Abstract
Human in vivo models of primary hyperparathyroidism (HPT), humoral hypercalcemia of malignancy (HHM) or lactational bone mobilization for more than 48 hours have not been described previously. We therefore developed seven-day continuous infusion models using hPTH(1–34) and hPTHrP(1–36) in healthy human adult volunteers. Study subjects developed sustained mild increases in serum calcium (10.0 mg/dl), with marked suppression of endogenous PTH(1–84). The maximal tolerated infused doses over a seven-day period (2 and 4 pmol/kg/hr, for PTH and PTHrP, respectively) were far lower than in prior, briefer human studies (8–28 pmol/kg/hr). In contrast to prior reports using higher PTH and PTHrP doses, both 1,25(OH)2D and TmP/GFR remained unaltered with these low doses, despite achievement of hypercalcemia and hypercalciuria. As expected, bone resorption increased rapidly, and reversed promptly with cessation of the infusion. However, in contrast to events in primary HPT, bone formation was suppressed by 30–40% for the seven days of the infusions. With cessation of PTH and PTHrP infusion, bone formation markers abruptly rebounded upward, confirming that bone formation is suppressed by continuous PTH or PTHrP infusion. These studies demonstrate that continuous exposure of the human skeleton to PTH or PTHrP in vivo recruits and activates the bone resorption program, but causes sustained arrest in the osteoblast maturation program. These events would most closely mimic and model events in HHM. Although not a perfect model for lactation, the increase in resorption and the rebound increase in formation with cessation of the infusions is reminiscent of the maternal skeletal calcium mobilization and reversal that occur following lactation. The findings also highlight similarities and differences between the model and HPT.
Keywords: Parathyroid Hormone, PTH-Related Protein, Humoral Hypercalcemia of Malignancy, Hyperparathyroidism, Lactation
Introduction
PTH and PTHrP are similar in structure, and are associated with similar, but not identical, pathophysiological syndromes, primary hyperparathyroidism (HPT) and humoral hypercalcemia of malignancy (HHM), respectively. Both human PTH and PTHrP are secreted as large pro-hormones which are then metabolized into a number of active forms of which hPTH(1–34) and hPTHrP(1–36) have been employed in studies in humans and rodents, as well as in vitro (1,2). hPTH(1–34) and hPTHrP(1–36) bind to, and signal via, a common PTH-PTHrP G-protein-coupled receptor, the PTH-1 receptor or “PTH1R” (3). They have been shown to signal via the same intracellular signaling pathways, and until recently, were thought to bind with identical affinity and signal with identical potency to the PTH1R. More recently, Dean et al (4) have demonstrated that while both peptides associate with the PTH1R with equivalent affinity and rapidity, hPTH(1–34) remains bound to the PTH1R, is internalized and continues to signal for more than an hour despite the removal of ligand (4). In contrast, PTHrP(1–36) dissociates from the PTH1R and ceases signaling almost immediately after removal of PTHrP from the perfusion medium (4).
In normal physiology, PTH and PTHrP play distinct functions (1,5). PTH is a classical endocrine peptide hormone, produced exclusively by the parathyroid glands. Its principal function is to maintain normal serum calcium homeostasis, through its direct actions on the skeleton and kidney, and indirect actions on the intestine. In contrast, PTHrP is a ubiquitously expressed, locally acting paracrine, autocrine and intracrine factor that variously regulates growth, survival, and differentiation in the myriad of cell types in its local environment. Unlike PTH, with only rare exceptions, PTHrP normally does not enter the systemic circulation. One exception is the normal systemic or endocrine secretion of PTHrP by the mammary gland during lactation, whereby PTHrP activates osteoclastic bone resorption, and also activates renal calcium retention, so that large quantities of skeletally derived calcium are available to be transported into milk (6).
Overproduction of the two hormones produces similar, but distinct, pathophysiological syndromes, HPT and HHM. In both syndromes, patients display hypercalcemia, hypercalciuria, hypophosphatemia, phosphaturia, and increases in osteoclastic bone resorption (7–10). On the other hand, osteoblastic bone formation is increased in HPT, but reduced in HHM (7–10). Also, circulating 1,25(OH)2D is increased in HPT but reduced in HHM (7,8). Why continuous secretion in vivo of two similar peptides that share a common receptor should produce opposite effects on two fundamental calcitropic peptide target organs has never been clear.
In this regard, lactation and HHM are particularly intriguing, because both of these conditions are associated with strikingly rapid bone loss. Normal lactating women lose up to 10% of their skeletal mass during six months of lactation (6,11). Patients with HHM, may lose 50% of their skeletal mass in a matter of a few months (7,9). In contrast, many or most patients with HPT do not lose bone mass rapidly but gradually over a period of years (12–14). These differences presumably reflect the tightly coupled increases in bone resorption and formation that occur in HPT, in contrast to the markedly uncoupled bone turnover in patients with HHM (7,9). Some degree of uncoupling must also occur in lactating women, since marked bone loss occurs, but confirmatory quantitative bone histomorphometry has never been performed during human lactation. Thus, the explanation for the complete uncoupling of resorption and formation by PTHrP in HHM, as compared to the tight coupling that occurs in HPT remains enigmatic despite some 30 years of study (7–9).
In an effort to understand how or why turnover can be coupled in response to one hormone but uncoupled in response to a similarly acting one, we have developed subacute 6-, 12- and 48-hour infusion models using PTH and PTHrP in healthy human volunteers (15–18). We found that infusion of both peptides causes hypercalcemia, phosphaturia, and increases in osteoclastic bone resorption. Surprisingly, we found that PTHrP, despite being reported to bind to the receptor with equivalent potency, failed to stimulate 1,25(OH)2D production with the efficiency of PTH, appearing some two-fold less potent than PTH (15–18). We also found that both peptides, when infused continuously for 12–48 hours, suppress markers of bone formation (17,18), as is seen in HHM (7,9,10) but in contrast to events in HPT (9,12,13). While this had been reported for short-term PTH infusions (19–21), it had never previously been reported for PTHrP.
Reasoning that increases in 1,25(OH)2D and in bone formation by both PTH and PTHrP would likely occur eventually, but might require more prolonged infusions, we have developed, for the first time, parallel seven-day continuous infusion models for both PTH and PTHrP. Here we report a number of surprising and novel observations. These help to further define the normal physiological and pathophysiological actions of PTH and PTHrP. Finally, they provide important tools for future human studies.
Methods
Study Subjects
Twenty-two healthy normal young adults between the ages of 24 and 35 years, recruited from the local community, participated in this study (Table 1). Exclusion criteria included subjects of African-American descent, smokers or anyone with a chronic disease such as cardiac, vascular, pulmonary, endocrine, musculoskeletal, hematologic or malignancy, BMI>30, were anemic, had a history of alcohol or drug abuse, had baseline hypertension. Subjects were required to have normal screening labs (serum calcium, albumin, phosphorous, creatinine, 25(OH)D, iPTH(1–84), and hemoglobin/hematocrit), a negative urine drug screen and a normal physical exam. All procedures were approved in advance by the University of Pittsburgh Institutional Review Board, and all subjects provided written informed consent.
Table 1.
Baseline Demographics (± SD)
| PTHrP 2 (n=3) |
PTHrP 4+ (n=6) |
PTHrP 5 (n =3) |
PTH 2+ (n=5) |
PTH 4 (n=5) |
|
|---|---|---|---|---|---|
| AGE | 31.0±6.1 | 28.1±3.1 | 27.3±2.9 | 26.4±1.7 | 29.8±3.2 |
| HEIGHT (cm) | 170.9±7.5 | 169.0±10.0 | 170.9±5.9 | 172.8±5.8 | 171.5±8.9 |
| WEIGHT (kg) | 69.0±12.9 | 66.4±10.9 | 71.6±16.3 | 74.1±15.5 | 72.4±9.6 |
| BMI (kg/m2) | 23.5±3.0 | 23.4±4.2 | 24.4±5.0 | 24.7±4.3 | 24.5±1.16 |
| MALE/FEMALE | 2/1 | 3/3 | 2/1 | 2/3 | 4/1 |
| CREATININE (mg/dl) | 1.13±0.06 | 0.83±0.05 | 0.77±0.06 | 1.04±0.24 | 1.12±0.25 |
| CALCIUM (mg/dl) | 9.50±0.20 | 9.78±0.40 | 9.63±0.32 | 9.70±0.17 | 9.56±0.48 |
| PHOSPHORUS (mg/dl) | 3.73±0.25 | 3.38±0.44 | 3.47±0.51 | 4.12±0.70 | 3.46±0.26 |
| ALBUMIN (g/dl) | 4.37±0.32 | 4.30±0.46 | 4.37±0.55 | 4.50±0.21 | 4.36±0.35 |
| PTH (1–84) (pg/ml) | 33.7±20.4 | 21.5±10.0 | 47.3±4.2 | 23.6±9.6 | 30.8±9.3 |
| 25 VIT D (ng/ml) | 30.0±9.6 | 48.8±47.7 | 32.0±8.5 | 34.2±16.0 | 25.0±6.7 |
Maximum tolerated dose; no statistically significant differences between PTHrP 4 and PTH 2 groups (p≥.05)
Study Design
This study was designed as a standard dose-escalation, dose-finding pilot study: the primary outcome measures were safety outcomes (hypercalcemia, hypophosphatemia, hemodynamic measurements, symptoms) during a progressive dose escalation. Secondary outcome measures were serum intact PTH(1–84), bone turnover markers, tubular maximum for phosphorus (TmP/GFR), plasma 1,25(OH)2D and urinary calcium excretion measures.
Subjects initially were assigned to receive either PTH or PTHrP at 2 pmol/kg/hr, one quarter the minimal dose used in prior studies of shorter duration (15–21). A modified Fibronacci dose escalation scheme was employed such that three subjects were scheduled to receive the initial 2 pmol/kg/hr dose. If no dose-limiting toxicity (DLT, see below) occurred, three more subjects were infused at the next higher dose, 4 pmol/kg/hr, for seven days. If DLT again did not occur, the dose was further increased until DLT occurred. If DLT occurred in one of the three subjects, three more were studied at that dose. When DLT occurred in > two subjects at a given dose, the prior dose was defined as the maximal tolerated dose (MTD) or the dose was de-esclated in 1 pmol/kg/hr increments.
DLT was defined as achieving one major criterion or two minor criteria. The major criteria were defined as symptomatic orthostatic hypotension (systolic BP fall >30 mm/hg), tachycardia (pulse > 120), hypertension (systolic BP >160 mm/hg on two occasions), hypercalcemia (serum calcium ≥ 12 mg/dl), and hypophosphatemia (serum phosphorous < 1.5 mg/dl). Minor criteria included symptoms such as flushing, nausea, abdominal or muscle cramps, dizziness, lightheadedness, palpitations, etc. Subjects were asked if they were experiencing any of these or additional symptoms prior to each blood draw. If any symptoms were present, the subject was asked to grade it on a scale of 0–3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). Any symptom rated ≥ 2 was considered a significant minor criterion.
PTHrP and PTH Peptides
Human PTH(1–34) (referred to hereafter as “PTH”) and human PTHrP(1–36) (referred to hereafter as “PTHrP”) were prepared using solid phase synthesis at the W.M. Keck Peptide Synthesis Facility at Yale University and purified using preparative scale reversed-phase HPLC as described previously (15–18,23–25). Purity was defined using analytical scale RP-HPLC and mass spectrometry. Peptide was sterile-filtered and aliquotted into individual sterile vials, lyophilized and stored at −80 until the day of use. Vials were tested for sterility, peptide content, and bioactivity as described in detail previously (15–18,23–25). PTH and PTHrP were approved for use by the FDA under INDs # 60,979 and # 49,175, respectively.
Human Infusion Protocol
On the day of study, subjects were admitted to the University of Pittsburgh Clinical Translational Research Center (CTRC), and an indwelling central catheter was inserted and attached to a continuous infusion pump. For the inpatient, overnight portions of the study, a Baxter Auto Syringe A550 pump (Baxter Healthcare, Round Lake, MN) was used. The PTH or PTHrP infusions were commenced at 11:00 AM on Day 1 of the study. Every 48 hours, fresh aliquots of PTH or PTHrP were suspended in 100–200 cc of 0.9% NaCl containing 1.0 cc of the study subject’s own blood, then loaded into sterile plastic syringes and refrigerated until use. Syringes were replaced at least every 24 hours, as described previously (16–18), and the infusion was continued for seven days. On days 3 to 6 of the infusion, after 7:00 AM labs, a second AM void was obtained, and if hemodynamically stable and not significantly hypercalcemic (7:00 AM serum calcium <11.0 mg/dl), subjects were allowed to leave the hospital between 9:00 am and 3:00 pm for up to six hours. To facilitate this, subjects were switched to a miniaturized continuous infusion pump (CADD-Micro Ambulatory Infusion Pump model 5900, SIMS Deltec, Saint Paul, MN), and provided with a cellular telephone to contact the research staff should the need arise. Meals contained 15% protein, 55% carbohydrate, 30% fat, 400 mg calcium and 800 mg phosphorus/day and were prepared on the CTRC, including bag lunches in the event that subjects elected to leave the CTRC during the day time.
Measurements
Vital signs were obtained every eight hours, including supine and standing blood pressure and pulse. Blood was obtained at multiple times each day as depicted in the Figures, and as discussed below. A second morning void was also obtained each day for calcium, phosphorus and creatinine. On the last day of the study, a 24 hour urine was also obtained for calcium and creatinine. Finally, 10 days after completion of the study, subjects returned for a follow-up visit for a final blood test as depicted in the Figures.
Analyses
Serum and urine calcium, serum ionized calcium, phosphorus, creatinine, plasma 25(OH)D and serum iPTH(1–84) were measured in the University of Pittsburgh Medical Center Clinical Laboratories. Ionized calcium samples were collected aerobically in a heparin sodium tube and transported on ice for immediate analysis of whole blood on an ABL700 Series instrument which automatically corrected the sample for pH (Radiometer ABL700 Series reference manual, Radiometer, Copenhagen, Denmark, 2009). Fractional excretion of calcium and TmP/GFR were calculated as described previously (15–18,24). 1,25(OH)2D was assayed using an RIA as described (26). Amino-terminal telopeptides of procollagen 1 (P1NP), serum amino-terminal telopeptide of collagen-1 (sNTX), and carboxy-terminal telopeptide of collagen 1 (CTX) were measured using commercial kits from Orion Diagnostics RIA (intra-assay CV 4.3%, inter-assay CV 7.7%) (Espoo, Finland), Osteomark ELISA (intra-assay CV 4.2%, inter-assay CV 9.6%), (Ostex International, Seattle, WA), and Crosslaps ELISA (intra-assay CV 3.8%, inter-assay CV 8.4) (Nordic Bioscience Diagnostics, Inc., Herlev, Denmark), respectively. Bone-specific alkaline phosphatase (BSAP) was measured using commercial kits from Ostase EIA (intra-assay CV 3.6%, inter-assay CV 7.2%) (Hybritech Incorporated, Fullerton, CA, USA). The safety labs (calcium, ionized calcium, phosphorous, and creatinine) were all run immediately after collection, while aliquots were frozen and batched together at the completion of the study for all other measurements.
Statistical Methods
Frequencies and percentages were used to describe the gender distribution by peptide-dose group, while measures of central tendency and dispersion were used to describe continuous type descriptors and the baseline values of endpoints by group. To assess for possible group imbalances, groups were compared using the F-test from an analysis of variance (or Kruskal-Wallis test, if non-normally distributed) for continuous type descriptors and the baseline values of endpoints (e.g., BMI, serum calcium, P1NP). The differences in the change in the five groups over time on serum calcium; fractional calcium excretion; plasma 1,25(OH)2D; serum phosphorous; TmP/GFR; endogenous PTH(1–84); and the percentage change in biomarkers (CTX, NTX, BSAP, serum osteocalcin, and P1NP) relative to baseline values were analyzed using repeated measures analysis with a linear mixed modeling approach that included fixed between-subjects effects for the peptide-dose group, fixed within-subjects effects for time, and their two way interactions. Subject was treated as a random effect. The PROC MIXED procedure in SAS (version 9.2, SAS Institute, Cary, NC) was employed for fitting these linear mixed models. The level of statistical significance was set at 0.05 (two-tailed). Repeated measures analyses via linear mixed modeling were also performed focusing only the two MTD’s, PTHrP 4 and PTH 2 pmol/kg/hr.
Results
Baseline Demographics
The demographics of the study subjects are summarized in Table 1. As can be seen, there were 13 male and 9 female participants with a mean age of 28 years. There were no significant differences in age, BMI, or other parameters between the groups at the two MTDs (defined below).
Serum Total and Ionized Calcium
The mean total serum calcium in each group is shown in Fig. 1. The mean baseline calcium was 9.65 mg/dl ± 0.07 mg/dl. Among the subjects receiving PTH at a dose of 2 pmol/kg/hr, five completed the study, with one dropping out on Day 2 because of infiltration of their intravenous line. The 2 pmol/kg/hr dose yielded a statistically significant increase in serum calcium (mean serum calcium during the infusion = 10.0 mg/dl) (p < 0.0001). DLT did not occur, and per protocol, the dose was therefore escalated to 4 pmol/kg/hr. Subjects receiving this dose displayed a similar calcemic response (10.0 mg/dl), but in this group, DLT occurred in two subjects: one developed a serum calcium > 12.0 mg/dl on Day 3 (PTH was terminated) another developed tachycardia (pulse = 148 bpm), but was able to complete the study. Based on these considerations, a dose of 2 pmol/kg/hr of hPTH(1–34) was identified as the MTD.
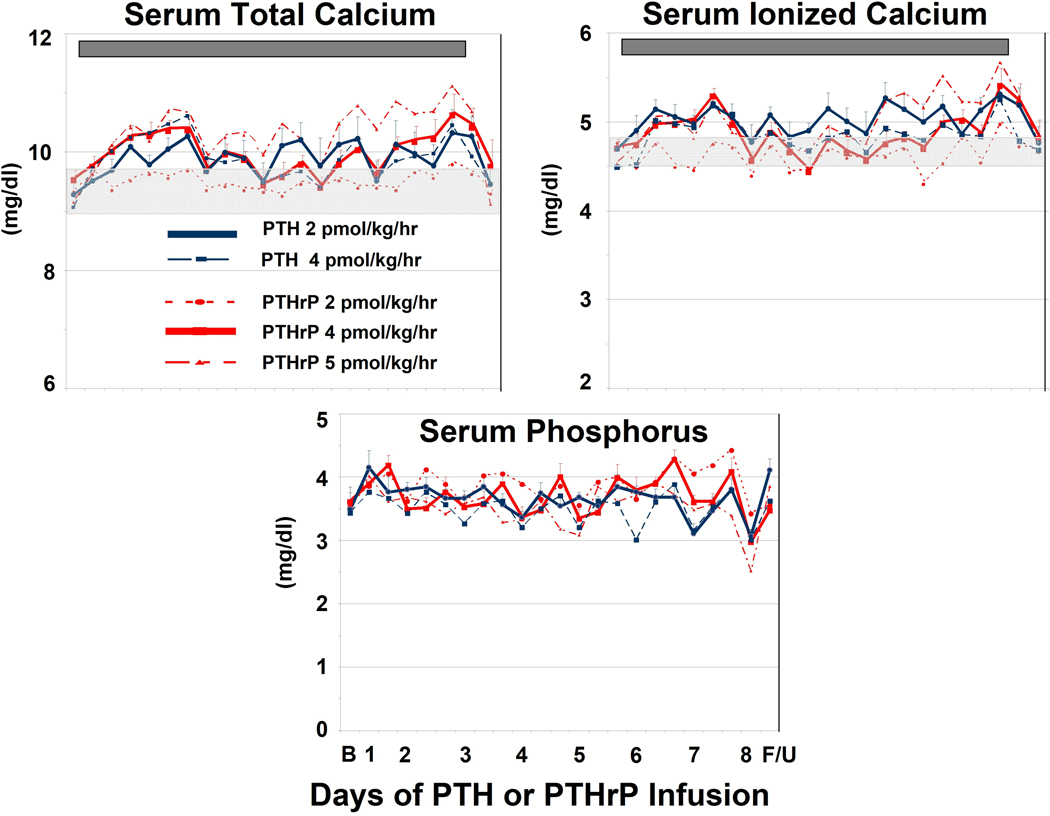
Figure 1. Changes in Serum Calcium, Phosphorus.
Total Serum Calcium (Upper Left). The legend describes the line colors and symbols for the five patient groups. The light grey bar in the middle of the panel indicates the baseline calcium concentration (9.65 ± 0.07 mg/dl) (mean ± SD). The dark grey bar in the top of the panel indicates the duration of the PTH and PTHrP infusions. Vertical bars indicate SEM. There was no statistical difference among the groups at baseline (p = 0.3) but there was a statistically significant increase over time in the 2 pmol/kg/hr PTH and 4 pmol/kg/hr PTHrP groups (p <0.0001). There was no statistically significant difference in calcemic response between these two groups. Ionized Serum Calcium. (Upper Right). There was no difference among the groups at baseline, but there was a statistically significant increase in time for both the 2 pmol/kg/hr PTH and 4 pmol/kg/hr PTHrP groups (p <0.0001). Serum Phosphorus (Lower Panel). There were no significant differences in serum phosphorus at baseline, nor important changes during the infusions.
Among subjects receiving PTHrP, the 2 pmol/kg/hr dose had little effect on total serum calcium (Fig. 1), and produced no DLTs. Per protocol, the dose was escalated to 4 pmol/kg/hr. This group developed a statistically significant (p < 0.0001) calcemic response (mean serum calcium during the infusion = 10.0 mg/dl), comparable to the 2 pmol/kg/hr dose of PTH. A total of six subjects at 4 pmol/kg/hr (three initially and three after the 5 pmol group was tested) completed the study without DLT. Accordingly, per protocol, after the initial three subjects, the PTHrP dose was escalated to 6 pmol/kg/hr, in two additional subjects. Among these two, one had a sustained serum calcium approaching the 12.0 mg/dl level and one developed DLT by minor criteria. Since the required three subjects were not achieved at this dose, these subjects were excluded from further analysis. Accordingly, an additional group was recruited to receive PTHrP at a dose of 5 pmol/kg/hr. Among these three subjects, one reached DLT based on minor criteria and a second was terminated on Day 4 for sustained hypertension. An additional three subjects were then studied at 4 pmol/kg/hr, which was defined as the MTD for PTHrP.
Fig. 1 also displays the ionized serum calcium and phosphorus. As can be seen, the ionized calcium results are similar to those observed for total serum calcium, and support selection of 2 pmol/kg/hr of PTH and 4 pmol/kg/hr as the MTDs (p < 0.0001). Infusion of PTHrP and PTH at the doses administered had no significant effect on serum phosphorus (Fig 1) or creatinine (data not shown).
Calcitropic Hormones
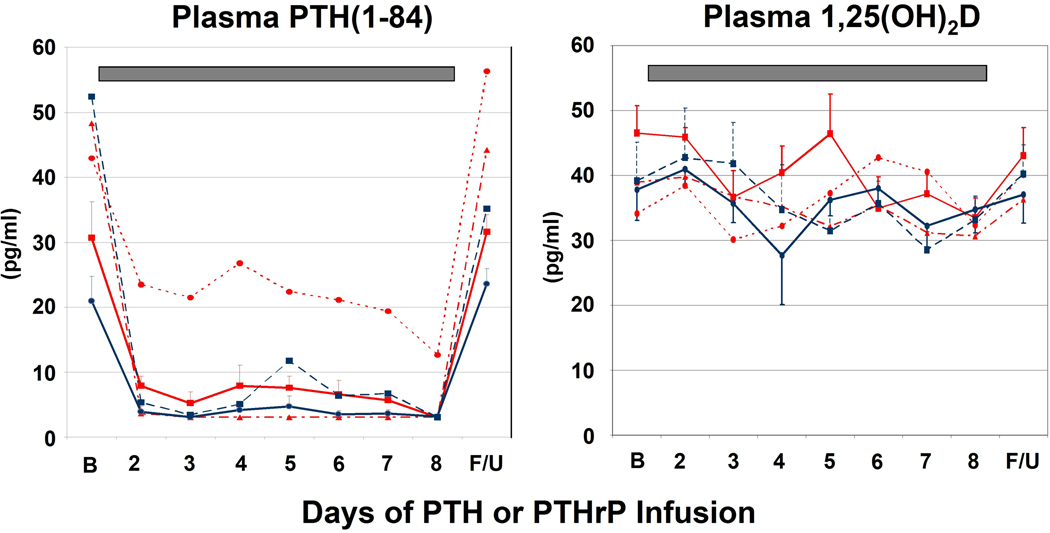
Endogenous serum PTH(1–84) declined significantly in all groups (Fig. 2) even the sub-hypercalcemic (2 pmol/kg/hr) dose of PTHrP.
Figure 2. Changes in Endogenous PTH(1–84) and Plasma 1,25(OH)2D.
See Figure 1 for explanation of bar, line and symbol colors. Endogenous serum PTH declined rapidly and robustly during the PTH and PTHrP infusions, even in the PTHrP 2 pmol/kg/hr group in which serum calcium did not appreciably change. Plasma 1,25(OH)2D did not increase in response to the infusions, and actually decreased by Day 8 for the PTHrP 4 pmol/kg/hr group (p = 0.01).
Although prior studies have demonstrated that infusion at higher doses of PTH (8–16 pmol/kg/hr) and PTHrP (8–28 pmol/kg/hr) induce marked increases in plasma 1,25(OH)2D (15–18,21,23), the current, lower doses produced no change in plasma 1,25(OH)2D for PTH or an actual decline by Day 8 for PTHrP (p = 0.01) (Fig. 2), despite clear changes in serum calcium and other measures (see below).
Renal Mineral Handling
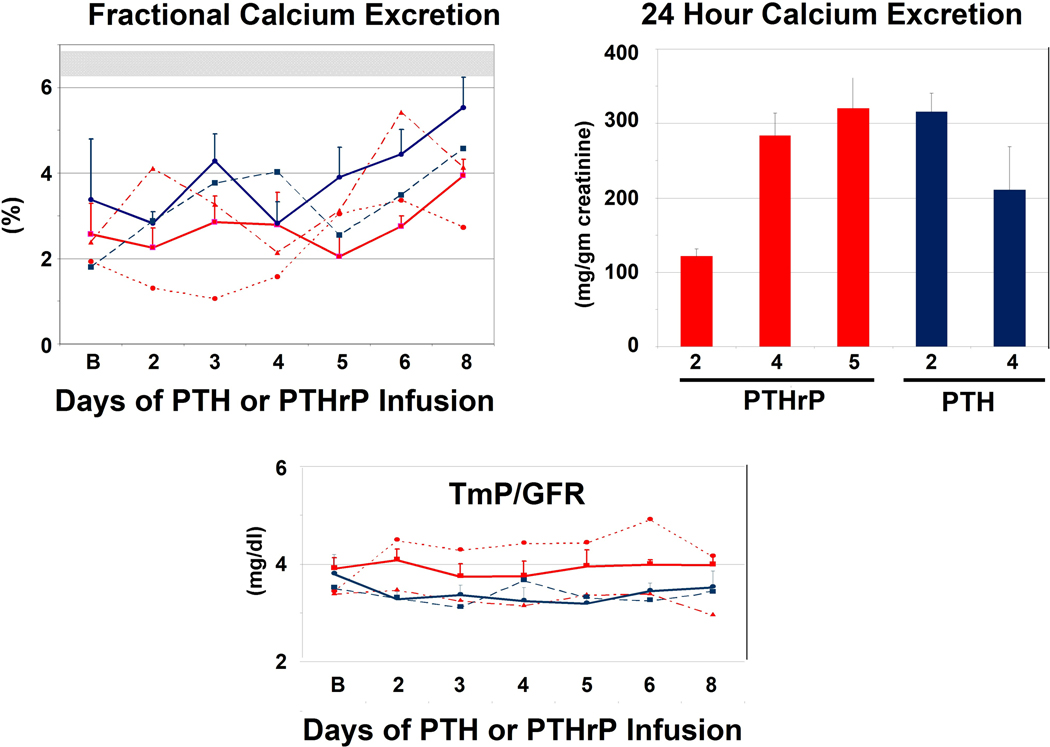
Fractional calcium excretion increased significantly in the 2 pmol/kg/hr PTH and 4 pmol/kg/hr PTHrP groups (p = 0.002) (Fig. 3), reflecting increases in total and ionized serum calcium, and therefore the filtered load of serum calcium. Twenty-four hour urinary calcium excretion (Fig. 3) was normal in the subjects receiving 2 pmol/kg/hr PTHrP. In contrast, it was at the upper limits of normal and comparable in the other groups, as expected by their increases in serum calcium and reductions in PTH(1–84). As with the serum phosphorus values, but in contrast with higher PTH and PTHrP doses in prior studies, the TmP/GFR did not change with these low doses of PTH and PTHrP (p = 0.61) (Fig. 3).
Figure 3. Changes in Renal Calcium and Phosphorus Excretion.
See Figure 1 for explanation of bar, line and symbol colors. Fractional Calcium Excretion (Upper Left). The upper light bar represents the fractional calcium excretion (mean ±SD = 6.5) in response to a calcium infusion in which the serum calcium was “clamped” at 10.3 mg/dl, and endogenous PTH suppressed by infusion of calcium chloride (16). Fractional calcium excretion increased significantly (p = 0.002) during the infusion, reflecting the increase in filtered calcium load, but was far below the 6.5% range observed with similar degrees of hypercalcemia and filtered load observed with the calcium clamp, revealing comparable anti-calciuric effects of PTH and PTHrP even at these low doses. 24 Hour Calcium Excretion (Upper Right). 24 hour calcium excretion, expressed as calcium per gm of creatinine, obtained at the conclusion of the study approached the hypercalciuric range. The mean 24-hour urine creatinine in the two MTD groups were 1.06 ± 0.3 and 1.49 ± 0.1 for the PTH and PTHrP groups, respectively. TmP/GFR (Bottom Panel). There were no changes in TmP/GFR, indicating that these low doses of PTH and PTHrP did not induce phosphaturia.
Bone Turnover
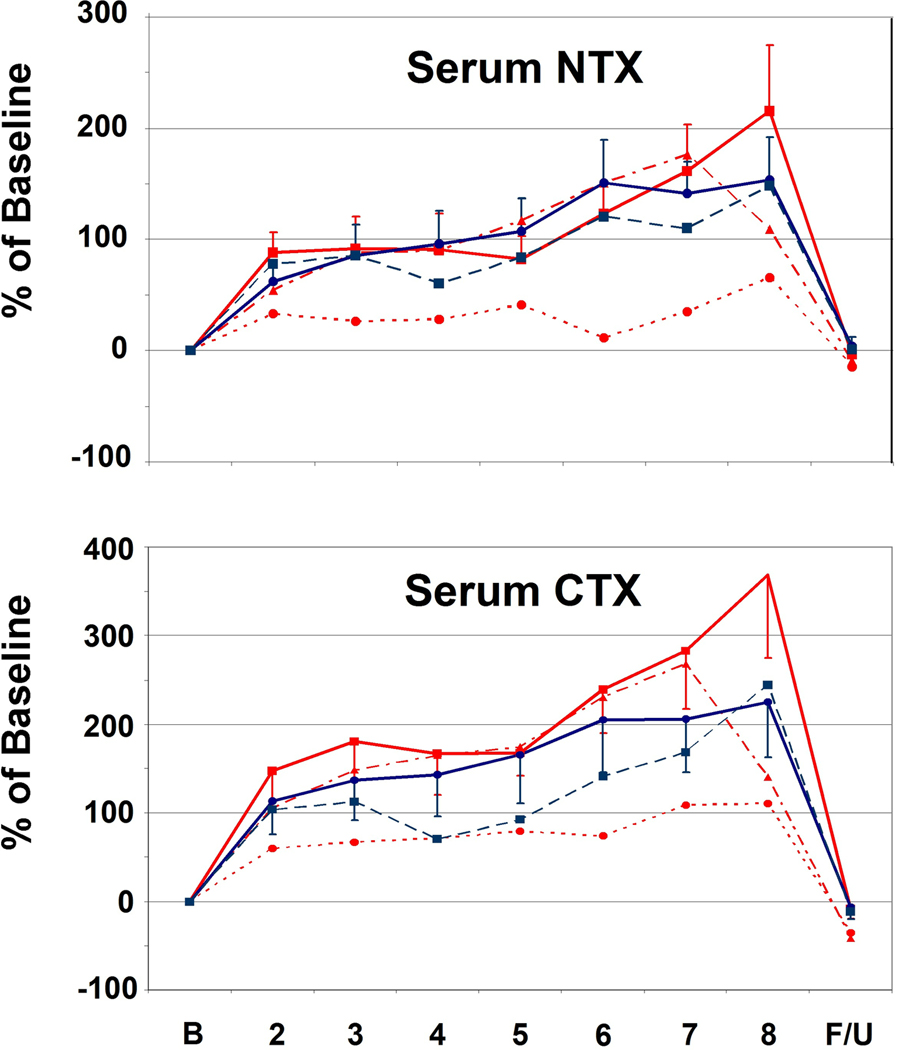
With respect to bone resorption, both serum CTX and NTX increased significantly in the face of both PTH and PTHrP infusion, even with the lowest, sub-calcemic, 2 pmol/kg/hr dose of PTHrP (Fig. 4A). Also, bone resorption reversed rapidly, returning to normal when PTH and PTHrP infusions were discontinued.
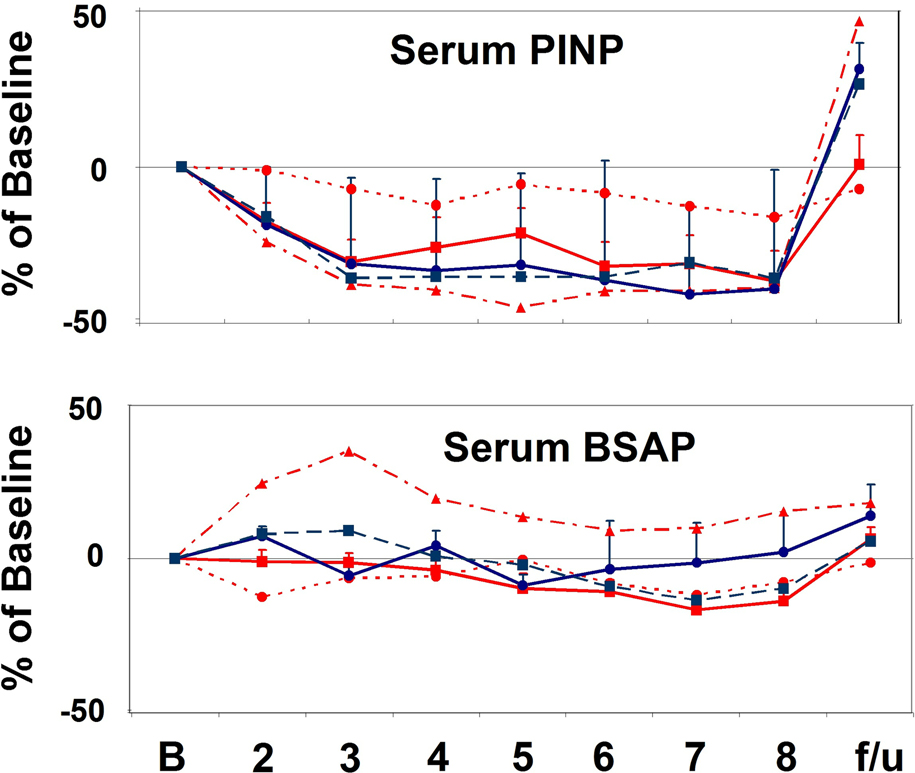
Figure 4.
A. Changes in Bone Resorption Markers. See Figure 1 for explanation of bar, line and symbol colors. Both serum NTX and CTX increased rapidly, and significantly in response to low-dose PTH and PTHrP infusion (p < 0.008 and p < 0.0001, respectively), and declined abruptly with cessation of the infusions. B. Changes in Bone Formation Markers. Serum P1NP was suppressed and remained so throughout the 7-day infusions(p< 0.0001), and promptly rebounded with cessation of the infusions (p=0.01). BSAP also declined significantly during the course of the study (p = 0.0001), but less robustly than for P1NP. The significant decline is mostly attributable to the PTHrP 4 pmol/kg/hr group (P=0.001).
In contrast, bone formation, as assessed using serum P1NP (Fig. 4B), declined and remained suppressed for the entire infusion (p = 0.0001). At the MTD’s for the two peptides, BSAP also declined significantly during the course of the study (p = 0.0001) (Fig. 4B), but less robustly than for P1NP, likely reflecting its lower sensitivity as a measure of bone formation. The decline in BSAP was significant in the PTHrP 4 pmol/kg/hr group (p = 0.001) but not the PTH 2 pmol/kg/hr group when looking at the groups individually. When the PTH or PTHrP infusions were discontinued, P1NP rapidly returned to normal and even overshot the baseline (p = 0.01).
Discussion
Important differences between the clinical HPT and HHM syndromes were defined some 30 years ago, but the reasons for these differences remain incompletely understood. The mechanisms that lead to bone loss and restitution associated with lactation are also incompletely understood. One obstacle to fully understanding these syndromes is that rodent models do not faithfully mimic the human syndromes in all regards. Additionally, human infusion studies with PTH and PTHrP have been relatively brief, lasting only 6–48 hours (15–22). This contrasts to primary HPT, to HHM, and to normal lactation which typically last for months or years. Since PTH1R modulation, recycling, signaling and expression as well as physiological and pathophysiological regulation of renal and skeletal responses to PTH and PTHrP likely take far longer than 6–48 hours to achieve steady state, we sought to develop longer models of PTH and PTHrP infusion into healthy human volunteers that might effectively and faithfully mimic human HPT, HHM and possibly lactation, and also do so in a way that was also safe and well tolerated. To the best of our knowledge, prior infusion studies of this duration have never been performed in humans.
The first necessary steps in developing such models would be to define the infused dose ranges of PTH and PTHrP that would be safe but effective in modeling HPT and HHM, and to develop a delivery system that is compatible with subacute PTH and/or PTHrP infusions. In this report, we describe such systems and safe infused dose ranges. We also define, for this infusion model, the lower dosing threshold of PTH and PTHrP for driving increases in circulating 1,25(OH)2D in humans with intact parathyroids. Importantly, we demonstrate that a seven day infusion of PTH and PTHrP continues to suppress P1NP - arguably the best marker for bone formation - despite markedly activating bone resorption. Finally, we demonstrate that cessation of PTH or PTH infusion leads to a rapid increase in bone formation, as demonstrated by a remarkable rebound, and even overshoot, in circulating P1NP.
The Optimal Peptide Doses
The lowest dose of PTH or PTHrP employed in earlier studies was 8 pmol/kg/hr over 6–48 hours (15–22), (~ 25 ug/day), of PTH (1–34) or PTHrP(1–36). Since earlier studies suggested that serum calcium was on the ascent during 6–48 hour infusions of PTH or PTHrP at the 8 pmol/kg/hr dose (15–18), we were concerned that week-long infusions at this dose might result in dangerous hypercalcemia. Accordingly, we selected a lower dose, 2 pmol/kg/hr, as a starting dose for this pilot study. Surprisingly, this proved to be the MTD for PTH.
PTHrP at 2 pmol/kg/hr produced measurable effects on endogenous PTH(1–84) and bone resorption, but marginal effects on serum calcium. The decline in PTH(1–84) may reflect a small increase in serum calcium or potential inhibitory effects on PTH secretion (27). An escalated dose of 4 pmol/kg/hr resulted in increases in serum calcium that were both safe and comparable to those observed with 2 pmol/kg/hr of PTH. Higher doses were associated with significant hypercalcemia and/or adverse effects. Four pmol/kg/hr was therefore selected as the MTD for PTHrP.
Thus, as noted in earlier studies (15–18,23), PTHrP appeared to be slightly weaker than PTH(1–34) in inducing hypercalcemia. This is not likely due to differences in bioactivity or stability between the two peptides, since SaOS2 adenylyl cyclase bioassays and peptide content confirmed their equivalence, and since measurement of circulating hPTH (1–34) and hPTHrP (1–36) concentrations with the best available assays suggested that circulating levels of PTHrP if anything, exceed those of PTH in this model (18). Thus, this calcemic dose difference presumably reflects intrinsic differences in how the two peptides interact with the PTH1R in their various target organs, as suggested by Dean (4). Finally, with regard to the pattern of hypercalcemia, it is interesting to note that the serum calcium consistently appeared to decline during the middle portion of the study. This did not correlate with inpatient versus outpatient status or other variables, and remains unexplained.
Differential Activation Thresholds for Hypercalcemia, Phosphaturia, 1,25(OH)2D and Bone Resorption in Humans
Prior studies in humans, using supraphysiological doses of PTH and PTHrP demonstrate that PTH and PTHrP are both capable of activating bone resorption, phosphaturia, renal calcium retention, and 1,25(OH)2D production (15–23). However, none of these studies have carefully explored the minimal dose thresholds required to activate these responses. Here, we show that, in this model, the minimal calcemic dose of PTHrP is 4 pmol/kg/hr, and the minimal calcemic dose of PTH is less than 2 pmol/kg/hr. Interestingly, all of these doses activated osteoclastic bone resorption, as assessed by sCTX and sNTX, but none induced phosphaturia or measurable increases in circulating 1,25(OH)2D. (It is important to note that some of the second morning urine samples were not fasting. This increases the variability in the TmP/GFR calculations and may have diminished the ability to measure significant changes in this small study.) With these qualifications, these studies therefore suggest that the thresholds for induction of phosphaturia and 1,25(OH)2D production are different than those for activation of bone resorption and induction of hypercalcemia using this model.
Suppression of Bone Formation
One of the principal goals of the study was to determine whether bone formation, which had declined in earlier, briefer studies, would eventually reverse, and increase, as occurs in HPT, or would continue to decline and remain suppressed, as occurs in HHM. We observed that even when administered for as long as seven days, bone formation remained suppressed, and showed no signs of becoming activated, as would be expected in HPT.
These observations support several possible interpretations. It is possible to argue that if the PTH or PTHrP infusions had continued for a longer period of time, bone formation would certainly have rebounded. In support of this hypothesis, primary HPT, with its characteristic increase in bone formation, has been present for many months or years before it is diagnosed (12,13), and even intermittent administration of PTH as a treatment of osteoporosis requires two weeks to observe an increase in bone formation markers (28,29).
On the other hand, many studies in animals and in vitro demonstrate that continuous exposure of bone-derived mesenchymal stem cells to PTH or PTHrP causes an arrest in differentiation at a pre-osteoblast stage (30–34) that lasts at least three weeks or longer (33) and suggest that the characteristic “marrow fibrosis” observed in HPT may represent a partial arrest of osteoblast differentiation (30,34). More specifically, Turner et al have suggested that continuous exposure of the skeleton to PTH recruits bone mesenchymal stem cells into the osteoblast lineage, but arrests them at the pre-osteoblast stage where they are morphologically indistinguishable from marrow fibroblasts (30,34). Thus, continuous exposure of the skeleton to PTH creates the appearance of marrow fibrosis with cells that may in fact be pre-osteoblasts.
Rebound of Bone Formation on Withdrawal of PTH or PTHrP
The largest surprise in the current study was the rapid and profound rebound, indeed overshoot, of bone formation (P1NP) following cessation of the PTH and PTHrP infusions. This observation is entirely reminiscent of the rebound osteoblast differentiation, function and mineralization following withdrawal of continuously administered PTH or PTHrP observed in vitro by others (31–33) suggesting that continuous PTH or PTHrP exposure recruits osteoblast precursors and induces their partial differentiation, but prevents them from completing their entire differentiation program. It also may suggest that the rapid increase in bone density that follows parathyroidectomy in HPT (12–14) may reflect a combination not only of mineralization of increased pre-existing osteoid, but also a component of release of pre-osteoblasts, such that they complete their differentiation program, and thereby increase new bone formation rates. Finally, Fig. 4 is very reminiscent of events at the end of lactation, when bone resorption abruptly ceases, and bone mass rapidly accumulates in a matter of a few months to pre-lactation levels (6,11). We hypothesize that in lactation, continuous PTHrP production by the mammary gland drives resorption, and recruits osteoblast precursors, but prevents them from completely differentiating. This allows uncoupled bone resorption during lactation as a source of calcium for lactational milk production. In this scenario, when lactation ceases, systemic PTHrP production ceases, and resorption abruptly declines, suddenly freeing pre-existing osteoblast precursors from arrest, so that they can robustly re-mineralize the maternal skeleton, as has been so well demonstrated (6,11,30–33).
Fidelity as a Model of HHM
The PTHrP infusion studies provide an almost perfect model of HHM, as it reproduces all of the features of HHM: it causes bone resorption, suppression of bone formation, inhibition of calcium excretion, phosphaturia and suppression of endogenous PTH. Interestingly, while 1,25(OH)2D concentrations are markedly reduced in HHM, they are also declining here in response to PTHrP, although they remain in the normal range. The reasons for this quantitative difference may be that additional factors {e.g., more severe hypercalcemia, secretion of tumor-derived cytokines, greater chronicity, or PTHrP secretory species other than PTHrP(1–36)} may suppress renal 1-α-hydroxylase in HHM, or that it may require longer than one week for 1,25(OH)2D concentrations to decline. Clarifying these issues will be of interest for future studies.
Fidelity as a Model of Lactation
The PTHrP infusion studies also provide a tool with which to partially model human lactation. Some features of this model are identical to those described in rodent and human lactation (6): continuous production and secretion of low levels of PTHrP into the maternal circulation, activation of bone resorption, prevention of renal calcium losses through PTHrP-mediated anti-calciuric effects, and mobilization of large amounts of calcium from the skeleton to serve as a source for milk production and neonatal skeletal mineralization. On the other hand, there are a number of differences between this model and lactation. First, the pregnancy-associated rise in estrogen and progesterone, and their sudden fluxes that accompany parturition are absent in this model. Second, these subjects lack milk production (and thus a “sink” for the excess skeletal calcium removal). This may possibly explain why hypercalcemia did develop here, but does not occur in lactation. This hypercalcemia and high filtered load, despite an only limited change in FECa, likely account for the elevated 24 urine calcium excretion in this model, as compared to the hypocalciuria typical lactation (6). (Parenthetically, it would have been ideal to have been ideal to have collected a baseline 24 hour urine for calcium in addition to the final 24 hour collection). In addition, P1NP is decreased in this model as compared to the variable increases or decreases in formation markers reported by others during human lactation (35–38). Third, serum phosphorus increases in lactation (6), but did not in our system, perhaps reflecting the fact that prolactin is increased in lactation but not in this model. And fourth, most importantly, precisely what happens to bone formation in humans during and at the cessation of lactation remains enigmatic at present. Bone biopsies to elucidate these issues will likely never be performed in human lactation. However, the kind of modeling described here may permit the development of human experimental paradigms that will clarify whether bone formation is reduced and/or arrested in lactation, as suggested here, or increased as suggested by other studies (35–38).
Fidelity as a Model of HPT
In some ways, this approach models primary HPT: mild hypercalcemia, bone resorption, increases in renal calcium retention contributing to hypercalcemia, but with net hypercalciuria, reflecting the increased renal calcium filtered load. These features mimic mild primary HPT, and over time would be predicted to lead to skeletal mineral loss, also characteristic of some patients with HPT. At higher doses of PTH, 1,25(OH)2D is increased and the TmP/GFR decreases (18) but these did not occur in this lower dose model. In contrast to these similarities with HPT, there is another striking difference: bone formation is regularly observed to be increased in HPT (12,13,39), whereas in the current model, bone formation is reduced with seven days of continuous PTH infusion (Fig. 5). As noted above for PTHrP, it is possible that the seven-day PTH infusion is simply not a sufficient duration to activate bone formation, since it is known that increases in bone formation markers requires some two weeks of daily injections with PTH(1–34) (28,29). On the other hand, in HPT, PTH is secreted from its neuroendocrine secretory granules in a pulsatile manner (40–42), in contrast to the continuous infusion model here, or the continuous secretion of PTHrP in lactation and in HHM, by mammary epithelium and cancers which lack neuroendocrine secretory granules or machinery. Importantly, the tools developed here will permit future, longer and also pulsatile vs. intermittent studies to clarify these issues and develop better models of HPT.
This study has a number of limitations. First, although the number of subjects studied was relatively large for this class of study, the numbers of subjects in the individual dose groups was relatively small. Despite this limitation, the long duration of the study with sampling of multiple time points allowed for attaining robust statistical significance. Also, with the definition of appropriate and safe doses of PTH and PTHrP over prolonged time periods described herein, power analyses can now be performed for future studies designed to address some of the questions alluded to above.
Second, this study was largely limited to Caucasians and included no African-Americans. This was intentional, since it is well known that bone turnover, renal calcium handling, PTH concentrations and vitamin D metabolism all differ in African-Americans as compared to Caucasians (43,44). Having developed the continuous infusion methodology, and having defined the requisite doses, duration, and group sizes, it is now be possible to pursue similar studies in African-Americans to explore the biology underlying the ethnic differences noted above.
Third, we examined only a single time point for follow-up: 10 days after discontinuation of the PTH and PTHrP infusions. We do not know whether indices of bone formation rose earlier and were declining, or would peak at a later time point, nor how long the apparent increase in formation might persist. This will need to be ascertained in future studies.
Fourth, while we have taken all standard precautions to ensure and document stability of the peptides as infused (15–18), in the current absence of sensitive and specific assays for PTHrP(1–36) and hPTH(1–34), we could not measure the circulating concentrations of each peptide at the multiple timepoints examined. The consistent increases in serum calcium and bone resorption markers in this and prior studies (15–18) support the likelihood that the peptide are stable and active as administered.
And fifth, this study did not include a control saline infusion group, raising the question as to whether the changes in PTH(1–84), markers of bone turnover and lack of change in 1,25(OH)2D or TmP/GFR might reflect adaptive responses to the study diet. This seems unlikely, since in our prior study which did include a saline-infused control group on the same diet (16), there were no changes in PTH(1–84) or TmP/GFR (16). Also, the low calcium (400 mg) study diet, if anything, might have increased PTH(1–84) and therefore reduced TmP/GFR. It also seems unlikely that a 400 mg calcium/800 mg phosphorus diet would induce a two-three-fold increase in resorption markers and suppress bone formation by 40% in the face of a decrease in endogenous PTH(1–84).
In summary, we have defined the dose responses and safety profiles of PTH and PTHrP infusion in humans with intact parathyroids, with 2 pmol/kg/hr for hPTH(1–34) and 4 pmol/kg/hr for hPTHrP(1–36) being optimal. We have shown that these chronic studies can serve as excellent models for elucidating the physiology and pathophysiology of HHM, and at least partially model events in human HPT as well as lactational bone loss and its subsequent recovery. We have made the surprising observation that continuous exposure of the human skeleton to PTH or PTHrP for one week leads to sustained, but reversible suppression of bone formation. We have also provided the first clear evidence of a rebound increase in bone formation in human skeletons exposed continuously to PTH or PTHrP. These techniques and approaches will be useful for deepening and expanding our understanding of the biology of human lactation and the pathophysiology of HPT and HHM.
Acknowledgements
We wish to thank the staff of the University of Pittsburgh CTRC and CTSI who were essential to this study. We thank Dr. William Crowley at the Massachusetts General Hospital in Boston for many helpful discussions in the design phase of these studies. We thank Dr. Mohamed Virgi in the University of Pittsburgh Medical Center for analytical advice and support. We also wish to thank the members of our DSMB, including Drs. Elizabeth Shane, Susan Greenspan, David Roodman and Steven Wisniewski. This work was funded by NIH grants R-01 DK 073039 and DK 51081, and CTSA UL1 RR024153 and M-01 RR000056.
Footnotes
Disclosure
Dr. Stewart is a member of Osteotrophin LLC. Dr. Hollis is a consultant for DiaSorin Corp. Dr. Horwitz is a consultant for Merck. All other authors have no disclosures.
Contributor Information
Mara J. Horwitz, Email: horwitz@pitt.edu.
Mary Beth Tedesco, Email: Tedesco@pitt.edu.
Susan M. Sereika, Email: ssereika@pitt.edu.
Linda Prebehala, Email: lprebeh@pitt.edu.
Caren M. Gundberg, Email: caren.gundberg@yale.edu.
Bruce W. Hollis, Email: hollisb@musc.edu.
Alessandro Bisello, Email: alb138@pitt.edu.
Adolfo Garcia-Ocaña, Email: ocana@pitt.edu.
Raquel M. Carneiro, Email: raquelmorais@hotmail.com.
Andrew F. Stewart, Email: stewarta@pitt.edu.
References
- 1.Nissenson RA, Juppner H. Parathyroid Hormone. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Seventh Edition. Washington D.C.: American Society for Bone and Mineral Research; 2008. pp. 123–126. [Google Scholar]
- 2.Wu T, Vasavada R, Yang K, Massfelder T, Ganz M, Abbas SK, Care AD, Stewart AF. Structural and physiologic characterization of the mid-region secretory form of PTHrP. J Biol Chem. 1996;271:24371–24381. doi: 10.1074/jbc.271.40.24371. PubMed ID: 8798692. [DOI] [PubMed] [Google Scholar]
- 3.Juppner H, Gardella TJ, Brown EM, Kronenberg HM, Potts JT. Parathyroid hormone and parathyroid hormone-related peptide in the regulation of calcium homeostasis and bone development. In: DeGroot L, editor. Endocrinology. Fifth Edition. Philadelphia: Elsevier Saunders; 2006. pp. 1377–1417. [Google Scholar]
- 4.Dean T, Vilardaga J-P, Potts JT, Gardella TJ. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endo. 2008;22:156–166. doi: 10.1210/me.2007-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wysolmerski JJ. Parathyroid hormone-related protein. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Seventh Edition. Washington D.C: American Society for Bone and Mineral Research; 2008. pp. 127–133. [Google Scholar]
- 6.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18:832–872. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 7.Stewart AF, Broadus AE. Malignancy-associated hypercalcemia. In: DeGroot L, Jameson LJ, editors. Endocrinology. Fifth edition. Philadelphia: W.B. Saunders; 2006. pp. 1555–1565. [Google Scholar]
- 8.Stewart AF, Horst R, Deftos LJ, Cadman EC, Lang R, Broadus AE. Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and non-humoral groups. N Engl J Med. 1980;303:1377–1383. doi: 10.1056/NEJM198012113032401. PubMed ID: 6253785. [DOI] [PubMed] [Google Scholar]
- 9.Stewart AF, Vignery A, Silvergate A, Ravin ND, LiVolsi V, Broadus AE, Baron R. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J Clin Endo Metab. 1982;55:219–227. doi: 10.1210/jcem-55-2-219. PubMed ID: 7085851. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama K, Fukumoto S, Takeda S, Takeuchi Y, Ishikawa T, Miura M, Hata K, Hane M, Tamura Y, Tanaka Y, Kitaoka M, Obara T, Ogata E, Matsumoto T. Differences in bone and vitamin D metabolism between primary hyperparathyroidism and malignancy-associated hypercalcemia. J Clin Endocrinol Metab. 1996;81:607–611. doi: 10.1210/jcem.81.2.8636276. [DOI] [PubMed] [Google Scholar]
- 11.Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. N Engl J Med. 1997;337:523–528. doi: 10.1056/NEJM199708213370803. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg SJ, Bilezikian JP. Primary Hyperparathyroidism. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Seventh Edition. Washington D.C.: American Society for Bone and Mineral Research; 2008. pp. 302–306. [Google Scholar]
- 13.Silverberg SJ, Bilezikian JP. Primary Hyperparathyroidism. In: DeGroot L, editor. Endocrinology. Fifth Edition. Philadelphia: Elsevier Saunders; 2006. pp. 1533–1554. [Google Scholar]
- 14.Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezkian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 2000;341:1249–1255. doi: 10.1056/NEJM199910213411701. PMID 10528034. [DOI] [PubMed] [Google Scholar]
- 15.Everhart-Caye M, Inzucchi SE, Guinness-Henry J, Mitnick MA, Stewart AF. Parathyroid hormone-related protein(1–36) is equipotent with parathyroid hormone(1–34) in humans. J Clin Endocrinol Metab. 1996;81:199–208. doi: 10.1210/jcem.81.1.8550752. PubMed ID: 8550752. [DOI] [PubMed] [Google Scholar]
- 16.Syed MA, Horwitz MJ, Tedesco MB, Garcia-Ocaña A, Wisniewski SR, Stewart AF. Parathyroid hormone-related protein (1–36) stimulates renal tubular calcium reabsorption in normal human volunteers: implications for the pathogenesis of humoral hypercalcemia of malignancy. J Clin Endocrinol Metab. 2001;86:1525–1531. doi: 10.1210/jcem.86.4.7406. PubMed ID: 11297578. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz MJ, Tedesco MB, Sereika S, Hollis B, Garcia-Ocaña A, Stewart AF. Direct comparison of sustained infusion of hPTHrP(1–36) versus hPTH(1–34) on serum calcium, plasma 1,25(OH)2 vitamin D concentrations and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab. 2003;88:1603–1609. doi: 10.1210/jc.2002-020773. PubMed ID: 12679445. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz MJ, Tedesco MB, Sereika SM, Syed MA, Garcia-Ocaña A, Bisello A, Hollis BW, Rosen CJ, Wysolmerski JJ, Dann P, Gundberg CM, Stewart AF. Continuous infusion of parathyroid hormone versus parathyroid hormone-related protein in humans: discordant effects on 1,25(OH)2 vitamin D and prolonged suppression of bone formation. J Bone Min Res. 2005;20:1792–1803. doi: 10.1359/JBMR.050602. PubMed ID: 16160737. [DOI] [PubMed] [Google Scholar]
- 19.Simon LS, Slovik Dm, Neer RM, Krane SM. Changes in serum levels of type I and III procollagen extension peptides during infusion of human parathyroid hormone fragment (1–34) J Bone Miner Res. 1988;3(2):241–246. doi: 10.1002/jbmr.5650030218. [DOI] [PubMed] [Google Scholar]
- 20.Leder BZ, Smith MR, Fallon MA, Lee M-LT, Finkelstein JS. Effects of gonadal steroid suppression on skeletal sensitivity to parathyroid hormone in men. J Clin Endocrinol Metab. 2001;86:511–516. doi: 10.1210/jcem.86.2.7177. [DOI] [PubMed] [Google Scholar]
- 21.Cosman F, Shen V, Fang X, Seibel M, Ratcliffe A, Lindsay R. Estrogen protection against bone-resorbing effects of parathyroid hormone infusion: assessment by use of biochemical markers. Ann Int Med. 1993;118:337–343. doi: 10.7326/0003-4819-118-5-199303010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Slovik DM, Adams JS, Neer RM, Holick MF, Potts JT. Deficient production of 1,25-Dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981;305:372–374. doi: 10.1056/NEJM198108133050704. [DOI] [PubMed] [Google Scholar]
- 23.Stewart A, Mangin M, Wu T, Goumas D, Insogna KL, Burtis WJ, Broadus AE. A synthetic human parathyroid hormone-like protein stimulates bone resorption and causes hypercalcemia in rats. J Clin Invest. 1988;81:596–600. doi: 10.1172/JCI113358. PubMed ID: 3339131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry JG, Mitnick MA, Dann PR, Stewart AF. Parathyroid hormone-related protein(1–36) is biologically active when administered subcutaneously to humans. J Clin Endocrinol Metab. 1997;82:900–906. doi: 10.1210/jcem.82.3.3811. PubMed ID: 9062504. [DOI] [PubMed] [Google Scholar]
- 25.Plotkin H, Gundberg C, Mitnick M, Stewart AF. Dissociation of bone formation from resorption during two-week treatment with hPTHrP(1–36) in humans: potential as an anabolic therapy for osteoporosis. J Clin Endocrinol Metab. 1998;83:2786–2791. doi: 10.1210/jcem.83.8.5047. PubMed ID: 9709948. [DOI] [PubMed] [Google Scholar]
- 26.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. Quantification of circulating 1,25(OH)2D by RIA with an 125-I-labled tracer. Clin Chem. 1996;42:586–592. [PubMed] [Google Scholar]
- 27.Cosman F, Shen V, Herrington B, Lindsay R. Response of the parathyroid gland to infusion of human parathyroid hormone (1–34): demonstration of suppression of endogenous secretion using immunoradiometric intact PTH-(1–84) assay. J Clin Endocrinol and Metab. 1991;73:1345–1351. doi: 10.1210/jcem-73-6-1345. [DOI] [PubMed] [Google Scholar]
- 28.Cosman F, Greenspan SL. Parathyroid Hormone Treatment for Osteoporosis. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Seventh Edition. Washington D.C.: American Society for Bone and Mineral Research; 2008. pp. 244–249. [Google Scholar]
- 29.Hodsman AB, Fraher LJ, Ostbye T, Adachi JD, Steer BM. An evaluation of several biochemical markers for bone formation and resorption in a protocol utilizing cyclical parathyroid hormone and calcitonin therapy for osteoporosis. J Clin Inves. 1993;91:1138–1148. doi: 10.1172/JCI116273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobnig H, Turner RH. Effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology. 1997;138:4607–4612. doi: 10.1210/endo.138.11.5505. [DOI] [PubMed] [Google Scholar]
- 31.van der Horst G, Farih-Sips H, Lowik CW, Karperien M. Multiple mechanisms are involved in inhibition of osteoblast differentiation by PTHrP and PTH in KS483 Cells. J Bone Miner Res. 2005;20:2233–2244. doi: 10.1359/JBMR.050821. [DOI] [PubMed] [Google Scholar]
- 32.Ishizuya T, Yokose S, Hori M, Noda T, Suda T, Yoshiki S. Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest. 1997;99:2961–2970. doi: 10.1172/JCI119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YH, Liu Y, Buhl K, Rowe DW. Comparison of the action of transient and continuous PTH on primary osteoblast cultures expressing differentiation stage-specific GFP. J Bone Miner Res. 2005;20:5–14. doi: 10.1359/JBMR.041016. [DOI] [PubMed] [Google Scholar]
- 34.Lotinun S, Sibonga JD, Turner RT. Evidence that the cells responsible for marrow fibrosis in a rat model of hyperparathyroidism are pre-osteoblasts. Endocrinology. 2005;146:4074–4081. doi: 10.1210/en.2005-0480. PMID 15947001. [DOI] [PubMed] [Google Scholar]
- 35.Sowers M, Eyre D, Hollis BW, Randolph JF, Shapiro B, Jannausch ML, Crutchfield M. Biochemical markers of bone turnover in lactating and nonlactating postpartum women. J Clin Endocrinol Metab. 1995;80:2210–2216. doi: 10.1210/jcem.80.7.7608281. [DOI] [PubMed] [Google Scholar]
- 36.Csaba M, Bhattoa HP, Bettembuk P, Balogh A. The effects of pregnancy and lactation on hormonal status and biochemical markers of bone turnover. Eur J Obstet Gynecol Reprod Biol. 2003;106(2):209–213. doi: 10.1016/s0301-2115(02)00237-3. [DOI] [PubMed] [Google Scholar]
- 37.Kalkwarf HJ, Specker BL, Ho M. Effects of Calcium Supplementation on Calcium Homeostasis and bone Turnover in Lactating Women. J Clin Endocrinol and Metab. 1999;84(2):464–470. doi: 10.1210/jcem.84.2.5451. PubMed ID 1002402. [DOI] [PubMed] [Google Scholar]
- 38.Cross NA, Hillman LS, Allen SH, Krause GF. Changes in Bone Mineral Density and Markers of Bone Remodeling during Lactation and Postweaning in Women Consuming High Amounts of Calcium. J Bone Miner Res. 1995;10(9):1312–1320. doi: 10.1002/jbmr.5650100907. PubMed ID 7502702. [DOI] [PubMed] [Google Scholar]
- 39.Dempster DW, Parisien M, Silverbrg SJ, Liang X-G, Schnitzer M, Shen V, Shane E, Kimmel DB, Lindsay R, Bilezikian JP. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab. 1999;84:1562–1566. doi: 10.1210/jcem.84.5.5652. [DOI] [PubMed] [Google Scholar]
- 40.Prank K, Nowlan SJ, Harms HM, Kloppstech M, Brabant G, Hesch R-D. Time series prediction of parathyroid hormone concentration. J Clin Invest. 1995;95:2910–2919. doi: 10.1172/JCI117998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitt CP, Obry J, Feneberg R, Veldhuis J, Mehls O, Ritz B, Schaefer F. Beta adrenergic blockade augments pulsatile PTH secretion in humans. JASN. 2003;14:3245–3250. doi: 10.1097/01.asn.0000101240.47747.7f. [DOI] [PubMed] [Google Scholar]
- 42.Chapotot F, Gronfier C, Speigel K, Luthringer R, Brandenberger G. Relationships between intact PTH and 24-hour profiles, sleep-wake cycle, and sleep electroencephalographic activity in man. J Clin Endocrinol Metab. 1996;81:3759–3765. doi: 10.1210/jcem.81.10.8855835. [DOI] [PubMed] [Google Scholar]
- 43.Cauley JA, Palermo L, Vogt M, Ensrud KE, Ewing S, Hochberg M, Nevitt MC, Black DM. Prevalent verbral fractures in black women and white women. J Bone Miner Res. 2008;23:1458–1467. doi: 10.1359/JBMR.080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cosman F, Nieves J, Dempster D, Lindsay T. Vitamin D economy in blacks. J Bone Miner Res. 2007;22(Suppl 2):v34–v38. doi: 10.1359/jbmr.07s220. [DOI] [PubMed] [Google Scholar]