygonum avicular extract induced 50% cell death at the concentration of 300 ng/µl and at the highest concentration of Polygonum avicular (400 ng/µl), the cell viability was 3% (Figs. 2a and 2b).
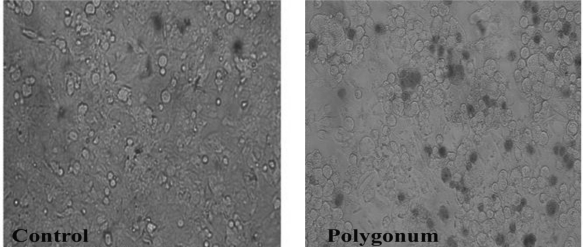
Figure 1.
Cell viability of the MCF-7 cells treated at concentrations of 50, 100, 150, 200, 250, 300,350 and 400 ng/µl of Polygonum avicular (right) versus control group (left).
Figure 2.
a: Cytotoxic effects of Polygonum avicular at concentrations of 50, 100, 150, 200, 250, 300,350 and 400 ng/µl on MCF-7 cells versus control group (P<0.001), b: Cytotoxic effects of polygonum avicular at various concentrations of 50, 100, 150, 200, 250, 300,350 and 400 ng/µl on MCF-7 cells comparing to the control group following incubation for different time lengths (P<0.001).
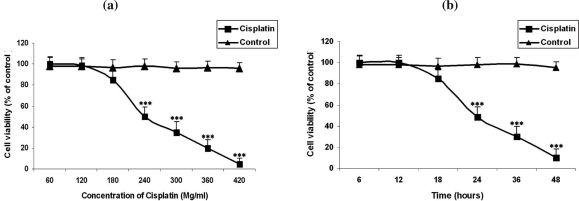
Figure 3.
a: Cytotoxic effects of cisplatin at concentrations of 60, 120,180, 240, 300, 360 and 420 µg/ml on MCF-7 cells versus control group (P<0.001). b: Cytotoxic effects of cisplatin at various concentrations of 5060, 120,180, 240, 300, 360 and 420 µg/ml on MCF-7 cells comparing to the control group following incubation for different time lengths (P<0.001)
Interestingly, cisplatin affected the viability of the cells at higher concentrations i.e. more than 180 µg/ml (Figs. 3a and 3b).
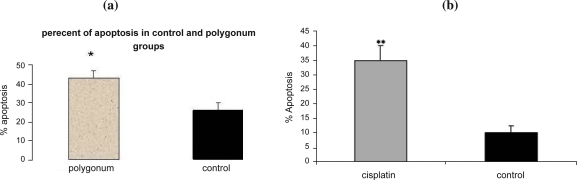
Induction of apoptosis following treatment with Polygonum avicular
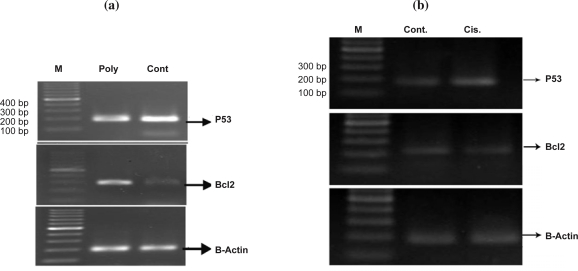
The results showed that apoptosis occurred significantly in 43.08% of the cells treated with Polygonum aviculare (p<0.05) extract compared to the control group (26.97%, Fig. 4a). Consistent with the above results, apoptosis was also induced by cisplatin but in lower extent (Fig. 4b). RT-PCR results revealed that Polygonum avicular induced an increase in the expression of P53 mRNA at the concentration of 100 ng/µl whereas the expression level of the Bcl-2 gene was reduced (Fig. 5a). Following treatment of the cells with 100µg/ml cisplatin for 48 hrs, p53 expression was up-regulated but there were no changes in the expression of the Bcl-2 gene (Fig. 5b).
Figure 4.
a: Effect of polygonum avicular on the apoptosis of MCF-7 cells represented in percent with flowcytometry. Apoptosis in Polygonum treated group increased significantly comparing to control (P<0.05). b: Effect of cisplatin on the apoptosis of MCF-7 cells represented in percent with flowcytometry. Apoptosis in cisplatin treated group increased significantly comparing to control (P<0.05).
Figure 5.
a: Expression of the P53 and Bcl-2 genes in MCF-7 cells in treated and control groups. Expression of P53 and Bcl-2 were determined by semiquantitative RT-PCR. P53 expression was up- regulated in Polygonum group comparing to the control. Bcl-2 expression was down-regulated in Polygonum group comparing to the control. Poly (Polygonum avicular), Cont (Control). b: Expression of the P53 and Bcl-2 genes in MCF-7 cells in treated and control groups. Following treatment of the cells with 100µg/ml cisplatin for 48 hrs, p53 expression was up-regulated but any changes in the expression of the Bcl2 gene was not observed. cis (cisplatin), Cont (Control).
DISCUSSION
Application and mechanism of action of of anticancer medicinal herbs is becoming popular day by day because of their effective antitumorproperties (1, 10). The data presented in this study demonstrated that Polygonum aviculare extract has strong inhibitory effects on cell proliferation and induces apoptotic cell death in the MCF-7 breast cancer cells.
Organic compounds such as phenolics that are found in Polygonum avicularis at high amounts exert various biological effects including antioxidant and anti tumor activities and have a wide range of medicinal properties (6).
It has been shown that the Bcl-2 family plays an important regulatory role in apoptosis, either as inhibitor (Bcl-2) or as activator (Bax). Also, evidence indicates that antiapoptotic Bcl-2 proteins act to stabilize mitochondrial membrane integrity by preventing cytochrome c release and subsequent caspase activation (11). After DNA damage in a normal cell, P53 plays an important role as an inhibitor to prevent cell proliferation (12). Considering the results of this study, it is suggested that Polygonum avicular might exert its anticancer properties through up-regulation of apoptotic genes like P53. In addition since, reactive oxygen species have numerous pathological effects such as lipid and, protein peroxidations, DNA damage and cellular degeneration causing cardiovascular disease, ageing, cancer, inflammatory diseases, and a variety of other disorders (7). It seems the Polygonum avicular might prevent or inhibit breast cancer because of its scavenger capability. Although from the results of this study it might be anticipated that Polygonum avicular is a cancer chemopreventive agent, but more detailed investigations concerning its application as a supplementary anticancer agent is necessary.
ACKNOWLEDGMENT
This study was supported by grant (Number 54/9176) from Research Deputy of Tabriz Medical University, Iran.
REFERENCES
- 1.Vickers A. Botanical medicines for the treatment of cancer: rationale, overview of current data, and methodological considerations for phase I and II trials. Cancer Invest. 2002;20:1069–1079. doi: 10.1081/cnv-120005926. [DOI] [PubMed] [Google Scholar]
- 2.Freddie B, Peter M, Maxwell P. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6:229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang B, Rosano JM, Cheheltani R, Achary MP, Kiani MF. Towards a targeted multi-drug delivery approach to improve therapeutic efficacy in breast cancer. Expert Opin Drug Deliv. 2010;7:1159–1173. doi: 10.1517/17425247.2010.513968. [DOI] [PubMed] [Google Scholar]
- 4.Abdolmohammadi MH, Fouladdel SH, Shafiee A, Amin GH, Ghaffari SM, Azizi E. Anticancer effects and cell cycle analysis on human breast cancerT47D cells treated with extracts of Astrodaucus persicus (Boiss.) Drude in comparison to doxorubicin. DARU. 2008;16:112–118. [Google Scholar]
- 5.Chin-Yuan H, Yu-Pei C, Jeli C. Antioxidant activity of extract from Polygonum aviculare L. Biol Res. 2007;40:13–21. doi: 10.4067/s0716-97602007000100002. [DOI] [PubMed] [Google Scholar]
- 6.Teodor R, Brînduşa R, Sabina R. VALORIFICATION OF HERBS IN PHYTOTHERAPY-AN ALTERNATIVE OF CHEMICAL TREATMENTS IN AGRICULTURE. Envioron Engine Manage J. 2008;7:579–588. [Google Scholar]
- 7.Essick EE, Sam F. Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxid Med Cell Longev. 2010;3:168–177. doi: 10.4161/oxim.3.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster FM, Streuli CH. Inhibitor of apoptosis proteins in breast cancer. Breast Can Res. 8:9. doi: 10.1186/bcr2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habibi Roudkenar M, Halabian R, Mohammadi Roushandeh A, Ebrahimi M, Nekogoftar M, Bahmani P, Jahanian-Najafabadi A, Shokrgozar MA. lipocalin 2 regulation by thermal stresses: Protective role of Lcn2/NGAL against cold and heat stresses. Exp Cell Res. 2009;315:3140–3151. doi: 10.1016/j.yexcr.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy-from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 11.Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006;43:1–67. doi: 10.1080/10408360500295626. [DOI] [PubMed] [Google Scholar]
- 12.Labi V, Erlacher M, Krumschnabel G, Manzl C, Tzankov A, Pinon J, Egle A, Villunger A. Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev. 2010;24:1602–1607. doi: 10.1101/gad.1940210. [DOI] [PMC free article] [PubMed] [Google Scholar]