Abstract
Background
Anorexia nervosa (AN) is prevalent in adolescents and is associated with decreased bone mineral accrual at a time critical for optimizing bone mass. Low bone mineral density (BMD) in AN is a consequence of nutritional and hormonal alterations, including hypogonadism and low estradiol levels. Effective therapeutic strategies to improve BMD in adolescents with AN have not been identified. Specifically, high estrogen doses given as an oral contraceptive do not improve BMD. The impact of physiological estrogen doses that mimic puberty on BMD has not been examined.
Subjects and Methods
We enrolled 110 girls with AN and 40 normal-weight controls (C) 12–18y of similar maturity. Subjects were studied for 18 months. Mature AN [bone age (BA) ≥15 y; n=96] were randomized to transdermal 100mcg 17β-estradiol (with cyclic progesterone) or placebo for 18m. Immature AN (BA <15y; n=14) were randomized to incremental low dose oral ethinyl-estradiol (3.75mcg daily from 0–6m, 7.5mcg from 6–12m, 11.25mcg from 12–18m) to mimic pubertal estrogen increases, or placebo for the 18m duration.
Results
All BMD measures assessed by dual energy x-ray absorptiometry (DXA) were lower in AN than C. At baseline, AN randomized to estrogen (AN E+) did not differ from those randomized to placebo (AN E−) for age, maturity, height, BMI, amenorrhea duration and BMD parameters. Spine and hip BMD Z-scores increased over time in the AN E+ compared with AN E− group, even after controlling for baseline age and weight.
Conclusion
Physiological estradiol replacement increases spine and hip BMD in girls with AN.
Keywords: Anorexia nervosa, adolescents, bone density, bone turnover, bone metabolism, estrogen, transdermal, IGF-1
Introduction
Anorexia nervosa (AN), a condition characterized by low weight and hypogonadism (1), is prevalent in adolescence (2) and associated with decreased bone mineral density (BMD) and bone accrual (3,4). It occurs at a time when normal bone accrual is essential to optimizing peak bone mass, an important determinant of future fracture risk. About 0.2–1% of adolescent girls suffer from AN, and 50% have BMD Z-scores of <−1 at ≥1 bone sites (5). In contrast to normal-weight girls, AN girls do not increase bone mass prospectively, leading to a continued decrease in Z-scores (3,6).
Low BMD in AN results from nutritional deficiencies and associated hormonal alterations, including hypogonadism, low insulin like growth factor-1 (IGF-1) and relative hypercortisolemia (5–8). Weight and menstrual recovery can improve BMD; however, residual deficits persist (3). Therefore, it is important to develop therapeutic strategies that increase bone accrual, while patients and their providers continue to work towards recovery.
Although estrogen deficiency is an important determinant of low BMD in AN, administration of estrogen-progesterone combination pills (such oral contraceptive pills) providing relatively high estrogen doses, does not improve BMD (9–11). In addition, oral dehydroepiandrosterone (DHEA) (12) or bisphosphonates (13) do not increase spine BMD in AN girls after controlling for weight changes, although a study in adults with AN did indicate beneficial effects of bisphosphonates (14). Of importance, the relatively high estrogen doses in oral contraceptives suppress IGF-1 secretion, an important bone trophic hormone (15–17). Therefore, a possible reason for the lack of effectiveness of oral estrogen in increasing BMD in AN is that it further suppresses IGF-1, a hormone already decreased in this condition (18). In contrast, low oral estrogen doses that mimic early pubertal increases in estrogen (19,20) and transdermal replacement doses of estrogen are not IGF-1 suppressive (15–17). The impact of physiological estrogen administration (that does not suppress IGF-1) on bone accrual has not been investigated in AN.
We performed a randomized, double-blind, placebo-controlled study to examine the impact of physiological estrogen replacement on BMD in girls with AN. We hypothesized that physiological estrogen administration would cause increases in BMD measures in AN girls to approximate changes in normal-weight controls. Our primary endpoint was the change in spine BMD Z-scores.
Subjects and Methods
Subject Selection
The study was performed at the Clinical Research Center of Massachusetts General Hospital (MGH), Boston, MA, USA, and the Clinical Investigation Unit at the Hospital for Sick Children (SickKids), Toronto, ON, Canada. We enrolled 150 AN girls (110 at MGH and 40 at SickKids) and 88 normal-weight controls 12–18 years old. All AN subjects were screened by the study psychiatrist to confirm they met DSM-IV criteria, including having amenorrhea for at least three months preceding study participation. All girls with AN were under the care of multidisciplinary treatment teams organized by their primary providers. The study did not assume the clinical care of the subjects. Exclusion criteria included other diseases affecting bone metabolism (including untreated thyroid disease, premature ovarian failure, diabetes, cancer, pituitary, renal disease or bone fracture within the past six months), use of prescription medications affecting bone metabolism within three months, suicidality, psychosis or substance abuse, and hematocrit <30 %, potassium <3.0 mmol/L, or glucose <50 mg/dl. No control subject had a past or present history of an eating disorder.
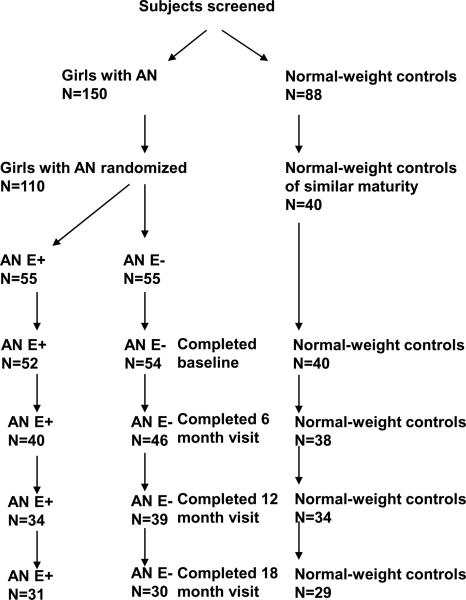
Following the screening visit, 110 AN girls (83 at MGH and 27 at SickKids) and 40 normal-weight controls (BMI 10th–90th percentiles) of similar maturity [Tanner stage and bone age (BA)] were enrolled. The remaining either did not qualify or agree to participate (Figure 1).
Figure 1.
Numbers of adolescent girls with anorexia nervosa and normal-weight controls recruited for the study and attrition over the 18-month course of the study.
AN subjects were recruited through eating disorder providers and treatment centers, and controls through mailings to local pediatricians, and advertisements in area periodicals. Informed assent and consent were obtained from subjects <18 years and their parents, and informed consent from subjects 18 years old. All subjects with AN were enrolled in active outpatient treatment programs that included behavioral therapy to promote weight gain. The Institutional Review Board of Partners HealthCare, Boston, and the Research Ethics Board at SickKids, Toronto, approved the study.
Experimental Protocol
At the screening visit, a history and physical examination were completed and blood drawn for complete blood count (CBC), potassium, glucose, thyroid stimulating hormone (TSH) and follicle stimulating hormone (FSH). A urine pregnancy test was performed. BA was assessed by a single pediatric endocrinologist blinded to the randomization sequence (21). Qualifying AN subjects at MGH and SickKids were randomized, double-blind, to physiological estrogen replacement (AN E+) or placebo (AN E−) by the MGH Research Pharmacy based on a pre-determined computer generated randomization sequence. Mature AN (BA≥15 years, N= 96) were randomized to transdermal 17-β estradiol (100mcg patch applied twice weekly; Novartis Pharmaceuticals, Inc.) continuously over the study duration or identical placebo. Girls randomized to the active estradiol patch also received medroxyprogesterone 2.5mg daily for 10 days each month, while girls randomized to the placebo patch received placebo medroxyprogesterone pills for 10 days each month. Immature AN (BA<15 years, N=14) were randomized to escalating doses of oral ethinyl estradiol (3.75mcg daily for the first six months, 7.5mcg daily for the second six months, and 11.25mcg daily for the last six months) or placebo for 18 months (ethinyl estradiol in these small doses was formulated by the Research Pharmacy of MGH). Estrogen doses were based on studies of physiologic estrogen replacement in immature hypogonadal girls to preserve height potential (22,23). Normal-weight controls were followed for 18 months without intervention at the same time points and per the same study protocol as randomized subjects with AN. All subjects were given 1200 mg calcium carbonate and 400 IU vitamin D daily.
Every two months an interval history, physical examination and pregnancy test were performed. At these visits, we assessed compliance with study medications with verbal questionnaires and by collecting calendars provided to subjects for recording missed study medication doses. We also collected all used and unused patches and pills. Groups did not differ for compliance with study medications. We used dual-energy x-ray absorptiometry (DXA) to assess BMD (spine and hip), and body composition at baseline, 6, 12 and 18 months. Baseline blood was obtained for 25(OH) vitamin D [25(OH)D], parathyroid hormone (PTH), estradiol, IGF-1, leptin, N-terminal propeptide of type 1 procollagen (P1NP), and C-terminal crosslinked peptides (CTX). IGF-1, leptin, P1NP and CTX were also assessed at follow-up. Controls were studied in the first 10 days of their menstrual cycles. Five girls with AN randomized to placebo resumed menses during the course of the study.
Anthropometric Measurements
Subjects were weighed in a hospital gown and height measured on a wall-mounted stadiometer (average of three measurements). BMI and percentage of ideal body weight (%IBW) were calculated.
Bone Density and Body Composition Measurements
We used DXA (Hologic 4500A densitometer, version 11.2; Waltham, MA) to assess BMD at the spine (L1–4) and hip, and body composition, including fat and lean mass. We calculated lumbar spine bone mineral apparent density (LBMAD) (a height adjusted measure of spine BMD) using published methods (24). Because of lack of standards, Z-scores for LBMAD are not reported. In addition, given the lack of validated standards for hip BMAD calculations in children, we adjusted hip BMD for height in our statistical analysis as an alternative approach. Coefficients of variation (CV) for spine and hip BMD are 0.8–1.1%, and for fat and lean mass 1.0–2.1%.
DXA scanners at MGH and SickKids were cross-calibrated using a Hologic spine and whole-body phantom from Synarc Inc. (San Francisco, CA). Each site completed 10 scans of each phantom. Synarc Inc. evaluated performance, consistency and cross-calibration between sites; no adjustments were necessary.
Biochemical Analysis
Screening labs (CBC, potassium, glucose, TSH and FSH) were assessed by the hospital laboratory. We used a chemiluminescent immunoassay to measure PTH (Beckman Coulter, Fullerton, CA; detection limit 1 pg/ml, intra-assay CV 1.6–2.6%), an IRMA to measure IGF-I (Diagnostic Systems Laboratories, Inc, Webster, TX; detection limit 2.06 ng/mL, intra-assay CV 3.9%), an ELISA to measure leptin (Millipore, St. Charles, MO; detection limit 0.5 ng/mL, intra-assay CV 2.6–4.6%), and a RIA to measure estradiol (Diagnostic Systems Laboratories, Inc., Webster, TX; limit of detection 2.2 pg/ml, intra-assay CV 6.5–8.9%) and P1NP (Orion Diagnostica, Espoo, Finland; detection limit 2 ng/ml, intra-assay CV of 6.5–10.2%). An ISYS Autoanalyzer was used to assess CTX (Immunodiagnostic Systems Inc., Scottsdale, AZ; detection limit 0.023 ng/mL, intra-assay CV 3.2%) and 25(OH)D (Immunodiagnostics Systems Inc, Fountain Hills, AZ; limit of detection 3.6 ng/mL, intra-assay CV 5.5–12.1%). Samples were stored at −80 C until analysis, and run in duplicate.
Statistical Analysis
A p value of <0.05 on a two-tailed test was used to indicate significance. Our data were normally distributed and did not require transformations. Baseline characteristics of AN and controls, and AN E+ versus AN E− were compared using the Student t-test (reported as mean±SE). Our primary endpoint was the prospective change in LBMD Z-scores in AN E+ versus AN E−. For our primary longitudinal analysis, the first step was to determine models of longitudinal data collected. This applied to BMD and body composition measures. We checked for linearity by examining data from individual subjects, and linearity over time held without requiring transformation. Next, LBMD and hip BMD Z-scores were analyzed by a mixed model analysis of variance (PROC MIXED), a common method for analyzing longitudinal data. We assumed that each subject had a different linear trajectory, and tested that the mean trajectory was different in AN E+ versus AN E−. This model has a fixed time and time-treatment interaction, and a random intercept and time term; the term of primary interest being the time-treatment interaction. An advantage of this method is that it allowed us to use data from subjects dropping out early, who still added data to the analysis. In a subset analysis, we analyzed the mature girls with AN separately, given that this subset comprised the majority of girls with AN. Given that the immature group included only 14 girls with AN, we did not analyze this subset separately.
For our secondary analyses, we compared absolute and percent changes in BMD measures and in BMD Z-scores in AN E+ versus AN E− at 6, 12 and 18 months using the Student t-test. In comparing AN E+ versus AN E−, we controlled for baseline chronological age and weight changes using multivariate analysis, given that these are known determinants of prospective changes in BMD measures over time. We confirmed that age and weight changes were predictive of subsequent changes in BMD measures using simple linear (Pearson's) correlation. In addition, we controlled for other covariates including height, years since menarche, duration of amenorrhea, and duration since diagnosis. We also compared changes in the two AN groups versus normal-weight controls at 6, 12 and 18 months using ANOVA followed by the Dunnett test to adjust for multiple comparisons. Finally, we used the Fisher Exact Test to compare proportions of AN girls who did or did not receive estrogen for reported adverse effects.
Results
Baseline Characteristics
Baseline characteristics are shown in Table 1. AN did not differ from controls for height and maturity (BA and Tanner stage). Calcium and vitamin D intake, 25(OH)D and PTH levels were higher, whereas estradiol, IGF-1 and leptin were lower in AN than controls. AN E+ did not differ from AN E− at baseline for characteristics reported in Table 1. In addition, girls with AN who completed the study did not differ from non-completers for age, bone age, weight, height, BMI, percent ideal body weight, pubertal stage, duration of amenorrhea, exercise activity, calcium or vitamin D intake, fat mass, lean mass, percent body fat, spine BMD and spine BMD Z-scores, spine BMAD, levels of vitamin D, PTH, estradiol, IGF-1, urinary cortisol and leptin levels (p>0.05). The completers had higher hip BMD and corresponding Z-scores than non-completers (p=0.03 and 0.01).
Table 1.
Baseline Characteristics of Adolescent Girls with Anorexia Nervosa and Normal-Weight Controls
| Controls (n=40) | Anorexia Nervosa (n=110) | P (Controls vs. AN) | |
|---|---|---|---|
| Age (y) | 15.6±0.2 | 16.5±0.2 | 0.001 |
| Bone age (y) | 15.8±0.2 | 16.2±0.1 | Ns |
| Weight (kg) | 57.9±1.4 | 47.2±0.5 | <0.0001 |
| Height (cm) | 164.3±0.9 | 164.3±0.6 | Ns |
| BMI (kg/m2) | 21.4±0.5 | 17.4±0.1 | <0.0001 |
| %IBW (BMI) | 106.3±2.4 | 84.6±0.6 | <0.0001 |
| Tanner stage (breasts) | 4.7±0.1 | 4.6±0.1 | Ns |
| Tanner stage (pubic hair) | 4.6±0.1 | 4.5±0.1 | Ns |
| Amenorrhea duration (y) | - | 0.90±0.08 | - |
| Exercise activity (h) | 16.5±1.7 | 16.8±1.3 | Ns |
| Calcium intake (mg) | 1180±85 | 2040±84 | <0.0001 |
| Vitamin D intake (IU) | 191±28 | 554±28 | <0.0001 |
| DXA Measures | |||
| Fat mass (kg) | 15.9±0.8 | 8.9±0.3 | <0.0001 |
| Lean mass (kg) | 42.0±0.8 | 37.7±0.4 | <0.0001 |
| Percent body fat | 26.2±0.8 | 18.2±0.5 | <0.0001 |
| Lumbar BMD (g/cm2) | 0.972±0.016 | 0.907±0.010 | 0.0009 |
| Lumbar BMD Z-score | 0.136±0.1476 | −0.623±0.098 | <0.0001 |
| Lumbar BMAD (g/cm3) | 0.152±0.0025 | 0.140±0.001 | <0.0001 |
| Hip BMD (g/cm2) | 0.974±0.0189 | 0.887±0.011 | <0.0001 |
| Hip BMD Z-score | 0.277±0.1793 | −0.644±0.098 | <0.0001 |
| Biochemical Parameters | |||
| Vitamin D (ng/ml) | 23.1±1.6 | 31.8±0.9 | <0.0001 |
| PTH (pg/ml) | 11.2±1.8 | 20.3±1.9 | 0.004 |
| Estradiol (pg/ml) | 66.2±13.3 | 39.6±5.4 | 0.03 |
| IGF-1 (ng/ml) | 361.6±16.2 | 239.3±11.3 | <0.0001 |
| Leptin (ng/ml) | 12.4±1.0 | 4.6±0.4 | <0.0001 |
| P1NP (ng/ml) | 200.8±23.8 | 110.6±13.9 | 0.0007 |
| CTX (ng/ml) | 1.01±0.06 | 0.78±0.04 | 0.002 |
Ns: not significant
Changes in Bone Density Parameters
AN E+ had greater increases in BMD Z-scores at the spine and hip than AN E− (p=0.044 and 0.040 respectively) (Table 2) in our primary analysis, an intent-to-treat analysis, using a mixed model ANOVA to analyze longitudinal data. Differences between the groups persisted after controlling for co-variates including age and weight changes. In a subset analysis, when only mature girls with AN were examined (who received transdermal estradiol versus placebo), AN E+ had greater increases in BMD Z-scores at the spine than AN E− (p=0.03) and trended to have greater increases in hip BMD Z-scores (p=0.07).
Table 2.
Mixed Model Analysis of Variance for Longitudinal Data (BMD Z-scores at the different sites) (AN E+ versus AN E−)
| Effect | Group | Parameter estimate | Standard Error | T-value | P | P† | |
|---|---|---|---|---|---|---|---|
| LBMD Z-scores | Visit*Group | AN E− | −0.0695 | 0.0342 | −2.03 | 0.045 | 0.046 |
| Visit*Group | AN E+ | 0 | - | - | |||
| Hip BMD Z-scores | Visit*Group | AN E− | −0.0660 | 0.0318 | −2.08 | 0.041 | 0.037 |
| Visit*Group | AN E+ | 0 | - | - |
p value after controlling for covariates (age and weight)
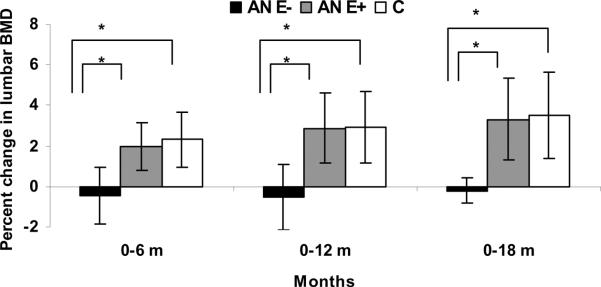
BMD changes were predicted inversely by baseline age and positively by weight changes (data not shown). In addition, BMD changes were predicted inversely by height and years since menarche. On secondary analyses to quantify prospective changes, AN E+ had greater increases in absolute LBMD, percent change LBMD, percent change LBMAD, and absolute LBMD Z-scores at all skeletal sites at all time points measured compared with AN−E even after adjusting for baseline age and change in weight. In addition, AN+E had greater increases in absolute and percent change hip BMD and absolute hip BMD Z-scores at 18m compared with the AN−E group (Table 3). Changes in LBMD, LBMAD and hip BMD at 6, 12 and 18m were lower in AN E− versus normal-weight controls (p<0.05) but comparable in AN E+ versus controls (percent change in spine BMD in the three groups is shown in Figure 2).
Table 3.
Changes in BMD Measures in Treated AN (AN E+), Untreated AN (AN E−) and Normal-weight Controls (C)
| AN E+ | AN E− | AN E+ vs. E− | Controls | P (ANOVA) 3 groups | |||||
|---|---|---|---|---|---|---|---|---|---|
| p† | p‡ | p†† | p‡‡ | p# | |||||
| Bone Density Changes | |||||||||
| Δ LBMD 6-0 (g/cm2) | 0.015±0.004 | −0.006±0.004 | 0.003 | 0.003 | 0.0003 | 0.0002 | 0.0003 | 0.021±0.003a | <0.0001 |
| % Δ LBMD 6-0 | 1.772±0.530 | −0.529±0.497 | 0.004 | 0.005 | 0.0003 | 0.0003 | 0.0003 | 2.283±0.361a | <0.0001 |
| Δ LBMD Z 6-0 | 0.043±0.044 | −0.155±0.040 | 0.004 | 0.006 | 0.0003 | 0.0003 | 0.0002 | 0.079±0.030a | <0.0001 |
| Δ LBMD 12-0 (g/cm2) | 0.020±0.006 | −0.002±0.007 | 0.004 | 0.002 | 0.003 | 0.0007 | 0.0004 | 0.030±0.006a | 0.002 |
| % Δ LBMD 12-0 | 2.513±0.796 | −0.072±0.821 | 0.004 | 0.002 | 0.003 | 0.001 | 0.0006 | 3.315±0.623a | 0.005 |
| Δ LBMD Z 12-0 | 0.027±0.060 | −0.218±0.059 | 0.003 | 0.002 | 0.002 | 0.001 | 0.0007 | 0.077±0.048a | 0.0005 |
| Δ LBMD 18-0 (g/cm2) | 0.021±0.009 | 0.002±0.011 | 0.02 | 0.02 | 0.03 | 0.04 | 0.02 | 0.042±0.008a | 0.01 |
| % Δ LBMD 18-0 | 2.611±1.047 | 0.307±1.144 | 0.01 | 0.01 | 0.02 | 0.03 | 0.01 | 4.483±0.890a | 0.02 |
| Δ LBMD Z 18-0 | −0.026±0.078 | −0.236±0.091 | 0.05 | 0.05 | 0.05 | 0.05 | 0.03 | 0.099±0.068a | 0.01 |
| Δ LBMAD 6-0 (g/cm3) | 0.003±0.001 | −0.001±0.001 | 0.004 | 0.002 | 0.003 | 0.001 | 0.002 | 0.004±0.001a | <0.0001 |
| % Δ LBMAD 6-0 | 1.988±0.580 | −0.560±0.550 | 0.004 | 0.002 | 0.002 | 0.001 | 0.001 | 2.390±0.369a | 0.0001 |
| Δ LBMAD 12-0 (g/cm3) | 0.003±0.001 | −0.000±0.001 | 0.008 | 0.005 | 0.01 | 0.008 | 0.005 | 0.004±0.001a | 0.02 |
| % Δ LBMAD 12-0 | 2.226±0.802 | −0.109±0.832 | 0.01 | 0.007 | 0.02 | 0.01 | 0.008 | 2.727±0.657a | 0.02 |
| Δ LBMAD 18-0 (g/cm3) | 0.003±0.001 | 0.000±0.001 | 0.07 | 0.07 | 0.006 | 0.01 | 0.004 | 0.006±0.001a | 0.01 |
| % Δ LBMAD 18-0 | 1.919±0.944 | 0.232±0.812 | 0.049 | 0.05 | 0.004 | 0.01 | 0.003 | 3.987±0.944a | 0.02 |
| Δ Hip BMD 6-0 (g/cm2) | −0.001±0.004 | −0.006±0.004 | 0.12 | 0.11 | 0.07 | 0.08 | 0.07 | 0.012±0.003a | 0.006 |
| % Δ Hip BMD 6-0 | −0.027±0.438 | −0.559±0.492 | 0.16 | 0.16 | 0.09 | 0.10 | 0.098 | 1.221±0.321a | 0.02 |
| Δ Hip BMD Z 6-0 | −0.002±0.037 | −0.077±0.041 | 0.08 | 0.07 | 0.06 | 0.06 | 0.06 | 0.096±0.032a | 0.006 |
| Δ Hip BMD 12-0 (g/cm2) | 0.005±0.008 | −0.004±0.007 | 0.08 | 0.06 | 0.12 | 0.04 | 0.04 | 0.016±0.004a | 0.06 |
| % Δ Hip BMD 12-0 | 0.617±0.870 | −0.299±0.734 | 0.10 | 0.07 | 0.16 | 0.06 | 0.03 | 1.715±0.379 | 0.12 |
| Δ Hip BMD Z 12-0 | −0.080±0.069 | −0.193±0.055 | 0.08 | 0.06 | 0.12 | 0.049 | 0.04 | −0.009±0.034a | 0.048 |
| Δ Hip BMD 18-0 (g/cm2) | −0.001±0.008 | −0.013±0.011 | 0.02 | 0.02 | 0.06 | 0.04 | 0.04 | 0.021±0.006a | 0.02 |
| % Δ Hip BMD 18-0 | 0.004±0.837 | −1.178±1.93 | 0.047 | 0.02 | 0.08 | 0.06 | 0.06 | 2.175±0.650a | 0.04 |
| Δ Hip BMD Z 18-0 | −0.177±0.063 | −0.331±0.089 | 0.03 | 0.02 | 0.07 | 0.045 | 0.049 | −0.016±0.056a | 0.01 |
Δ: Difference in; 6-0: At 6 months compared with baseline; 12-0: At 12 months compared with baseline; 18-0: At18 months compared with baseline
P value after controlling for baseline age and weight changes
P value after controlling for baseline age, weight changes and height
P value after controlling for baseline age, weight changes and years since menarche
P value after controlling for baseline age, weight changes and duration of amenorrhea
P value after controlling for baseline age, weight changes, height, years since menarche and duration of amenorrhea
P<0.05 compared with AN E−
b P<0.05 compared with AN E+
Figure 2.
Percent change in lumbar spine bone mineral density (LBMD) in adolescent girls with anorexia nervosa (AN) randomized to placebo (AN E−) (black bars), girls with AN randomized to estrogen (AN E+) (gray bars) and normal-weight controls (C) (white bars). AN E+ had significant increases in LBMD at 6, 12 and 18 months compared with AN E−. When compared with C, AN E− had significant decreases in LBMD at 6, 12 and 18 months, whereas AN E+ did not differ from C for changes in BMD over time. Analysis was performed for differences between means for pairs * p<0.05
We also examined differences in AN E+ versus AN E− after controlling for (i) baseline age, height and weight changes, for (ii) baseline age, years since menarche and weight changes, for (iii) baseline age, duration of amenorrhea and weight changes, and finally for (iv) baseline age, height, years since menarche, duration of amenorrhea and weight changes (Table 3). Differences between AN E+ and AN E− became even stronger for changes in LBMD and LBMAD measures and for most hip BMD measures after controlling for baseline age, height, years since menarche, duration of amenorrhea and weight changes. Data did not differ when duration of amenorrhea was replaced by duration since diagnosis of AN. Similarly, addition of changes in lean mass to the multivariate model did not change the results and are not reported.
Changes in Biochemical Parameters
AN E+ did not differ from AN E− over 18 months for changes in levels of IGF-1 (8.4±32.6 vs. 6.2±26.0 ng/ml, p=0.96) and leptin (3.0±1.8 vs. 1.7±0.8 ng/ml, p=0.36). Levels of CTX, a marker of bone resorption, decreased more in AN E+ than in AN E− over the 18 months, although the difference was not statistically significant (−0.25±0.09 vs. −0.12±0.10 ng/ml, p=0.35). Changes in P1NP, a marker of bone formation, also did not differ in AN E+ versus AN E− (−2.9±22.5 vs. −10.9±27.0 ng/ml, p=0.83).
Changes in Weight and Body Composition Parameters
AN E+ did not differ from AN E− for prospective changes in weight (p=0.50), BMI (p=0.22), lean mass (p=0.20) or percent fat mass (p=0.25) in our primary analysis, an intent-to-treat analysis, using a mixed model ANOVA. Similarly, when mature girls with AN were analyzed, AN E+ did not differ from AN E− for changes in weight (p=0.46), BMI (p=0.26), lean mass (p=0.21) or percent fat mass (p=0.24). No differences were observed in AN E+ versus AN E− for changes in height over the study duration within mature girls and immature girls. Additionally, on secondary analysis (Table 4), AN E+ did not differ from AN E− for changes in weight, BMI, fat and lean mass over 6, 12 and 18 months.
Table 4.
Changes in Body Composition Parameters in Treated AN (AN E+) versus Untreated AN (AN E−)
| AN E+ | AN E− | P | |
|---|---|---|---|
| Δ Weight 6-0 | 0.27±0.11 | 0.29±0.14 | 0.90 |
| Δ Weight 12-0 | 3.35±0.97 | 4.83±0.77 | 0.23 |
| Δ Weight 18-0 | 4.63±1.02 | 4.18±0.99 | 0.75 |
| Δ BMI 6-0 | 0.98±0.23 | 1.14±0.20 | 0.59 |
| Δ BMI 12-0 | 1.07±0.36 | 1.63±0.29 | 0.23 |
| Δ BMI 18-0 | 1.57±0.38 | 1.47±0.36 | 0.84 |
| Δ Fat mass 6-0 | 1.11±0.38 | 1.76±0.41 | 0.26 |
| Δ Fat mass 12-0 | 1.31±0.68 | 2.92±0.53 | 0.06 |
| Δ Fat mass 18-0 | 2.01±0.88 | 2.90±0.63 | 0.41 |
| Δ Lean mass 6-0 | 1.63±0.31 | 1.02±0.33 | 0.18 |
| Δ Lean mass 12-0 | 1.60±0.49 | 2.00±0.45 | 0.55 |
| Δ Lean mass 18-0 | 1.82±0.56 | 1.72±0.60 | 0.90 |
Δ: Difference in; 6-0: At 6 months compared with baseline; 12-0: At 12 months compared with baseline; 18-0: At18 months compared with baseline
Adverse Effects of Study Medications
Adverse events did not differ between groups (Table 5).
Table 5.
Adverse Effects in Treated AN (AN E+) and Untreated AN (AN E−)
| AN E+ | AN E− | |
|---|---|---|
| Admissions to the hospital for low weight and/or bradycardia | 21.2% | 29.6% |
| Nausea/vomiting | 25.0% | 14.8% |
| Dizziness | 11.5% | 18.5% |
| Headaches | 17.3% | 25.9% |
| Bloating | 32.7% | 29.6% |
| Constipation | 7.7% | 7.4% |
| Breast tenderness | 25.0% | 16.7% |
| Premenstrual symptoms | 1.9% | 0% |
| Mood swings | 1.9% | 0% |
| Increased vaginal discharge | 23.1% | 16.7% |
| Excessive or irregular bleeding | 23.1% | 16.7% |
| Irritation from contact lenses | 3.9% | 11.1% |
| Perceived increase in facial hair | 5.8% | 1.9% |
| Perceived loss of scalp hair | 13.5% | 9.3% |
| Worsening depression | 5.8% | 9.3% |
| Erythema at patch application site | 31.1% | 35.4% |
| Vasovagal symptoms | 0% | 1.9% |
| Calf pain (not associated with deep vein thrombosis) | 1.9% | 0% |
*p<0.05
Discussion
We demonstrate for the first time that physiological estrogen replacement increases spine and hip BMD in girls with AN in a randomized placebo-controlled study.
Adolescents with AN lack estrogen at a time when physiologic estrogen secretion is critical for optimizing bone accrual. Low BMD is common in AN (5), and bone accrual is profoundly impaired (3,6) resulting in lower than optimal peak bone mass, which may impair future bone health. Adolescence is a relatively narrow window in time in which to maximize bone accrual, and deficits incurred at this time may lead to permanent deficits in peak bone mass. In fact, adult women who develop AN during adolescence have lower BMD than those developing the condition in adult life, even when duration of amenorrhea is comparable (25). Consequently, it is important to identify therapeutic strategies to improve bone accrual in AN during adolescence.
Consistent with previous studies, AN girls had lower spine and hip BMD and corresponding Z-scores, and lower levels of bone turnover markers than normal-weight controls. Importantly, these differences were observed despite higher calcium and vitamin D intake and higher 25(OH)D levels in AN, as reported in earlier studies (26). AN is associated with a nutritionally acquired resistance to GH with low IGF-1 (7), in contrast to high IGF-1 levels typically seen during puberty (27). Another hormonal alteration in AN that may impact BMD and bone accrual is low leptin (28,29). AN girls in this study had lower lean mass, estradiol, IGF-1 and leptin than controls.
Although recovery of weight and menses is the optimal strategy for improving bone accrual in AN, studies indicate that weight gain and menses recovery is not sufficient to normalize bone accrual (3,26,30). Importantly, recovery can be difficult to attain and sustain, despite concentrated efforts of a multidisciplinary treatment team. In this study, only five girls in the placebo-treated group resumed regular menses.
Oral estrogen has been effectively used as replacement therapy in normal-weight hypogonadal adolescents (e.g. girls with Turner syndrome) for many years (22,31). However, multiple studies show that oral estrogen (given as an oral contraceptive), is not effective in increasing BMD in AN (9–11). Although the reason for lack of beneficial effects of oral estrogen on BMD in AN remains speculative, possibilities include non-physiologic dosing and/or suppression of systemic IGF-1 by oral estrogen, as in post-menopausal women (15). This is particularly an issue in AN, a condition already associated with low IGF-1 (7). Use of transdermal estrogen, which does not suppress IGF-1, should be a more effective replacement strategy (15). In fact, a recent exploratory study in girls with Turner syndrome demonstrated that transdermal estrogen caused greater increases in BMD than did oral estrogen (31). Additionally, small incremental doses of oral estrogen in early puberty (to mimic the early pubertal rise in estrogen) do not suppress IGF-1 (19,20) and could be beneficial to bone.
Based on these data, we hypothesized that administration of replacement doses of transdermal estrogen to mature AN girls (BA≥15 years) and small incremental doses of oral estrogen to younger AN girls (with potential to grow based on BA<15 years), should be effective in increasing BMD. Our results support our hypothesis, and we show that physiological estrogen replacement over 18-months is effective in increasing spine and hip BMD Z-scores in AN girls 12–18 years. We also demonstrate that spine and hip BMD changes are lower in AN E− compared with normal-weight controls, whereas AN E+ do not differ from controls for bone accrual. Overall, AN E+ had greater increases in spine and hip BMD and corresponding Z-scores than AN E−. These results became even stronger after controlling for important covariates including baseline age, weight changes, height, years since menarche, duration of amenorrhea or duration since diagnosis.
Changes in IGF-1 levels did not differ in AN E+ versus AN E− consistent with our hypothesis that physiological estrogen replacement would not suppress IGF-1 levels. Levels of CTX, a marker of bone resorption, decreased in girls with AN randomized to estrogen, but the change was not significant when compared with girls with AN randomized to placebo, likely because levels of bone turnover markers were already very suppressed in girls with AN compared with normal-weight girls at baseline, as also reported in earlier studies (32). A further suppression of bone resoprtion markers following estrogen administration may be difficult to appreciate in a maximally suppressed state of bone turnover. Levels of P1NP, a bone formation marker, did not differ between the groups.
Importantly, we found no difference in prospective changes in weight, lean or fat mass, or in leptin levels, in AN E+ versus AN E−, indicating that physiological estrogen replacement does not cause weight or body composition changes, which may be reassuring to AN girls and enhance compliance. Additionally, adverse events did not differ in AN E+ versus AN E−. Of note, attrition in our study population was high, but consistent with other treatment studies in adolescents with AN (33,34).
In order to normalize and `catch-up' BMD over time, girls with AN may need to not only gain bone mass at a rate comparable to controls (as in this study), but may need to surpass controls. In order for complete catch-up, other hormonal alterations in AN may need to be addressed. For example, it may be important to raise IGF-1 through weight recovery or rhIGF-1 administration. One study in normal-weight women with hypothalamic amenorrhea reported that leptin replacement caused menses resumption in five of eight women, with associated increases in bone formation markers (35). However, these women had subjective reductions in appetite and lost significant weight. Leptin replacement is thus not a good strategy for improving BMD in AN, in whom low leptin is likely adaptive.
Another possible strategy to increase BMD in AN is to use bisphosphonates. Our group has recently reported an improvement in BMD in adults with AN randomized to bisphosphonates (14). However, caution is necessary in considering bisphosphonates in adolescents given their long half-life, and also because bisphosphonates are associated with marked reductions in bone turnover, already low in adolescents with AN. In one trial of 12-months of alendronate in AN girls, there was no improvement in spine BMD after controlling for weight changes, although some effect at the femoral neck was reported (13).
Our study indicates that physiological estrogen replacement is effective in prospectively halting BMD reductions in AN girls, and may be considered a therapeutic option in girls refractory to weight and menstrual recovery despite ongoing multi-disciplinary therapy. Further studies are necessary to determine strategies that result in complete catch-up of BMD and normalization of peak bone mass acquisition in young women with AN.
Acknowledgements
We thank the skilled nursing and bionutrition staff of the Clinical Research Center of Massachusetts General Hospital, Boston, MA, USA, and Clinical Investigation Center of the Hospital for Sick Children, Toronto, ON, USA, for their help with carrying out this study. Finally, we thank our subjects, without whom this study would not have been possible.
This work was supported by National Institutes of Health Grants R01 DK 062249, K23 RR018851, M01-RR-01066 and 1 UL1 RR025758-03.
Footnotes
Disclosures The authors state that they have no conflicts of interest.
Bibliography
- 1.Diagnostic and statistical manual of mental disorders. 3rd. ed. American Psychiatric Association; Washington, D.C.: 1987. [Google Scholar]
- 2.Lucas AR, Beard CM, O'Fallon WM, Kurland LT. 50-year trends in the incidence of anorexia nervosa in Rochester, Minn.: a population-based study. Am J Psychiatry. 1991;148(7):917–22. doi: 10.1176/ajp.148.7.917. [DOI] [PubMed] [Google Scholar]
- 3.Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, Lockhart P, Cord J, Herzog DB, Katzman DK, Klibanski A. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008;93(4):1231–7. doi: 10.1210/jc.2007-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachrach LK, Katzman DK, Litt IF, Guido D, Marcus R. Recovery from osteopenia in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 1991;72(3):602–6. doi: 10.1210/jcem-72-3-602. [DOI] [PubMed] [Google Scholar]
- 5.Misra M, Aggarwal A, Miller KK, Almazan C, Worley M, Soyka LA, Herzog DB, Klibanski A. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;114(6):1574–83. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- 6.Soyka L, Misra M, Frenchman A, Miller K, Grinspoon S, Schoenfeld D, Klibanski A. Abnormal bone mineral accrual in adolescent girls with anroexia nervosa. J Clin Endocrinol Metab. 2002;87:4177–85. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 7.Misra M, Miller K, Bjornson J, Hackman A, Aggarwal A, Chung J, Ott M, Herzog D, Johnson M, Klibanski A. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88 doi: 10.1210/jc.2003-030532. in press. [DOI] [PubMed] [Google Scholar]
- 8.Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, Neubauer G, Herzog DB, Klibanski A. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89(10):4972–4980. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 9.Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15(3):135–43. doi: 10.1016/s1083-3188(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 10.Munoz MT, Morande G, Garcia-Centenera JA, Hervas F, Pozo J, Argente J. The effects of estrogen administration on bone mineral density in adolescents with anorexia nervosa. Eur J Endocrinol. 2002;146(1):45–50. doi: 10.1530/eje.0.1460045. [DOI] [PubMed] [Google Scholar]
- 11.Strokosch GR, Friedman AJ, Wu SC, Kamin M. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: a double-blind, placebo-controlled study. J Adolesc Health. 2006;39(6):819–27. doi: 10.1016/j.jadohealth.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Gordon CM, Grace E, Emans SJ, Feldman HA, Goodman E, Becker KA, Rosen CJ, Gundberg CM, LeBoff MS. Effects of Oral Dehydroepiandrosterone on Bone Density in Young Women with Anorexia Nervosa: A Randomized Trial. J Clin Endocrinol Metab. 2002;87(11):4935–4941. doi: 10.1210/jc.2002-020545. [DOI] [PubMed] [Google Scholar]
- 13.Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, Hertz S, Shenker IR. Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90(6):3179–85. doi: 10.1210/jc.2004-1659. [DOI] [PubMed] [Google Scholar]
- 14.Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, Herzog D, Klibanski A. Effects of Risedronate and Low-Dose Transdermal Testosterone on Bone Mineral Density in Women with Anorexia Nervosa: A Randomized, Placebo-Controlled Study. J Clin Endocrinol Metab. 2011 Apr 27; doi: 10.1210/jc.2011-0380. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab. 1991;72(2):374–81. doi: 10.1210/jcem-72-2-374. [DOI] [PubMed] [Google Scholar]
- 16.Cardim H, Lopes C, Gianella-Neto D, da Fonseca A, Pinotti J. The insulin like growth factor-I system and hormone replacement therapy. Fertil Steril. 2001;75:282–7. doi: 10.1016/s0015-0282(00)01691-5. [DOI] [PubMed] [Google Scholar]
- 17.Kam G, Leung K, Baxter R, Ho K. Estrogens exert route and dose dependent effects on insulin-like growth factor (IGF)- binding protein-3 and the acid labile subunit of the IGF ternary complex. J Clin Endocrinol Metab. 2000;85:1918–22. doi: 10.1210/jcem.85.5.6527. [DOI] [PubMed] [Google Scholar]
- 18.Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84(12):4489–96. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- 19.Moll GW, Jr., Rosenfield RL, Fang VS. Administration of low-dose estrogen rapidly and directly stimulates growth hormone production. Am J Dis Child. 1986;140(2):124–7. doi: 10.1001/archpedi.1986.02140160042027. [DOI] [PubMed] [Google Scholar]
- 20.Ross JL, Cassorla FG, Skerda MC, Valk IM, Loriaux DL, Cutler GB., Jr. A preliminary study of the effect of estrogen dose on growth in Turner's syndrome. N Engl J Med. 1983;309(18):1104–6. doi: 10.1056/NEJM198311033091806. [DOI] [PubMed] [Google Scholar]
- 21.Greulich W, Pyle S. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford University Press; Stanford: 1959. [Google Scholar]
- 22.Beckett PR, Copeland KC, Flannery TK, Sherman LD, Abrams SA. Combination growth hormone and estrogen increase bone mineralization in girls with Turner syndrome. Pediatr Res. 1999;45(5 Pt 1):709–13. doi: 10.1203/00006450-199905010-00017. [DOI] [PubMed] [Google Scholar]
- 23.Quigley CA, Crowe BJ, Anglin DG, Chipman JJ. Growth hormone and low dose estrogen in Turner syndrome: results of a United States multi-center trial to near-final height. J Clin Endocrinol Metab. 2002;87(5):2033–41. doi: 10.1210/jcem.87.5.8477. [DOI] [PubMed] [Google Scholar]
- 24.Katzman D, Bachrach L, Carter D, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–9. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 25.Biller B, Saxe V, Herzog D, Rosenthal D, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68(3):548–54. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- 26.Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, Klibanski A. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87(9):4177–85. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 27.Cara J, Rosenfield R, Furlanetto R. A longitudinal study of the relationship of plasma somatomedin-C concentration to the pubertal growth spurt. Am J Dis Child. 1987;141(5):562–4. doi: 10.1001/archpedi.1987.04460050104041. [DOI] [PubMed] [Google Scholar]
- 28.Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289(3):E373–81. doi: 10.1152/ajpendo.00041.2005. [DOI] [PubMed] [Google Scholar]
- 29.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34(3):376–83. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Audi L, Vargas DM, Gussinye M, Yeste D, Marti G, Carrascosa A. Clinical and biochemical determinants of bone metabolism and bone mass in adolescent female patients with anorexia nervosa. Pediatr Res. 2002;51(4):497–504. doi: 10.1203/00006450-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Nabhan ZM, Dimeglio LA, Qi R, Perkins SM, Eugster EA. Conjugated oral versus transdermal estrogen replacement in girls with Turner syndrome: a pilot comparative study. J Clin Endocrinol Metab. 2009;94(6):2009–14. doi: 10.1210/jc.2008-2123. [DOI] [PubMed] [Google Scholar]
- 32.Misra M, Soyka LA, Miller KK, Herzog DB, Grinspoon S, De Chen D, Neubauer G, Klibanski A. Serum osteoprotegerin in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2003;88(8):3816–22. doi: 10.1210/jc.2003-030088. [DOI] [PubMed] [Google Scholar]
- 33.le Grange D, Lock J. The dearth of psychological treatment studies for anorexia nervosa. Int J Eat Disord. 2005;37(2):79–91. doi: 10.1002/eat.20085. [DOI] [PubMed] [Google Scholar]
- 34.Lock J, Agras WS, Bryson S, Kraemer HC. A comparison of short- and long-term family therapy for adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2005;44(7):632–9. doi: 10.1097/01.chi.0000161647.82775.0a. [DOI] [PubMed] [Google Scholar]
- 35.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant Human Leptin in Women with Hypothalamic Amenorrhea. N Engl J Med. 2004;351(10):987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]